Abstract
The leather industry is currently between two opposing paths: on the one hand, recent legislative trends in terms of the eco-sustainability of industrial processes are leading leather manufacturing towards the development of cleaner production methods; on the other hand, the spread of new alternative materials to leather is driving the leather industry to improve its competitiveness by developing new innovative and high-quality products. Leather finishing is one of the most important phases of leather production, and is capable of improving its quality and organoleptic properties. However, this phase is characterized by the use of polluting chemical products, such as volatile organic compounds, potentially toxic crosslinking agents, and hardly biodegradable resins. In this context, this research work aims to develop a finishing formulation capable of giving leather the durability and quality properties required by the market, while at the same time, being eco-sustainable. Specifically, the aim of the present work is to suggest a new finishing formulation in terms not only of green technology but also of a circular production flow, by recovering solid leather wastes. The developed finishing system is based on the application of collagen, extracted from tanned wastes through an enzymatic treatment, to be cross-linked and bound to the leather surface. This new bio-based leather finish is compared to a resin-based leather finish, and shows the same quality standards as those requested by the market.
1. Introduction
Leather finishing is the last step of the leather manufacturing processes and is responsible for the final properties and aesthetic characteristics of leather: hydrophobicity, color fastness, rub resistance, gloss, and color homogeneity; these properties are directly linked to the composition of finishing formulations [1,2].
Finishing involves two coating steps: the application of a base coat and of a top coat. The base coat mainly involves the application of solvents and binders able to interact with the surface of crust leather, thus modifying its chemical properties and allowing the fixation of pigments and dyes. The top coat protects the finished leather surface by means of lacquers or polyurethanes [3]. To achieve high performance, the base and top coats need to be highly compatible; the base coat, indeed, acts as a bridge between the leather and the top coat.
Finishing formulations also comprise film-forming agents, which can be classified into resin-based and protein-based formulations. Resin-based formulations confer high-standard properties, such as color fastness and hydrophobicity, although they often contain polluting and toxic compounds [1].
Protein-based formulations provide a more natural appearance, with casein being the most widely used and well-known binder for this type of finishing. Casein-finished leather is characterized by brightness and thermostability; however, it presents low water resistance and rub fastness [4].
Several methods to improve the properties of protein-based formulations have been developed, mainly based on cross-linking agents capable of increasing film-forming capability [5].
As a result, current leather finishing technologies must be innovated to increase product quality while reducing polluting and hazardous chemicals [6,7,8,9]. Therefore, the leather industry must develop new bio-based systems for finishing, aiming at more ecologically-friendly production processes. Developing new bio-based finishing systems with highly compatible base and top coats, as well as highly compatible finishing formulations and leather surfaces, is the most challenging objective in this field [10].
In this perspective, collagen-based formulations are of interest, since collagen is highly compatible with leather [11] and is capable of conferring resistance to leather without affecting its natural appearance. Furthermore, when applied as a finishing agent, collagen does not require a high degree of purity, so it can be isolated from a wide range of sources. From a circularity point of view, collagen derived from solid leather waste can be used effectively in finishing formulations. As reported by Popa et al., indeed, collagen polypeptides were used as a substitute for casein in leather finishing formulations; the hydrolyzed polypeptides were treated with glutaraldehyde to increase the hydrophobicity of the formulation [12].
In the present study, collagen, previously enzymatically extracted from leather-tanned wastes, was used as an ingredient for the base and top coats of leather finishing formulations. Contrary to the studies reported in the literature, the extracted collagen was treated with transglutaminase to cross-link with casein, in order to combine the finishing properties of casein to collagen’s binding properties and compatibility with leather. The formulations were first prepared as films to test their properties, and then, sprayed on the leather. The developed formulations were compared to a standard resin-based finishing formulation, in terms of the physical properties of the leather and the biodegradability of the solutions.
2. Materials and Methods
2.1. Materials
Vegetable-tanned bovine shavings, organic-tanned bovine shavings, and mineral-tanned bovine shavings were collected from the Italian Leather Research Institute (Veneto district, Italy).
Bovine crust leather was supplied by the A3 Leather Innovation Center (Igualada, Spain).
Casein from bovine milk was purchased from Sigma-Aldrich (Milan, Italy). Microbial transglutaminase Activa WM was provided by Ajinomoto (Hamburg, Germany).
Luron Binder U, Melio Resin A-943, Aqualen Top 2006, Aqualen Top DC-2060, and metallic dye and pigment were supplied by Stahl (Parets del Vallès, Spain).
Silicon was provided by Pielcolor (Parets del Vallès, Spain).
2.2. Extraction of Collagen
Vegetable-tanned bovine shavings, organic-tanned bovine shavings, and mineral-tanned bovine shavings were processed through an enzymatic treatment to extract collagen, as previously reported [13].
2.3. Film Formation
Aqueous solutions of extracted collagen and casein (total solids value 30%) were prepared by varying the collagen/casein mass ratio (0:1, 1:1, 2:1, 5:1, 10:1, and 1:0) and by adding 0 or 50 U/g of transglutaminase. Consequently, 5 mL of the solutions were cast into a silicon plate (5 cm × 3 cm) and air-dried in a ventilated incubator at room temperature for 48 h. A Softness Tester (ST300 IUP/36, SDL Atlas, SC, USA) was used to test the hardness of the films.
2.4. BOD and COD Analyses
Determination of the chemical oxygen demand (COD) was performed according to UNI EN ISO 6060:1986 [14]; the samples were treated using potassium dichromate and incubated at 160 °C for 30 min (NANOCOLOR CBS 1500 analysis system, MACHEREY-NAGEL).
The determination of biochemical oxygen demand (BOD) was performed according to UNI EN ISO 5815-1 [15]; the sample to be tested was serially diluted in seeded water and stored in the dark at RT for 5 days; after the incubation period, the oxygen concentration was measured.
2.5. Application of Finishing Formulations
The finishing formulations are shown in Table 1 and Table 2 Leather samples (25 cm × 20 cm) were sprayed twice with the base coat formulations using a spray gun, dried at 40 °C in a heater, and then, pressed at 70 °C at 100 kg/cm2 for 2 s. The base coat was applied and dried again, twice. Leather samples were then sprayed twice with the top coat formulations, dried at 40 °C in a heater, and then, pressed at 75 °C at 100 kg/cm2 for 2 s. Finished leather was stored for 5 days to let the finishing settle.

Table 1.
Base coat formulations.

Table 2.
Top coat formulations.
2.6. Mechanical Tests
Softness was measured as the distention of the leather produced via pressing (in the range of 5.5 and 6.1 N) using a Softness tester ST300A, according to UNI EN ISO 17235:2015 [16].
Color fastness in response to water spotting was performed, according to UNI EN ISO 15700:1998 [17], by depositing a water drop on the leather surface and evaluating leather damage after 24 h of settling.
Color fastness to artificial light, according to UNI EN ISO 105-B02 [18], was assessed by noting the color change of the leather surface after exposure to a Xenon lamp for 24 h.
According to UNI EN ISO 11640:2018 [19], color fastness to rubbing was performed by swiping dry or wet felt on the leather surface for 150 cycles (dry condition) or 50 cycles (wet condition), using a Rub Fastness Tester (Veslic Giuliani, Torino, Italy); after the test, the damage to the leather surface and color transfer to the felt were evaluated.
3. Results and Discussion
3.1. Film Formation
Wet-white-tanned shavings were enzymatically treated to extract collagen; the extracted collagen maintained its structure and, despite the presence of metals and polyphenols, it was able to undergo both enzymatically and chemically mediated cross-linking [13].
The optimal conditions for an enzymatic cross-linking reaction between collagen and casein were previously selected: we incubated the collagen and casein (mass ratio 10:1) with 50 U/g of transglutaminase for 4 h at 40 °C, so that nearly 80% of the cross-linked collagen was obtained [13].
Collagen-based films were developed to assess their applicability to leather finishing. The hardness of the films was measured according to the Shore A scale, whose values are commonly used to report the hardness of materials in a range from 0 to 100, where 0 and 100 are related to extra-soft and extra-hard materials, respectively. Assuming that the hardness of films is proportional to the cross-linking degree [20], different mass ratios of collagen and casein were tested to study their influence on film properties. All films are transparent and glossy, but they present different hardness values; condition 3 of Table 3 was selected on the basis of its lower Shore value.

Table 3.
Properties and hardness values of films (photos 4 cm × 1.5 cm, magnification 1×), obtained by incubating collagen and casein (0:1, 1:1, 2:1, 5:1, 10:1, and 1:0) with 50 U/g transglutaminase.
The 2:1 collagen/casein solution was selected to replace the synthetic resins in a standard resin-based finishing formulation (Table 1 and Table 2); thus, the films of the finishing agents were developed (in proportion to each other, as reported in Table 1 and Table 2).
From the data concerning the film properties (Table 4), it is clear that solutions containing both collagen and casein are compatible with both polyurethanes and acrylic resins, since all the films appear to be homogeneously distributed. When collagen and casein are added to polyurethanes, the finishing films become more appreciably transparent, a valuable property in leather finishing; moreover, the films based solely on collagen and casein show interesting properties even though their hardness increases.
3.2. Biodegradability
When the aim is to develop a new eco-friendly finishing system, the biodegradability of the formulation plays a key role. The origin of the collagen used, i.e., tanned shavings, involves the presence of contaminants that may affect its biodegradability [13]. Therefore, to assess its environmental impact, the biodegradability of the selected collagen and casein solution (2:1) was measured as the ratio between the biochemical oxygen demand (BOD) and chemical oxygen demand (COD) [21]. When comparing collagen/casein biodegradability with that of the polyurethanes and acrylic resins used in the standard finishing formulation, it was found that collagen/casein solutions were more biodegradable than resin-based formulations (Table 5), especially when compared to Polyurethane 2 and acrylic resins that are classified in the toxic zone, according to their levels of biodegradability [22].

Table 5.
Biodegradability (BOD/COD ratio) of collagen/casein solution, Polyurethane 1, Polyurethane 2, and acrylic resins of finishing formulations.
3.3. Application of Collagen and Casein to Finishing Formulations
The leather samples were treated by combining each base coat with each top coat formulation (Table 2 and Table 3). The physical properties of the finished leather were measured and are reported in Table 6.

Table 6.
Physical properties of finished leather.
All samples show very high color fastness to dry rubbing, but only samples 1, 4, 7, and 10 maintain the same values in wet conditions. Wet rub fastness is commonly due to the protective action of the top coat [23], as demonstrated by the results of samples 1, 4, and 7, which were treated with the top coat standard formulation, assuring high performance. However, when resins are completely replaced by collagen and casein (sample 10), the high compatibility between the base and top coats assures high wet rub fastness.
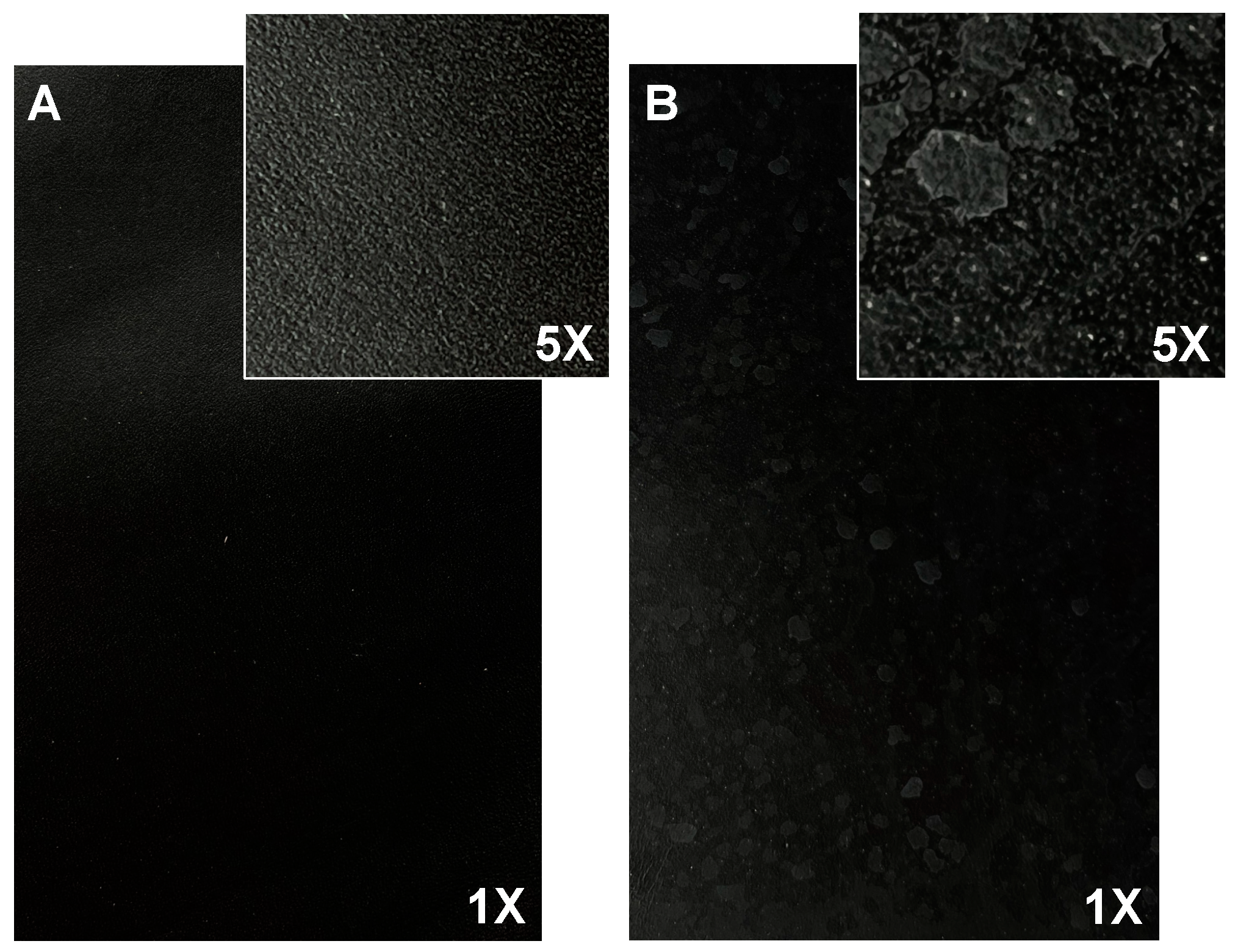
The magnification images of the samples show that sample 10 (Figure 1B) presents white spots on its surface, possibly caused by the accumulation of salts present in the extracted collagen stock solution.
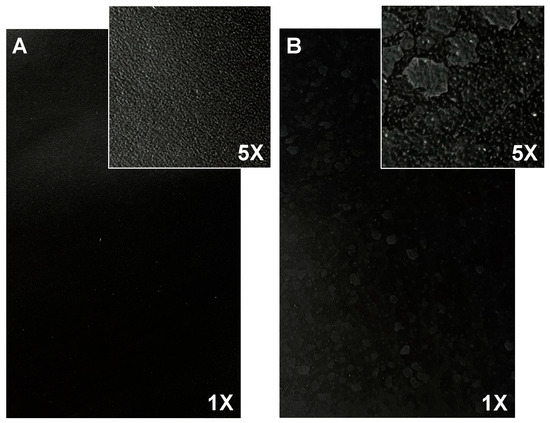
Figure 1.
Finished leather surface (photos 17 cm × 10 cm, magnification 1× and 5×) of (A) sample 1 and (B) sample 10.
3.4. Optimization of Developed Finishing Formulation
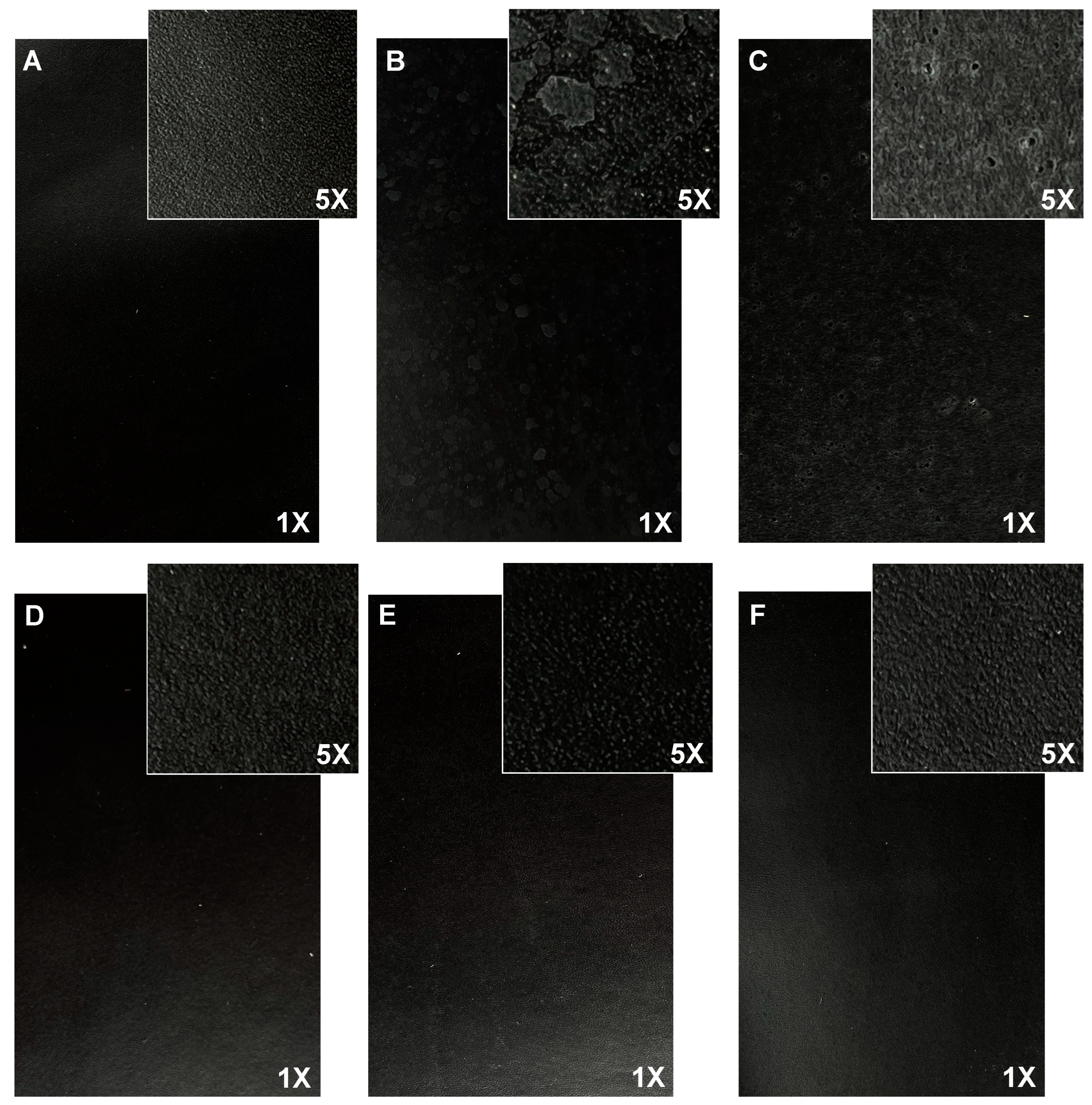
Although very suitable physical properties were achieved in sample 10 without adding any resins to the finishing treatment, sample 10 (BC3 + TC3) was studied in more detail. To avoid the formation of white spots on the leather surface, the total solids content (TSC) of the collagen/casein solution was gradually decreased. Panels D, E, and F of Figure 2 indicate that after decreasing the total solids content in the treatment to below 20%, no white spots were formed.
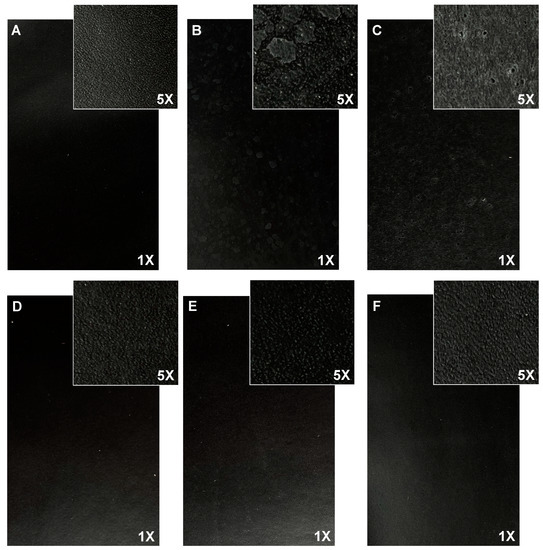
Figure 2.
Finished leather surface (photos 17 cm × 11 cm, magnification 1× and 5×) of (A) BC + TC; (B) BC3 (30%TSC) + TC3 (30%TSC); (C) BC3 (20%TSC) + TC3 (20%TSC); (D) BC3 (12%TSC) + TC3 (7%TSC); (E) BC3 (10%TSC) + TC3 (7%TSC); and (F) BC3 (7%TSC) + TC3 (10%TSC).
When looking at the physical properties of all the samples (Table 7), the total solids content appears to affect the color fastness, as well. In particular, when dosed at a concentration of less than 12% in the base coat (samples E and F), the color fastness to wet rubbing and water spots is diminished. Therefore, sample D treated with 12% base coat and 7% top coat, was selected due to the absence of white spots on its surface and for its physical properties, comparable to those of the reference (sample A—standard formulation).

Table 7.
Physical properties of leather treated with (A) BC + TC (standard formulation), (B) BC3 (30% TSC) + TC3 (30% TSC), (C) BC3 (20% TSC) + TC3 (20% TSC), (D) BC3 (12% TSC) + TC3 (7% TSC), (E) BC3 (10% TSC) + TC3 (7% TSC), and (F) BC3 (7% TSC) + TC3 (12% TSC).
To increase the softness of the leather treated with this improved bio-based formulation, the concentration of enzymes, related to the cross-linking solution, was decreased from 50 U/g, as in the initial formulation, to 5 U/g, thus reducing the cross-linking degree between collagen and casein.
Decreasing the amount of enzyme used, indeed, increases softness, but when the amount of transglutaminase is lower than 15 U/g, the color fastness severely worsens (Table 8).

Table 8.
Physical properties of finished leather with (A) BC + TC; (B) BC3 (12% TSC) + TC3 (7% TSC) 50 U/g TGase; (C) BC3 (12% TSC) + TC3 (7% TSC) 25 U/g TGase; (D) BC3 (12% TSC) + TC3 (7% TSC) 15 U/g TGase; (E) BC3 (12% TSC) + TC3 (7% TSC) 10 U/g TGase; and (F) BC3 (12% TSC) + TC3 (7% TSC) 5 U/g TGase.
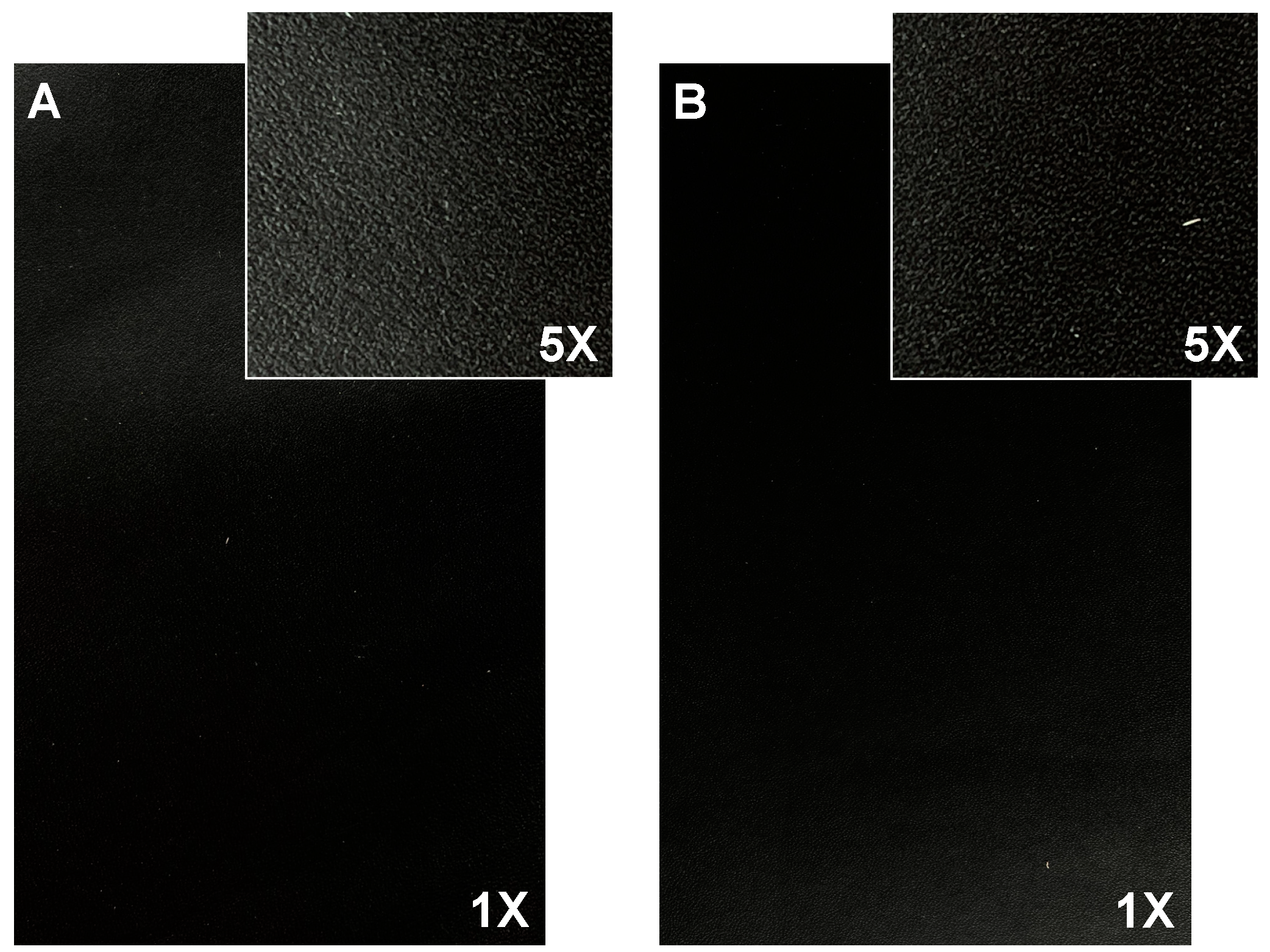
The selected finishing formulation (BC3 (12% TSC) + TC3 (7% TSC) + 15 U/g of TGase) allows for the manufacture of leathers with the same surface properties as the standard formulation (Figure 3), according to the quality criteria reported in the UNI EN ISO 10826:2020 [24].
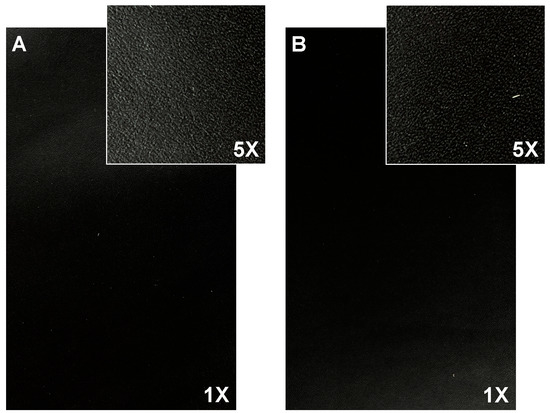
Figure 3.
Finished leather surface (photos 17 cm × 11 cm, magnification 1× and 5×) of (A) standard finishing formulation (BC + TC) and (B) developed finishing formulation (BC3 (12% TSC) + TC3 (7% TSC) + 15U/g TGase).
By optimizing both the composition of the bio-based finishing formulation and the amount of the enzymatic cross-linker, it was possible to completely replace polyurethanes and acrylic resins in leather finishing.
3.5. Preliminary Cost Analysis
To assess the economic feasibility of the new bio-based finishing formulation, a preliminary cost analysis was performed [25]. In Table 9, the costs of producing 1 kg of standard and bio-based formulations are reported and compared. The bio-based formulation is nearly 60% more expensive than the standard formulation, due to the high costs of collagen, enzyme, and casein extraction. However, this cost analysis was performed on a laboratory scale, with high-purity reagents.

Table 9.
Cost analysis of the production of 1 kg of standard and bio-based finishing formulations.
4. Conclusions
The reduction of chemicals and the valorization of solid wastes are challenging and complex issues in the transition of the leather industry towards cleaner production. In the present study, collagen extracted from leather solid wastes was used to develop a new bio-based finishing formulation.
In finishing operations, the developed system allows for the replacement of recalcitrant polyurethanes and acrylic resins with waste-extracted collagen through its enzymatic cross-linking with casein.
Physical softness and color fastness tests showed that this formulation was proven innovative, eco-sustainable, and capable of conferring the same quality standards to leather as resin-based formulations.
Author Contributions
Conceptualization, investigation, methodology, validation, formal analysis, visualization, and writing—original draft, M.G. Validation, supervision, and writing—review and editing, A.B. Conceptualization, validation, visualization, supervision, project administration, writing—original draft, and writing—review and editing, G.S. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry for University and Research (MUR) under the National Operative Program (PON): Programma Operativo Nazionale Ricerca e Innovazione 2014–2020, Fondo Sociale Europeo (FSE), Azione I.1 “Dottorati Innovativi con caratterizzazione Industriale”, as a part of the doctoral project “Green Chemistry and Circular Economy as alternative strategies for the traditional leather manufacturing industry”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, Y.; Hu, J.; Xin, Z. Facile Preparation of High Solid Content Waterborne Polyurethane and Its Application in Leather Surface Finishing. Prog. Org. Coatings 2019, 130, 8–16. [Google Scholar] [CrossRef]
- Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Recent Trends in Leather Making: Processes, Problems, and Pathways. Crit. Rev. Environ. Sci. Technol. 2005, 35, 37–79. [Google Scholar] [CrossRef]
- Tamilselvi, A.; Jayakumar, G.C.; Sri Charan, K.; Sahu, B.; Deepa, P.R.; Kanth, S.V.; Kanagaraj, J. Extraction of Cellulose from Renewable Resources and Its Application in Leather Finishing. J. Clean. Prod. 2019, 230, 694–699. [Google Scholar] [CrossRef]
- Dong, Q.; Hsieh, Y. Lo Acrylonitrile Graft Copolymerization of Casein Proteins for Enhanced Solubility and Thermal Properties. J. Appl. Polym. Sci. 2000, 77, 2543–2551. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Q.; Li, J.; Du, G.; Chen, J. Characterization of Gelatin and Casein Films Modified by Microbial Transglutaminase and the Application as Coating Agents in Leather Finishing. J. Am. Leather Chem. Assoc. 2012, 107, 13–20. [Google Scholar]
- Ollé, L.; Bacardit, A.; Borràs, M.D.; Morera, J.M.; Cobos, M.; Borràs, E. Binders Cross-Linked with Polyaziridine. Study of Cross-Linked Polymers for Aqueous Finishing. Part Iii: Influence of a Cationic Pre-Bottom. J. Soc. Leather Technol. Chem. 2009, 93, 91–96. [Google Scholar]
- Bacardit, A.; Ollé, L.; Borràs, M.D.; Cobos, M.; Jericó, A.; Solé, O. Aqueous Finishing with Polycarbodiimide Cross-Linked Binders. J. Soc. Leather Technol. Chem. 2010, 94, 117–123. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic Hazards of Leather Industry and Technologies to Combat Threat: A Review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Dettmer, A.; Schacker Dos Anjos, P.; Gutterres, M. Enzymes in Leather Industry. J. Am. Leather Chem. Assoc. 2013, 108, 146–158. [Google Scholar]
- Pahlawan, I.F.; Sutyasmi, S.; Griyanitasari, G. Hydrolysis of Leather Shavings Waste for Protein Binder. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Dalian, China, 28–29 September 2019; Volume 230. [Google Scholar]
- Haines, B.M.; Barlow, J.R. The Anatomy of Leather. J. Mater. Sci. 1975, 10, 525–538. [Google Scholar] [CrossRef]
- Popa, E.; Balau Mindru, I.; Pruneanu, M.; Balau Mindru, T. Potential Use of Collagen Hydrolysates from Chamois Leather Waste as Ingredient in Leather Finishing Formulations. Ann. Univ. Oradea Fascicle Text. Leatherwork 2018, 17, 203–209. [Google Scholar]
- Gargano, M.; Florio, C.; Amoresano, A.; Sannia, G.; Lettera, V. Leather Industry towards Circular Economy: Enzymatic Extraction of Potential High Added-Value Products from Tanned Wastes. J. Environ. Manage. 2023. [Google Scholar]
- International Organization for Standardization International Standard, ISO 6060:1989. Water Quality–Determination of the Chemical Oxygen Demand. 1989.
- International Organization for Standardization International Standard, ISO5815-1. Water Quality -Determination of Biochemical Oxygen Demand After n Days (BODn)−Part 1: Dilution and Seeding Method with Allylthiourea Addition 2019.
- IULTCS International Union of Leather Technologists and Chemists Societies International Standard, ISO 17235:2015 | IULTCS/IUP 36. Leather—Physical and Mechanical Tests—Determination of Softness 2015.
- IULTCS International Union of Leather Technologists and Chemists Societies International Standard, ISO 15700:1998 | IULTCS/IUF 420 Leather—Tests for Colour Fastness—Colour Fastness to Water Spotting 1998.
- International Standard, ISO 105-B02:2014 Textiles—Tests for Colour Fastness—Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test 2014.
- IULTCS International Union of Leather Technologists and Chemists Societies International Standard, ISO 11640:2018 | IULTCS/IUF 450 Leather—Tests for Colour Fastness—Colour Fastness to Cycles of to-and-Fro Rubbing 2018.
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Samudro, G.; Mangkoedihardjo, S. Review on Bod, Cod and Bod/Cod Ratio: A Triangle Zone for Toxic, Biodegradable and Stable Levels. Int. J. Acad. Res. 2010, 2, 235–239. [Google Scholar]
- Bader, A.C.; Hussein, H.J.; Jabar, M.T. BOD: COD Ratio as Indicator for Wastewater and Industrial Water Pollution. Int. J. Spec. Educ. 2022, 37, 2022. [Google Scholar]
- Zehra, B.; Rub Nawaz, H.; Solangi, B.A.; Nadeem, U.; Zeeshan, M. Preparation and Evaluation of Non-Toxic Top-Coatings for Leather to Minimize Pollutants in Leather Finishing Process. Int. J. Renew. Energy Eng. Res. 2020, 1, 11–15. [Google Scholar]
- UNI- Ente Nazionale Italiano di Unificazione International Standard, UNI 1086:2020 Leather — Characteristics and requirements of leather for leather goods and accessories, 2020.
- Tavoosi, Y.; Behin, J. Unhairing of Bovine Hide Using Wastewater from Merox Unit of Oil Refinery: Techno-Environmental Aspect. Environ. Sci. Pollut. Res. 2022, 29, 28180–28193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).