Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition
Abstract
:1. Introduction
2. Experiments
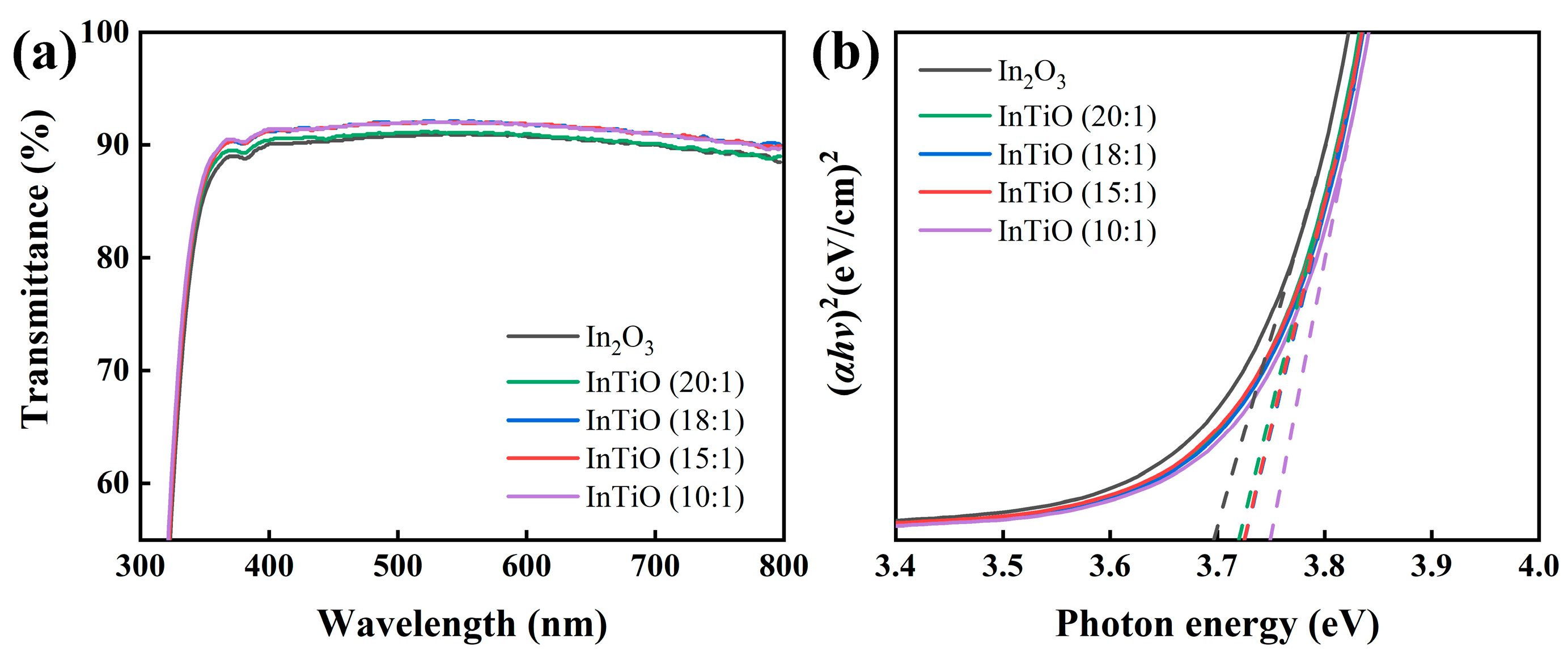
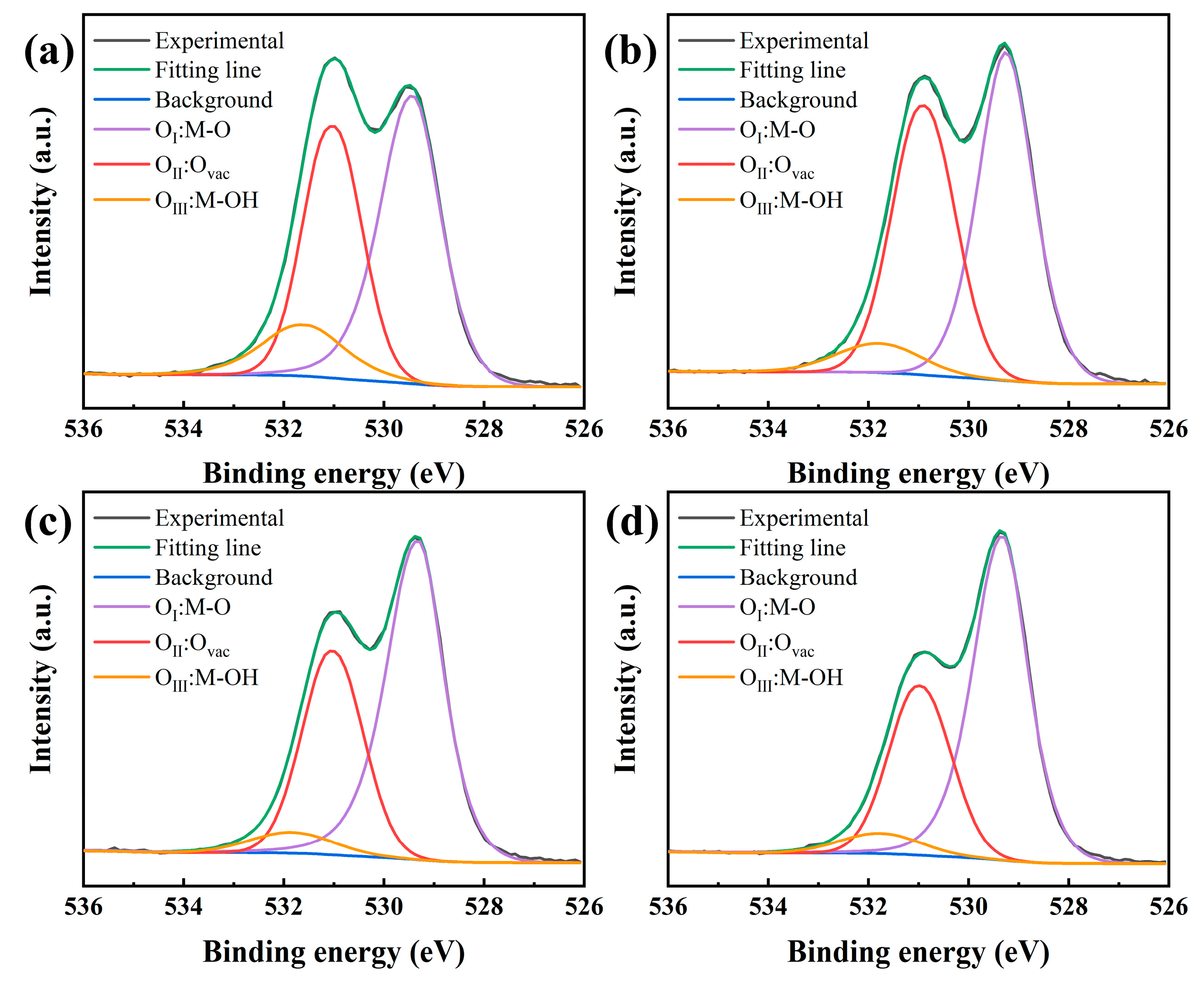
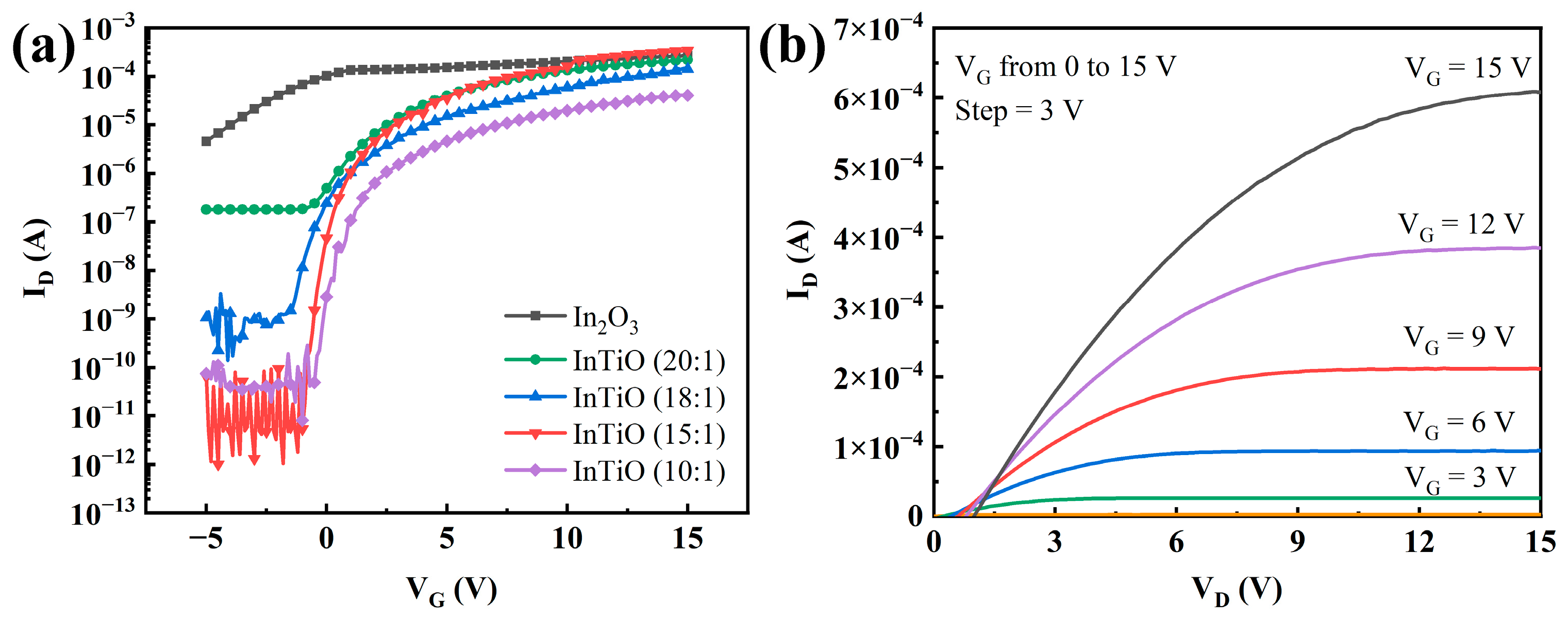
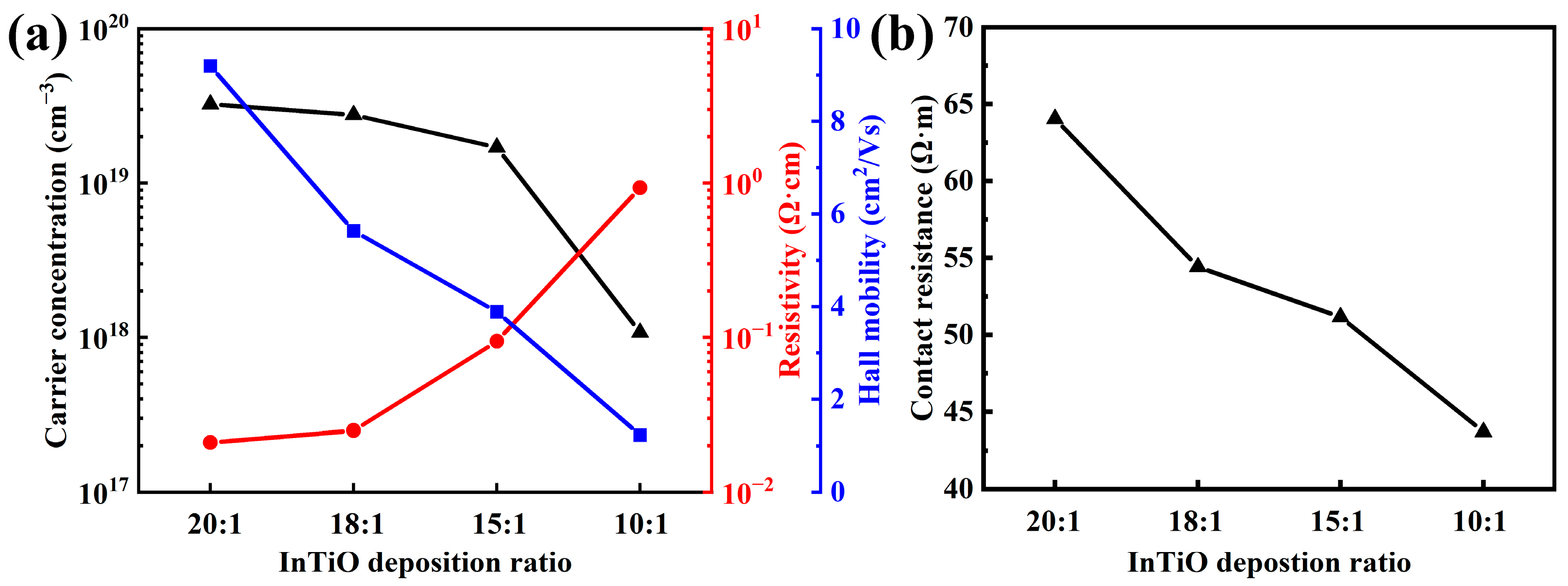
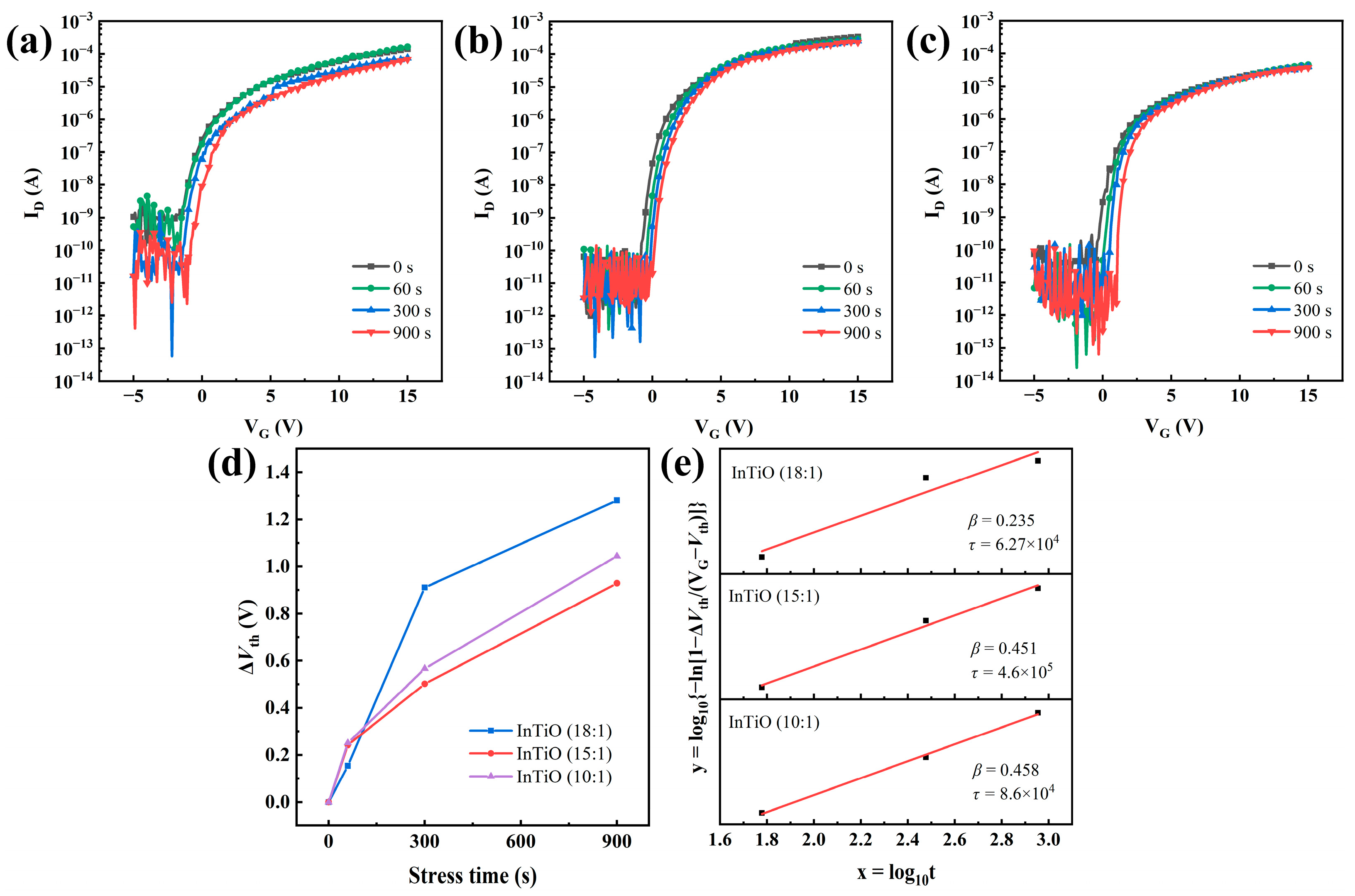
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellmer, K. Past achievements and future challenges in the development of optically transparent electrodes. Nat. Photonics 2012, 6, 809–817. [Google Scholar] [CrossRef]
- Liu, A.; Liu, G.; Shan, F.; Zhu, H.; Xu, S.; Liu, J.; Shin, B.; Lee, W. Room-temperature fabrication of ultra-thin ZrOx dielectric for high-performance InTiZnO thin-film transistors. Curr. Appl. Phys. 2014, 14, S39–S43. [Google Scholar] [CrossRef]
- Arai, T. Oxide-TFT technologies for next-generation AMOLED displays. J. Soc. Inf. Disp. 2012, 20, 156–161. [Google Scholar] [CrossRef]
- Weiher, R.; Ley, R. Optical properties of indium oxide. J. Appl. Phys. 1966, 37, 299–302. [Google Scholar] [CrossRef]
- Bel Hadj Tahar, R.; Ban, T.; Ohya, Y.; Takahashi, Y. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.; Kim, J.; Yun, S.J.; Jung, K.H.; Park, M.A. Photoinduced Synaptic Behavior of InxTiyO Thin Film Transistors. Adv. Electron. Mater. 2021, 7, 2001049. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fu, L.; Ouyang, J.; Yang, H. 3D ordered macro–mesoporous indium doped Al2O3. CrystEngComm 2013, 15, 6046–6053. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, T.; Avis, C.; Lee, S.-H.; Jang, J. Stable and high-performance indium oxide thin-film transistor by Ga doping. IEEE Trans. Electron Devices 2016, 63, 1078–1084. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Li, J.; Zhong, D.-Y.; Huang, C.-X.; Zhang, J.-H.; Li, X.-F.; Jiang, X.-Y.; Zhang, Z.-L. Mg Doping to Simultaneously Improve the Electrical Performance and Stability of MgInO Thin-Film Transistors. IEEE Trans. Electron Devices 2017, 64, 2216–2220. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, H.-K. Characteristics of sputtered Al-doped ZnO films for transparent electrodes of organic thin-film transistor. Thin Solid Film. 2011, 519, 8018–8022. [Google Scholar] [CrossRef]
- Li, Z.; Meng, Y.; Wang, C.; Cui, Y.; Yao, Z.; Shin, B.; Liu, G.; Shan, F. Enhancement-mode field-effect transistors based on Ti-doped In2O3 nanowires fabricated by electrospinning. J. Phys. D Appl. Phys. 2019, 52, 225102. [Google Scholar] [CrossRef]
- Kim, J.-H.; Son, D.-H.; Park, S.-N.; Kim, D.-H.; Sung, S.-J.; Jung, E.-A.; Ha, K.; Kang, J.-K. Effects of Ti addition on sol-gel derived InO and InZnO thin film transistors. Curr. Appl. Phys. 2012, 12, e24–e28. [Google Scholar] [CrossRef]
- Sheng, J.; Han, K.-L.; Hong, T.; Choi, W.-H.; Park, J.-S. Review of recent progresses on flexible oxide semiconductor thin film transistors based on atomic layer deposition processes. J. Semicond. 2018, 39, 011008. [Google Scholar] [CrossRef]
- Ding, X.; Yang, J.; Qin, C.; Yang, X.; Ding, T.; Zhang, J. Nitrogen-doped ZnO film fabricated via rapid low-temperature atomic layer deposition for high-performance ZnON transistors. IEEE Trans. Electron Devices 2018, 65, 3283–3290. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Janicek, P.; Nandi, D.K.; Slang, S.; Bouska, M.; Oh, H.; Shong, B.; Kim, S.-H. Low-temperature growth of crystalline Tin (II) monosulfide thin films by atomic layer deposition using a liquid divalent tin precursor. Appl. Surf. Sci. 2021, 565, 150152. [Google Scholar] [CrossRef]
- Bak, Y.G.; Park, J.W.; Park, Y.J.; Ansari, M.Z.; NamGung, S.; Cho, B.Y.; Kim, S.-H.; Lee, H.Y. In-Zn-Sn-O thin film based transistor with high-k HfO2 dielectric. Thin Solid Film. 2022, 753, 139290. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Wu, Q.; Dussarrat, C.; Qi, J.; Zhu, W.; Ding, X.; Zhang, J. High-performance 1-V ZnO thin-film transistors with ultrathin, ALD-processed ZrO2 gate dielectric. IEEE Trans. Electron Devices 2019, 66, 3382–3386. [Google Scholar] [CrossRef]
- Sheng, J.; Hong, T.; Lee, H.-M.; Kim, K.; Sasase, M.; Kim, J.; Hosono, H.; Park, J.-S. Amorphous IGZO TFT with high mobility of∼ 70 cm2/(V s) via vertical dimension control using PEALD. ACS Appl. Mater. Interfaces 2019, 11, 40300–40309. [Google Scholar] [CrossRef]
- Park, H.-W.; Park, K.; Kwon, J.-Y.; Choi, D.; Chung, K.-B. Effect of active layer thickness on device performance of tungsten-doped InZnO thin-film transistor. IEEE Trans. Electron Devices 2016, 64, 159–163. [Google Scholar] [CrossRef]
- Abliz, A.; Huang, C.-W.; Wang, J.; Xu, L.; Liao, L.; Xiao, X.; Wu, W.-W.; Fan, Z.; Jiang, C.; Li, J. Rational design of ZnO: H/ZnO bilayer structure for high-performance thin-film transistors. ACS Appl. Mater. Interfaces 2016, 8, 7862–7868. [Google Scholar] [CrossRef] [PubMed]
- Rim, Y.S.; Chen, H.; Zhu, B.; Bae, S.H.; Zhu, S.; Li, P.J.; Wang, I.C.; Yang, Y. Interface engineering of metal oxide semiconductors for biosensing applications. Adv. Mater. Interfaces 2017, 4, 1700020. [Google Scholar] [CrossRef]
- Kamarulzaman, N.; Kasim, M.F.; Rusdi, R. Band gap narrowing and widening of ZnO nanostructures and doped materials. Nanoscale Res. Lett. 2015, 10, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.T.; Renault, O.; Aventurier, B.; Rodriguez, G.; Barnes, J.P.; Templier, F. Analysis of IGZO thin-film transistors by XPS and relation with electrical characteristics. J. Disp. Technol. 2013, 9, 770–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Yang, J.; Ding, X.; Zhang, J. Solution-processed yttrium-doped IZTO semiconductors for high-stability thin film transistor applications. IEEE Trans. Electron Devices 2019, 66, 5170–5176. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.; Zhang, Y.; Che, B.; Ding, X.; Zhang, J. Improved gate bias stressing stability of IGZO thin film transistors using high-k compounded ZrO2/HfO2 nanolaminate as gate dielectric. Mol. Cryst. Liq. Cryst. 2018, 676, 65–71. [Google Scholar] [CrossRef]
- Liu, G.; Liu, A.; Zhu, H.; Shin, B.; Fortunato, E.; Martins, R.; Wang, Y.; Shan, F. Low-Temperature, Nontoxic Water-Induced Metal-Oxide Thin Films and Their Application in Thin-Film Transistors. Adv. Funct. Mater. 2015, 25, 2564–2572. [Google Scholar] [CrossRef]
- Kamiya, T.; Hosono, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Du Ahn, B.; Shin, H.S.; Kim, H.J.; Park, J.-S.; Jeong, J.K. Comparison of the effects of Ar and H 2 plasmas on the performance of homojunctioned amorphous indium gallium zinc oxide thin film transistors. Appl. Phys. Lett. 2008, 93, 203506. [Google Scholar] [CrossRef]
- Gamerith, S.; Klug, A.; Scheiber, H.; Scherf, U.; Moderegger, E.; List, E.J. Direct ink-jet printing of Ag–Cu nanoparticle and Ag-precursor based electrodes for OFET applications. Adv. Funct. Mater. 2007, 17, 3111–3118. [Google Scholar] [CrossRef]
- John, R.A.; Chien, N.A.; Shukla, S.; Tiwari, N.; Shi, C.; Ing, N.G.; Mathews, N. Low-temperature chemical transformations for high-performance solution-processed oxide transistors. Chem. Mater. 2016, 28, 8305–8313. [Google Scholar] [CrossRef]


| Devices | Vth (V) | μ (cm2/Vs) | Ion/Ioff | SS (V/dec.) |
|---|---|---|---|---|
| InTiO (20:1) | −1.12 | 9.38 | 103 | 1.43 |
| InTiO (18:1) | −0.52 | 8.73 | 105 | 0.74 |
| InTiO (15:1) | 0.17 | 7.69 | 107 | 0.13 |
| InTiO (10:1) | 0.29 | 1.26 | 105 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Li, P.; Chen, Z.; Xu, H.; Fu, C.; Ding, X.; Zhang, J. Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition. Coatings 2023, 13, 605. https://doi.org/10.3390/coatings13030605
Yang B, Li P, Chen Z, Xu H, Fu C, Ding X, Zhang J. Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition. Coatings. 2023; 13(3):605. https://doi.org/10.3390/coatings13030605
Chicago/Turabian StyleYang, Bing, Pingping Li, Zihui Chen, Haiyang Xu, Chaoying Fu, Xingwei Ding, and Jianhua Zhang. 2023. "Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition" Coatings 13, no. 3: 605. https://doi.org/10.3390/coatings13030605
APA StyleYang, B., Li, P., Chen, Z., Xu, H., Fu, C., Ding, X., & Zhang, J. (2023). Effect of Titanium Cation Doping on the Performance of In2O3 Thin Film Transistors Grown via Atomic Layer Deposition. Coatings, 13(3), 605. https://doi.org/10.3390/coatings13030605





