Hard Carbons Derived from Phenyl Hyper-Crosslinked Polymers for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures for Hyper-Crosslinked Polymers and Hard Carbon Materials
2.3. Structural Characterization
2.4. Electrochemical Measurement
3. Results
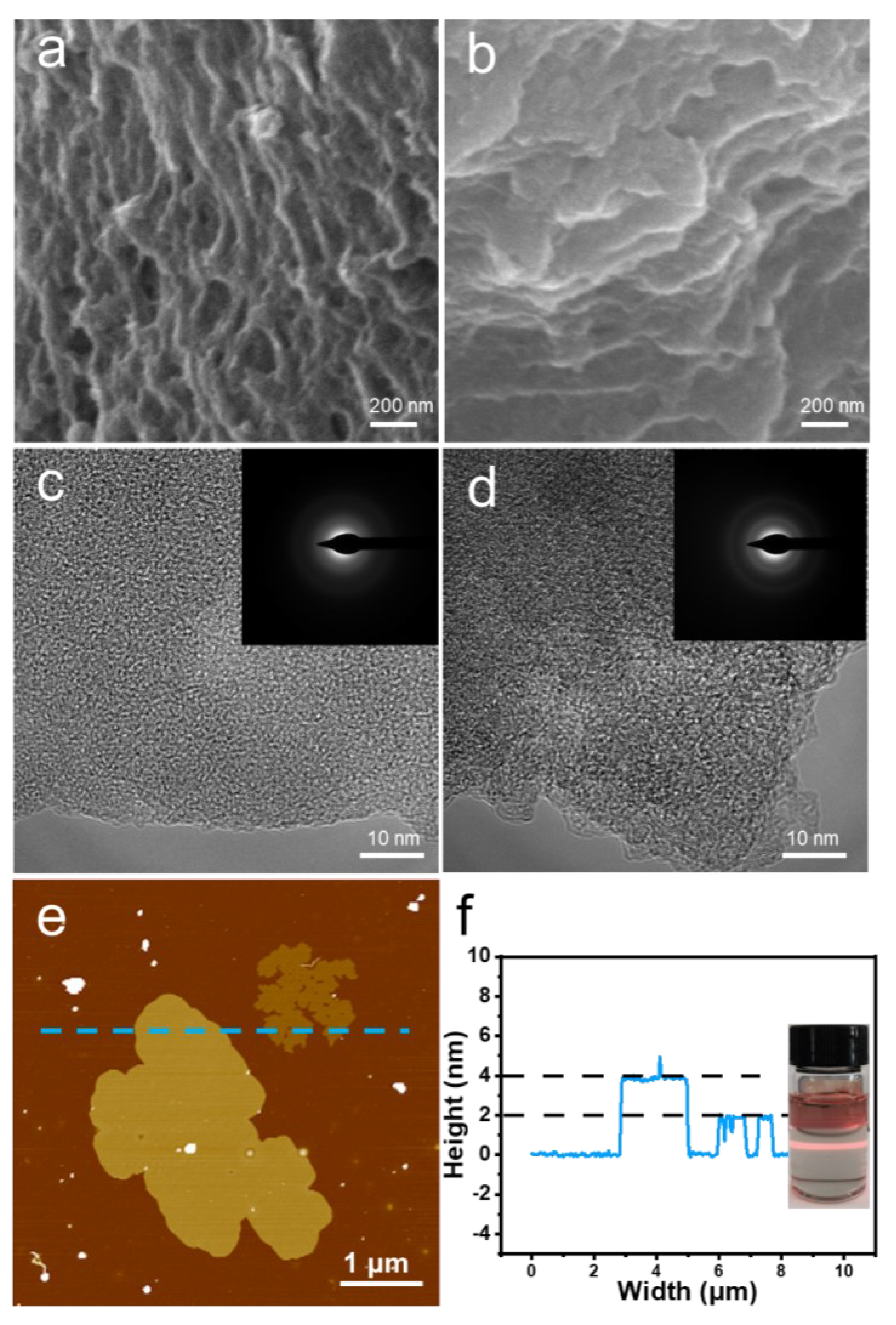
3.1. The Structure and Morphology Characterization of the Materials
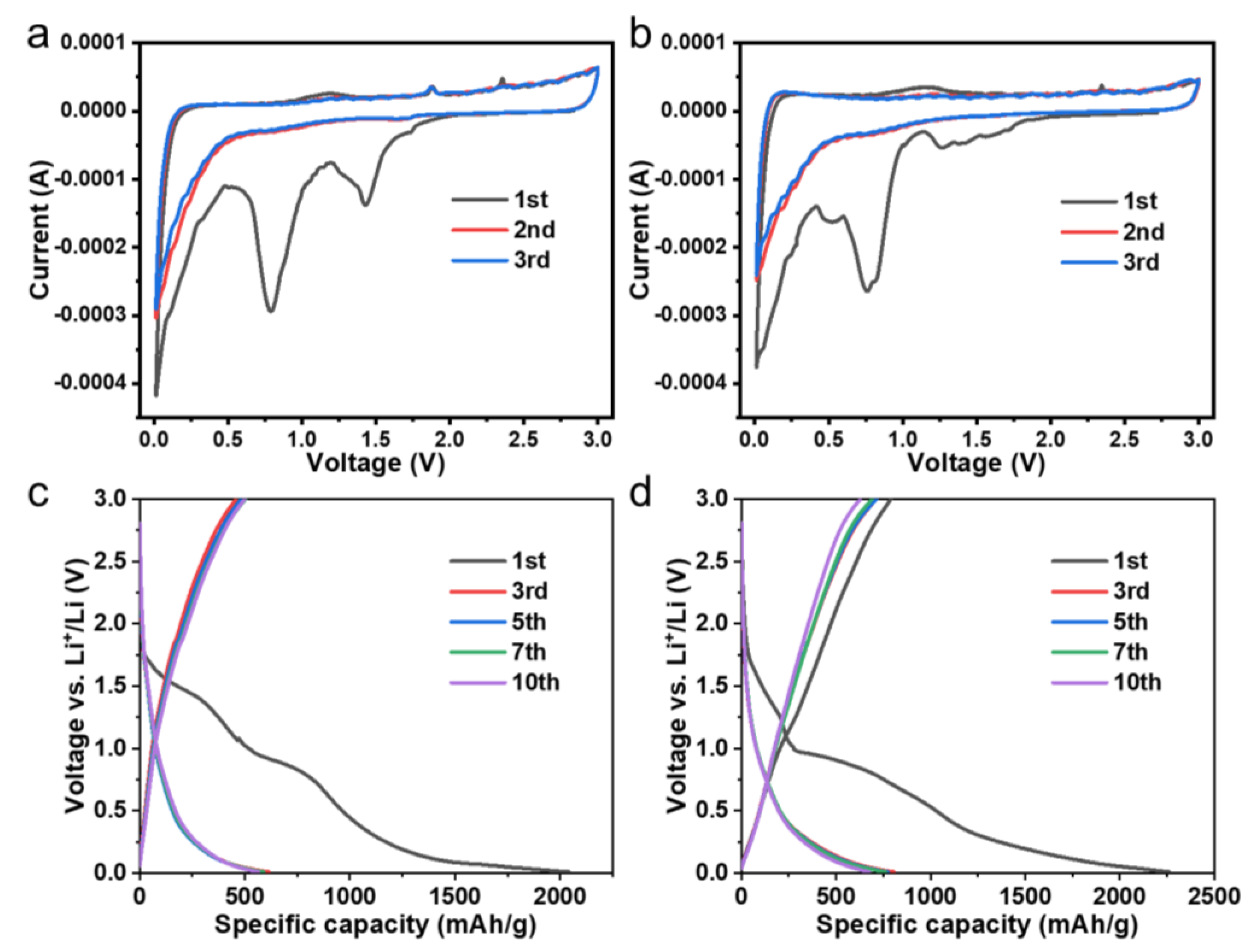
3.2. Li Ion Storage Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Liu, Q.; Fang, Y.; Teng, C.; Liu, X.; Fang, P.; Tong, Y.; Lu, X. Boosting Zn-Ion Energy Storage Capability of Hierarchically Porous Carbon by Promoting Chemical Adsorption. Adv. Mater. 2019, 31, 1904948. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Zhang, X.; Abdolhosseinzadeh, S.; Sheng, H.; Lan, W.; Pakdel, A.; Heier, J.; Nüesch, F. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes): Synthesis, Properties, and Electrochemical Energy Storage Applications. Energy Environ. Mater. 2020, 3, 29–55. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Zeng, S.; Zhou, L.; Xu, W.; Zheng, D.; Liu, J.; Zeng, Y.; Lu, X. An ultrathin defect-rich Co3O4 nanosheet cathode for high-energy and durable aqueous zinc ion batteries. J. Mater. Chem. A 2019, 7, 21678–21683. [Google Scholar] [CrossRef]
- Zhang, C.; Park, S.-H.; O’Brien, S.E.; Seral-Ascaso, A.; Liang, M.; Hanlon, D.; Krishnan, D.; Crossley, A.; McEvoy, N.; Coleman, J.N.; et al. Liquid exfoliation of interlayer spacing-tunable 2D vanadium oxide nanosheets: High capacity and rate handling Li-ion battery cathodes. Nano Energy 2017, 39, 151–161. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Pan, L.; Cullen, C.P.; Liu, Y.; Pakdel, A.; Long, D.; Yang, J.; McEvoy, N.; Duesberg, G.S.; et al. In Situ Formed Protective Barrier Enabled by Sulfur@Titanium Carbide (MXene) Ink for Achieving High-Capacity, Long Lifetime Li-S Batteries. Adv. Sci. 2018, 5, 1800502. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Schneider, R.; Verma, A.; Heier, J.; Nüesch, F.; Zhang, C. Turning Trash into Treasure: Additive Free MXene Sediment Inks for Screen-Printed Micro-Supercapacitors. Adv. Mater. 2020, 32, 2000716. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards practical lithium-metal anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S. Synthesis of hard carbon from argan shells for Na-ion batteries. J. Mater. Chem. A 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.-J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef] [PubMed]
- Beda, A.; Taberna, P.-L.; Simon, P.; Matei Ghimbeu, C. Hard carbons derived from green phenolic resins for Na-ion batteries. Carbon 2018, 139, 248–257. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Fujimoto, H.; Tokumitsu, K.; Mabuchi, A.; Chinnasamy, N.; Kasuh, T. The anode performance of the hard carbon for the lithium ion battery derived from the oxygen-containing aromatic precursors. J. Power Sources 2010, 195, 7452–7456. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Ni, J.; Huang, Y.; Gao, L. A high-performance hard carbon for Li-ion batteries and supercapacitors application. J. Power Sources 2013, 223, 306–311. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent Development of Hypercrosslinked Microporous Organic Polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, Q.; Yan, C.; Su, C.; Li, A. Nitrogen-doped porous carbon nanoparticle derived from nitrogen containing conjugated microporous polymer as high performance lithium battery anode. J. Alloys Compd. 2017, 714, 204–212. [Google Scholar] [CrossRef]
- Yang, X.; Wei, C.; Sun, C.; Li, X.; Chen, Y. High performance anode of lithium-ion batteries derived from an advanced carbonaceous porous network. J. Alloys Compd. 2017, 693, 777–781. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, R.; Wang, X.; Xu, Y.; Wang, F.; Ren, W.; Wang, Y.; Su, F.; Jiang, J.-X. Porous carbons derived from hypercrosslinked porous polymers for gas adsorption and energy storage. Carbon 2017, 114, 608–618. [Google Scholar] [CrossRef]
- Li, C.; Kong, D.; Wang, B.; Du, H.; Zhao, J.; Dong, Y.; Xie, Y. Conjugated microporous polymer derived N, O and S co-doped sheet-like carbon materials as anode materials for high-performance lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2022, 134, 104293. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Bai, Y.; Zhang, Y. Low temperature synthesis and superior lithium storage properties of fluorine-rich tubular porous carbon. J. Alloys Compd. 2022, 901, 163657. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Lian, F.; Xu, Z.; Wang, X. Synthesis and lithium storage properties of nitrogen-containing hard carbon from conjugated microporous polymers. Ionics 2022, 28, 2623–2633. [Google Scholar] [CrossRef]
- Wang, X.; Mu, P.; Zhang, C.; Chen, Y.; Zeng, J.; Wang, F.; Jiang, J.X. Control Synthesis of Tubular Hyper-Cross-Linked Polymers for Highly Porous Carbon Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 20779–20786. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Zhao, J.; Zhang, G.; Wang, J.; Zhang, D.; Li, K. Template-Free Synthesis of Honeycomblike Porous Carbon Rich in Specific 2–5 nm Mesopores from a Pitch-Based Polymer for a High-Performance Supercapacitor. ACS Sustain. Chem. Eng. 2018, 7, 2116–2126. [Google Scholar] [CrossRef]
- Xu, F.; Han, H.; Qiu, Y.; Zhang, E.; Repich, H.; Qu, C.; Yu, H.; Wang, H.; Kaskel, S. Facile regulation of carbon framework from the microporous to low-porous via molecular crosslinker design and enhanced Na storage. Carbon 2020, 167, 896–905. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, C.; Shu, Y.; Jiang, S.; Xia, Q.; Chen, L.; Jin, S.; Hussain, I.; Cooper, A.I.; Tan, B. Layered microporous polymers by solvent knitting method. Sci. Adv. 2017, 3, e1602610. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-L.; Huang, Y.-Q.; Tang, X.-Y.; Xu, J.; Peng, C.; Tan, Y.-Z. Two-dimensional extended π-conjugated triphenylene-core covalent organic polymer. J. Mater. Chem. A 2019, 7, 3066–3071. [Google Scholar] [CrossRef]
- Li, C.; Guo, X.; Du, H.; Zhao, J.; Liu, L.; Yuan, Q.; Fu, C. The synthesis of the D-A-type polymers containing benzo[1,2-b:6,5-b′]dithiophene-4,5-dione unit, their composites with carbon, and the lithium storage performances as electrode materials. J. Solid State Electrochem. 2021, 25, 1847–1859. [Google Scholar] [CrossRef]
- Piedboeuf, M.-L.C.; Job, N.; Aqil, A.; Busby, Y.; Fierro, V.; Celzard, A.; Detrembleur, C.; Léonard, A.F. Understanding the Influence of Surface Oxygen Groups on the Electrochemical Behavior of Porous Carbons as Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 36054–36065. [Google Scholar] [CrossRef]
- Ni, B.; Li, Y.; Chen, T.; Lu, T.; Pan, L. Covalent organic frameworks converted N, B co-doped carbon spheres with excellent lithium ion storage performance at high current density. J. Colloid Interface Sci. 2019, 542, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, J.; Huang, S.; Chen, Z.; Lu, C.; Yang, C.; Zhai, G.; Zhu, J.; Zhuang, X. Porphyrinic conjugated microporous polymer anode for Li-ion batteries. J. Power Sources 2022, 531, 231340. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Ma, Y.; Xu, Y.; Chang, G. Intermolecular channel expansion induced by cation-π interactions to enhance lithium storage in a crosslinked π-conjugated organic anode. J. Power Sources 2020, 449, 227551. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Gong, Z.; Xie, J.; Lu, T.; Pan, L. Nitrogen and sulfur co-doped vanadium carbide MXene for highly reversible lithium-ion storage. J. Colloid Interface Sci. 2021, 587, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhou, H. Towards sustainable and versatile energy storage devices: An overview of organic electrode materials. Energy Environ. Sci. 2013, 6, 2280–2301. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, W.; Cheng, A.; Li, Z.; Li, L.; Zhang, H. Novel hyper-crosslinked polymer anode for lithium-ion batteries with highly reversible capacity and long cycling stability. Electrochim. Acta 2018, 281, 162–169. [Google Scholar] [CrossRef]
- Jiang, G.; Qiu, Y.; Lu, Q.; Zhuang, W.; Xu, X.; Kaskel, S.; Xu, F.; Wang, H. Mesoporous Thin-Wall Molybdenum Nitride for Fast and Stable Na/Li Storage. ACS Appl. Mater. Interfaces 2019, 11, 41188–41195. [Google Scholar] [CrossRef]

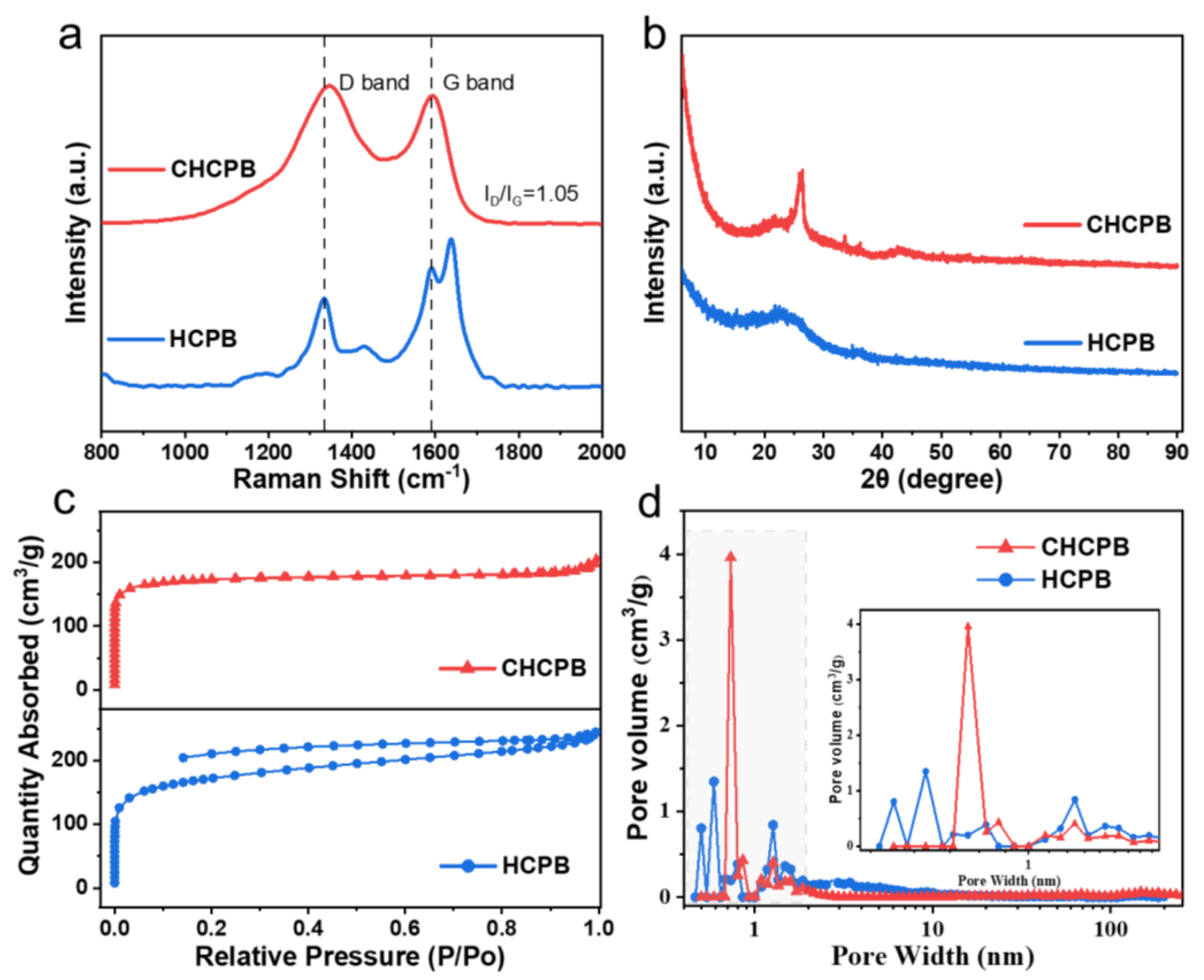


| Sample | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vtotal (cm3 g−1) |
|---|---|---|---|---|
| HCPB | 593 | 0.15 | 0.20 | 0.38 |
| CHCPB | 583 | 0.23 | 0.07 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Tian, X.; Song, Y.; Yang, T.; Ma, Z.; Gong, X.; Wang, C. Hard Carbons Derived from Phenyl Hyper-Crosslinked Polymers for Lithium-Ion Batteries. Coatings 2023, 13, 421. https://doi.org/10.3390/coatings13020421
Guo Z, Tian X, Song Y, Yang T, Ma Z, Gong X, Wang C. Hard Carbons Derived from Phenyl Hyper-Crosslinked Polymers for Lithium-Ion Batteries. Coatings. 2023; 13(2):421. https://doi.org/10.3390/coatings13020421
Chicago/Turabian StyleGuo, Ziyang, Xiaodong Tian, Yan Song, Tao Yang, Zihui Ma, Xiangjie Gong, and Chao Wang. 2023. "Hard Carbons Derived from Phenyl Hyper-Crosslinked Polymers for Lithium-Ion Batteries" Coatings 13, no. 2: 421. https://doi.org/10.3390/coatings13020421
APA StyleGuo, Z., Tian, X., Song, Y., Yang, T., Ma, Z., Gong, X., & Wang, C. (2023). Hard Carbons Derived from Phenyl Hyper-Crosslinked Polymers for Lithium-Ion Batteries. Coatings, 13(2), 421. https://doi.org/10.3390/coatings13020421






