Abstract
This paper underlined how the naturally occurring amygdalin (Amy) and raffinose (Raf) can inhibit the corrosion of aluminum in 0.1 M sodium hydroxide utilizing various tools at fixed temperatures. The obtained outcomes designated that the rate of corrosion of Al was set to rise with the rising alkali. The evaluated greater values of inhibition efficiencies (%IEs) of Amy and Raf (reaching 89% and 92%, respectively, at doses of 500 ppm) indicated that such compounds were efficient inhibitors against aluminum corrosion. Such %IE values depended on the concentrations and the structures of the inhibitors. The examined compounds played as mixed-kind inhibitors with a trivial cathodic priority. At similar concentrations, the average %IE values of raffinose were discovered to be faintly greater than those of amygdalin. The %IE values were reduced with the rising temperature. The acquired high values of %IEs were understood to be a result of the effective adsorption of the molecules of the examined compounds on the aluminum surface and the construction of a defensive film, and this adsorption was in agreement with the Langmuir adsorption isotherm. The thermodynamic and kinetic parameters were evaluated and debated. The kinetics of inhibition by the tested compounds were also investigated. The mechanisms of Al corrosion and its inhibition were discussed. The results acquired from the three utilized tools with respect to the values of %IEs were set to be in a good agreement with each other, confirming the validity of the obtained results of the existing study. Computational studies for the interactions between Amy and Raf molecules at the Al (1 1 1) surface were found to be consistent with the experimental results. The quadratic model of response surface methodology (RSM) modeling was used to expertly evaluate the relationships between the input parameters and the expected response (output).
1. Introduction
Metals undergo a natural process called metallic corrosion that changes them into their ionic states in a solution or the solid phase, degrading the useful properties of metals [1,2,3,4,5]. Just being exposed to certain substances or conditions, moisture in the air, and numerous metals can cause corrosion [6,7,8]. Special compounds known as corrosion inhibitors are used to stop or slow down metal corrosion [9,10,11,12]. In particular, amygdalin and raffinose were selected to investigate their effectiveness as corrosion inhibitors for Al because they are readily available, naturally occurring, green compounds. A cyanogenic glycoside called amygdalin is present in many plants, including apples, peaches, and plums. Raffinose is a trisaccharide that is present in several vegetables and is used as a prebiotic, a food or beverage additive, and a moisturizer and smoother for the skin. Due to its good physicochemical qualities, aluminum is one of the most often used metals in our lives [13]. In many applications and in practically all industries, aluminum is regarded as one of the most appealing materials [14]. It is corrosion-resistant because a thin oxide film has been created on its surface to stop further deterioration [15]. Although this type of oxide film shields the surface of aluminum from aggressive ions and/or certain media, it can be destroyed, which can cause the aluminum to corrode [16]. Aluminum corrodes quickly as a result of the built oxide layer dissolving at pH values higher or lower than four [17]. In general, alkaline media, notably sodium and potassium hydroxide solutions, are conducive to the corrosion of aluminum and some of its alloys [18]. Al is exposed to alkaline solutions in many applications, including alkaline etching and alkaline air batteries [19]. Al-air battery performance hinges on preventing Al corrosion in alkaline media, as this can otherwise lead to a self-discharge [20].
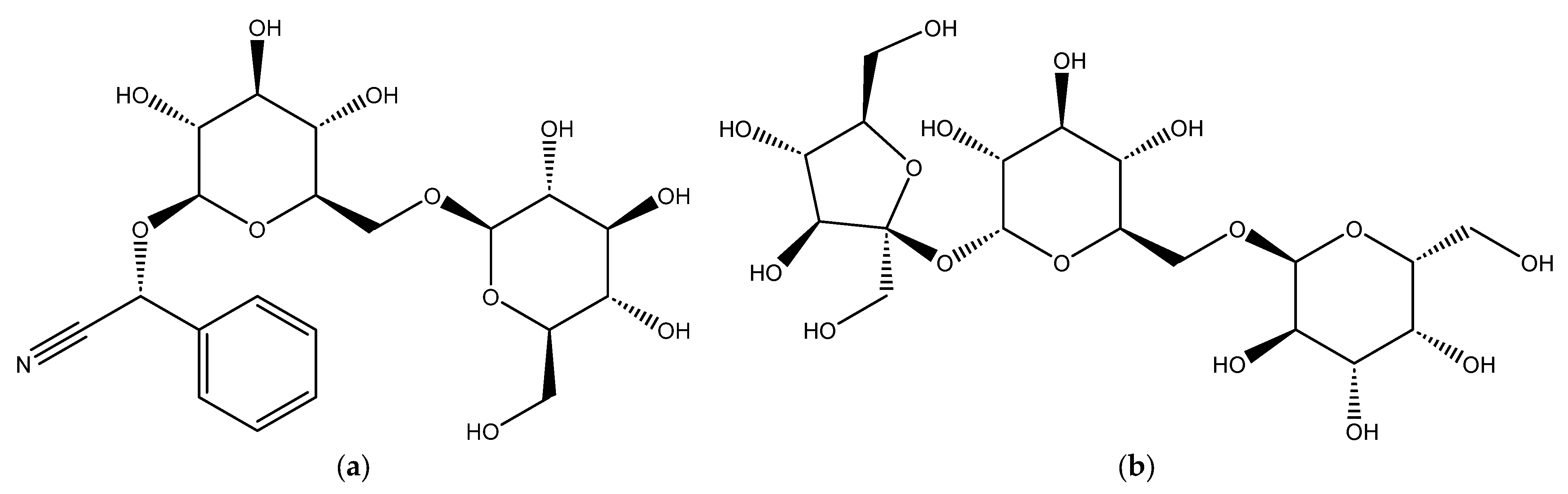
The primary objectives of the present research were to estimate the inhibition efficacy of two ecologically friendly natural compounds, amygdalin and raffinose, for Al corrosion in 0.1 M NaOH. The choice depended on their structures (Figure 1) as they have different functional groups which makes them used as typical corrosion inhibitors. Chemical, electrochemical, and microscopic tools were applied in this study. Thermodynamic and kinetic features were explored. The kinetics and mechanisms of the corrosion of aluminum and its inhibition by the examined compounds were also examined and debated. Using the response surface methodology (RSM) model, density functional theory (DFT), and MD simulation strategy, theoretical and statistical estimations were performed. The results of this study confirmed their high potential for Al corrosion protection.
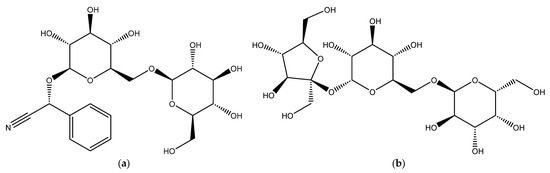
Figure 1.
Chemical structures of amygdalin and raffinose compounds, (a) Amygdalin (Amy), and (b) Raffinose (Raf).
2. Experimental
2.1. Materials
The 0.1 M NaOH solution was the tested corrosive media of the research. The 2 natural chemicals under study, amygdalin (C20H27NO11) and raffinose (C18H32O16) Sigma-Aldrich, St. Louis, MO, USA), were converted into fresh solutions using double-distilled water and employed in a range of doses from 100 to 500 mg/L. All tests were conducted on 99.9% pure aluminum samples (Saudi Arabia). For weight loss (WL) studies, sheets of aluminum with areas of around 14 cm2 were utilized, whereas cylindrical rods embedded in araldite with exposed surface areas of 1.0 cm2 were used for potentiodynamic polarization (PDP, and electrochemical impedance spectroscopy (EIS). The aluminum specimen surfaces were cleaned with double-distilled water, degreased with acetone, and polished with various emery paper grades before any runs. Then, the aluminum specimen was inserted into the 0.1 M NaOH solution and/or with a definite dose of the examined compounds at a fixed temperature in a thermostated system. Every experiment was repeated about 3 times under similar conditions for reproducibility.
2.2. Methods and Instruments
Many technologies were used in this investigation, including PDP, EIS, WL, and SEM (scanning electron microscope). Using a thermostated PGSTAT30 potentiostat/galvanostat (AUTOLAB, Metrohm, Riverview, FL, USA), the PDP and EIS tests were both recorded. Both the PDP and the EIS measurements made use of a cell with three electrodes. The cell had a working electrode made of an aluminum sheet, a counter electrode made of a Pt sheet, and a reference electrode made of saturated calomel (SCE). Before doing any experiments, the utilized cell and the working electrode were prepared, as stated in the report [21]. The PDP measurements were used with a scan rate of 2.0 mV/s and a potential range of 300 mV vs. SCE. Equation (1) [22] was used to calculate the inhibitory efficiency (%IE) and the surface coverage percentages (θ) of the tested chemicals:
where icorr and icorr are the corrosion current densities with and without the inhibitor, respectively (inh). The EIS measurements were made at OCP (open circuit potential)using AC signals with an amplitude of 4.0 mV and a frequency range from 100 kHz to 0.1 Hz (peak to peak). The values of %IEs were calculated using Equation (2) [23,24]:
where Rct and Rct(inh) are the charge transfer resistance values without and with the inhibitor, correspondingly.
In the WL experiments, the corrosion rate of aluminum (CR) was computed in mpy (mils penetration per year) via Equation (3) [25]:
where K is a constant (3.45 × 106), W is the WL in grams, A is the area of the Al sheet in cm2, t is the time in hours, and d is the density of Al. The values of %IEs of the examined compounds were calculated by Equation (4) [26]:
where the corrosion rates without and with the inhibitor, respectively, are CR and CRinh. The Al sheet was immersed in the corrosive solution and the tested compounds for 12 h in order to conduct SEM examinations. Following this time, the Al specimen was taken out of the testing solution, dried, and put up for SEM analysis. At a repetition voltage of 10.0 kV, the JEOL SEM model T-200, (Peabody, MA, USA), was used for these experiments.
2.3. Response Surface Methodology (RSM)
Using the RSM approach, which evaluated the interactions between independent variables and the expected response (%IE) based on gravimetric measurements, the experiments were created. The temperature and inhibitor concentrations served as the independent variables (input variables), and the inhibition effectiveness (%IE) of the two inhibitors under investigation served as the output variable (response). The inhibition efficiency is the dependent variable that a mathematical model generated. Using the ANOVA and R2 values, the performance of the RSM model was statistically predicted.
2.4. Computations for Quantum Chemistry
Utilizing Gaussian software version 9.0, quantum simulations were performed to investigate how the molecule structure affected the inhibition mechanism and effectiveness. All computations were performed using complete geometry optimization and the hybrid B3LYP functional level with a higher basis set, denoted by 6–31 G(d,p) [27]. From the border molecular orbitals of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the following formulas were used to derive many quantum chemical parameters:
where the absolute electronegativity of aluminum and inhibitors’ particles are denoted by (=3.23) eV mol−1 and , respectively. The absolute hardness of aluminum and the inhibitor molecule is denoted by = zero eV mol–1 and , respectively. The is the back donation and the number of transferred electrons is given by [28].
2.5. Molecular Dynamic (MD) Simulation
The corrosion system, which consisted of an aluminum oxide substrate and the adsorbates Amy and Raf, was constructed using the molecular dynamics module of BIOVIA’s Materials Studio 2020 program. The shape of the individual inhibitor molecules was optimized using Materials Studio’s DMol3 algorithms. Al2O3 (111), the most stable aluminum oxide surface, was chosen for this simulation. With a slab thickness of 1.0 nm, a supercell of (2.47 × 4.15 × 1.70 nm), and a vacuum of 5.0 nm, the molecular simulation work was completed. The simulations’ force field was the COMPASS force field.
3. Results and Discussion
3.1. PDP Measurements
3.1.1. OCP
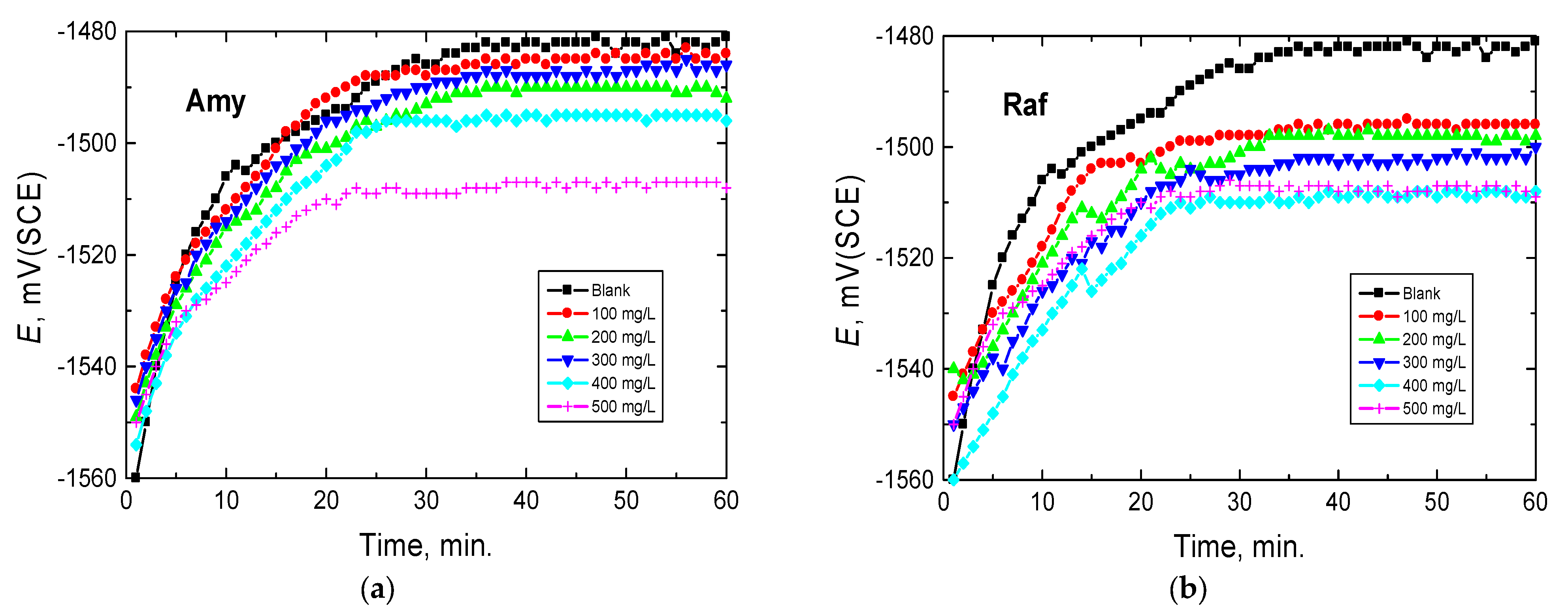
To underline the electrochemistry of aluminum in alkaline media, the time reliance of the open circuit potential, EOCP (OCP), was studied for a period of 60 min of the immersion of Al in 0.1 M NaOH and in the presence of several concentrations (100–500 mg/L) of amygdalin and raffinose at 298 K, as illustrated in Figure 2. Figure 2 shows that the potential of the aluminum electrode in the blank solution (0.1 M NaOH) moved towards more electropositive values at the initial ~30 min of immersion, interpreted by an anodic passivation caused by the formation or growth of insoluble Al2O3 or Al(OH)3 [29]. After this period, EOCP reached a fixed potential. Similar behavior was observed upon adding the inhibitors (at different concentrations) but the positive shifts in the OCP curves of Amy and Raf occurred in the initial ~20 min (Amy) and ~15 min (Raf) of immersion and steady states were attained in shorter times, compared with the blank solution. This behavior signified that the examined compounds covered the aluminum surface, and, hence, constructed a protective layer on it [30]. On the other hand, the examined compound caused the EOCP to move in the negative direction compared with the blank solution, suggesting that the inhibition of the cathodic reaction resulted in an accumulation of the electrons produced from the anodic reaction, resulting in more negative potentials for aluminum in the inhibited media [31].
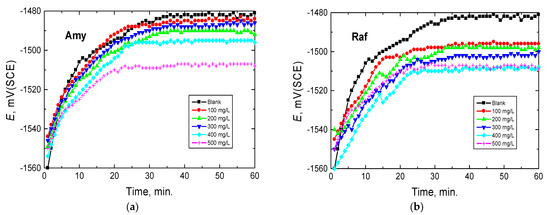
Figure 2.
Potential-time curves for Al in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf) at 298 K.
3.1.2. Effect of Corrosive Medium Concentration
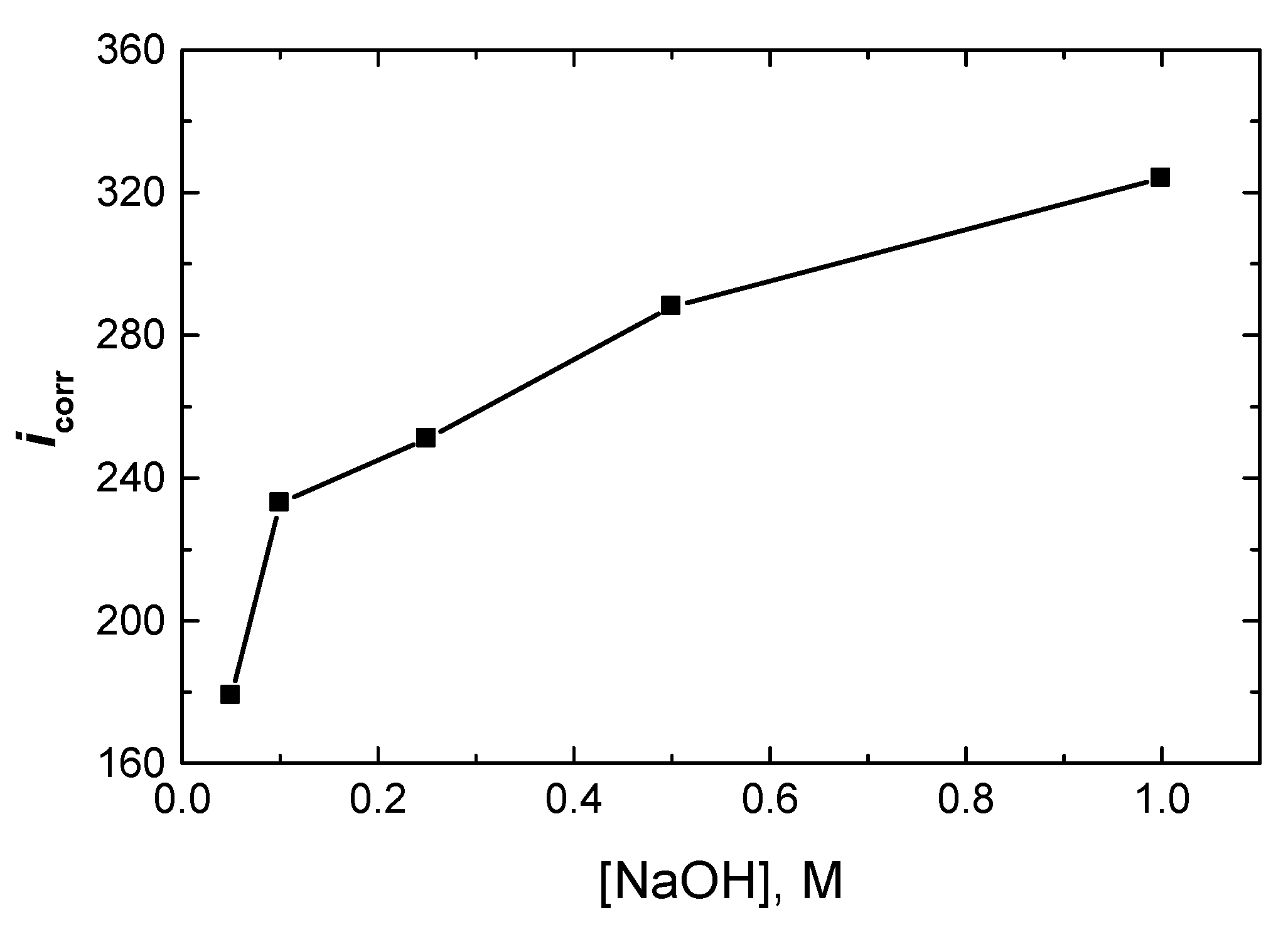
The PDP measurements were carried out at 298 K for Al in NaOH solutions (0.05 to 1.0 M). The corrosion parameters, including the cathodic and anodic Tafel slopes (c, a), polarization resistance (Rp), corrosion potential (Ecorr), corrosion current density (icorr), and Tafel plots (not shown here), were calculated and are listed in Table 1. The results showed that as [NaOH] rose, the value of icorr of Al increased (as shown in Figure 3), while icorr of Rp decreased, indicating that the corrosion of aluminum intensified.

Table 1.
Values of PDP parameters for Al corrosion in different [NaOH] at 298 K.
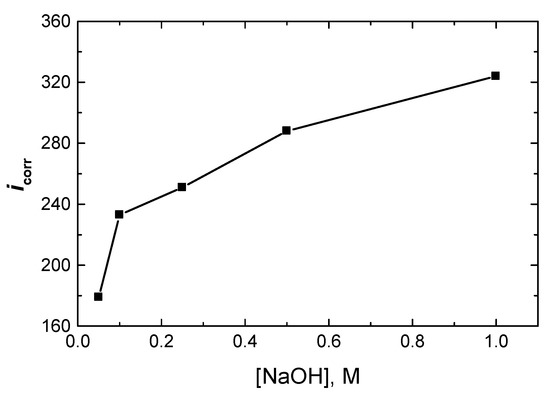
Figure 3.
Effect of [NaOH] on the corrosion rate density, icorr (µA/cm2), of Al at 298 K.
3.1.3. Effect of the Examined Compounds
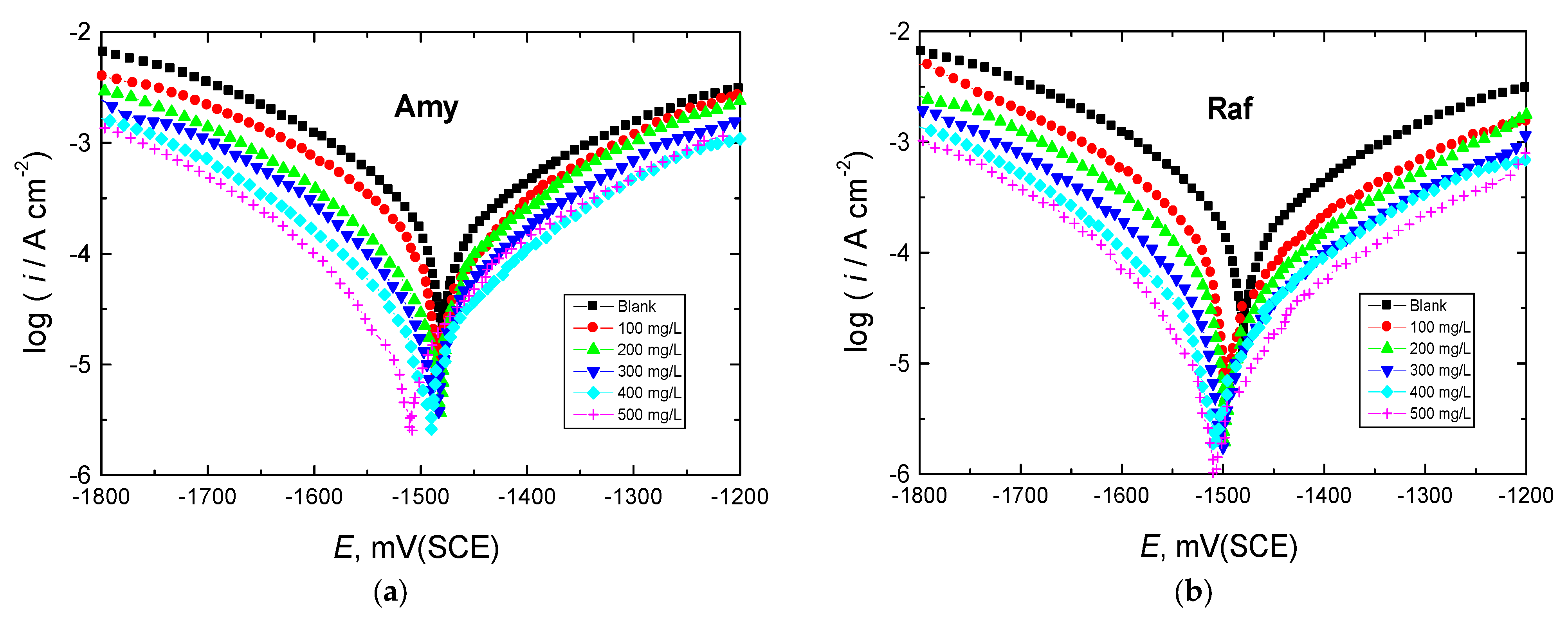
The Tafel graphs for Al in 0.1 M NaOH medium with varying amounts of the tested chemicals added are shown in Figure 4 at 298 K. After being evaluated, the PDP parameters are reported in Table 2. Figure 4 demonstrates how the Tafel curve was shifted to lower current densities by introducing the tested compounds to the corrosive medium. This behavior suggests that the rate of Al corrosion decreased. Due to the addition of the inhibitors, the Ecorr value in the blank solution was slightly shifted in the cathodic or negative direction, showing that these compounds were mixed-type inhibitors with a minor cathodic predominance (the measured shifts were less than +85 mV) [32]. In addition, the Rp value increased as the concentrations of the tested inhibitors rose, signifying corrosion inhibition, whereas the icorr value of aluminum declined. The computed values of %IEs in Table 2 increased with increasing doses of the examined inhibitors, and raffinose had a marginally better inhibition performance than amygdalin at comparable concentrations of both. The observed percentages of IEs were interpreted on the basis of the inhibitor molecules’ strong ability to bind to the aluminum surface, creating a shielding layer that protects the surface from the effects of the hostile solution [33].
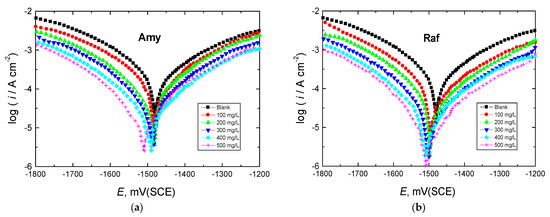
Figure 4.
PDP curves for Al corrosion in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf) at 298 K.

Table 2.
Values of PDP parameters for Al corrosion in 0.1 M NaOH medium and with Amy and Raf at 298 K.
3.2. EIS Measurements
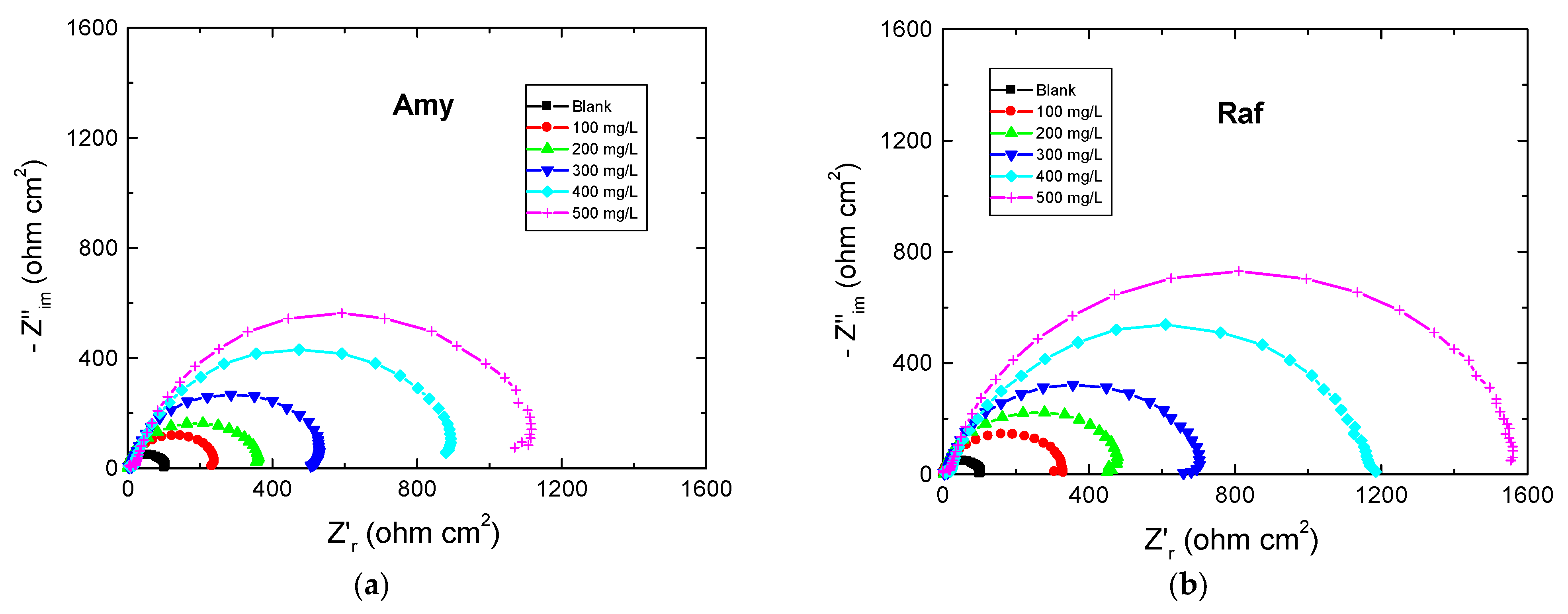
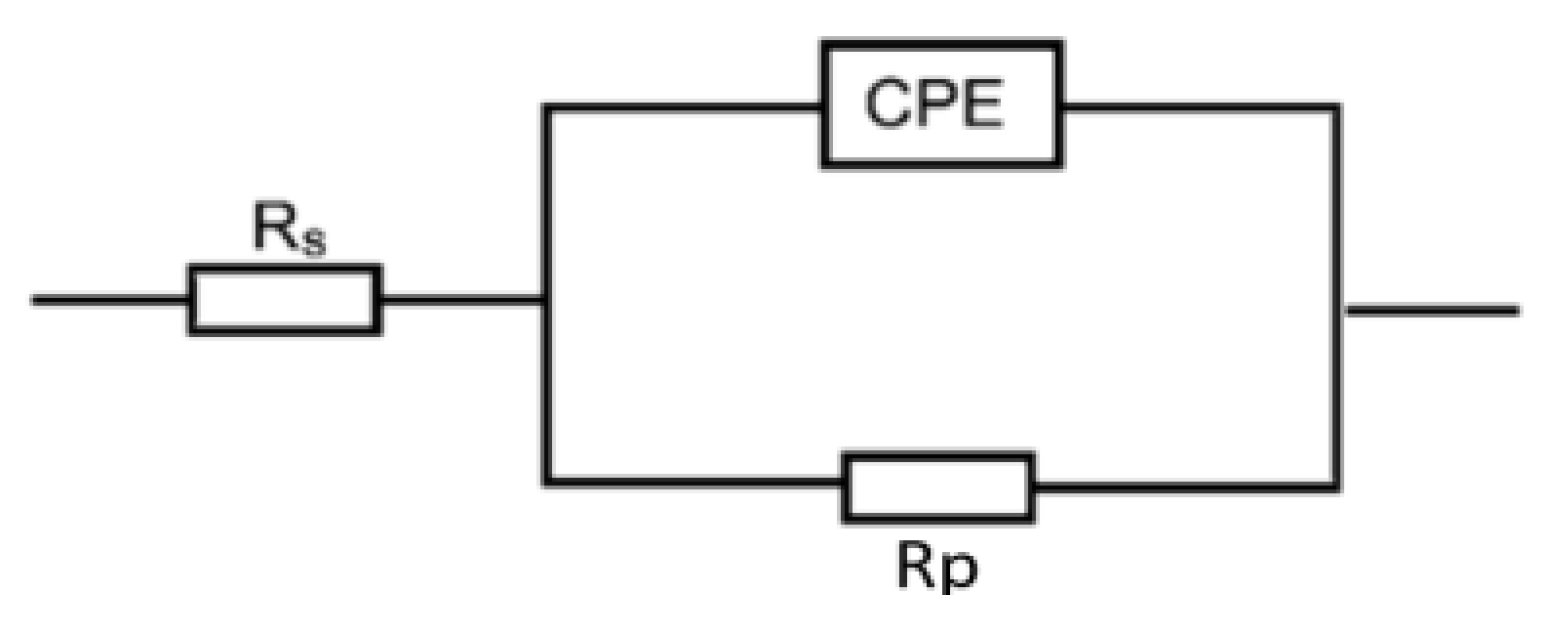
The EIS measurements are valuable techniques in the description of the adsorption behavior of the inhibitors’ molecules on the metal surface and, thus, the performance of the inhibition process. Figure 5 shows the EIS spectra represented as Nyquist plots of Al in 0.1 M NaOH medium and with amygdalin and raffinose at 298 K. The Nyquist plots consisted of only depressed capacitive loops which indicated that the adsorption of the tested compounds happened by masking the aluminum surface by their molecules [34]. The same Nyquist plots were obtained in both the absence and existence of the examined compounds, designating that adding these compounds did not change the corrosion mechanism [35]. The size of the capacitive loop recorded for the corrosive medium was expanded by increasing the inhibitors’ doses illuminating a reduction in the rate of corrosion rate of aluminum. On the other hand, the obtained EIS spectra were analyzed via an equivalent circuit as illustrated in Figure 6 comprising a solution resistance (Rs), polarization resistance (Rp), and constant phase element (CPE). Such EIS parameters were computed and listed in Table 3. Such table indicated that with the rising doses of the examined compounds the value of Rp increased, signifying the inhibition of the aluminum corrosion [36]. In this context, the %IE values were enhanced with augmenting the doses of the examined compounds, establishing that these compounds were efficient inhibitors for aluminum corrosion in the NaOH solution. As gained from the PDP tool, the values of %IEs of raffinose were slightly greater than those of amygdalin at the same concentrations.
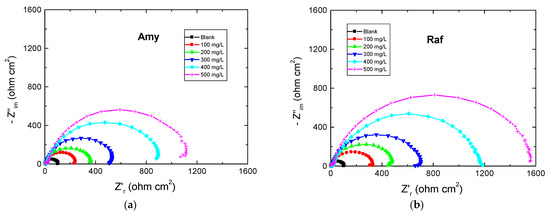
Figure 5.
Nyquist plots for Al corrosion 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf) at 298 K.
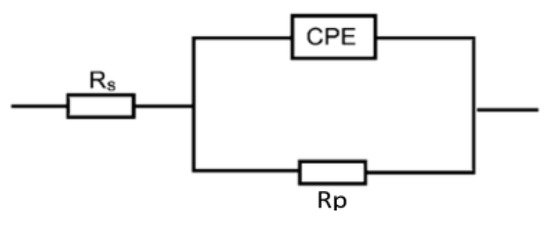
Figure 6.
Equivalent circuit employed to suit the obtained EIS spectra in Al corrosion in 0.1 M NaOH medium and with amygdalin and raffinose.

Table 3.
EIS results for Al corrosion in 0.1 M NaOH medium and with Amy and Raf at 298 K.
3.3. WL Measurements
3.3.1. Effect of Inhibitors’ Concentrations at Different Temperatures
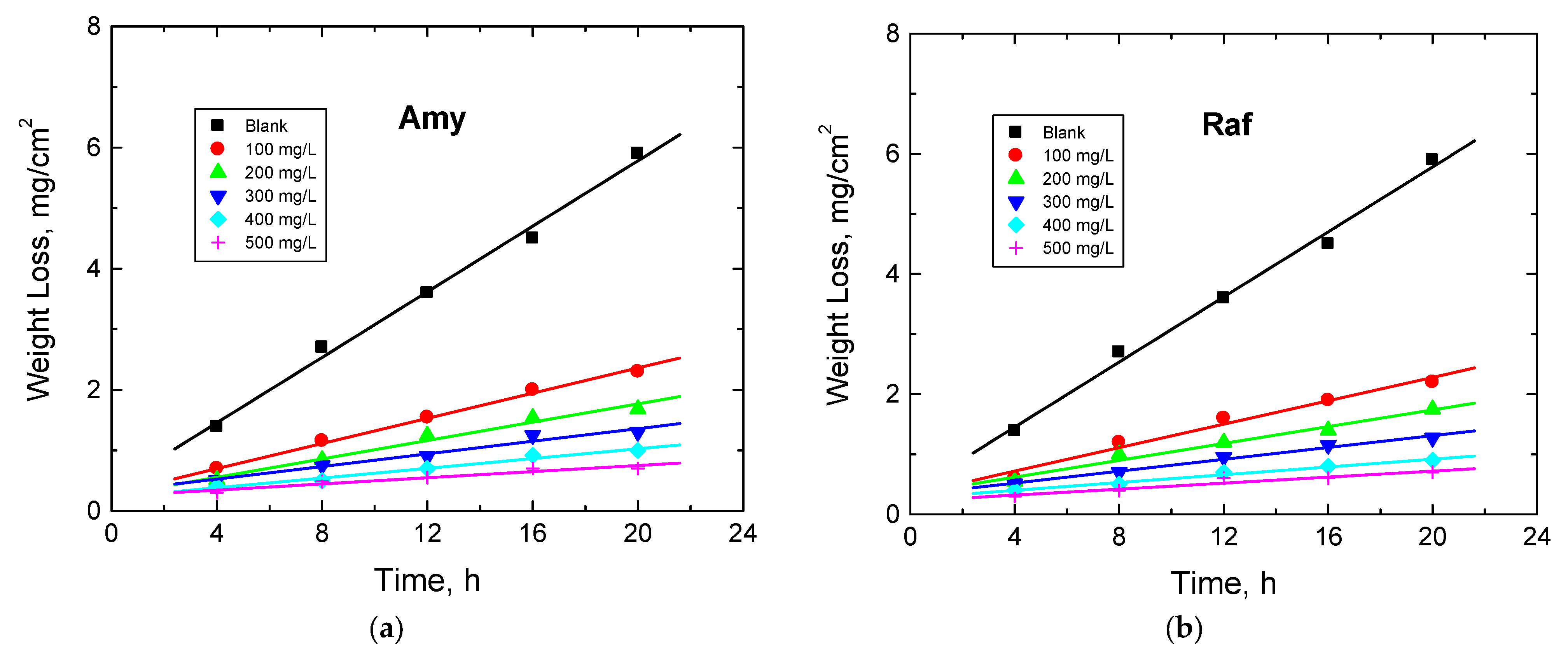
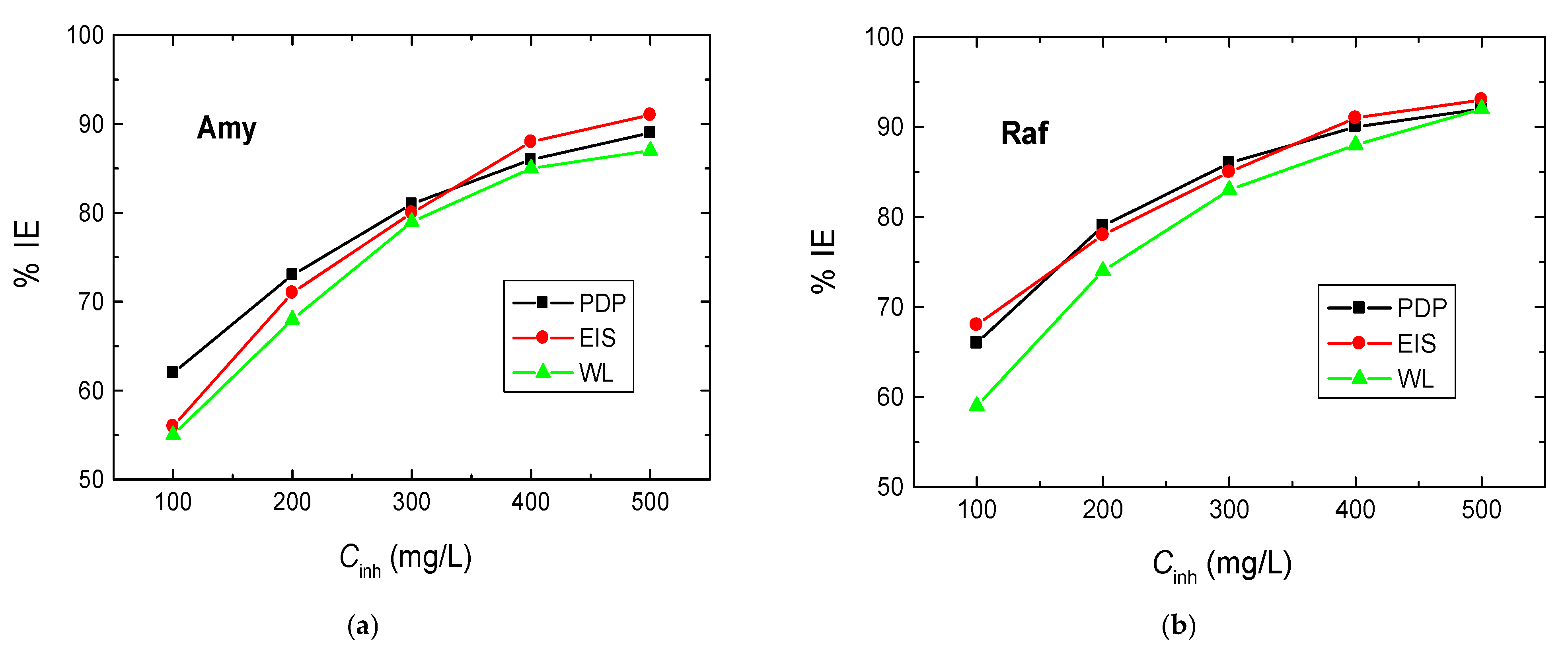
The WL experiments of Al sheets were performed in 0.1 M NaOH solution and with amygdalin and raffinose with various doses, viz. from 100 to 500 mg/L, at different temperatures (288–318 K). Figure 7 illustrates the WL—immersion time plots in the presence of the two examined compounds obtained at 298 K (as a representative example). The determined values of CR of Al, as well as the %IEs and θ values of the examined compounds at different temperatures, are presented in Table 4. These values showed that the CR values decreased and those of the %IEs increased with the compounds’ concentrations, signifying that these compounds were proficient inhibitors for the corrosion of aluminum in 0.1 M NaOH solution. As the temperature of the tested medium rose, the rate of corrosion of aluminum increased, while the IEs of the analyzed compounds decreased (Table 4). This behavior suggests that the adsorption of the molecules of the inhibitors was physical in character [37]. In agreement with the other utilized two techniques, PDP and EIS, the sequence of %IEs is: Raf > Amy. In addition, Figure 8 shows a comparison of the values of %IEs of the tested inhibitors with their doses acquired from all utilized techniques, PDP, EIS, and WL, at 298 K. This figure demonstrates the good accordance between all employed tools, illuminating the reasonableness of the findings.
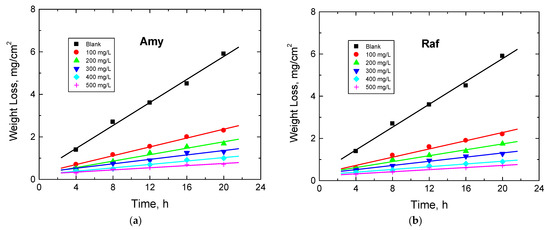
Figure 7.
Plots of WL vs. dipping time for Al corrosion in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf) at 298 K.

Table 4.
The mean values of corrosion rates (CRs) of Al in 0.1 M NaOH medium and with Amy and Raf at various temperatures.
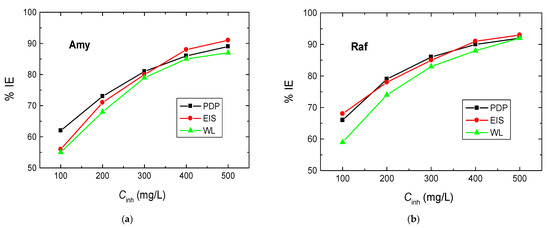
Figure 8.
Variation of %IEs of, (a) Amygdalin (Amy), and (b) Raffinose (Raf), with their concentrations in the Al corrosion in 0.1 M NaOH medium using PDP, EIS, and WL techniques.
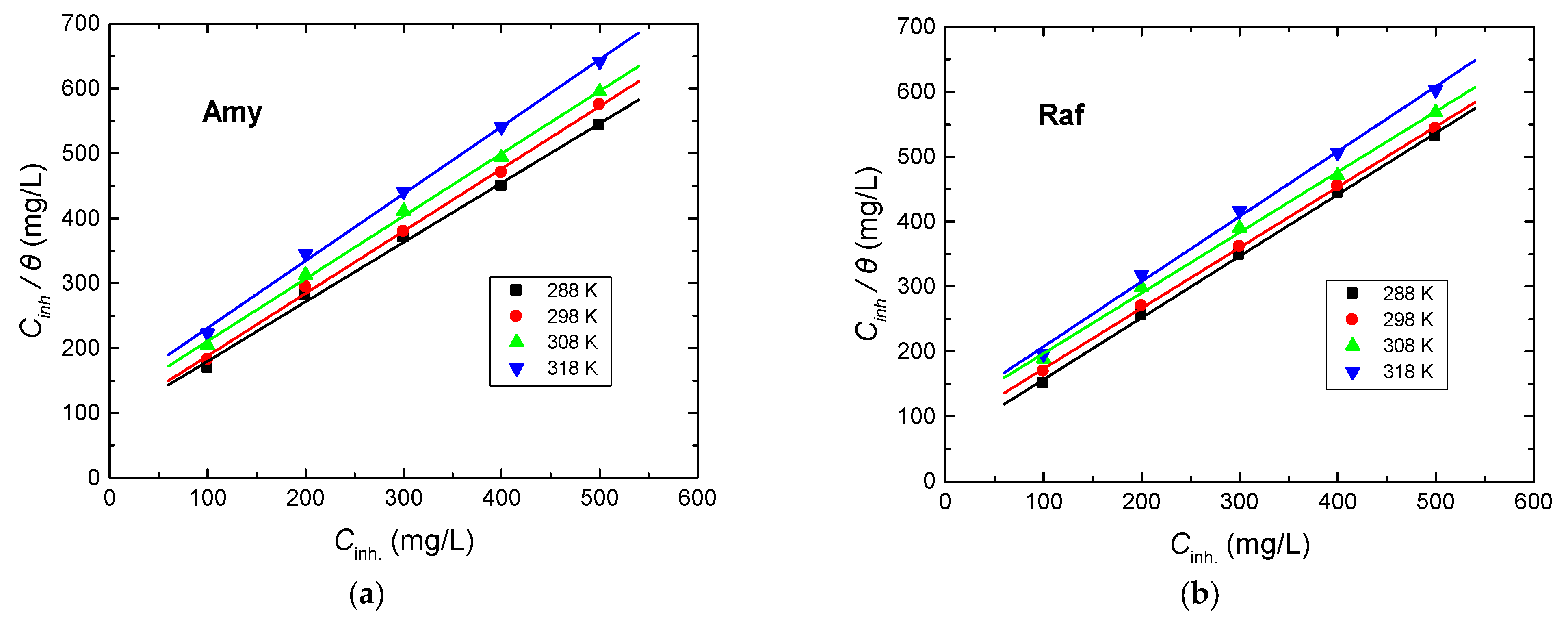
3.3.2. Adsorption Isotherms
Adsorption isotherms provide noteworthy reports about the interaction of the inhibitors’ molecules with the metal surface [38,39]. The gained results from the employed tools demonstrate that the examined compounds are efficient inhibitors for Al corrosion in the 0.1 M NaOH solution. The inhibition performances of these compounds were interpreted on the basis of the strong adsorption of these compounds on the metal surface, leading to the construction of a protective layer. The degrees of surface coverage (θ) values related to different inhibitor doses were utilized to explain the appropriate isotherm for the adsorption process, such as Freundlich, Temkin, Langmuir, Frumkin, etc. The graphs of Cinh/θ against the concentration of the examined inhibitor (Cinh) at different temperatures were linear with almost unit gradients, as illustrated in Figure 9. This finding signified that the adsorption process was in accordance with the Langmuir isotherm which is represented by Equation (12) [40,41]:
where Kads is the adsorption equilibrium constant. The values of Kads at different temperatures were calculated and are inserted in Table 5. The values of Kads reduced with the rising temperature, specifying a strong adsorption of the inhibitors’ molecules on the copper surface at lower temperatures; when the temperature became comparatively higher, the adsorbed molecules tended to desorb from the metal surface [42]. In addition, the values of Kads in the case of raffinose were higher than those acquired for amygdaline, confirming the superiority of raffinose in the inhibition performance in accordance with the obtained results from all utilized techniques.
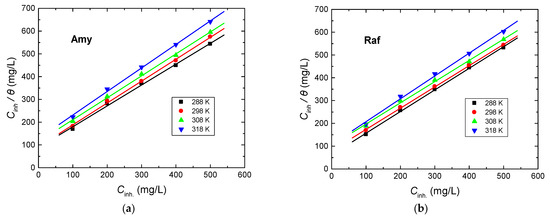
Figure 9.
Langmuir adsorption isotherms for, (a) Amygdalin (Amy), and (b) Raffinose (Raf), adsorbed on the Al surface in 0.1 M NaOH medium at various temperatures.

Table 5.
Values of Kads and thermodynamic parameters for Al corrosion inhibition in 0.1 M NaOH medium by Amy and Raf at various temperatures.
3.3.3. Thermodynamic Parameters
The thermodynamic parameters of the adsorption process, namely: standard free energy (), standard heat (), and standard entropy () were evaluated (as listed in Table 5) in order to present substantial information about the mechanisms of the corrosion process and its inhibition. The values of were computed at various temperatures using Equation (13) [42]:
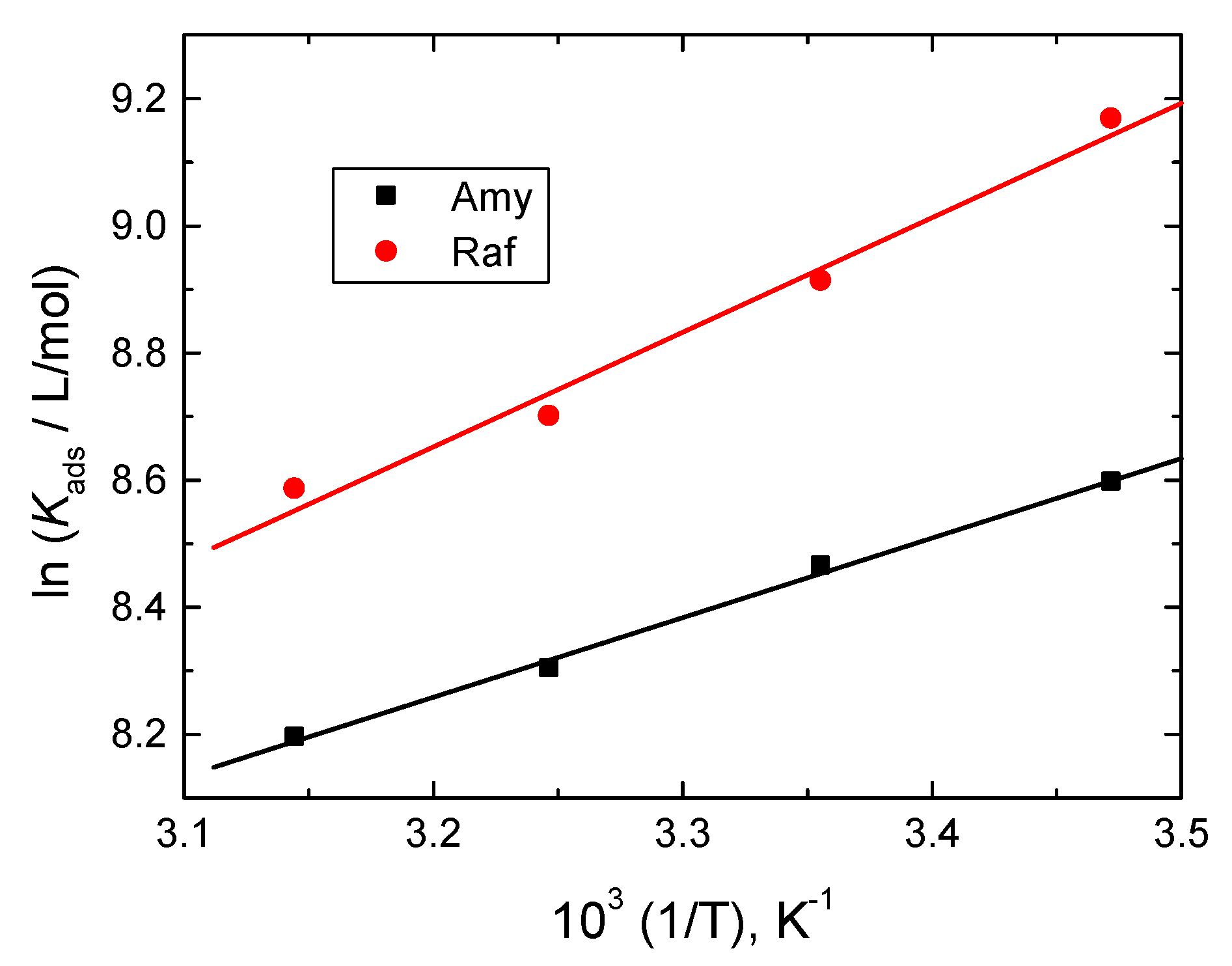
The inhibitor molecules were adsorbing spontaneously, according to the negative values of , and a strong protective layer formed on the aluminum surface. In addition, these results showed that the adsorption mechanism was heterogeneous, i.e., both physical and chemical. The van’t Hoff equation gave the values of [43]:
The plots of ln Kads versus 1/T were linear, as shown in Figure 10, and the values of were evaluated from the gradients of the straight lines and are also inserted in Table 5. The obtained negative values of indicated that the adsorption process was exothermic and physical in its nature.
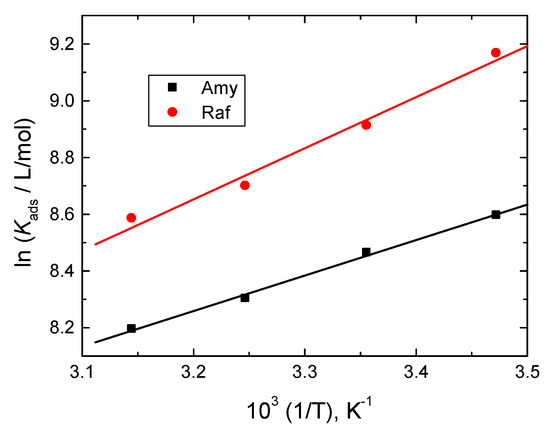
Figure 10.
Van’t Hoff plots for Amy and Raf adsorbed on Al surface in 0.1 M NaOH medium.
Also, the values of were computed via the Gibbs–Helmholtz equation, Equation (15):
The obtained positive values of signified an augmenting disorder of the inhibitors’ molecules throughout their adsorption on the Al surface [44].
3.3.4. Kinetic Parameters
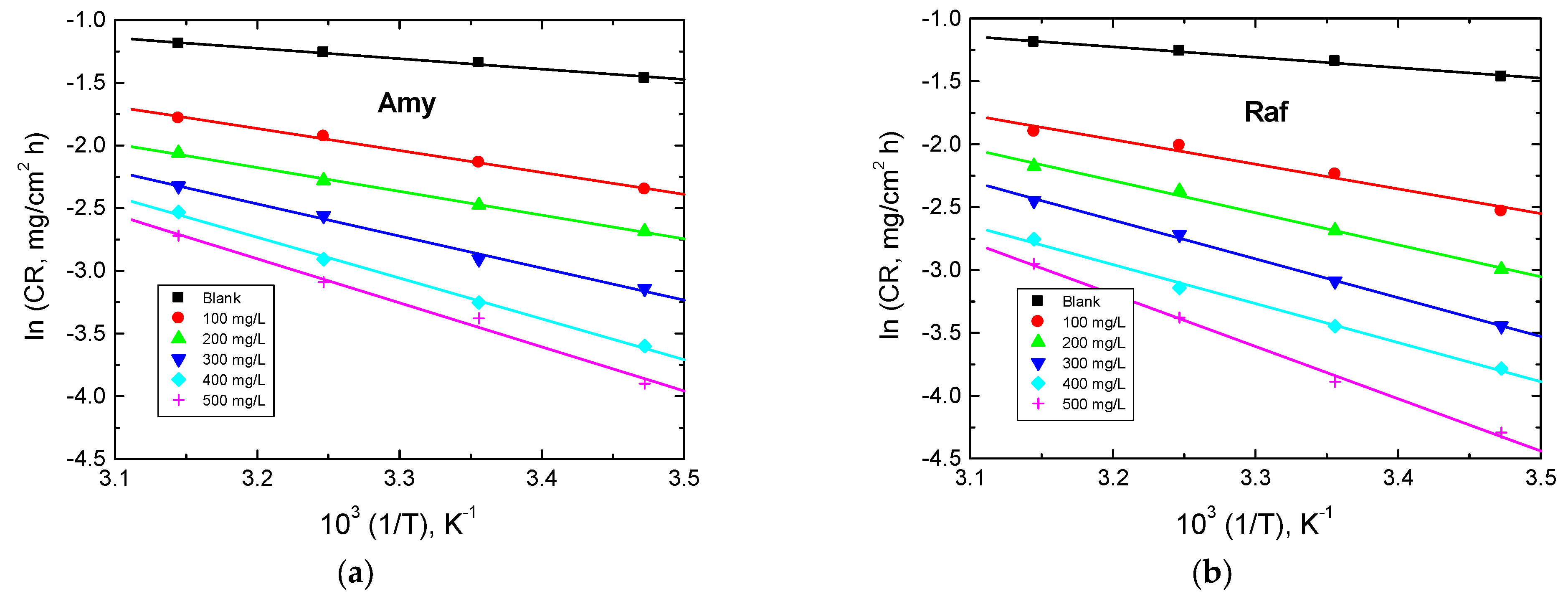
The effect of the temperature on the value of CR was inspected by Arrhenius equation, which gives the values of the activation energy () [45]:
Arrhenius plots (lnCR vs. 1/T) for aluminum in 0.1 M NaOH medium and in the existence of the examined compounds are shown in Figure 11. The values of were calculated and are listed in Table 6. The acquired values of in the existence of the examined compounds were set to be greater than those gained in the corrosive medium. These findings showed that the chemicals under study were adsorbed on the aluminum surface and provided a barrier between it and the corrosive medium [46]. The obtained values were less than 80 kJ/mol (required for chemical adsorption), signifying that the mechanism of adsorption was physical. These findings are in a good accordance with those based on the and values, indicating the consistency of the obtained results. The enthalpy (ΔH*) and entropy of activation (ΔS*) were determined using Equation (17) [47]:
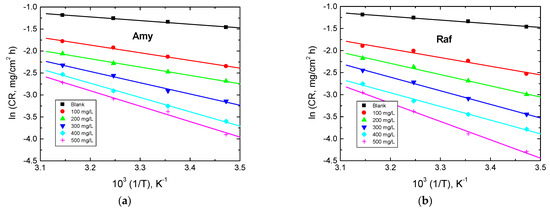
Figure 11.
Arrhenius plots for Al corrosion in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf).

Table 6.
Activation parameters for Al corrosion in 0.1 M NaOH medium and with Amy and Raf.
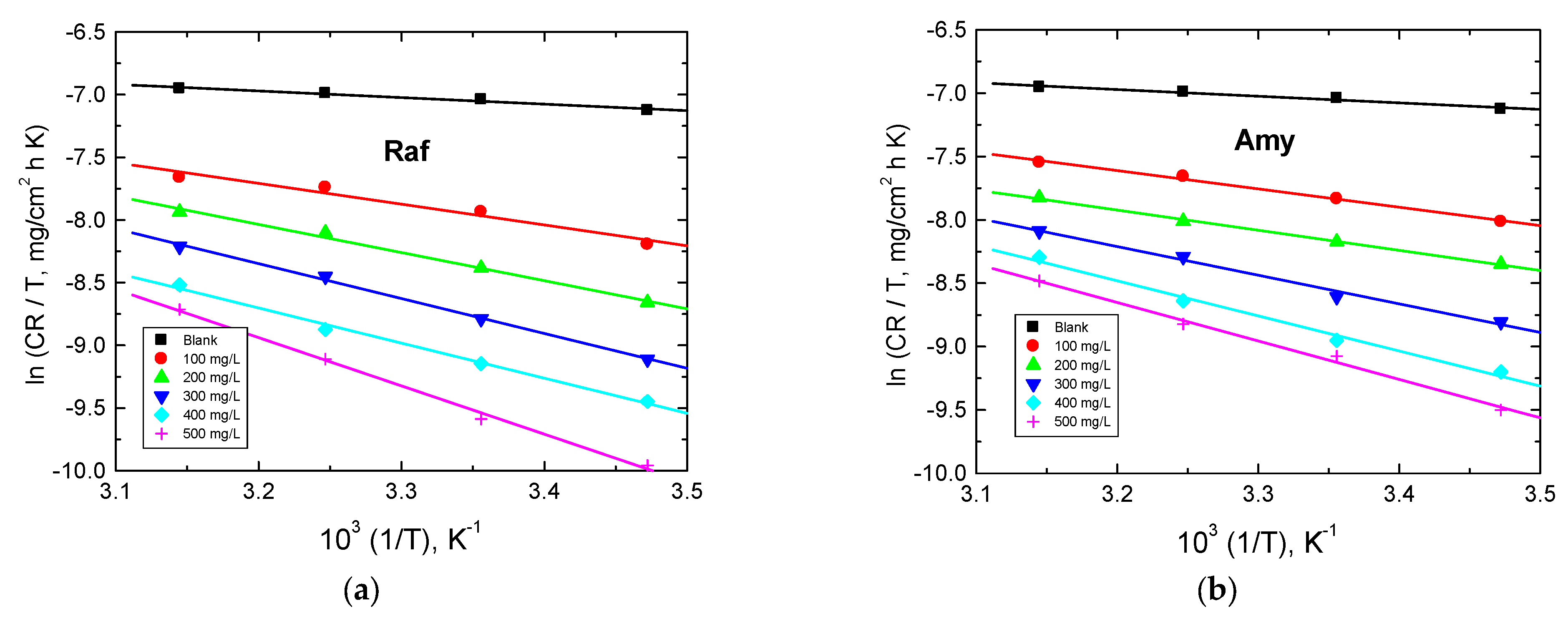
The plots of ln(CR/𝑇) vs. 1/T were linear, as shown in Figure 11. The values of both Δ𝐻* and Δ𝑆* were calculated from the plots in Figure 12 and are also inserted in Table 6. The obtained positive values of ΔH* indicated that the corrosion of aluminum was an endothermic process. Moreover, the higher negative values of ΔS* illuminated a great reduction in the disorder due to the formation of activated complexes [48].
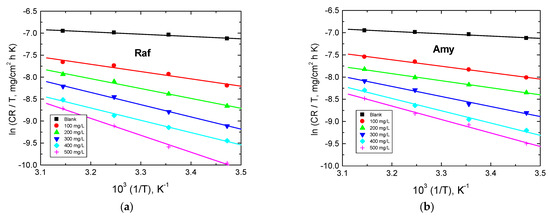
Figure 12.
Transition-state plots for Al corrosion in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf).
3.3.5. Kinetics of Aluminum Corrosion and Its Inhibition
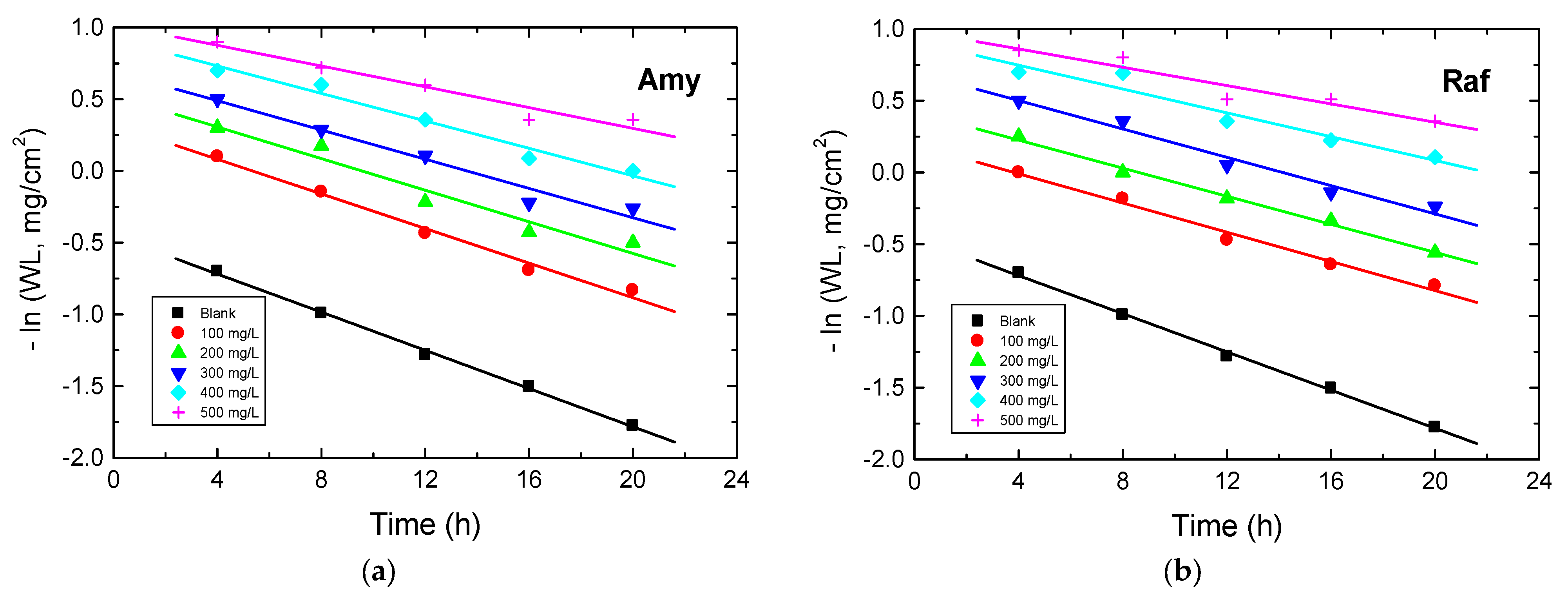
The kinetics of the corrosion of aluminum in 0.1 M NaOH medium before and after the addition of Amy and Raf were investigated at 298 K. When the values of −ln(WL) were plotted against the immersion times, straight lines were obtained (as shown Figure 13), signifying that the kinetics of the Al corrosion in the tested corrosive medium and its inhibition by the examined compounds were negative first-order processes. The values of the first-order rate constant, k1 (h−1), were computed from the gradients of such plots and are presented in Table 7. In addition, the half-life times (t1/2, h) were calculated (t1/2 = 0.693/k1) and are also presented in Table 7. The orders (n) of the inhibition of Al corrosion by the examined compounds regarding their concentrations (Cinh) were calculated from the following equation, which is plotted in Figure 14 [49]:
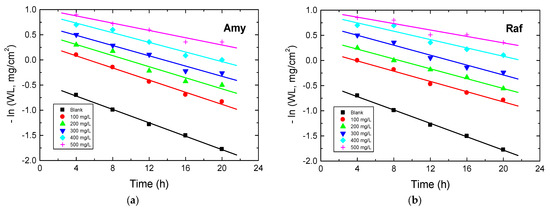
Figure 13.
First-order plots for Al corrosion in 0.1 M NaOH medium and with, (a) Amygdalin (Amy), and (b) Raffinose (Raf) at 298 K.

Table 7.
Values of k1 and t1/2 (half-life time) for Al corrosion in 0.1 M NaOH medium and with Amy and Raf at 298 K.
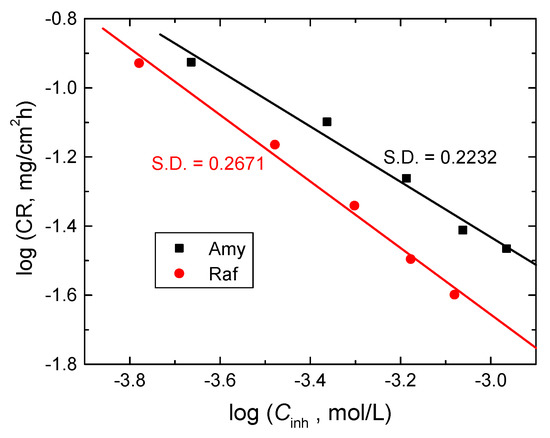
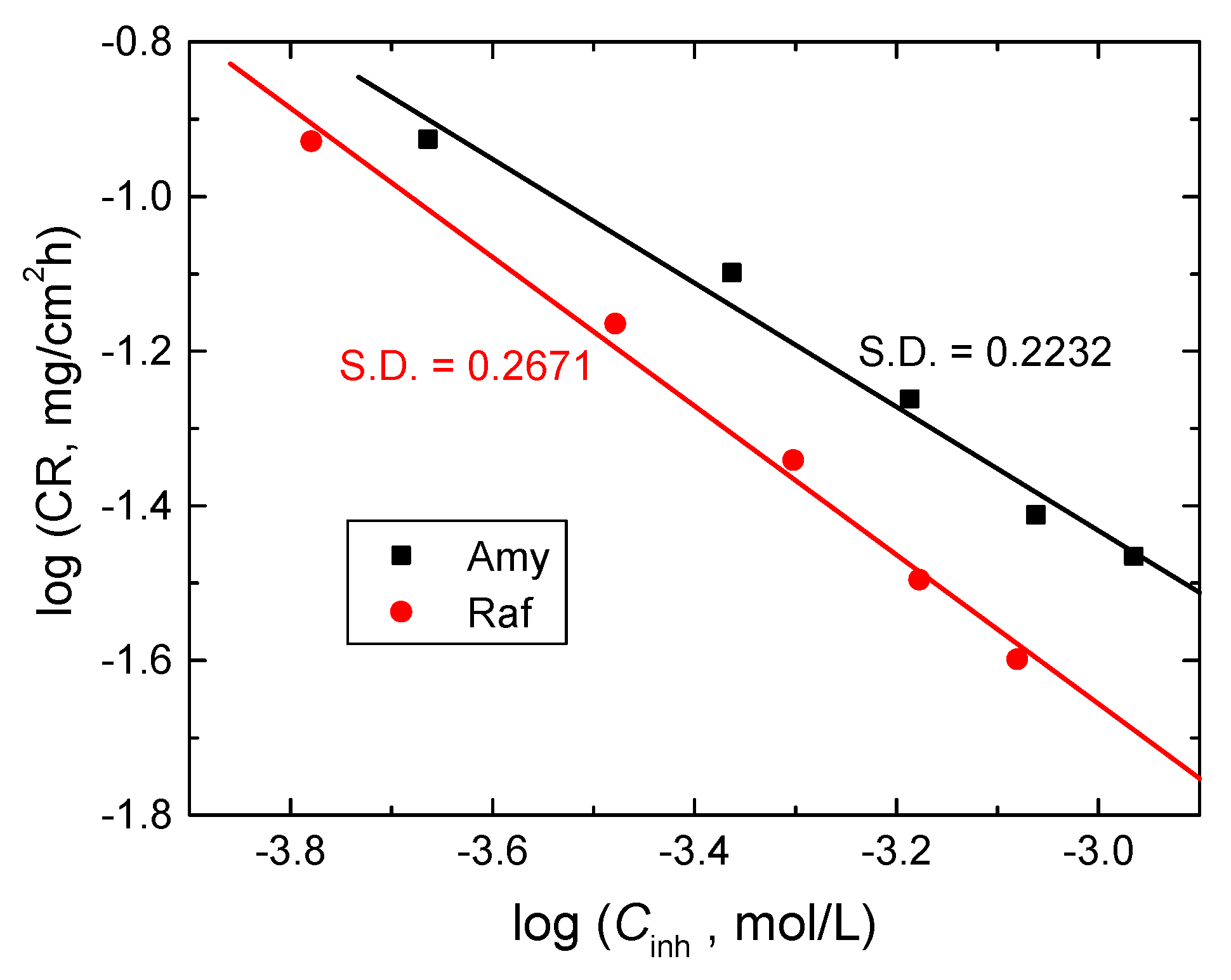
Figure 14.
log CR vs. log Cinh for Al corrosion inhibition in 0.1 M NaOH medium by Amy and Raf at 298 K. S.D. = standard deviation.
The evaluated values of n were −0.82 and −0.96 for amygdalin and raffinose, individually. These values prove that the inhibition of aluminum corrosion by the tested compounds was a negative first-order processes. Furthermore, the opposite behavior of the CRs with the doses of inhibitors and the negative values of n illuminated the good %IEs of the examined compounds [50].
3.4. Surface Examination
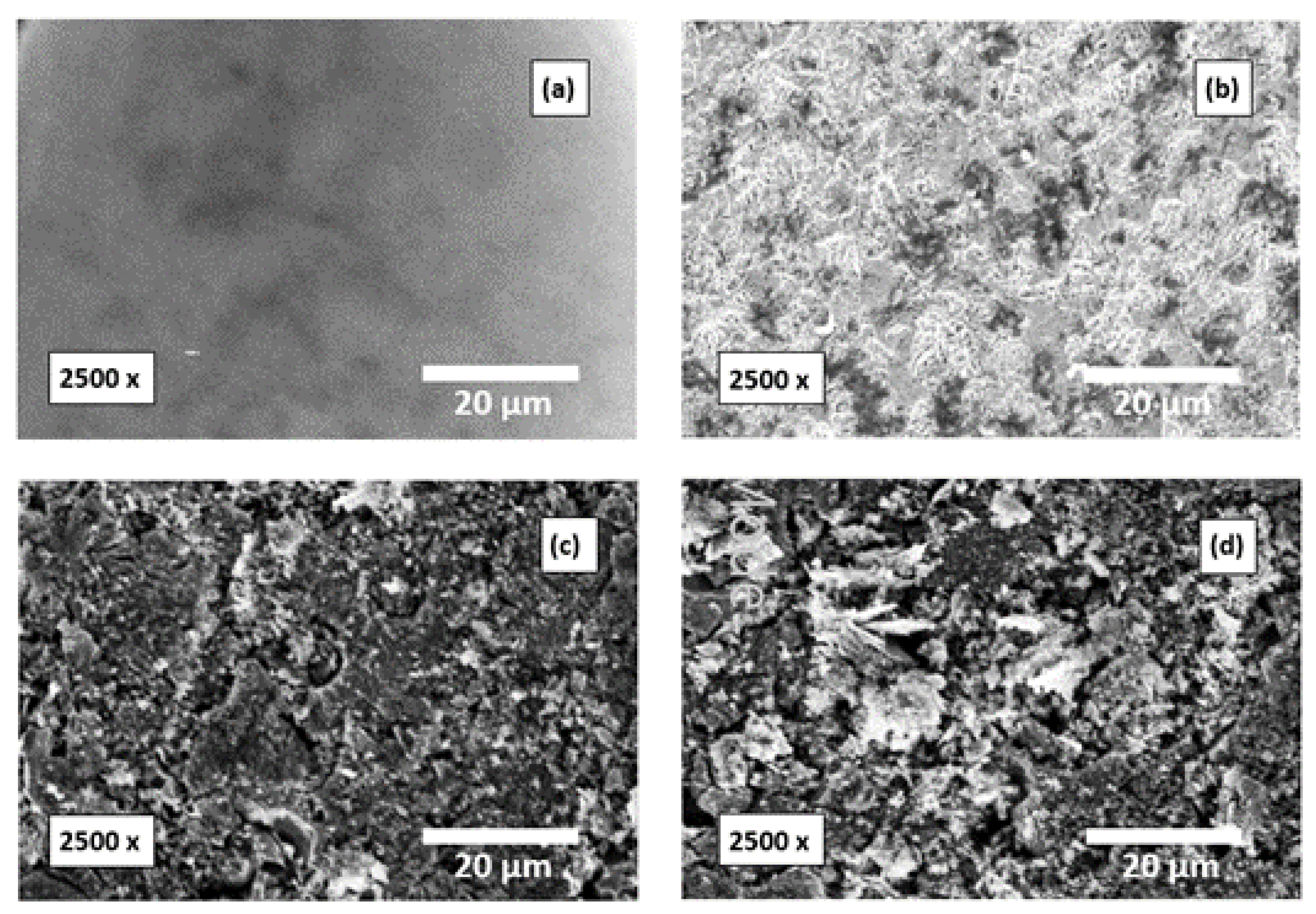
The SEM images of Al surfaces before and after immersion in the investigated corrosive medium without and with addition of a 500 mg/L of the tested compounds are presented in Figure 15. The degreased Al surfaces before and after dipping in 0.1 M NaOH medium (corrosive medium) for 12 h are shown in Figure 15a,b, correspondingly. Figure 15b shows the corroded Al surface. Figure 15c,d shows the Al surfaces after adding 500 mg/L of amygdalin and raffinose, respectively. These micrographs showed the vanishing of the corrosion in the Al surface and the surfaces were chiefly covered with the molecules of the examined compounds. This may be due to the powerful adsorption of such compounds’ molecules on the Al surface.
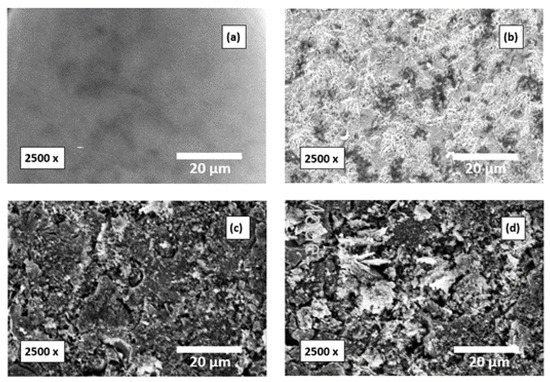
Figure 15.
SEM images (Mag. 2500×) of the surfaces of Al specimens; (a) prior to dipping, (b) after dipping in 0.1 M NaOH medium for 12 h, (c,d) after 12 h of dipping in 0.1 M NaOH with a 500 mg/L of amygdalin and raffinose, correspondingly.
3.5. Mechanisms of Aluminum Corrosion and Its Inhibition
It was reported that a thin stable passive oxide film was inherently formed in the air and that such oxide layer has a good adhesion on the Al surface, resulting in the protection of it from further corrosion, i.e., outstanding corrosion challenges [51]. However, the constructed oxide film chiefly deteriorates faster in lesser or greater pH media in the existing aggressive ions, such as Cl− ions, resulting in the rapid corrosion of aluminum [52].
The corrosion of Al was proposed [53] to occur by the migration of ions in the solution, followed by aluminum dissolution at the passive layer/solution interface. Generally, Al and some of its alloys are disposed to corrosion in alkaline media [54], particularly in KOH and NaOH solutions. The corrosion mechanism of Al in the alkaline solution was suggested by several researchers [55]. They reported that the first step in Al corrosion proceeds through the electrochemical formation of the hydroxide layer, Al(OH)3 [56], as presented in Equation (19):
This is followed by its dissolution as a partial anodic reaction by the attack of OH− ions to yield a soluble , as represented by Equation (20):
The combination of Equations (19) and (20) imparts the partial anodic dissolution of Al, as seen in Equation (21):
There are two conceivable partial cathodic reactions, Equations (22) and (23):
The corrosion reaction can be illuminated by merging Equations (21) and (22):
and/or by combining Equations (21) and (23):
Thus, as represented by Equation (25), Al corrosion in basic media proceeds with gas evolution. The inhibition of Al corrosion in alkaline medium by the examined compounds can be attributed to the effective adsorption of the compounds’ molecules on the Al surface. The adsorption of these molecules is due to the presence of electrons’ lone pairs on the O and N atoms and the π-bonds in aromatic rings included in the chemical structure of amygdalin and electrons’ lone pairs on O atoms in the raffinose structure. These sites are regarded as adsorption places that can form coordinate bonds with the empty orbitals on the Al surface, leading to the construction of a protecting film. In addition, the formation of donor–acceptor complexes or precipitates on the Al surface amongst electrons’ lone pairs and Al ions on the surface results in the corrosion inhibition of Al [57].
3.6. Surface Response Methodology (RSM)
To comprehensively evaluate the interactions between the input variables, inhibitors concentrations (A), and temperatures (B), the anticipated response inhibition efficiency R1 (for Amy) and R2 (for Raf) were used for RSM analyses to design the experiment with 20 experimental runs (Table 8). The experimental data were used to build a quadratic model equation that links the response (%IE) to the input variables using the RSM model. The following equations illustrate the regression model equation in terms of the coded factor:
%IE (Amy) = +8.72 + 1.01 A − 0.4132 B + 0.0525 AB − 0. 4613 A2 − 0. 0753 B2
%IE (Raf) = +8.93 + 0.9261 A − 0.3922 B + 0.1173 AB − 0. 4150 A2 − 0. 0262 B2

Table 8.
Design matrix and RSM outcome for Al corrosion inhibition in 0.1 M NaOH medium by the examined compounds.
The predictions regarding the reactions for specific amounts of each element can be made using these equations in terms of the coded factors. By default, the factors’ high levels are coded as +1 and their low levels as −1. By contrasting the factor coefficients, the coded equation can be used to determine the relative importance of the elements. The stability of the response (%IE) with respect to the process variables of inhibitor concentration (A) and temperature (B) within the examined range is demonstrated by both Equations (26) and (27). The factor is coded with a +1 for high levels and a −1 for low values. This implies that the combined effects are given a positive sign, and the competing operation is given a negative sign [58].
Table 9 lists the results of the analysis of variance of the ANOVA, which is also used to demonstrate that the suggested model is suitable for analyzing the experimental data [59]. The significant coefficient factors are those with p-values less than 0.05, and the insignificant coefficient factors are those with p-values more than 0.05. Table 9’s prediction indicates that the model terms A, B, C, AC, A2, B2, and C2 are important and were employed.

Table 9.
ANOVA result for quadratic regression model for Al corrosion inhibition in 0.1 M NaOH medium by the examined compounds.
Furthermore, the model’s significance and fitness were demonstrated by the ANOVA prediction of low p-values and high values of the model’s coefficient of determination (R2 = 0.9978 and 0.9933 for Amy and Raf, respectively). The adjusted R2 (0.9970 and 0.9845 for Amy and Raf, respectively) and anticipated R2 (0.9952 and 0.9909) values were both in agreement, demonstrating a strong degree of reliance between the observed and expected response values.
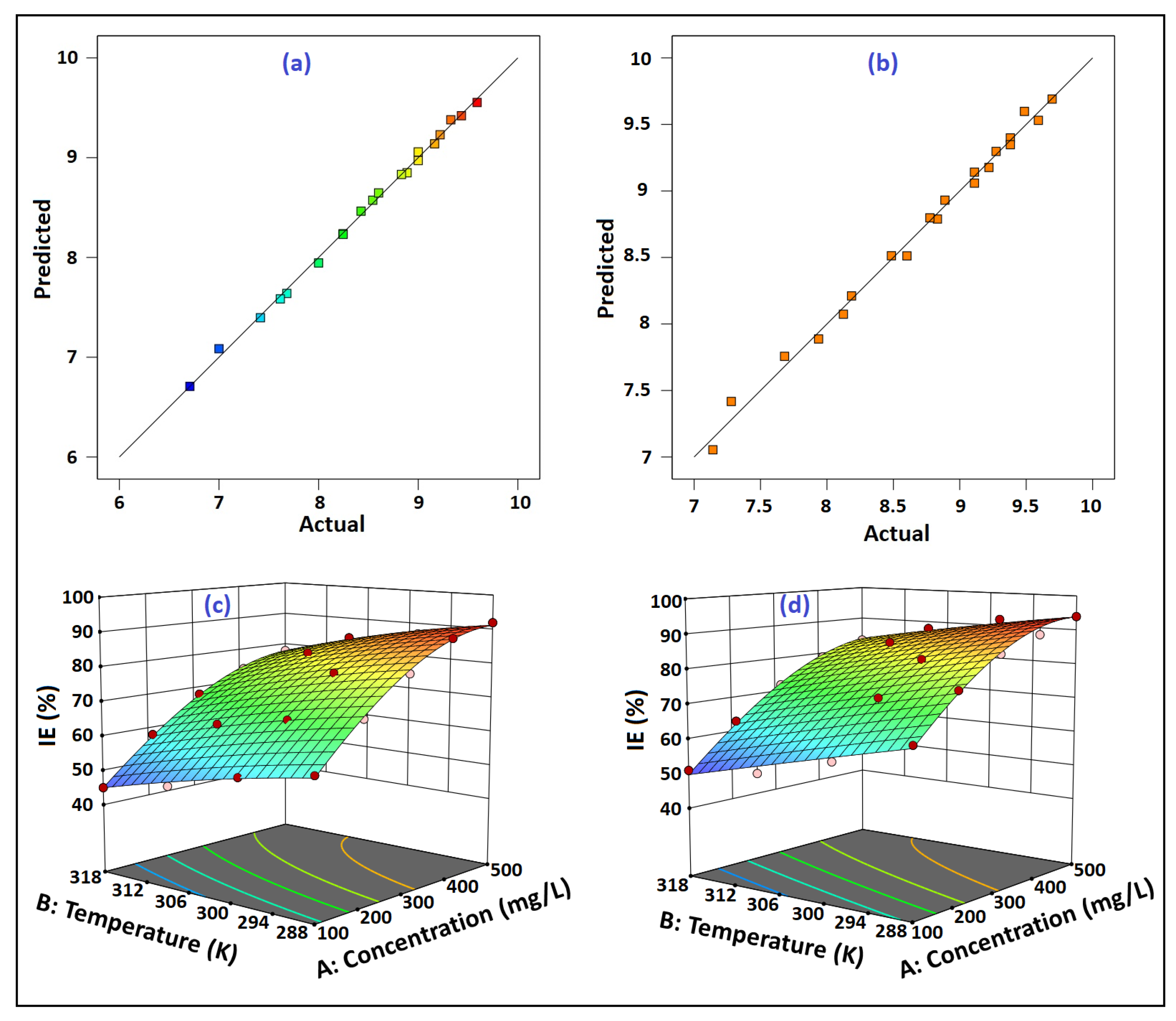
Figure 16a,b plotts the graph of predicted versus actual %IEs for Amy and Raf, respectively. These curves allowed the researchers to determine whether there was any association between the experimental and the anticipated %IEs. The anticipated versus the real %IEs plot showed a linear connection, demonstrating a strong correlation between the two measures of %IE. In light of the forecast, it may be concluded that the mathematical model was successful in predicting the outcome (reaction) for the experimental data.
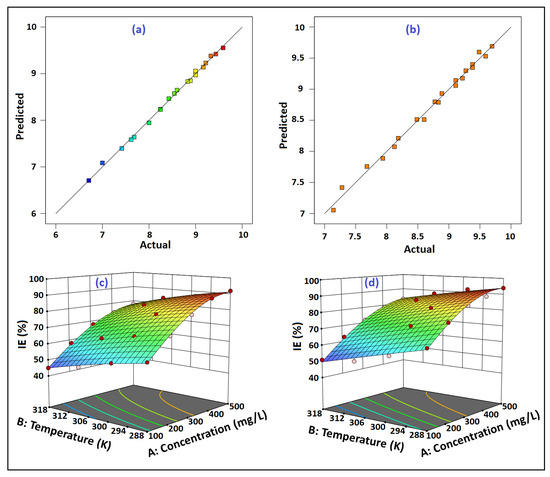
Figure 16.
The correlation between the experimental and the predicted %IE values for: (a) Amy and (b) Raf; 3D surface plots of outcome %IE for: (c) Amygdalin (Amy), and (d) Raffinose (Raf).
Three-dimensional surface plots were taken into consideration to anticipate the combined parameters that exhibited significant results on the corrosion inhibition process. Figure 16c,d shows that, although the %IE values fell with the rising temperature, the %IEs increased as the Amy and Raf concentrations rose. The increased temperature caused the inhibitor molecules that had been adsorbed to the metal surface to desorb, leaving the metal surface exposed to a corrosive attack [60]. Because there are enough molecules vying for attachment to the metal surface, a methodical rise in the inhibitor concentration encourages surface coverage and prevents the development of Al oxide. This demonstrated a substantial link between this finding and the experimental data.
3.7. Studies on Quantum Chemistry
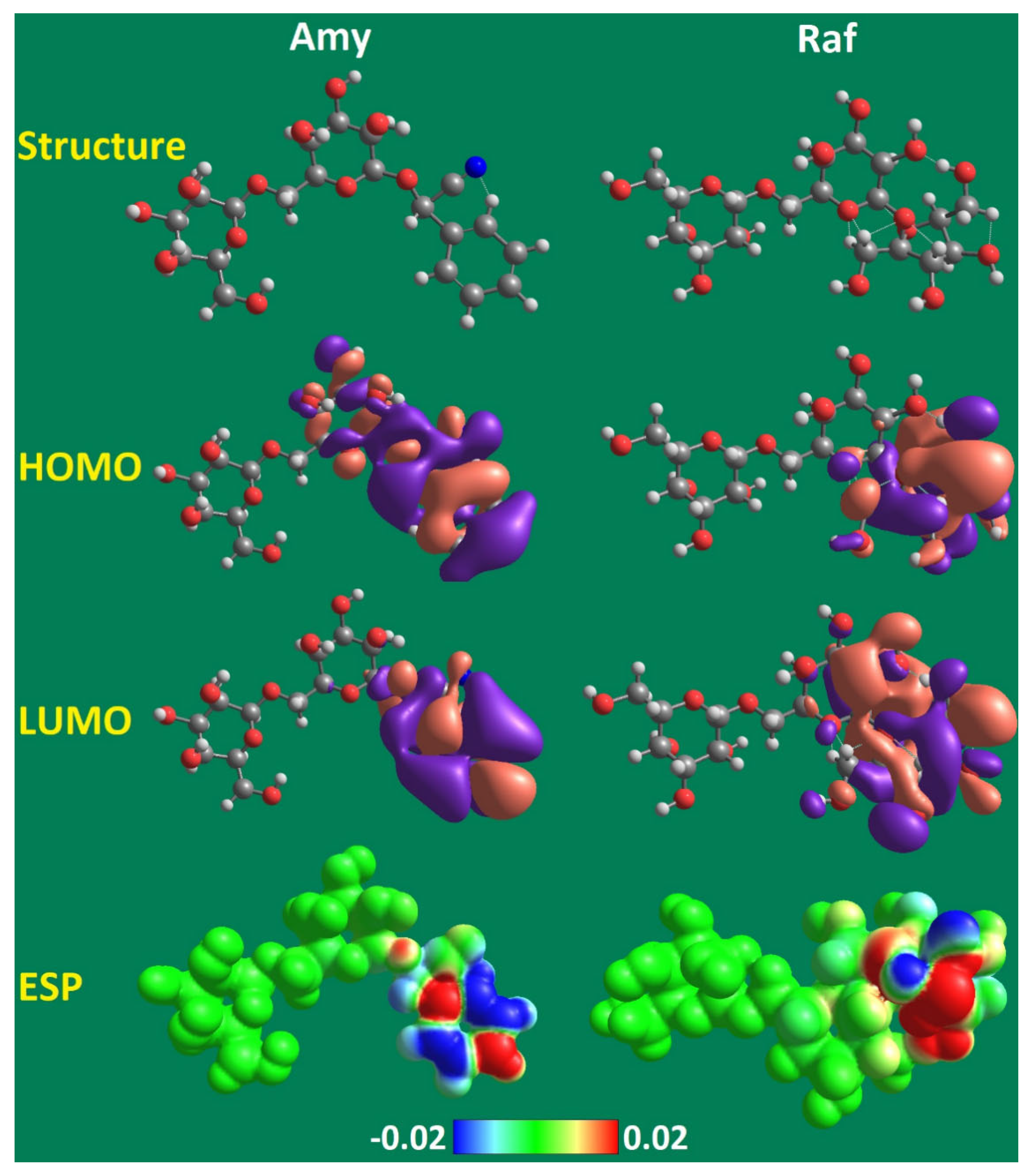
The efficiency of the researched inhibitors (Amy and Raf) has been investigated using a quantum chemical calculation, which has also been used to validate the experimental results from gravimetric and electrochemical studies. Figure 17 displays the optimized molecular structure, the electrostatic potential (ESP) map, and the frontier molecular orbitals of the HOMO and the LUMO generated using the B3LYP/6-31 G(d,p) approach. The distributions of the HOMO and LUMO electron densities at the aromatic phenylacetonitrile in the Amy molecule and at the tetrahydrofuran with the hydroxyl groups in Raf molecule are depicted in Figure 17. A thorough knowledge of the areas in the molecules where the electron-rich area (HOMO) provides charge electron densities and free electrons to metal atoms’ free orbitals, whilst the molecules with LUMO regions allow electrons in occupied orbits to be provided retroactively. The calculated quantum parameters are displayed in Table 10.
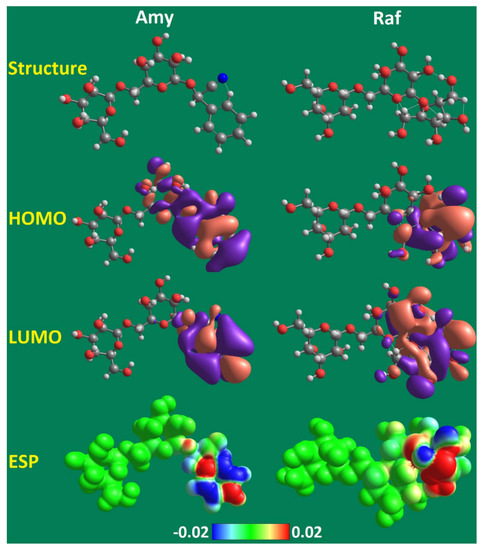
Figure 17.
Optimized structure, HOMO, LUMO, and ESP maps for the studied Amy and Raf given by the B3LYP/6–31G(d,p) method.

Table 10.
Calculated parameters for the Amy and Raf inhibitors obtained using the DFT method at the B3LYP/6–31G(d,p) basis set.
Amy, Raf, and their respective EHOMO values were high, whereas their respective ELUMO values were low, supporting the inhibitory efficiency found through experimentation. EHOMO was discovered to be 4.7 and 4.2 eV for Amy and Raf, respectively. This is caused by the hydroxyl-group-containing tetrahydrofuran in the Raf molecule and the aromatic phenylacetonitrile in the Amy molecule, as well as the increased electron densities of the lone pairs of heteroatoms. Additionally, Amy and Raf were discovered to have low ELUMO values of 0.9 and 1.3 eV, respectively, which point to excellent electron-acceptance capabilities [61].
A key indicator of the activity of an inhibitor molecule is the energy gap (ΔEGAP). Amy and Raf’s low ΔEGAP values of 3.8 and 2.9 eV, respectively, exhibit increased reactivity and facilitate organic molecule binding to the Al surface, resulting in enhanced inhibitory performance. The decline in ΔEGAP and ELUMO, as well as the rise in EHOMO, are associated with the improvement in the inhibitor efficiency [62].
Additionally, softness (σ) is a crucial metric that can indicate the inhibitor compounds’ capacity for adsorption. A strong contact with metal and a high %IE are linked to the softness and hardness values. Amy and Raf have softness values of 0.5 and 0.7 eV and hardness values of 1.9 and 1.5 eV, respectively. These can forecast the inhibitor compounds’ strong affinity for adsorption with the order of Amy < Raf, which is congruent with the findings of the experiments.
The ratio of electrons transferred (ΔN) between Amy, or Raf, inhibitors and the Al surface atoms revealed that N < 3.6 indicates that the inhibitors’ molecules contribute electrons to the aluminum interface and have a greater propensity to adsorb on the metal surface. This transferring of electrons occurs through donor-acceptor interactions of non-bonding electron pairs of the heteroatoms and π-electrons of the inhibitors molecules with the vacant P-orbitals of the aluminum atom.
On the other hand, it has been reported that an electronic back-donation process regulates the anticorrosive molecule-metallic surface process. The global hardness of the molecule is directly correlated with the change in energy, according to this theory. If η > 0 and < 0, this indicates that back-donation from the inhibitor molecule to metal is energetically preferred.
The results shown in Table 10 show that 0, indicating that the charge transfer from the Amy and Raf inhibitors to the Al surface is energetically beneficial and that the inhibitor’s efficacy increases with adsorption on the metallic surface. The experimental results in the tendency that Amy and Raf report below are in line with the estimated values for .
The ESP, which is created by the surfaces of the electron density, determines which parts of the molecule are electrophilic and which are nucleophilic. Figure 17 depicts the determination of Amy and Raf based on the ESP of the molecules. The light blue area denotes the absence of electrons, the yellow area denotes a slight excess of electrons, the green area denotes neutrality (zero potential), and the red area denotes the strongest repulsion. The blue area represents the partially positive charge and the strongest attractions. The spreading of the lone pairs of electrons at the oxygen and nitrogen atoms, which are depicted in red and the negative region at which they are located, leading to positive sites near the hydrogen and carbon atoms (blue). This makes it clear that Amy and Raf are the most vulnerable to electrophilic attack and have the greatest ability to attach to the surface of aluminum, which is in good agreement with both practical and theoretical investigations.
3.8. Molecular Dynamic (MD) Simulations
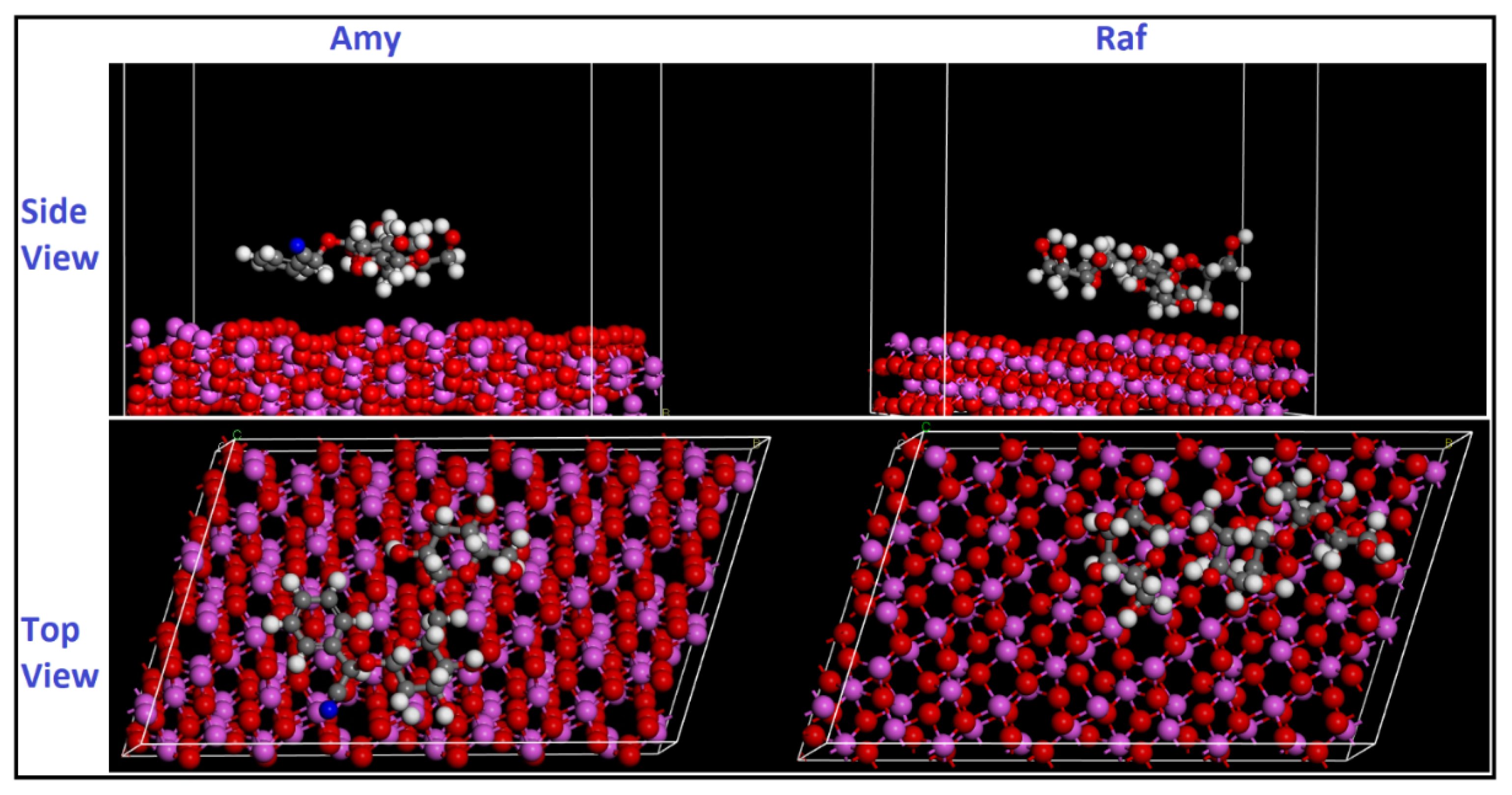
An important and innovative tool to study the interaction of inhibitors and metal surfaces is an MD simulation [63]. Through the use of MD simulations, the adsorption process of Amy and Raf on an aluminum surface was examined, as represented in Figure 18. The purpose of this study was to determine whether there is a significant relationship between experimental inhibition efficiencies and binding energies for the inhibitors examined in this study. Therefore, MD-simulations studies were used to predict the binding energies of Amy and Raf on the aluminum substrate. The following estimates of the interaction energies between the inhibitor molecules and the Al surface were made using MD simulations [64]:
where is the total energy of the Al substrate, is the total energy of the inhibitor chemical, and is the adsorption energy. The energy of the new system is represented as when the adsorption takes place between the compound and the Al surface. Inspection Table 11 indicates that Raf has the largest adsorption energy on the surface of Al (111), being more effective than Amy, which is consistent with the experimental findings. According to the computed binding energy values, the interactions between the molecules Amy or Raf with the aluminum surface are highly strong. It is significant to remember that the inhibitor adheres to the metal surface more readily and with greater efficiency the higher the binding energy value. The observed order of the increasing binding energies, Amy < Raf, is in good agreement with both the experimental evidence and DFT computations.
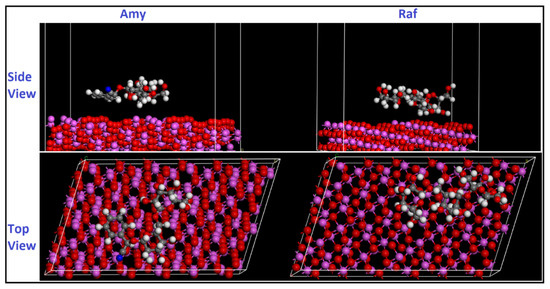
Figure 18.
Side and top views of the most stable low energy configurations for the adsorption of Amy and Raf on Al (111) surface obtained using MD simulations.

Table 11.
The adsorption and binding energies for Amy and Raf on Al (111) surface.
4. Conclusions
- The inhibition of Al corrosion in 0.1 M NaOH medium by amygdalin and raffinose was investigated at fixed temperatures using various techniques.
- The examined compounds were set to be efficient inhibitors against Al corrosion.
- The values of the inhibition efficiencies (%IEs) of the examined compounds depended on the concentrations and structures of these compounds.
- The compounds acted as mixed-kind inhibitors with a trivial cathodic priority.
- The gained values of %IEs of raffinose were slightly greater than amygdalin.
- The values of %IEs decreased with increasing the temperature.
- The gained high values of %IEs were attributed to the effective adsorption of the compounds’ molecules on the Al surface, which agreed with the Langmuir adsorption isotherm.
- Thermodynamic and kinetic parameters were evaluated and debated.
- The kinetics of corrosion inhibition by the examined compounds were investigated.
- The mechanisms of Al corrosion and its inhibition were discussed.
- The results acquired from all utilized tools were set to be in a good agreement with each other, confirming the validity of the obtained results of the existing study.
- The relationships among the involvement parameters and the anticipated comeback (output) have been expertly evaluated by means of the quadratic perfect using RSM.
- The findings of the theoretical studies on the Al(111) surface were consistent with the testing findings and statistical studies and demonstrated a significant interaction between the Amy and Raf molecules and the Al surface.
Author Contributions
Conceptualization, A.T. and A.F.; Methodology, A.T. and A.F.; Software, M.E.A.Z., M.M.S.S., A.I.A. and A.A.F.; Validation, A.T., A.F., N.A., M.M.S.S. and A.A.F.; Formal analysis, A.T., A.F., N.A. and A.A.F.; Investigation, A.T., A.F., N.A. and A.A.F.; Resources, A.I.A.; Data curation, A.T., A.F. and A.A.F.; Writing—original draft, A.T., A.F. and A.A.F.; Writing—review & editing, A.T., A.F., N.A., M.E.A.Z., M.M.S.S., A.I.A. and A.A.F.; Funding acquisition, A.T. and A.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for funding and supporting this work through Research Partnership Program no RP-21-09-76.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for funding and supporting this work through the Research Partnership Program no RP-21-09-76.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fawzy, A.; Toghan, A.; Alqarni, N.; Morad, M.; Zaki, M.E.A.; Sanad, M.M.S.; Alakhras, A.I.; Farag, A.A. Experimental and Computational Exploration of Chitin, Pectin, and Amylopectin Polymers as Efficient Eco-Friendly Corrosion Inhibitors for Mild Steel in an Acidic Environment. Kinetic, Thermodynamic, and Mechanistic Aspects. Polymers 2023, 15, 891. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Abdallah, M.; Zaafarany, I.A.; Ahmed, S.A.; Althagafi, I.I. Thermodynamic, Kinetic and Mechanistic Approach to the Corrosion Inhibition of Carbon Steel by New Synthesized Amino Acids-Based Surfactants as Green Inhibitors in Neutral and Alkaline Aqueous Media. J. Mol. Liq. 2018, 265, 276–291. [Google Scholar] [CrossRef]
- El-Ghamry, H.A.; Fawzy, A.; Farghaly, T.A.; Bawazeer, T.M.; Alqarni, N.; Alkhatib, F.M.; Gaber, M. Evaluation of the Efficiency of Divalent Cobalt and Copper Chelates Based on Isatin Derivatives and Thiosemicarbazide Ligands as Inhibitors for the Corrosion of Sabic Iron in Acidic Medium. Arab. J. Chem. 2022, 15, 103522. [Google Scholar] [CrossRef]
- Heikal, M.; Ali, A.; Ibrahim, B.; Toghan, A. Electrochemical and physico-mechanical characterizations of fly ash-composite cement. Constr. Build. Mater. 2020, 243, 118309. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; El-Beltagi, H.S.; Mohamed, M.E.M.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Fawzy, A.; Farghaly, T.A.; Al Bahir, A.A.; Hameed, A.M.; Alharbi, A.; El-Ossaily, Y.A. Investigation of Three Synthesized Propane Bis-Oxoindoline Derivatives as Inhibitors for the Corrosion of Mild Steel in Sulfuric Acid Solutions. J. Mol. Struct. 2021, 1223, 129318. [Google Scholar] [CrossRef]
- Toghan, A.; Khairy, M.; Huang, M.; Gadow, H. Electrochemical, surface analysis, and theoretical investigation of 3-hydroxy-5-(phenylamino)-4-(p-tolyldiazenyl)thiophen-2-yl)(phenyl)methanone as a corrosion inhibitor for carbon steel in a molar hydrochloric acid solution. Int. J. Electrochem. Sci. 2023, 18, 100070. [Google Scholar] [CrossRef]
- Alfakeer, M.; Abdallah, M.; Fawzy, A. Corrosion inhibition effect of expired ampicillin and flucloxacillin drugs for mild steel in aqueous acidic medium. Int. J. Electrochem. Sci. 2020, 15, 3283–3297. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Al Bahir, A. Expired Amoxicillin and Cefuroxime Drugs as Efficient Anticorrosives for Sabic Iron in 1.0 M Hydrochloric Acid Solution. Chem. Eng. Commun. 2022, 209, 158–170. [Google Scholar] [CrossRef]
- Farag, A.A.; Mohamed, E.A.; Toghan, A. The New Trends in Corrosion Control Using Superhydrophobic Surfaces: A Review. Corros. Rev. 2023, 41, 21–37. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Farag, A.A.; Badran, B.M. Corrosion Inhibitiob of Mild Steel Using Emulsified Thiazole Adduct in Different Binder Systems. Eurasian Chem. J. 2008, 10, 67–77. [Google Scholar]
- Toghan, A.; Khairy, M.; Huang, M.; Farag, A.A. Electrochemical, chemical and theoretical exploration of the corrosion inhibition of carbon steel with new imidazole-carboxamide derivatives in an acidic environment. Int. J. Electrochem. Sci. 2023, 18, 100072. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Revie, R.W. Uhlig’s Corrosion Handbook, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-08032-0. [Google Scholar]
- Elboujdaini, M.; Ghali, E.; Barradas, R.G.; Girgis, M. An Electrochemical Investigation of the Behaviour of Aluminum Alloys in Different Electrolytes. Corros. Sci. 1990, 30, 855–867. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0454-6. [Google Scholar]
- Xhanari, K.; Finšgar, M. Organic Corrosion Inhibitors for Aluminium and Its Alloys in Acid Solutions: A Review. RSC Adv. 2016, 6, 62833–62857. [Google Scholar] [CrossRef]
- Abdallah, M.; Hazazi, O.A.; Fawzy, A.; El-Shafei, S.; Fouda, A.S. Influence of N-Thiazolyl-2-Cyanoacetamide Derivatives on the Corrosion of Aluminum in 0.01 M Sodium Hydroxide. Prot. Met. Phys. Chem. Surfaces 2014, 50, 659–666. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Skeldon, P. Surface Texture Formed on AA2099 Al–Li–Cu Alloy during Alkaline Etching. Corros. Sci. 2013, 66, 292–299. [Google Scholar] [CrossRef]
- Egan, D.R.; Ponce de León, C.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in Electrode Materials and Electrolytes for Aluminium–Air Batteries. J. Power Sources 2013, 236, 293–310. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A. Inhibition Evaluation of Chromotrope Dyes for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and Kinetic Aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef]
- Shaban, M.M.; Negm, N.A.; Farag, R.K.; Fadda, A.A.; Gomaa, A.E.; Farag, A.A.; Migahed, M.A. Anti-Corrosion, Antiscalant and Anti-Microbial Performance of Some Synthesized Trimeric Cationic Imidazolium Salts in Oilfield Applications. J. Mol. Liq. 2022, 351, 118610. [Google Scholar] [CrossRef]
- Farag, A.A.; Eid, A.M.; Shaban, M.M.; Mohamed, E.A.; Raju, G. Integrated Modeling, Surface, Electrochemical, and Biocidal Investigations of Novel Benzothiazoles as Corrosion Inhibitors for Shale Formation Well Stimulation. J. Mol. Liq. 2021, 336, 116315. [Google Scholar] [CrossRef]
- Farag, A.A.; Badr, E.A. Non-Ionic Surfactant Loaded on Gel Capsules to Protect Downhole Tubes from Produced Water in Acidizing Oil Wells. Corros. Rev. 2020, 38, 151–164. [Google Scholar] [CrossRef]
- Tang, L.; Mu, G.; Liu, G. The Effect of Neutral Red on the Corrosion Inhibition of Cold Rolled Steel in 1.0 M Hydrochloric Acid. Corros. Sci. 2003, 45, 2251–2262. [Google Scholar] [CrossRef]
- Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H.M.A. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. [Google Scholar] [CrossRef]
- Farag, A.A.; Toghan, A.; Mostafa, M.S.; Lan, C.; Ge, G. Environmental Remediation through Catalytic Inhibition of Steel Corrosion by Schiff’s Bases: Electrochemical and Biological Aspects. Catalysts 2022, 12, 838. [Google Scholar] [CrossRef]
- Satpati, S.; Suhasaria, A.; Ghosal, S.; Dey, S.; Sukul, D. Interaction of Newly Synthesized Dipeptide Schiff Bases with Mild Steel Surface in Aqueous HCl: Experimental and Theoretical Study on Thermodynamics, Adsorption and Anti-Corrosion Characteristics. Mater. Chem. Phys. 2023, 296, 127200. [Google Scholar] [CrossRef]
- Nie, Y.; Gao, J.; Wang, E.; Jiang, L.; An, L.; Wang, X. An Effective Hybrid Organic/Inorganic Inhibitor for Alkaline Aluminum-Air Fuel Cells. Electrochim. Acta 2017, 248, 478–485. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Jafar Mazumder, M.A.; Ali, S.A.; Aljeaban, N.A.; Alharbi, B.G. Design and Synthesis of a Novel Corrosion Inhibitor Embedded with Quaternary Ammonium, Amide and Amine Motifs for Protection of Carbon Steel in 1 M HCl. J. Mol. Liq. 2020, 317, 113917. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Chen, S.; Xiao, Y.; Chen, S.; Zheng, P.; Wang, J.; Qu, J.-E. A Promising Hybrid Additive for Enhancing the Performance of Alkaline Aluminum-Air Batteries. Mater. Chem. Phys. 2021, 257, 123787. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Nathan, C.C. Corrosion Inhibitors; CC Nathan: Houston, TX, USA, 1973. [Google Scholar]
- Fawzy, A.; El-Sayed, R.; Al Bahir, A.; Morad, M.; Althagafi, I.; Althagafy, K. Assessment of New Designed Surfactants as Eco-Friendly Inhibitors for the Corrosion of Steel in Acidic Environment and Evaluation of Their Biological and Surface Features: Thermodynamic, Kinetic and Mechanistic Aspects. J. Adhes. Sci. Technol. 2022, 36, 1993–2019. [Google Scholar] [CrossRef]
- Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Springer US: Boston, MA, USA, 1970; ISBN 978-1-4615-8254-0. [Google Scholar]
- Reis, F.M.; de Melo, H.G.; Costa, I. EIS Investigation on Al 5052 Alloy Surface Preparation for Self-Assembling Monolayer. Electrochim. Acta 2006, 51, 1780–1788. [Google Scholar] [CrossRef]
- Quraishi, M.; Rawat, J. Inhibition of Mild Steel Corrosion by Some Macrocyclic Compounds in Hot and Concentrated Hydrochloric Acid. Mater. Chem. Phys. 2002, 73, 118–122. [Google Scholar] [CrossRef]
- Lorenz, W.J.; Mansfeld, F. Determination of Corrosion Rates by Electrochemical DC and AC Methods. Corros. Sci. 1981, 21, 647–672. [Google Scholar] [CrossRef]
- Amer, A.; Sayed, G.H.; Ramadan, R.M.; Rabie, A.M.; Negm, N.A.; Farag, A.A.; Mohammed, E.A. Assessment of 3-Amino-1H-1,2,4-Triazole Modified Layered Double Hydroxide in Effective Remediation of Heavy Metal Ions from Aqueous Environment. J. Mol. Liq. 2021, 341, 116935. [Google Scholar] [CrossRef]
- Wahab, M.M.A.; Sayed, G.H.; Ramadan, R.M.; Mady, A.H.; Rabie, A.M.; Farag, A.A.; Negm, N.A.; Mohamed, E.A. Synergistic Effects of Graphene Oxide Grafted with Barbituric Acid Nanocomposite for Removal of Heavy Metals from Aqueous Solution. Nanotechnol. Environ. Eng. 2022, 1–13. [Google Scholar]
- Abd El-Lateef, H.M.; Shaaban, S.; Khalaf, M.M.; Toghan, A.; Shalabi, K. Synthesis, experimental, and computational studies of water soluble anthranilic organoselenium compounds as safe corrosion inhibitors for J55 pipeline steel in acidic oilfield formation water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126894. [Google Scholar] [CrossRef]
- Shaban, S.M.; a Badr, E.; Shenashen, M.A.; Farag, A.A. Fabrication and Characterization of Encapsulated Gemini Cationic Surfactant as Anticorrosion Material for Carbon Steel Protection in Down-Hole Pipelines. Environ. Technol. Innov. 2021, 23, 101603. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Alakhras, A.I.; Farag, A.A. Electrochemical and Theoretical Examination of Some Imine Compounds as Corrosion Inhibitors for Carbon Steel in Oil Wells Formation Water. Int. J. Electrochem. Sci. 2022, 17, 2212108. [Google Scholar] [CrossRef]
- Farag, A.A. Oil-in-Water Emulsion of a Heterocyclic Adduct as a Novel Inhibitor of API X52 Steel Corrosion in Acidic Solution. Corros. Rev. 2018, 36, 575–588. [Google Scholar] [CrossRef]
- Jeeja Rani, A.T.; Sreelakshmi, T.; Joseph, A. Effect of the Addition of Potassium Iodide and Thiourea on the Corrosion Inhibition Effect of Aqueous Extract of Ayapana Triplinervis towards Mild Steel in HCl at Elevated Temperatures-Theoretical, Electrochemical and Surface Studies. J. Mol. Liq. 2022, 366, 120211. [Google Scholar] [CrossRef]
- Alqarni, N.; El-Gammal, B.; Fawzy, A.; Al Bahir, A.; Toghan, A. Investigation of Expired Ticarcillin and Carbenicillin Drugs for Inhibition of Aluminum Corrosion in Hydrochloric Acid Solution. J. Electrochem. Sci. 2022, 17, 2212113. [Google Scholar] [CrossRef]
- Mobin, M.; Parveen, M.; Huda; Aslam, R. Effect of Different Additives, Temperature, and Immersion Time on the Inhibition Behavior of L-Valine for Mild Steel Corrosion in 5% HCl Solution. J. Phys. Chem. Solids 2022, 161, 110422. [Google Scholar] [CrossRef]
- Douadi, T.; Hamani, H.; Daoud, D.; Al-Noaimi, M.; Chafaa, S. Effect of Temperature and Hydrodynamic Conditions on Corrosion Inhibition of an Azomethine Compounds for Mild Steel in 1M HCl Solution. J. Taiwan Inst. Chem. Eng. 2017, 71, 388–404. [Google Scholar] [CrossRef]
- Dehghani, A.; Ramezanzadeh, B. Rosemary Extract Inhibitive Behavior against Mild Steel Corrosion in Tempered 1 M HCl Media. Ind. Crops Prod. 2023, 193, 116183. [Google Scholar] [CrossRef]
- Toghan, A.; Gadow, H.; Dardeer, H.M.; Elabbasy, H. New promising halogenated cyclic imides derivatives as Potential Corrosion Inhibitors for Carbon Steel in Acidic Environment. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
- Sami, B.A. Highly Efficient Corrosion Inhibition of Carbon Steel in Aggressive Acidic Media with a Pyridazinium-Based Ionic Liquid. Int. J. Electrochem. Sci. 2013, 8, 10788–10804. [Google Scholar]
- Shetty, S.K.; Shetty, A.N. Eco-Friendly Benzimidazolium Based Ionic Liquid as a Corrosion Inhibitor for Aluminum Alloy Composite in Acidic Media. J. Mol. Liq. 2017, 225, 426–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, Y. Corrosion Inhibition of Aluminum in Hydrochloric Acid Solution by Alkylimidazolium Ionic Liquids. Mater. Chem. Phys. 2010, 119, 57–64. [Google Scholar] [CrossRef]
- Dražić, D.M.; Zečević, S.K.; Atanasoski, R.T.; Despić, A.R. The Effect of Anions on the Electrochemical Behaviour of Aluminium. Electrochim. Acta 1983, 28, 751–755. [Google Scholar] [CrossRef]
- Abdelshafi, N.S.; Sadik, M.A.; Shoeib, M.A.; Halim, S.A. Corrosion Inhibition of Aluminum in 1 M HCl by Novel Pyrimidine Derivatives, EFM Measurements, DFT Calculations and MD Simulation. Arab. J. Chem. 2022, 15, 103459. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Moon, S.-M. Corrosion Mechanism of Pure Aluminium in Aqueous Alkaline Solution. J. Solid State Electrochem. 2000, 4, 267–272. [Google Scholar] [CrossRef]
- Moon, S.; Pyun, S.-I. The Formation and Dissolution of Anodic Oxide Films on Pure Aluminium in Alkaline Solution. Electrochim. Acta 1999, 44, 2445–2454. [Google Scholar] [CrossRef]
- Prakashaiah, B.G.; Vinaya Kumara, D.; Anup Pandith, A.; Nityananda Shetty, A.; Amitha Rani, B.E. Corrosion Inhibition of 2024-T3 Aluminum Alloy in 3.5% NaCl by Thiosemicarbazone Derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium Strumarium Leaves Extracts as a Friendly Corrosion Inhibitor of Low Carbon Steel in Hydrochloric Acid: Kinetics and Mathematical Studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Olawale, O.; Ogunsemi, B.T.; Bello, J.O.; Ikubanni, P.P.; Ogundipe, S.J.; Abayomi, T.S. Optimisation and Modelling of Aluminium Corrosion Inhibition Using Almond Prunus Amydgdalus) Fruit Leaves Extract as Green Inhibitor in Hcl Acidic Medium. Int. J. Mech. Eng. Technol. 2018, 9, 1274–1285. [Google Scholar]
- Migahed, M.A.; Farag, A.A.; Elsaed, S.M.; Kamal, R.; Abd El-Bary, H. Corrosion Inhibition of Carbon Steel in Formation Water of Oil Wells by Some Schiff Base Non Ionic Surfactants. In Proceedings of the European Corrosion Congress EUROCORR 2009, Nice, France, 6–10 September 2009. [Google Scholar]
- Hashem, H.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M. Experimental and Theoretical Assessment of Benzopyran Compounds as Inhibitors to Steel Corrosion in Aggressive Acid Solution. J. Mol. Struct. 2022, 1249, 131641. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Hashem, H.E.; Azmy, E.M.; Negm, N.A.; Farag, A.A. Synthesis, Structural Analysis, and Inhibition Approach of Novel Eco-Friendly Chalcone Derivatives on API X65 Steel Corrosion in Acidic Media Assessment with DFT & MD Studies. Environ. Technol. Innov. 2021, 24, 101966. [Google Scholar] [CrossRef]
- Toghan, A.; Fawzy, A.; Al Bahir, A.; Alqarni, N.; Sanad, M.M.S.; Khairy, M.; Alakhras, A.I.; Farag, A.A. Computational Foretelling and Experimental Implementation of the Performance of Polyacrylic Acid and Polyacrylamide Polymers as Eco-Friendly Corrosion Inhibitors for Copper in Nitric Acid. Polymers 2022, 14, 4802. [Google Scholar] [CrossRef]
- Farag, A.A.; Abdallah, H.E.; Badr, E.A.; Mohamed, E.A.; Ali, A.I.; El-Etre, A.Y. The Inhibition Performance of Morpholinium Derivatives on Corrosion Behavior of Carbon Steel in the Acidized Formation Water: Theoretical, Experimental and Biocidal Evaluations. J. Mol. Liq. 2021, 341, 117348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).