Preparation and Super-Hydrophobic Mechanism Analysis of FAS-17-Modified SiO2/PDMS Coatings for High-Voltage Composite Insulators
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Instrument and Apparatus
2.3. Method
2.3.1. The Preparation of Nano-SiO2
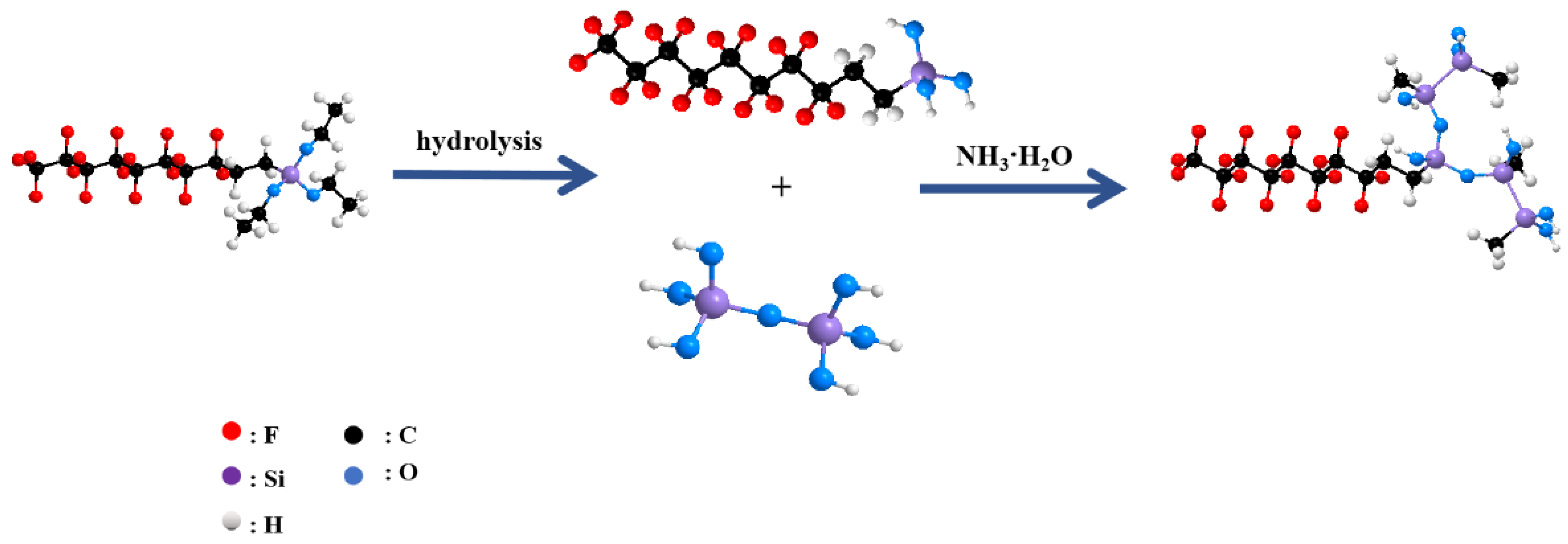
2.3.2. The Preparation of FAS-17-Modified Nano-SiO2
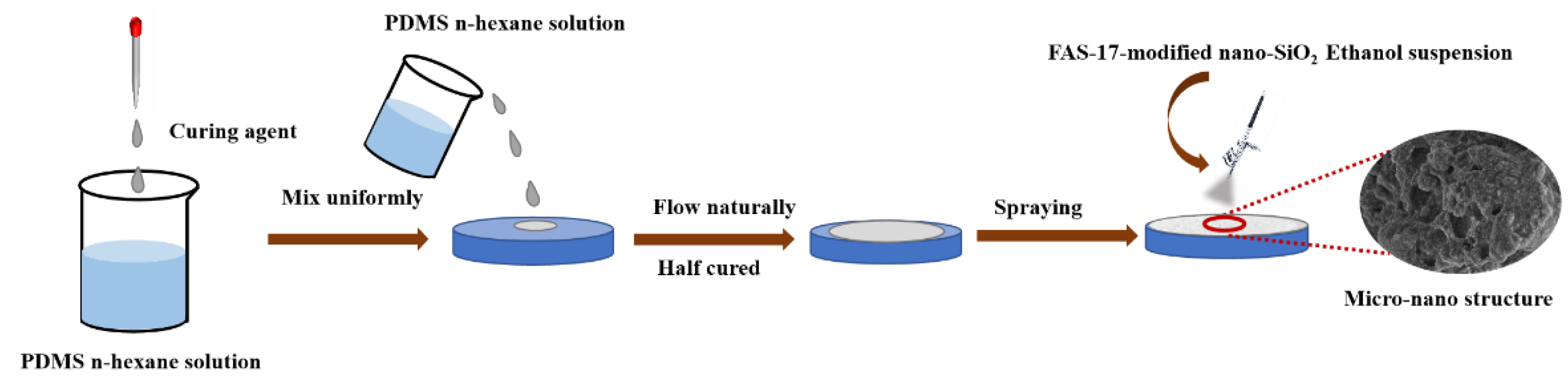
2.3.3. The Preparation of Super-Hydrophobic Coating
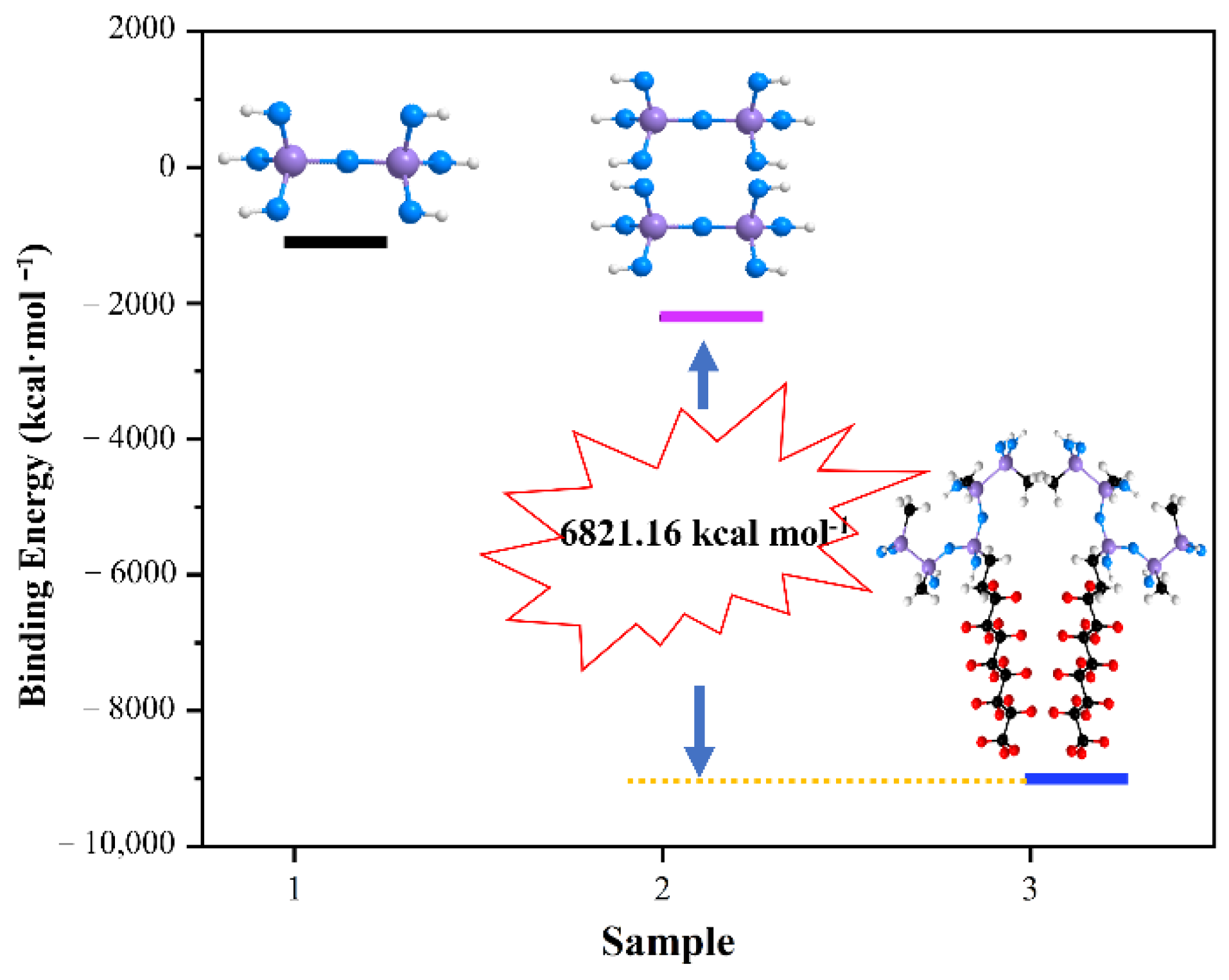
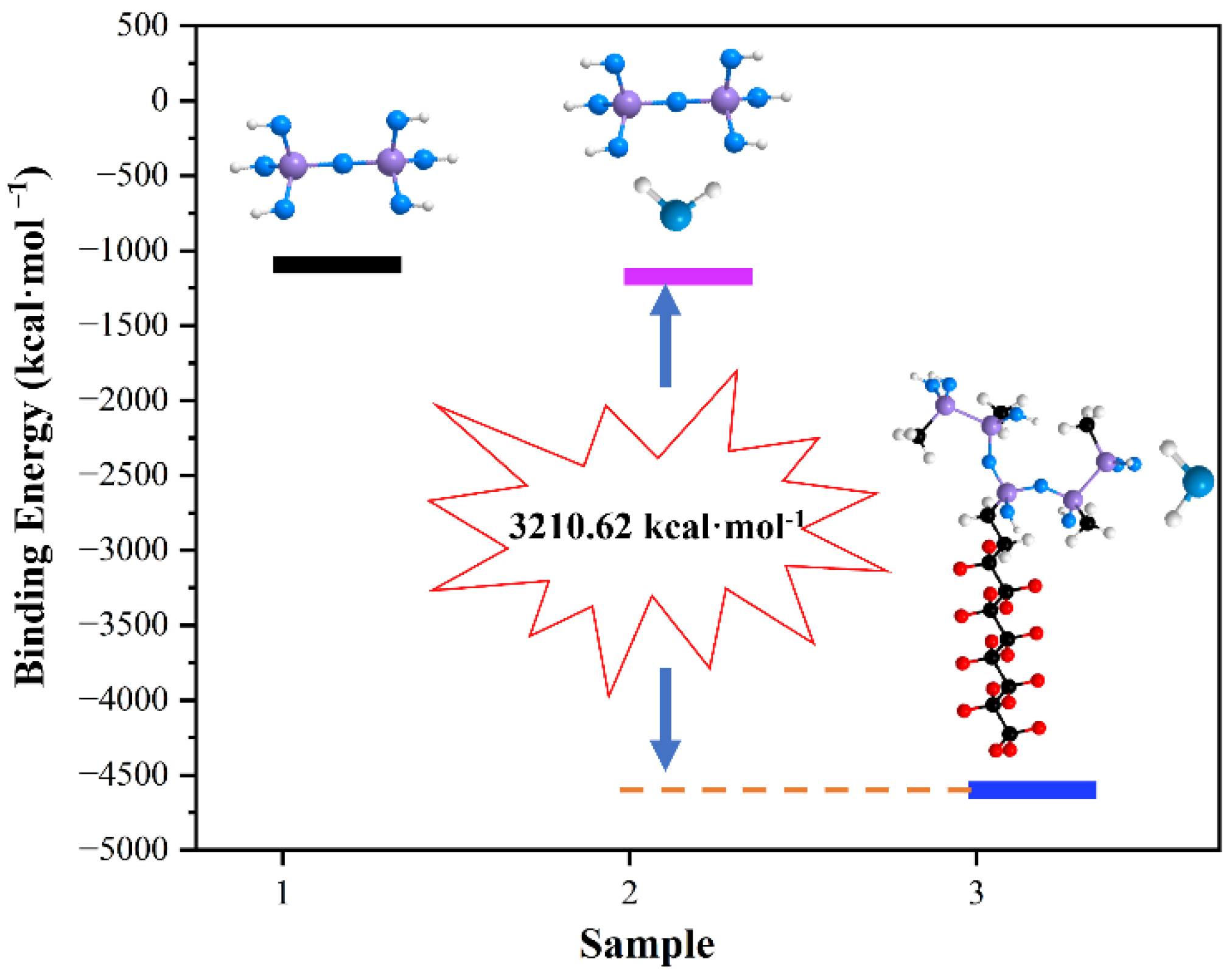
2.3.4. The Preparation to Calculate the Binding Energy
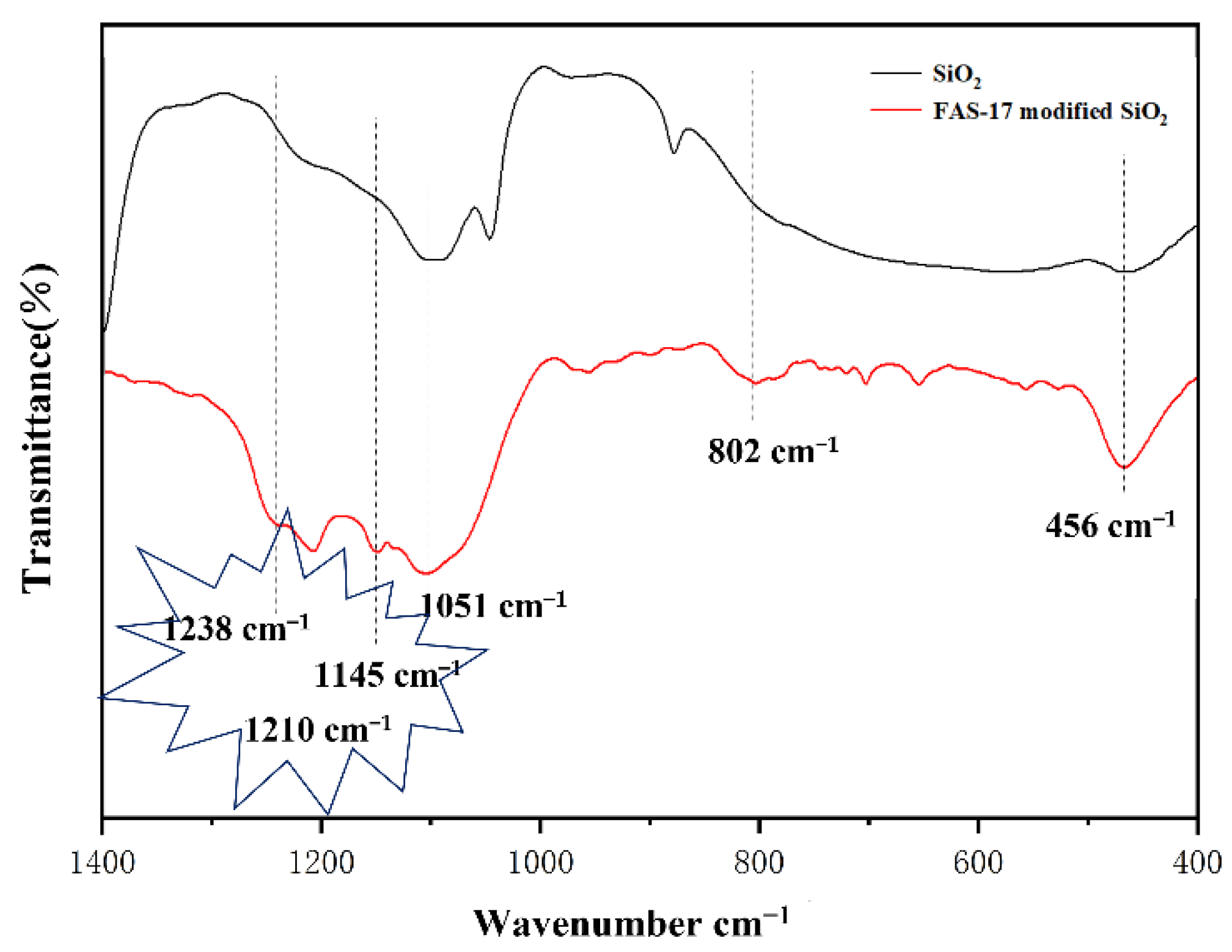
2.4. Characterization
2.5. Performance Testing
Power Grid Security Test
3. Results and Discussion
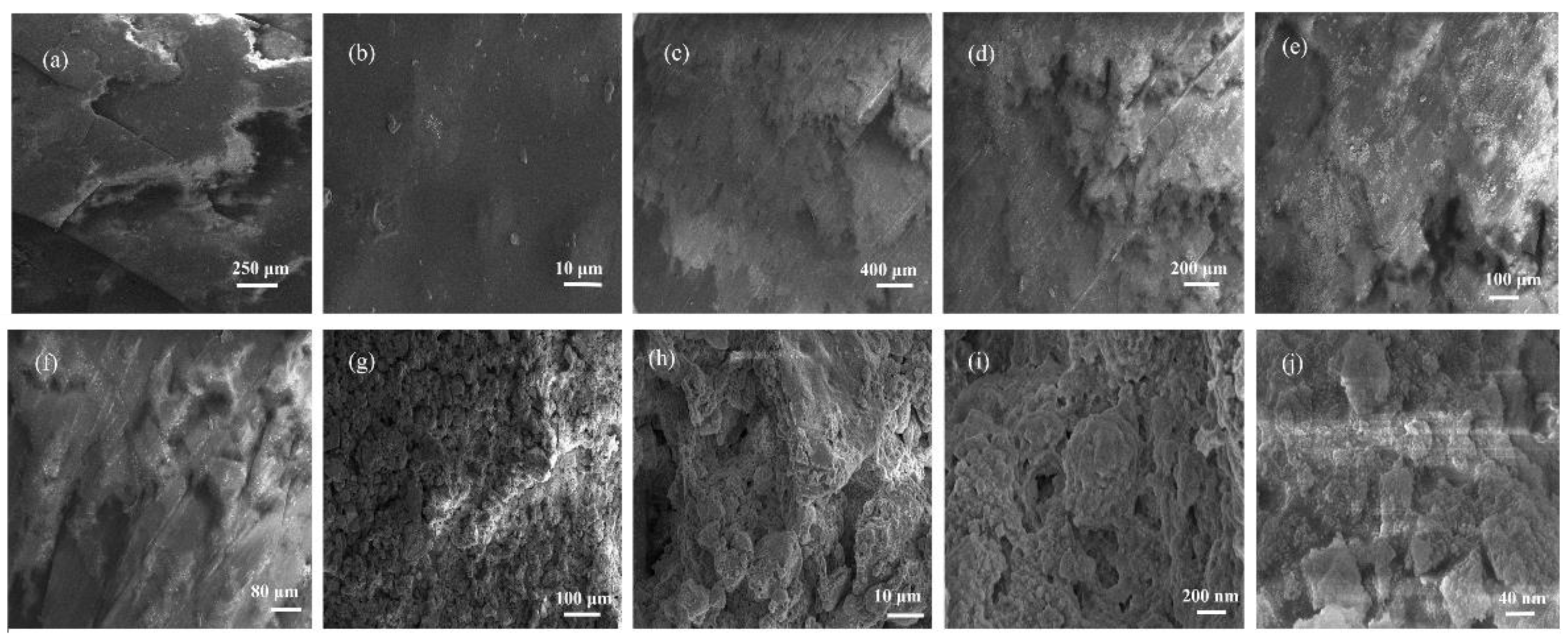
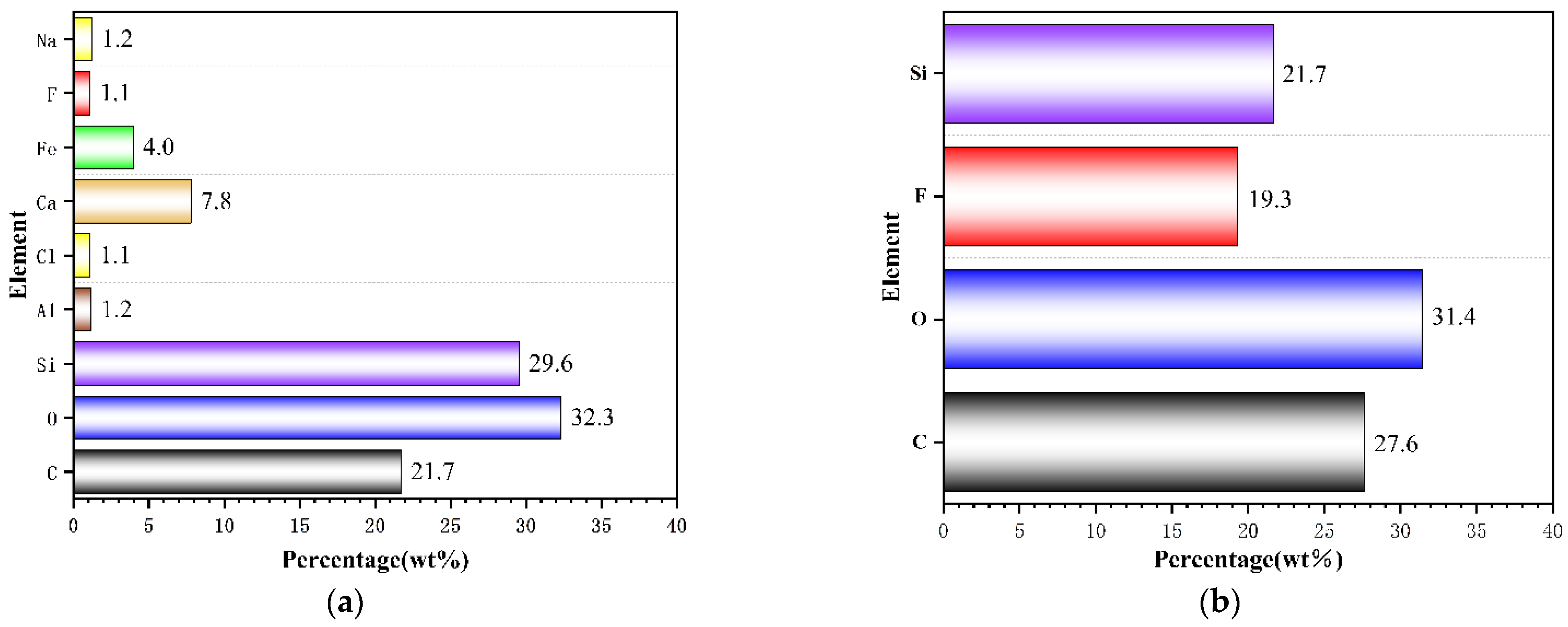
3.1. Analysis of Surface Morphology and Elemental Distribution
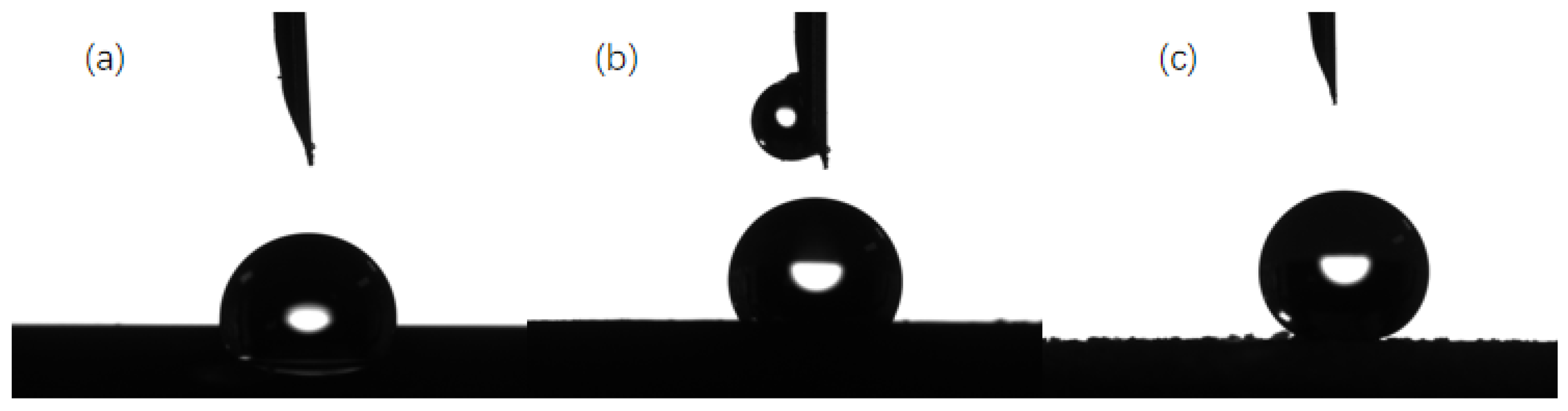
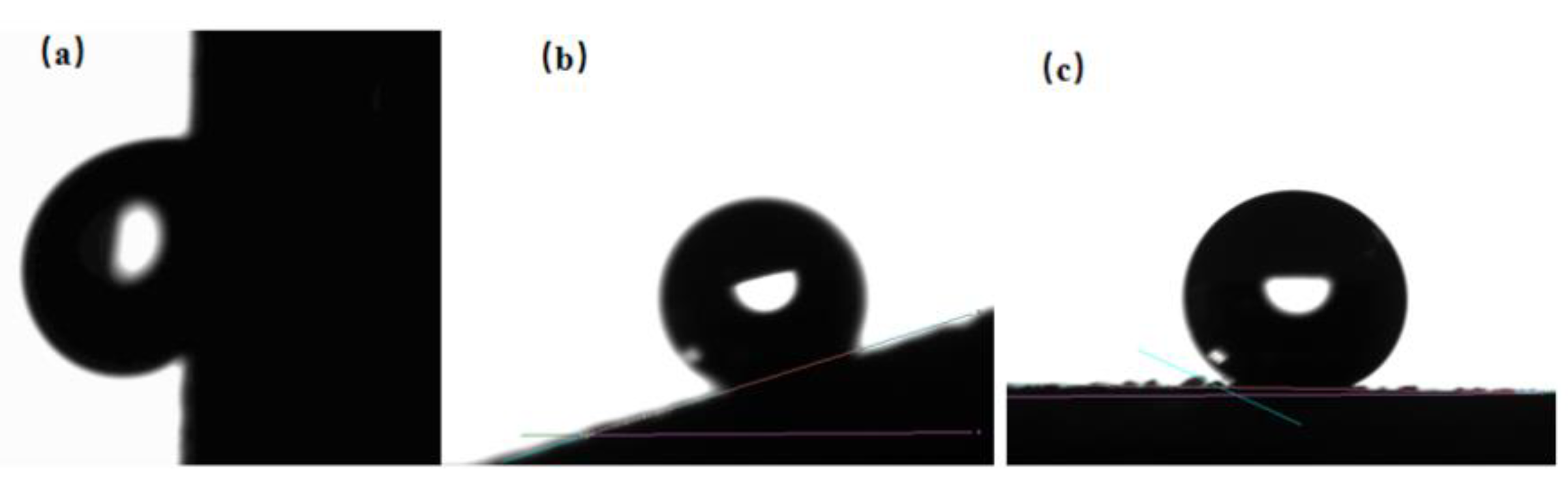
3.2. Analysis of Super-Hydrophobic Coating
3.3. SiO2 Agglomeration Mechanism and FAS-17-Modified Nano-SiO2 De-Agglomeration Mechanism
- (a)
- Particles approach the phase of motion;
- (b)
- Contact phase;
- (c)
- Agglomerative growth stage.
3.4. Super-Hydrophobic Mechanism
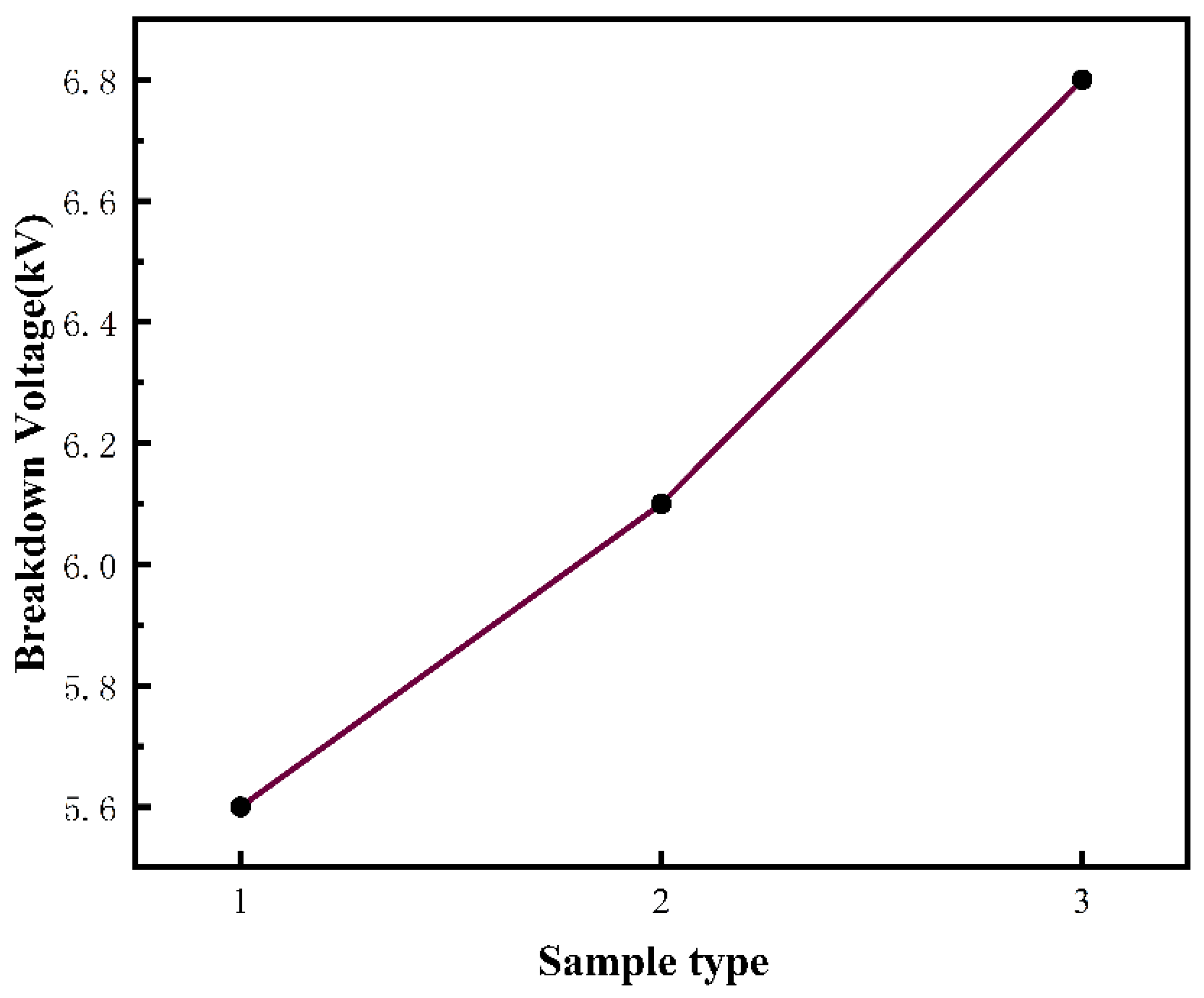
3.5. High-Voltage Safety Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Hu, Y.; Jiang, X.; Wu, Y.; Li, T.; Zhao, Y.; Rong, Y. Discharge development process and flashover performance of snow-accreted insulators under natural environment. Int. J. Electr. Power Energy Syst. 2023, 146, 108678. [Google Scholar] [CrossRef]
- Bahrami, A.; Yan, M.; Shahidehpour, M.; Pandey, S.; Vukojevic, A.; Paaso, E.A. Mobile and Portable De-Icing Devices for Enhancing the Distribution System Resilience Against Ice Storms: Preventive strategies for damage control. IEEE Electrif. Mag. 2021, 9, 120–129. [Google Scholar] [CrossRef]
- Gomila, D.; Carreras, B.A.; Reynolds-Barredo, J.M.; Colet, P.; Gomis-Bellmunt, O. Analysis of the blackout risk reduction when segmenting large power systems using lines with controllable power flow. Int. J. Electr. Power Energy Syst. 2023, 148, 108947. [Google Scholar] [CrossRef]
- Salem, A.A.; Lau, K.Y.; Abdul-Malek, Z.; Al-Gailani, S.A.; Tan, C.W. Flashover voltage of porcelain insulator under various pollution distributions: Experiment and modeling. Electr. Power Syst. Res. 2022, 208, 107867. [Google Scholar] [CrossRef]
- Wang, W.; Lu, H.; Li, C.; Deng, Y.; Lan, L.; Wen, X.; Luo, B.; Xiao, W. Study on the relationship between the characteristics of water droplets on the shed of composite insulators and the pollution flashover voltage. Energy Rep. 2022, 8, 1071–1077. [Google Scholar] [CrossRef]
- Li, Z.; Yin, F.; Cao, B.; Wang, L.; Shao, S.; Farzaneh, M. Pollution flashover performance of RTV coatings with partial damage. Int. J. Electr. Power Energy Syst. 2020, 121, 106102. [Google Scholar] [CrossRef]
- Mohammadnabi, S.; Rahmani, K. Influence of humidity and contamination on the leakage current of 230-kV composite insulator. Electr. Power Syst. Res. 2021, 194, 107083. [Google Scholar] [CrossRef]
- Liu, Y.; Zong, H.; Gao, S.; Du, B.X. Contamination deposition and discharge characteristics of outdoor insulators in fog-haze conditions. Int. J. Electr. Power Energy Syst. 2020, 121, 106176. [Google Scholar] [CrossRef]
- Mohammadi Savadkoohi, E.; Mirzaie, M.; Seyyedbarzegar, S.; Mohammadi, M.; Khodsuz, M.; Ghorbani Pashakolae, M.; Biazar Ghadikolaei, M. Experimental investigation on composite insulators AC flashover performance with fan-shaped non-uniform pollution under electro-thermal stress. Int. J. Electr. Power Energy Syst. 2020, 121, 106142. [Google Scholar] [CrossRef]
- Subba Reddy, B.; Ramamurthy, P.C. Analysis of in-service composite insulators used in overhead railway traction. Eng. Fail. Anal. 2020, 108, 104227. [Google Scholar] [CrossRef]
- Qiao, X.; Zhang, Z.; Jiang, X.; Sundararajan, R.; You, J. DC pollution flashover performance of HVDC composite insulator under different non-uniform pollution conditions. Electr. Power Syst. Res. 2020, 185, 106351. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Ma, Z.; Zhou, W.; Qin, Y. Numerical Analysis and Experimental Study of the Laser Cleaning of Ceramic Insulator Contamination. IEEE Access 2022, 10, 49285–49296. [Google Scholar] [CrossRef]
- Máša, V.; Horňák, D.; Petrilák, D. Industrial use of dry ice blasting in surface cleaning. J. Clean. Prod. 2021, 329, 129630. [Google Scholar] [CrossRef]
- Baghshahi, S.; Mohammadi, M.; Payrazm, S.; Aliabadizadeh, A. Hydrophobic nanocrystalline glazes based on cassiterite for self-cleaning outdoor power grid insulators. J. Eur. Ceram. Soc. 2021, 41, 5750–5754. [Google Scholar] [CrossRef]
- Plieva, M.; Gurieva, E.; Lysokon, E. Analysis of Different Modes of Cleaning Insulators of Air Transmission Lines in Mountain Conditions. In Proceedings of the 2020 International Ural Conference on Electrical Power Engineering (UralCon), Chelyabinsk, Russia, 22–24 September 2020. [Google Scholar]
- Mittal, T. Self-cleaning smart photocatalytic coatings for water treatment. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Lv, G.; Zhou, R.; Ma, R.; Hou, X.; Zhang, J.; Li, W. Fabrication of superhydrophilic self-cleaning SiO2–TiO2 coating and its photocatalytic performance. Ceram. Int. 2022, 48, 20033–20040. [Google Scholar] [CrossRef]
- Wang, K.; Yu, S.; Li, W.; Song, Y.; Gong, P.; Zhang, M.; Li, H.; Sun, D.; Yang, X.; Wang, X. Superhydrophobic and photocatalytic synergistic Self-Cleaning ZnS coating. Appl. Surf. Sci. 2022, 595, 153565. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Zou, Q.; Chen, J.; Zhao, X.; Xie, Y.; Hu, Y.; Yang, K. Abrasion mechanisms of superhydrophobic coating surfaces wetted in Wenzel state. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130585. [Google Scholar] [CrossRef]
- Chen, X.; Hu, L.; Du, Y. Anti-icing and anti-frost properties of structured superhydrophobic coatings based on aluminum honeycombs. Mater. Chem. Phys. 2022, 291, 126683. [Google Scholar] [CrossRef]
- Aparna, A.; Sethulekshmi, A.S.; Saritha, A.; Joseph, K. Recent advances in superhydrophobic epoxy based nanocomposite coatings and their applications. Prog. Org. Coat. 2022, 166, 106819. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A. Chapter 8-Superhydrophobic foul resistant and self-cleaning polymer coating. In Superhydrophobic Polymer Coatings; Samal, S.K., Mohanty, S., Nayak, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–203. [Google Scholar]
- He, G.; Wan, M.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of firm, superhydrophobic and antimicrobial PVDF@ZnO@TA@DT electrospun nanofibrous membranes for emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130962. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.-B.; Yang, W.; Zhao, Q.; Xu, Z.-M.; Yan, W.-M. Fabrication of stable and versatile superhydrophobic PTFE coating by simple electrodeposition on metal surface. Prog. Org. Coat. 2022, 172, 107090. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, H.; Wei, B.; Deng, C.; Bai, Y. Fabrication of UV-curable Anti-fouling coating based on fluorinated polyoxetane and long Side-Chain Polysilcone. Eur. Polym. J. 2022, 172, 111227. [Google Scholar] [CrossRef]
- Gupta, R.; Verma, R.; Kango, S.; Constantin, A.; Kharia, P.; Saini, R.; Kudapa, V.K.; Mittal, A.; Prakash, J.; Chamoli, P. A critical review on recent progress, open challenges, and applications of corrosion-resistant superhydrophobic coating. Mater. Today Commun. 2023, 34, 105201. [Google Scholar] [CrossRef]
- Singh, A.K. Surface engineering using PDMS and functionalized nanoparticles for superhydrophobic coatings: Selective liquid repellence and tackling COVID-19. Prog. Org. Coat. 2022, 171, 107061. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R.; Liu, J.; Zhang, Q. Robust PFMA/CNTs composite PDMS superhydrophobic film via SI-CuCRP method for efficient anti-icing. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130913. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, G.; Tong, Q.; Yang, W.; Hao, W. Fluorine-free superhydrophobic coatings from polydimethylsiloxane for sustainable chemical engineering: Preparation methods and applications. Chem. Eng. J. 2021, 426, 130829. [Google Scholar] [CrossRef]
- Armugam, A.; Teong, S.P.; Lim, D.S.W.; Chan, S.P.; Yi, G.; Yew, D.S.; Beh, C.W.; Zhang, Y. Broad spectrum antimicrobial PDMS-based biomaterial for catheter fabrication. Biomater. Res. 2021, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, N.; Li, J.; Wu, H.; Izuchukwu, N.K.; Ahmed, S.; Han, E.-H.; Liu, F. Enhanced anti-icing and anticorrosion properties of nano-SiO2 composite superhydrophobic coating constructed by a large-scale micropillar array approach. Prog. Org. Coat. 2023, 175, 107324. [Google Scholar] [CrossRef]
- Liang, T.; Guo, X.; Yuan, B.; Kong, S.; Huang, H.; Fu, D.; Zhang, F.; Xu, J.; Li, X. Design of functionalized α-Fe2O3 (III) films with long-term anti-wetting properties. Ceram. Int. 2020, 46, 6129–6135. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.; Wang, Y.; Li, Q.; Cui, S.; Nie, Z.; Wei, Q. Growing nearly vertically aligned ZnO nanorod arrays on porous α-Al2O3 membranes to enhance the separation of MTBE from aqueous solution. Sep. Purif. Technol. 2023, 311, 123239. [Google Scholar] [CrossRef]
- Khaled, U.; Beroual, A. Lightning impulse breakdown voltage of synthetic and natural ester liquids-based Fe3O4, Al2O3 and SiO2 nanofluids. Alex. Eng. J. 2020, 59, 3709–3713. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, J.; Chen, X.; Zhao, X.; Deng, J.; Zhang, G. Molecular-dynamics study on the thermodynamic properties of nano-SiO2 particle-doped silicone rubber composites. Comput. Mater. Sci. 2022, 212, 111571. [Google Scholar] [CrossRef]
- Liu, M.; Tan, X.; Li, X.; Geng, J.; Han, M.; Wei, K.; Chen, X. Transparent superhydrophobic EVA/SiO2/PTFE/KH-570 coating with good mechanical robustness, chemical stability, self-cleaning effect and anti-icing property fabricated by facile dipping method. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130624. [Google Scholar] [CrossRef]
- Du, J.; Yuan, H.; Xia, H.; Kou, H.; Zhang, Y.; Xing, W.; Zhang, C. Improvement of superhydrophobicity and durability of EP+PDMS/SiO2 composite coatings by adjusting laser curing powers. Mater. Chem. Phys. 2022, 289, 126428. [Google Scholar] [CrossRef]
- Dai, X.; Wei, Q.; Wang, Y.; Li, Q.; Cui, S.; Nie, Z. A novel strategy to enhance the desalination stability of FAS (fluoroalkylsilane)-modified ceramic membranes via constructing a porous SiO2@PDMS (polydimethylsiloxane) protective layer on their top. Chem. Eng. J. 2022, 435, 134757. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Biserova-Tahchieva, A.; Sansa-López, V.; Llorca-Isern, N. Superhydrophobic PDMS coated 304 stainless-steel mesh for the removal of HDPE microplastics. Prog. Org. Coat. 2022, 170, 107009. [Google Scholar] [CrossRef]
- Yang, Y.; Shan, L.; Shen, H.; Qiu, J. Manufacturing of robust superhydrophobic Wood surfaces based on PEG–Functionalized SiO2/PVA/PAA/Fluoropolymer hybrid transparent coating. Prog. Org. Coat. 2021, 154, 106186. [Google Scholar] [CrossRef]
- Sivolapov, P.; Myronyuk, O.; Baklan, D. Synthesis of Stober silica nanoparticles in solvent environments with different Hansen solubility parameters. Inorg. Chem. Commun. 2022, 143, 109769. [Google Scholar] [CrossRef]
- Kolb, C.G.; Lehmann, M.; Kulmer, D.; Zaeh, M.F. Modeling of the stability of water-based graphite dispersions using polyvinylpyrrolidone on the basis of the DLVO theory. Heliyon 2022, 8, e11988. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ran, B.; Liu, L. Determining the interaction energy of a quartz–kaolinite system at different pH levels by atomic force microscopy and extended DLVO theory. Powder Technol. 2022, 409, 117842. [Google Scholar] [CrossRef]
- de Lima, S.L.S.; Miguel, V.M.; Rosado, T.F.; Petri, M.V.; Gardener, J.; Avillez, R.; Rodrigues, T.S.; de Torresi, S.I.C.; Solorzano, G.; da Silva, A.G.M. Sized-controlled Pd nanoflowers by a non-classical growth mechanism combining the LaMer and DLVO theories and their catalytic activities. Mater. Today Commun. 2022, 33, 104397. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Shi, X.; Zhang, Z.; Wang, H.; Feng, L. SiO2 nanoparticle-based superhydrophobic spray and multi-functional surfaces by a facile and scalable method. Ceram. Int. 2019, 45, 15741–15744. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, H.; Mao, H.; Duan, M.; Wang, K.; Bao, N.; Zhao, Z.-P.; Li, H. Silica hollow spheres-based superhydrophobic PDMS composite membrane for enhanced acetone permselective pervaporation. Sep. Purif. Technol. 2023, 304, 122041. [Google Scholar] [CrossRef]

| Symbol | Meaning of the Symbols |
|---|---|
| A | Hamaker constant |
| R | Radius of the particles |
| εo | Permittivity of vacuum |
| ε | Dielectric constant of the solvent |
| ψ | Stern potential |
| k | Debye constant |
| zi | Charge number of the ionic species |
| i | Elementary charge |
| ci | Concentration of ion i (co- or counter-ions) at x = ∞ (in the bulk solution) |
| kB | Boltzmann constant |
| T | Temperature of the system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Dou, P.; Zhao, R.; Shi, Y.; Fu, G.; Shen, B. Preparation and Super-Hydrophobic Mechanism Analysis of FAS-17-Modified SiO2/PDMS Coatings for High-Voltage Composite Insulators. Coatings 2023, 13, 563. https://doi.org/10.3390/coatings13030563
Li C, Dou P, Zhao R, Shi Y, Fu G, Shen B. Preparation and Super-Hydrophobic Mechanism Analysis of FAS-17-Modified SiO2/PDMS Coatings for High-Voltage Composite Insulators. Coatings. 2023; 13(3):563. https://doi.org/10.3390/coatings13030563
Chicago/Turabian StyleLi, Chengqian, Peng Dou, Ruyi Zhao, Yurou Shi, Gaojie Fu, and Bin Shen. 2023. "Preparation and Super-Hydrophobic Mechanism Analysis of FAS-17-Modified SiO2/PDMS Coatings for High-Voltage Composite Insulators" Coatings 13, no. 3: 563. https://doi.org/10.3390/coatings13030563
APA StyleLi, C., Dou, P., Zhao, R., Shi, Y., Fu, G., & Shen, B. (2023). Preparation and Super-Hydrophobic Mechanism Analysis of FAS-17-Modified SiO2/PDMS Coatings for High-Voltage Composite Insulators. Coatings, 13(3), 563. https://doi.org/10.3390/coatings13030563





