Mechanism of Sodium Dodecyl Diphenyl Ether Disulfonate Filled Hydrotalcite Inhibiting the Photo-Degradation of Polyvinyl Chloride under Different Ranges of Ultraviolet Wavelength Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MADS Anion-Intercalated LDH
2.3. Preparation of LDHs/PVC Composite Materials
2.4. UV Photoaging Test
2.5. Characterization
3. Results and Discussion
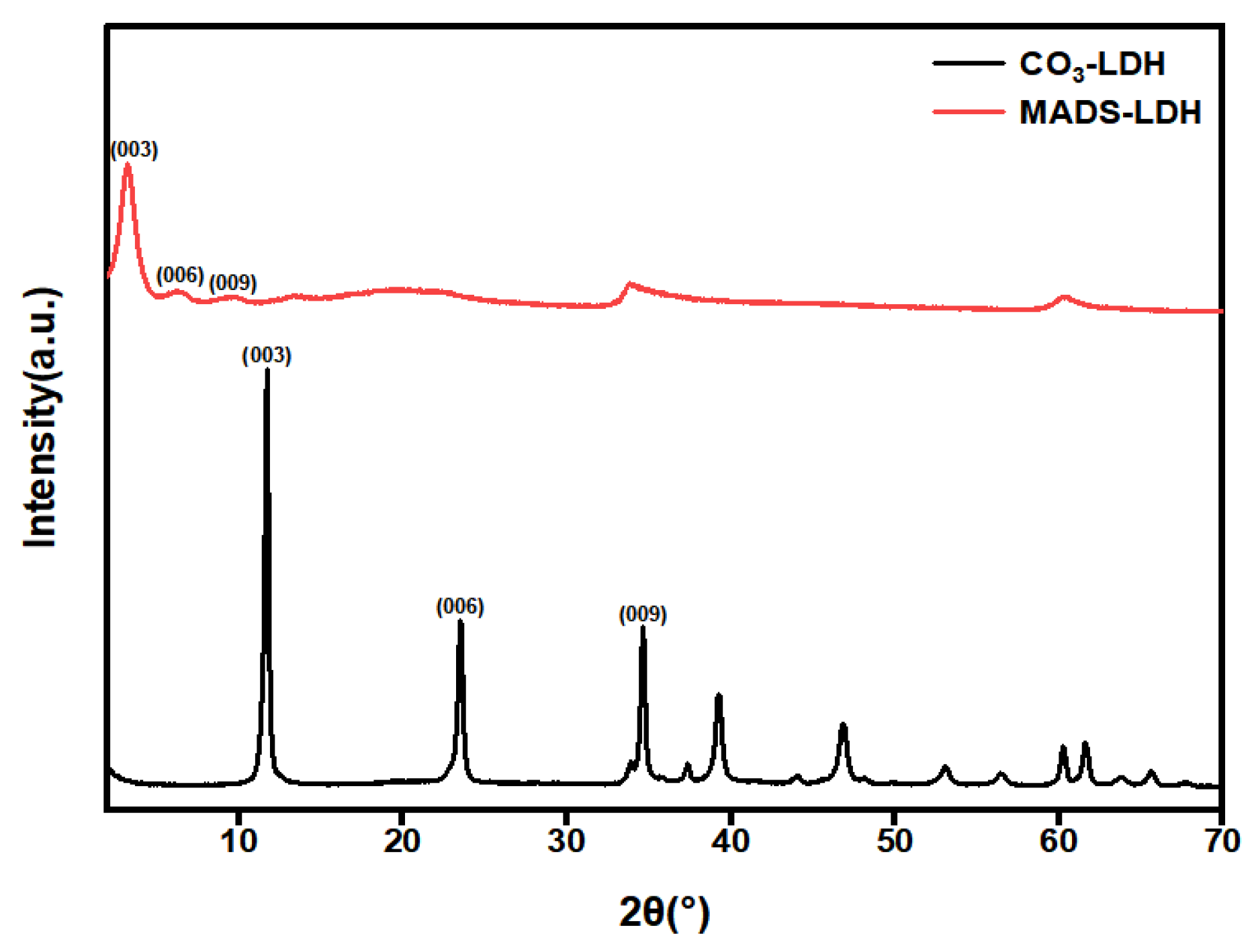
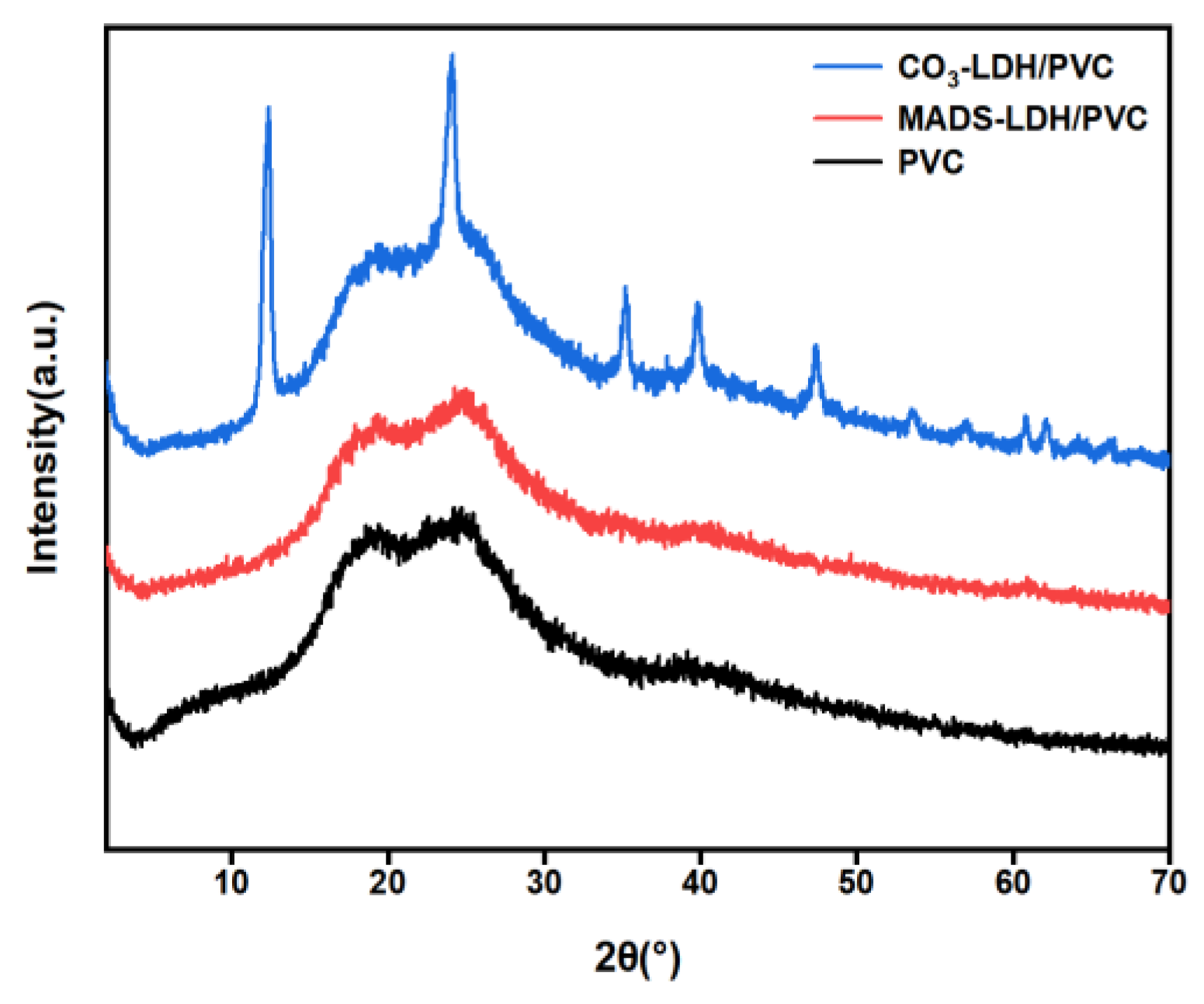
3.1. XRD Analysis
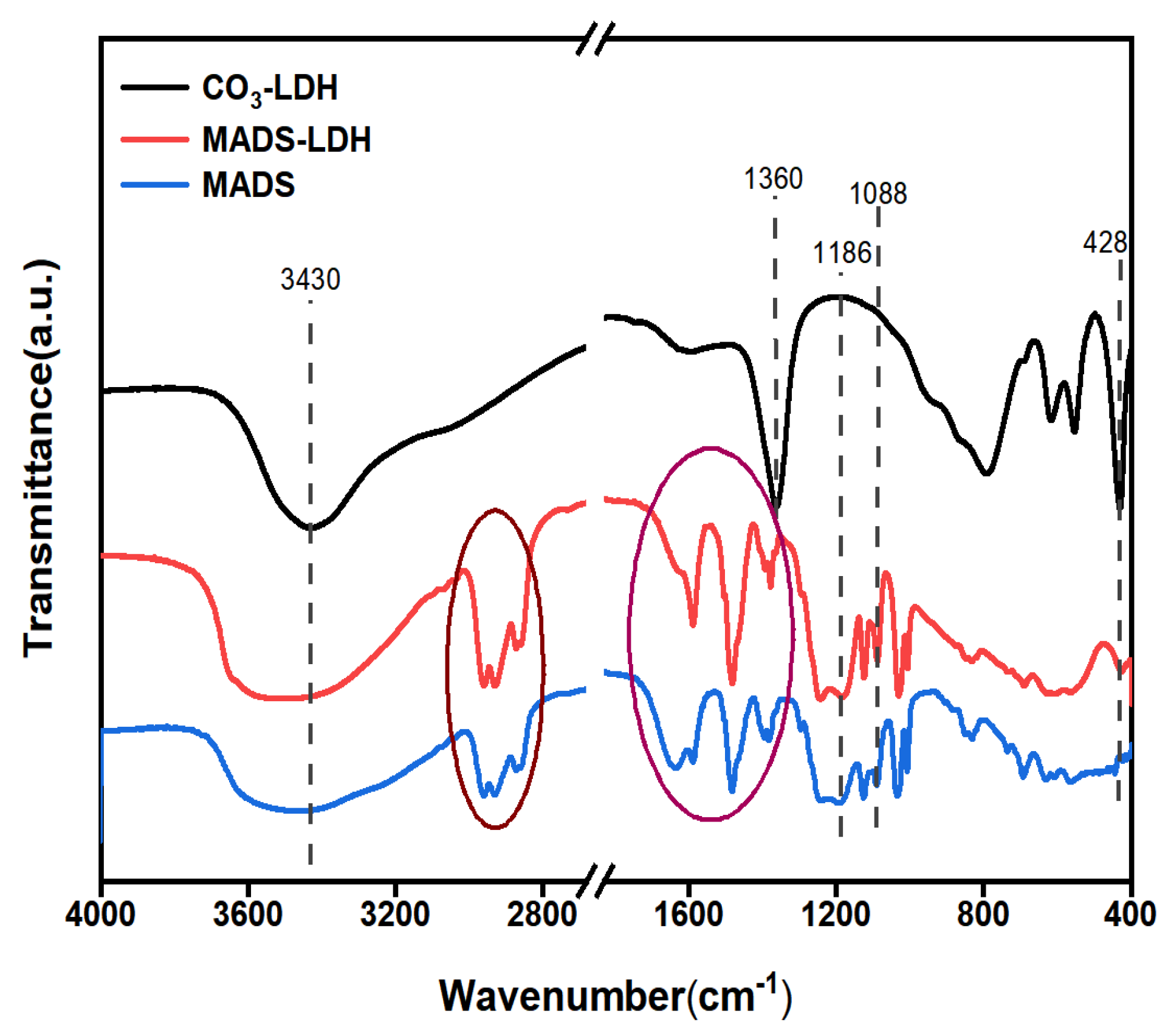
3.2. FT-IR Analysis
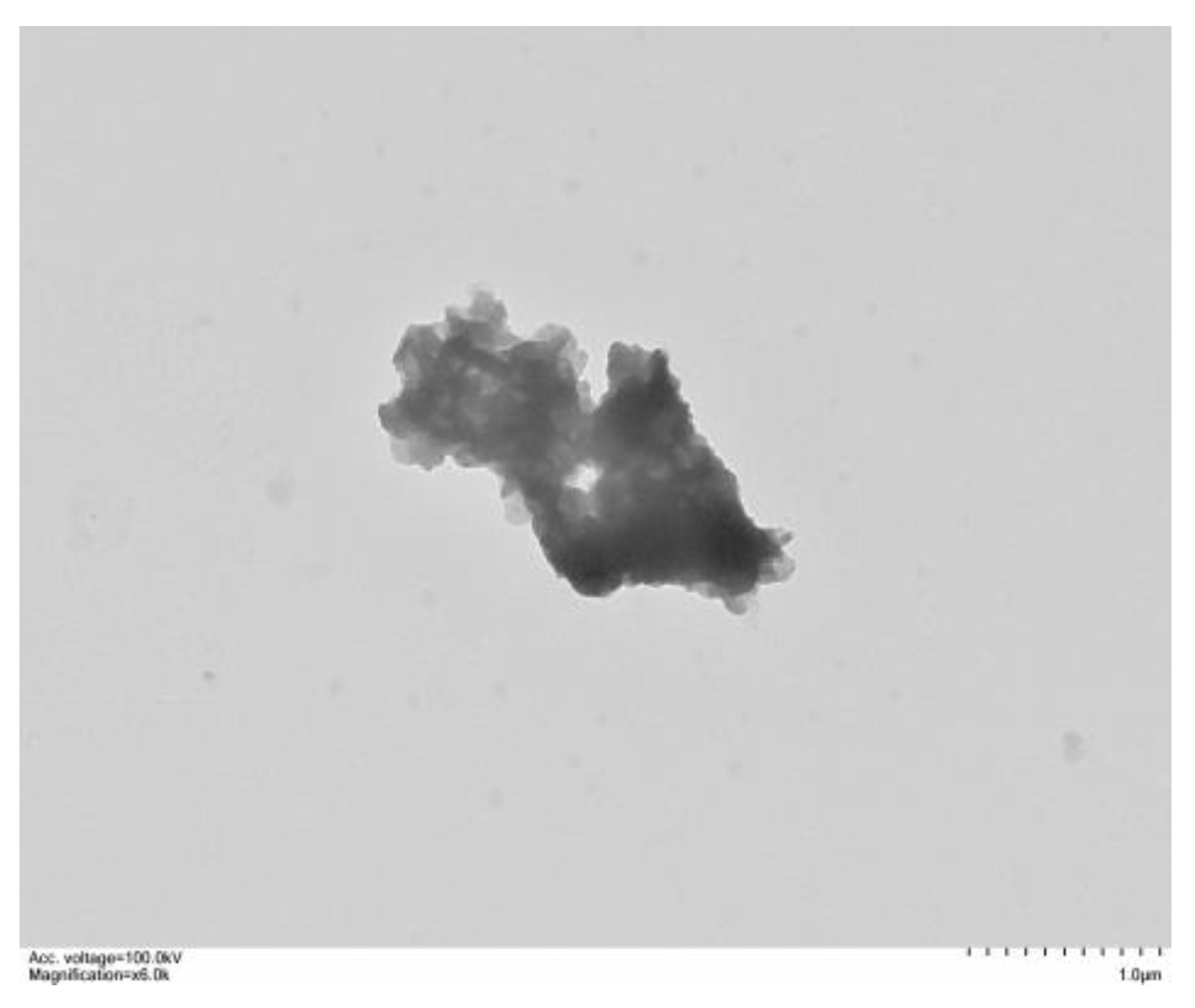
3.3. SEM and TEM Analysis
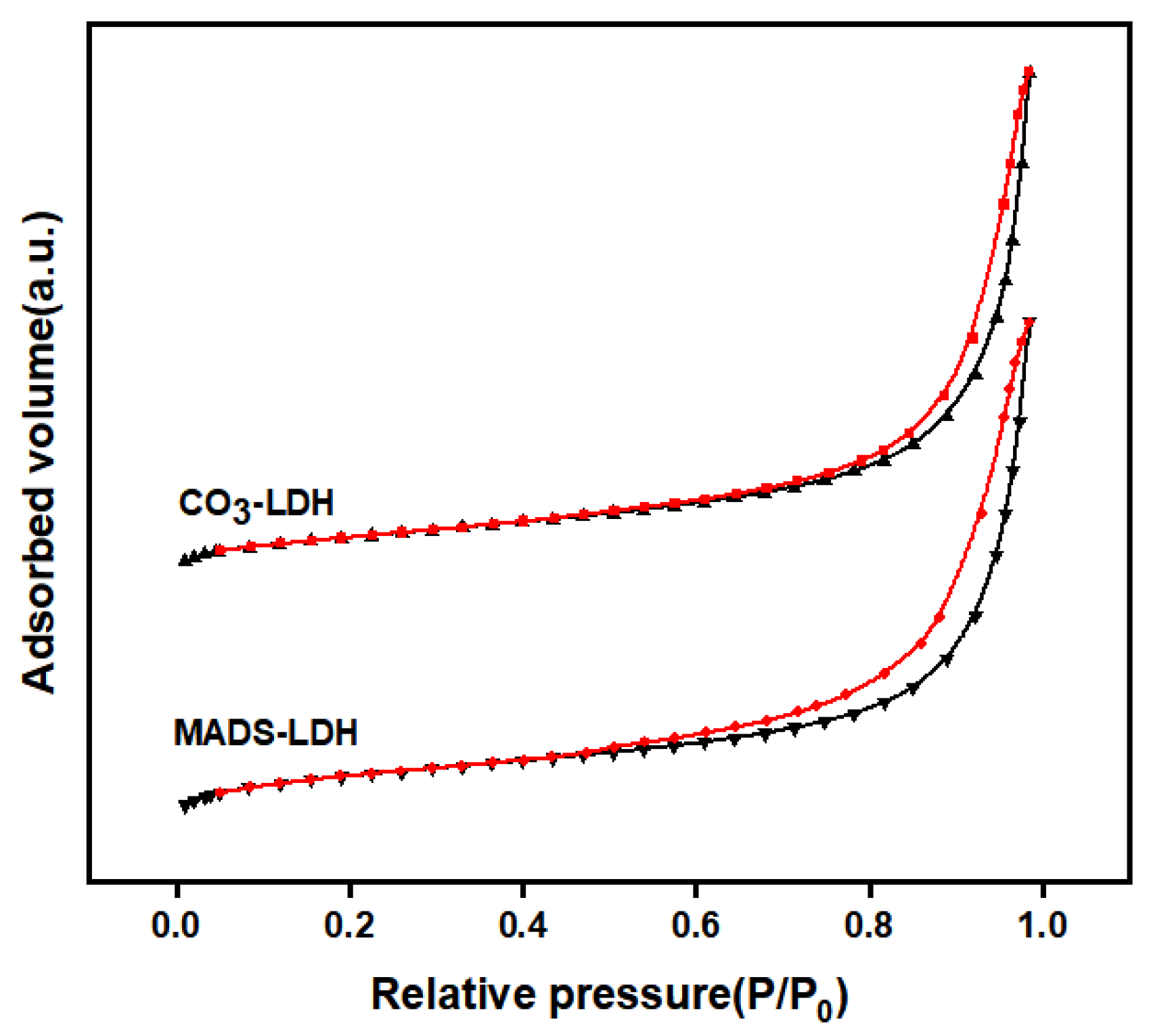
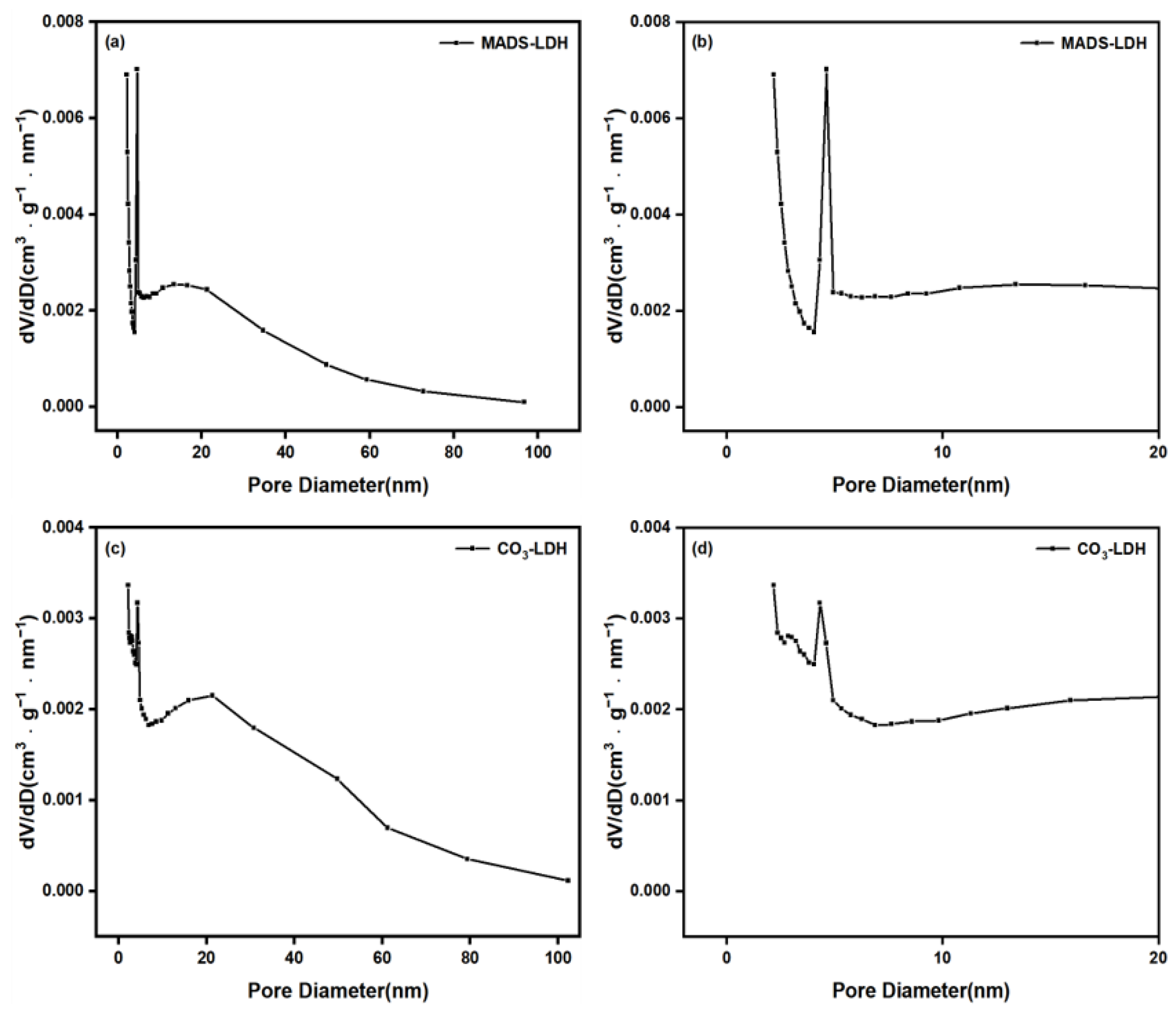
3.4. BET Analysis
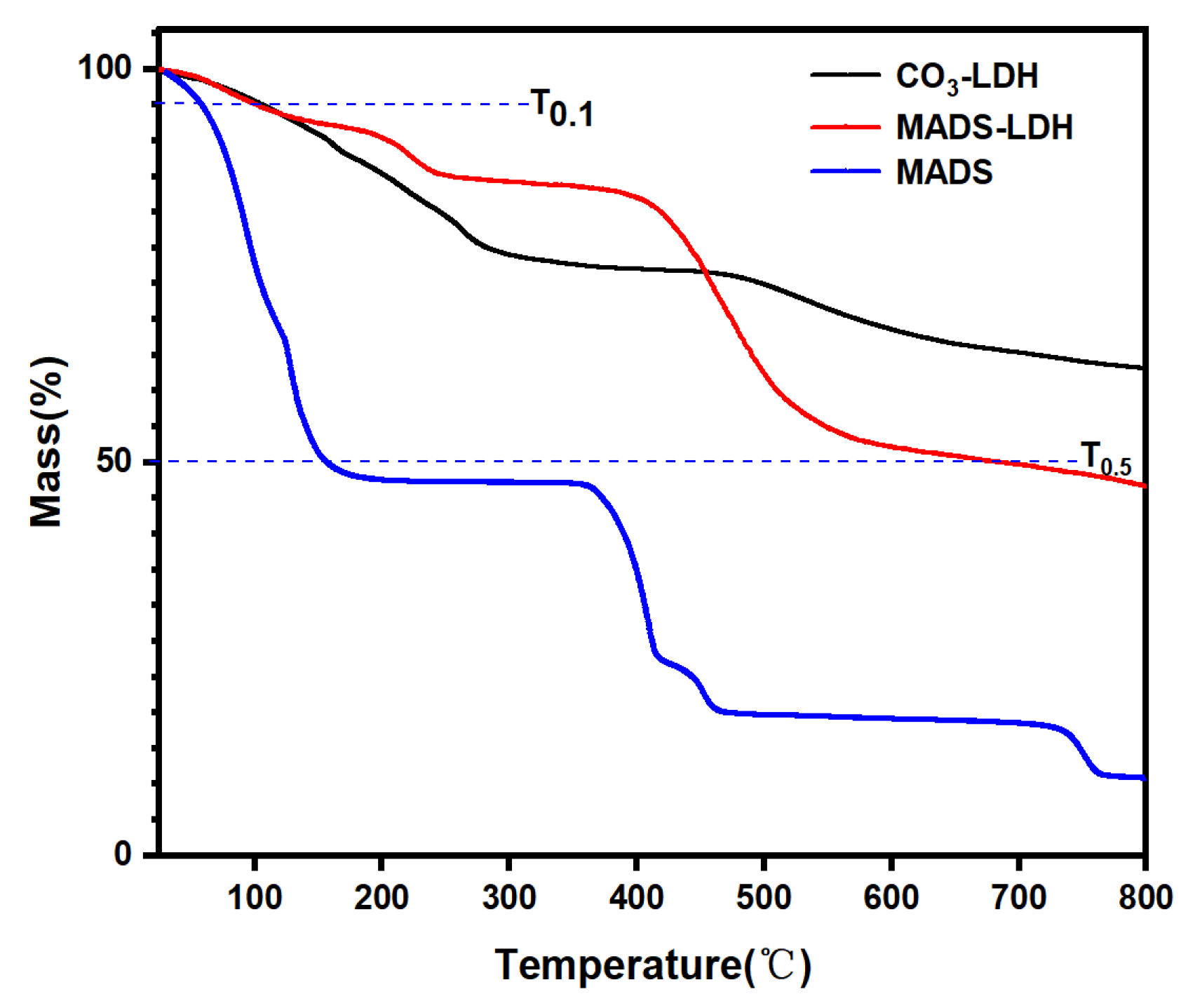
3.5. TGA Analysis
3.6. UV Absorption of CO3-LDH, MADS-LDH and MADS
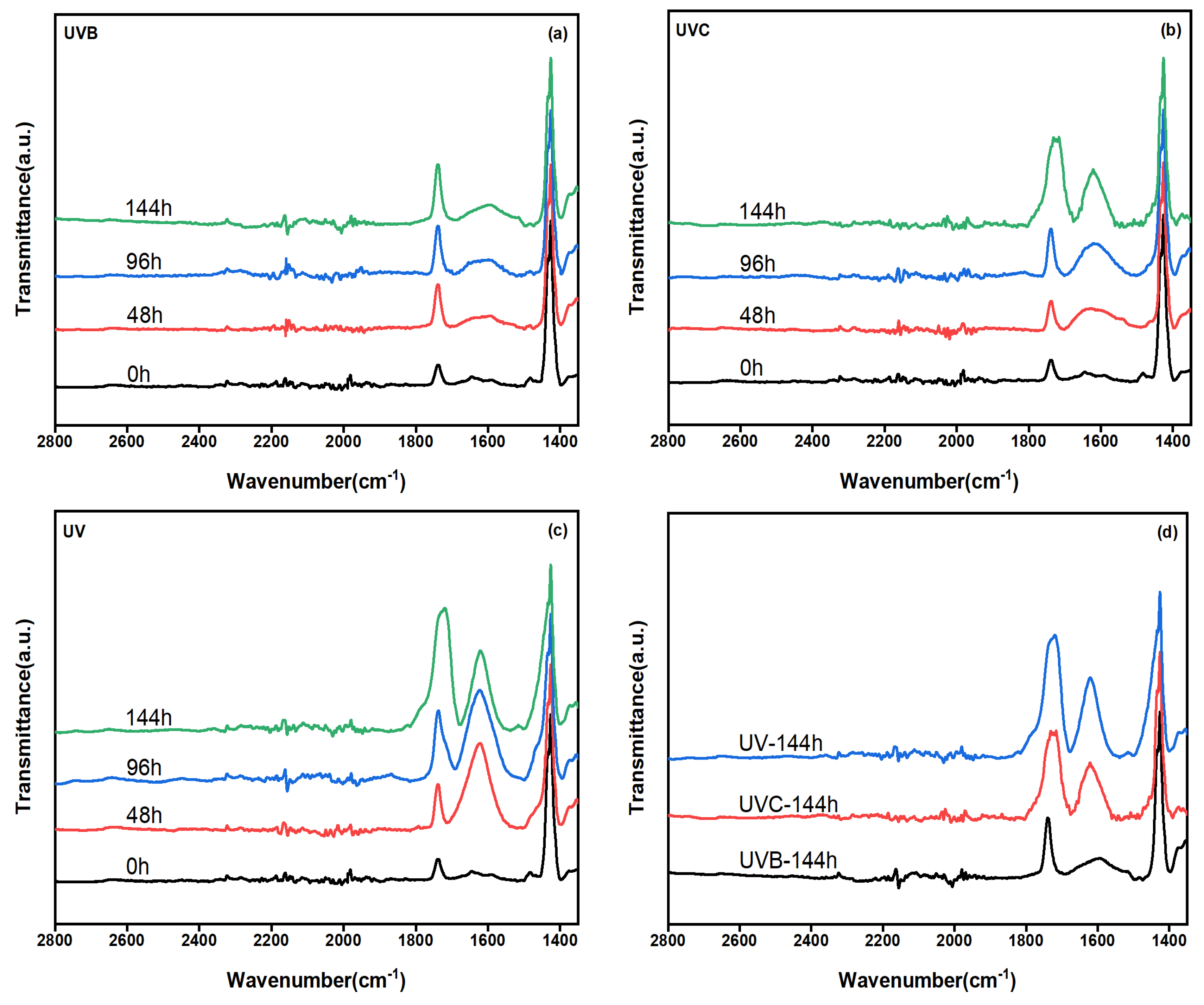
3.7. Effect of LDHs on the Structure of PVC Film Composite Materials
3.8. Film Color Change of LDHs/PVC Film Composite Materials during Photoaging
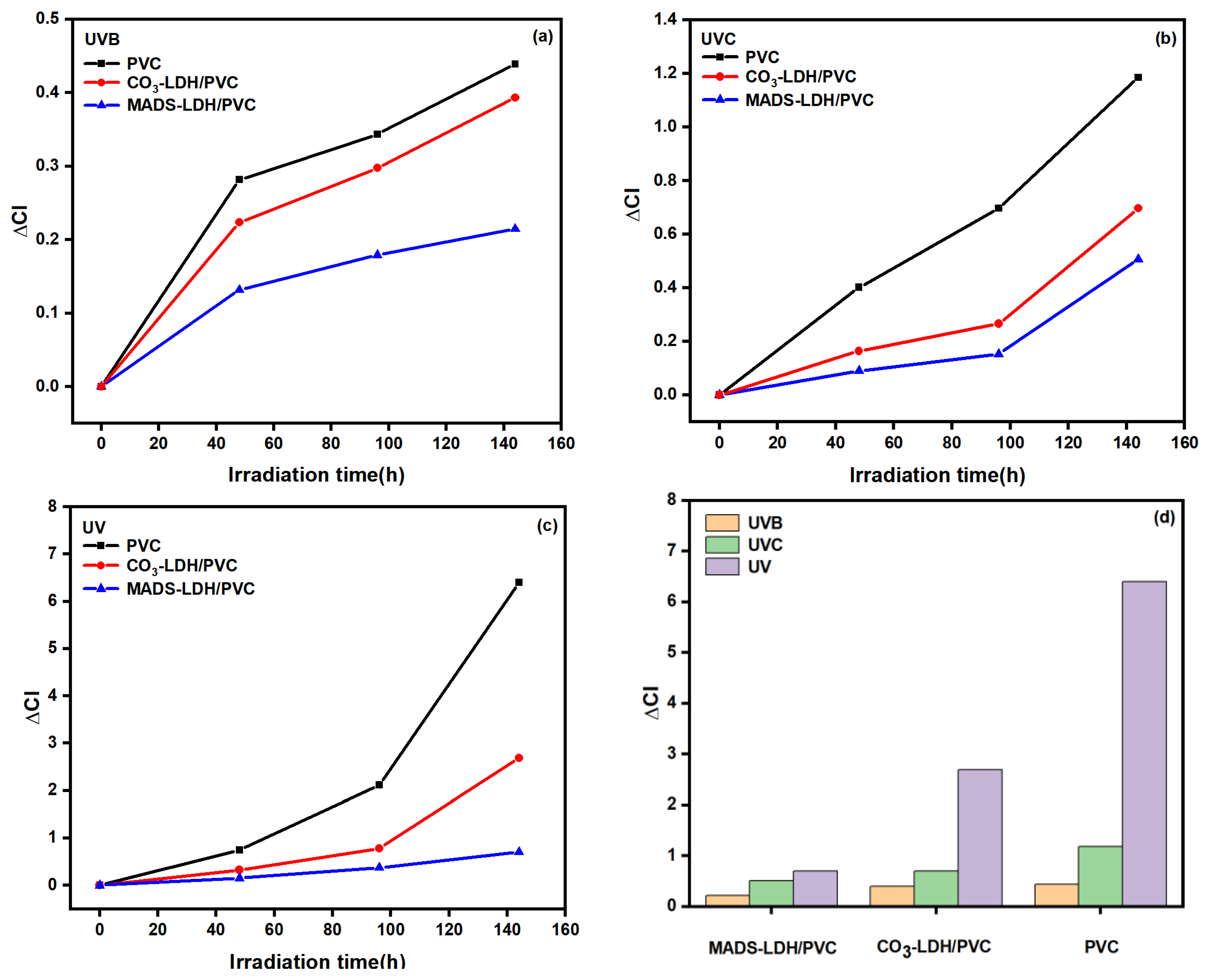
3.9. Light Stability of LDHs/PVC Film Composite Materials
4. Conclusions
- (1)
- The successful preparation of MADS-LDH was confirmed through XRD and FT-IR analysis, which presented a d003 peak position at 3.08°, a proportional relationship between planar spacing in (d003) = 2 (d006) = 3 (d009), and a single set of Bragg reflections (00l) (l = 3, 6, 9). Most of the characteristic absorption bands of MADS were detected in the FT-IR spectra of MADS-LDH.
- (2)
- The agglomeration of MADS-LDH nanosheet particles through stacking was observed using SEM and TEM. BET and TGA analysis indicated that the pore size of MADS-LDH is mesoporous with a homogeneous pore size distribution, and that the MADS anion intercalation created a hydrophobic environment within the layer, reducing the formation of interlayer crystalline water in MADS-LDH.
- (3)
- MADS-LDH/PVC film composite materials exhibited the least color change and the lowest degree of aging under both UVC and UV band irradiation compared to pristine PVC and CO3-LDH/PVC films. The color of PVC films changed significantly under both UVC and UV band irradiation, with similar changes in CO3-LDH/PVC films, but the degree of aging was relatively low.
- (4)
- The addition of MADS-LDH inhibited the generation of carbonyl groups in the MADS-LDH/PVC film composite materials during photoaging, resulting in lower values of both carbonyl index (ΔCl) and relative degradation rate (RDR) compared to pristine PVC and CO3-LDH/PVC films. The degree of influence of UVB, UVC, and UV bands on the photoaging of PVC film and LDHs/PVC film composite materials was found to be UV > UVC > UVB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kann, Y.; Billingham, N.C. Chemiluminescence is shedding light on degradation and stabilisation of plasticised poly (vinyl chloride). Polym. Degrad. Stab. 2004, 85, 957–966. [Google Scholar] [CrossRef]
- Belhaneche-Bensemra, N.; Ouazene, N. Study of the influence of atmospheric pollutants on the natural ageing of rigid polyvinyl chloride. Macromol. Symp. 2002, 180, 181–190. [Google Scholar] [CrossRef]
- Decker, C. Photostabilization of poly (vinyl chloride) by protective coatings. J. Vinyl Addit. Technol. 2001, 7, 235–243. [Google Scholar] [CrossRef]
- Belhaneche-Bensemra, N. Influence of atmospheric pollutants on the natural and artificial aging of rigid poly (vinyl chloride). J. Vinyl Addit. Technol. 2002, 8, 45–54. [Google Scholar] [CrossRef]
- Benavides, R.; Castillo, B.M.; Castañeda, A.O.; López, G.M.; Arias, G. Different thermo-oxidative degradation routes in poly (vinyl chloride). Polym. Degrad. Stab. 2001, 73, 417–423. [Google Scholar] [CrossRef]
- Mohapi, M.; Sefadi, J.S.; Mochane, M.J.; Magagula, S.I.; Lebelo, K. Effect of LDHs and Other Clays on Polymer Composite in Adsorptive Removal of Contaminants: A Review. Crystals 2020, 10, 957. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A.u. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Jabeen, S.; Wattoo, M.A.; Bashir, M.S.; Shah, S.S.A.; ur Rehman, A. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 2022, 145, 110008. [Google Scholar] [CrossRef]
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Jamshaid, M.; Nazir, M.A.; Najam, T.; Shah, S.S.A.; Khan, H.M.; Rehman, A.u. Facile synthesis of Yb3+-Zn2+ substituted M type hexaferrites: Structural, electric and photocatalytic properties under visible light for methylene blue removal. Chem. Phys. Lett. 2022, 805, 139939. [Google Scholar] [CrossRef]
- Balbin Tamayo, A.I.; Esteva Guas, A.M.; Pupim Ferreira, A.A.; Aucélio, R.Q.; Huertas Flores, J.O. Influence of molar fraction of Mg/Al, on the electrochemical behavior of hycrotalcite-epoxy-graphite composites. Mater. Chem. Phys. 2020, 253, 123392. [Google Scholar] [CrossRef]
- Scavetta, E.; Berrettoni, M.; Giorgetti, M.; Tonelli, D. Electrochemical characterisation of Ni/Al X hydrotalcites and their electrocatalytic behaviour. Electrochim. Acta 2002, 47, 2451–2461. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Z.; Kou, J. High-quality modification of general polypropylene by the synergistic effect of zinc adipate, hydrotalcite, and polypropylene (SP179). J. Appl. Polym. Sci. 2023, 140, e53704. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Z. Intercalated hydrotalcite-like materials and their application as thermal stabilizers in poly (vinyl chloride). J. Appl. Polym. Sci. 2017, 134, 44896. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yao, A.; Shi, Y.; Noor, N.; Zeb, A.; Li, M.; Li, H. Preparation and properties of hierarchical Al–Mg layered double hydroxides as UV resistant hydrotalcite. Mater. Chem. Phys. 2020, 256, 123630. [Google Scholar] [CrossRef]
- Zeng, R.; Tang, W.; Zhou, Q.; Liu, X.; Liu, Y.; Wang, S.; Chen, Z.; Yi, N.; Wang, Z.; Chen, J. Efficient adsorption of Pb (II) by sodium dodecyl benzene sulfonate intercalated calcium aluminum hydrotalcites: Kinetic, isotherm, and mechanisms. Environ. Sci. Pollut. Res. Int. 2022, 29, 46161–46173. [Google Scholar] [CrossRef]
- Bouraada, M.; Lafjah, M.; Ouali, M.; Demenorval, L. Basic dye removal from aqueous solutions by dodecylsulfate- and dodecyl benzene sulfonate-intercalated hydrotalcite. J. Hazard. Mater. 2008, 153, 911–918. [Google Scholar] [CrossRef]
- Li, Y.; Bi, H.Y.; Shen, S.L. Removal of bisphenol A from aqueous solution by a dodecylsulfate ion-intercalated hydrotalcite-like compound. Environ. Technol. 2012, 33, 1367–1373. [Google Scholar] [CrossRef]
- Bouraada, M.; Ouali, M.S.; de Ménorval, L.C. Dodecylsulfate and dodecybenzenesulfonate intercalated hydrotalcites as adsorbent materials for the removal of BBR acid dye from aqueous solutions. J. Saudi Chem. Soc. 2016, 20, 397–404. [Google Scholar] [CrossRef]
- Milagres, J.L.; Bellato, C.R.; Ferreira, S.O.; de Moura Guimaraes, L. Preparation and evaluation of hydrocalumite-iron oxide magnetic intercalated with dodecyl sulfate for removal of agrichemicals. J. Environ. Manag. 2020, 255, 109845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pi, H.; Guo, S. The mechanism for inorganic fillers accelerating and inhibiting the UV irradiation aging behaviors of rigid poly (vinyl chloride). J. Appl. Polym. Sci. 2011, 122, 2869–2875. [Google Scholar] [CrossRef]
- Ma, X.; Gao, H.; Lu, Y.; Liu, X.; Dang, L.; Xu, S. Application of β-diketone boron complex as an ultraviolet absorber in polyvinyl chloride film. Mater. Res. Express 2020, 7, 076403. [Google Scholar] [CrossRef]
- Yousufzai, A.; Zafar, M.J.; Hasan, S.U. Radical degradation of polyvinyl chloride. Eur. Polym. J. 1972, 8, 1231–1236. [Google Scholar] [CrossRef]
- Yu, J.J.; Jiang, Z.; Zhu, L.; Hao, Z.P.; Xu, Z.P. Adsorption/Desorption Studies of NOx on Well-Mixed Oxides Derived from Co−Mg/Al Hydrotalcite-like Compounds. J. Phys. Chem. B 2006, 110, 4291–4300. [Google Scholar] [CrossRef]
- Li, L.; Ma, R.; Ebina, Y.; Fukuda, K.; Takada, K.; Sasaki, T. Layer-by-Layer Assembly and Spontaneous Flocculation of Oppositely Charged Oxide and Hydroxide Nanosheets into Inorganic Sandwich Layered Materials. J. Am. Chem. Soc. 2007, 129, 8000–8007. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Evans, D.G.; Li, D. Layered and intercalated hydrotalcite-like materials as thermal stabilizers in PVC resin. J. Phys. Chem. Solids 2006, 67, 998–1001. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, K.; Zhao, J.; Kong, X.; Sun, Y.; Liu, X.; Zhang, Y.; Zeng, Q.; Zhang, H. Hexanedioic acid mediated surface–ligand-exchange process for transferring NaYF4: Yb/Er (or Yb/Tm) up-converting nanoparticles from hydrophobic to hydrophilic. J. Colloid Interface Sci. 2009, 336, 171–175. [Google Scholar] [CrossRef]
- Sampath, S.K.; Cordaro, J.F. Optical properties of zinc aluminate, zinc gallate, and zinc aluminogallate spinels. J. Am. Ceram. Soc. 1998, 81, 649–654. [Google Scholar] [CrossRef]
- Mannepalli, L.K.; Dupati, V.; Vallabha, S.J.; Sunkara, V.M. Synthesis of substituted guanidines using Zn–Al hydrotalcite catalyst. J. Chem. Sci. 2013, 125, 1339–1345. [Google Scholar] [CrossRef]
- Bai, L.; Liu, X.; Jiao, T.; Wang, Y.; Huo, Y.; Niu, J. Surface and Interfacial Properties of Mono and Didodecyl Diphenyl Ether Disulfonates. Tenside Surfactants Deterg. 2018, 55, 302–311. [Google Scholar] [CrossRef]
- Liu, X.; Niu, J.; Wang, X. Synthesis, Surface and Interfacial Properties of Dodecyl Diphenyl Oxide Disulfonate with Different Counterions. J. Surfactants Deterg. 2015, 18, 675–679. [Google Scholar] [CrossRef]
- Tao, Q.; Yuan, J.; Frost, R.L.; He, H.; Yuan, P.; Zhu, J. Effect of surfactant concentration on the stacking modes of organo-silylated layered double hydroxides. Appl. Clay Sci. 2009, 45, 262–269. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P.; Huang, Z.; Zhu, N.; Wu, J.; Li, P.; Dang, Z. Solar photocatalytic degradation of methylene blue by mixed metal oxide catalysts derived from ZnAlTi layered double hydroxides. Appl. Clay Sci. 2014, 95, 95–103. [Google Scholar] [CrossRef]
- Li, Z.-X.; Zeng, H.-Y.; Gohi, B.F.C.A.; Ding, P.-X. Preparation of CeO2-decorated organic-pillared hydrotalcites for the UV resistance of polymer. Appl. Surf. Sci. 2020, 507, 145110. [Google Scholar] [CrossRef]
- Sharma, D.; Sakthivel, A.; Michelraj, S.; Muthurasu, A.; Ganesh, V. Surfactant Intercalated Mono-metallic Cobalt Hydrotalcite: Preparation, Characterization, and its Bi-functional Electrocatalytic Application. ChemistrySelect 2020, 5, 9615–9622. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Elhalil, A.; Barka, N. Characteristics and mechanisms of methyl orange sorption onto Zn/Al layered double hydroxide intercalated by dodecyl sulfate anion. Sci. Afr. 2019, 6, e00216. [Google Scholar] [CrossRef]
- Wang, C.-X.; Pu, M.; Zhang, P.-H.; Gao, Y.; Yang, Z.-Y.; Lei, M. Structure Simulation and Host–Guest Interaction of Histidine-Intercalated Hydrotalcite–Montmorillonite Complex. Minerals 2018, 8, 198. [Google Scholar] [CrossRef]
- Ma, R.; Tang, P.; Feng, Y.; Li, D. UV absorber co-intercalated layered double hydroxides as efficient hybrid UV-shielding materials for polypropylene. Dalton Trans. 2019, 48, 2750–2759. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Wang, Y.; Evans, D.G.; Duan, X. Synthesis and characterization of a UV absorbent-intercalated Zn–Al layered double hydroxide. Polym. Degrad. Stab. 2006, 91, 789–794. [Google Scholar] [CrossRef]
- Kohno, Y.; Asai, S.; Shibata, M.; Fukuhara, C.; Maeda, Y.; Tomita, Y.; Kobayashi, K. Improved photostability of hydrophobic natural dye incorporated in organo-modified hydrotalcite. J. Phys. Chem. Solids 2014, 75, 945–950. [Google Scholar] [CrossRef]
- Miotto Menino, N.; da Silveira Salla, J.; do Nascimento, M.S.; Dallago, R.M.; Peralta, R.A.; Moreira, R.F. High-performance hydrophobic magnetic hydrotalcite for selective treatment of oily wastewater. Environ. Technol. 2021, 44, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, Z.-P.; Qin, Y.; Dai, J.; Zhang, T. Comparative Studies on Hydrophilic and Hydrophobic Segments Grafted Poly (vinyl chloride). Chin. J. Polym. Sci. 2018, 36, 604–611. [Google Scholar] [CrossRef]
- Owen, E.D. Photodegradation of Polyvinyl chloride. In Ultraviolet Light Induced Reactions in Polymers; American Chemical Society: Washington, DC, USA, 1976; Volume 25, pp. 208–219. [Google Scholar]
- Owen, J. Degradation and Stabilisation of PVC; Springer Science & Business Media: Cham, Switzerland, 2012. [Google Scholar]
- Zhang, X.; Pi, H.; Guo, S.; Fu, J. Influence of ultraviolet absorbers on the ultraviolet-irradiation behaviour of rigid poly vinyl chloride. Plast. Rubber Compos. 2016, 45, 352–361. [Google Scholar] [CrossRef]
- Liu, H.; Dong, L.; Xie, H.; Wan, L.; Liu, Z.; Xiong, C. Ultraviolet light aging properties of PVC/CaCO3 composites. J. Appl. Polym. Sci. 2013, 127, 2749–2756. [Google Scholar] [CrossRef]



| Parameter (nm) | CO3-LDH | MADS-LDH |
|---|---|---|
| d003 | 0.75 | 2.87 |
| d006 | 0.38 | 1.41 |
| d009 | 0.26 | 0.93 |
| d110 | 0.15 | 0.15 |
| Sample | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|
| CO3-LDH | 30.430 | 0.119 | 16.610 |
| MADS-LDH | 23.927 | 0.117 | 16.187 |
| Sample | T0.1 | ΔT0.1 | T0.5 | ΔT0.5 |
|---|---|---|---|---|
| MADS | 58 | − | 156 | − |
| CO3-LDH | 104 | 46 | >800 | >644 |
| MADS-LDH | 99 | 41 | 685 | 529 |
| UVB | UVC | UV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 0 h | 48 h | 96 h | 144 h | 48 h | 96 h | 144 h | 48 h | 96 h | 144 h | |
| Sample | |||||||||||
| PVC |  |  |  |  |  |  |  |  |  |  | |
| MADS-LDH/PVC |  |  |  |  |  |  |  |  |  |  | |
| CO3-LDH/PVC |  |  |  |  |  |  |  |  |  |  | |
| UVB | UVC | UV | ||||
|---|---|---|---|---|---|---|
| Sample | ΔCl | RDR | ΔCl | RDR | ΔCl | RDR |
| PVC | 0.439 | 100% | 1.185 | 100% | 6.401 | 100% |
| CO3-LDH/PVC | 0.393 | 89.522% | 0.697 | 58.819% | 2.687 | 41.978% |
| MADS-LDH/PVC | 0.215 | 48.975% | 0.507 | 42.785% | 0.705 | 11.014% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, E.; Liu, Y.; Yuan, H.; Cheng, X.; Zhong, Y.; He, J.; Lu, X. Mechanism of Sodium Dodecyl Diphenyl Ether Disulfonate Filled Hydrotalcite Inhibiting the Photo-Degradation of Polyvinyl Chloride under Different Ranges of Ultraviolet Wavelength Irradiation. Coatings 2023, 13, 985. https://doi.org/10.3390/coatings13060985
Zhou E, Liu Y, Yuan H, Cheng X, Zhong Y, He J, Lu X. Mechanism of Sodium Dodecyl Diphenyl Ether Disulfonate Filled Hydrotalcite Inhibiting the Photo-Degradation of Polyvinyl Chloride under Different Ranges of Ultraviolet Wavelength Irradiation. Coatings. 2023; 13(6):985. https://doi.org/10.3390/coatings13060985
Chicago/Turabian StyleZhou, Enguo, Yuan Liu, Huajin Yuan, Xiaoling Cheng, Yuanhong Zhong, Jiebing He, and Xi Lu. 2023. "Mechanism of Sodium Dodecyl Diphenyl Ether Disulfonate Filled Hydrotalcite Inhibiting the Photo-Degradation of Polyvinyl Chloride under Different Ranges of Ultraviolet Wavelength Irradiation" Coatings 13, no. 6: 985. https://doi.org/10.3390/coatings13060985






