Effect of Nb Content on Phase Transformation and Comprehensive Properties of TiNb Alloy Coating
Abstract
:1. Introduction
2. Materials and Methods
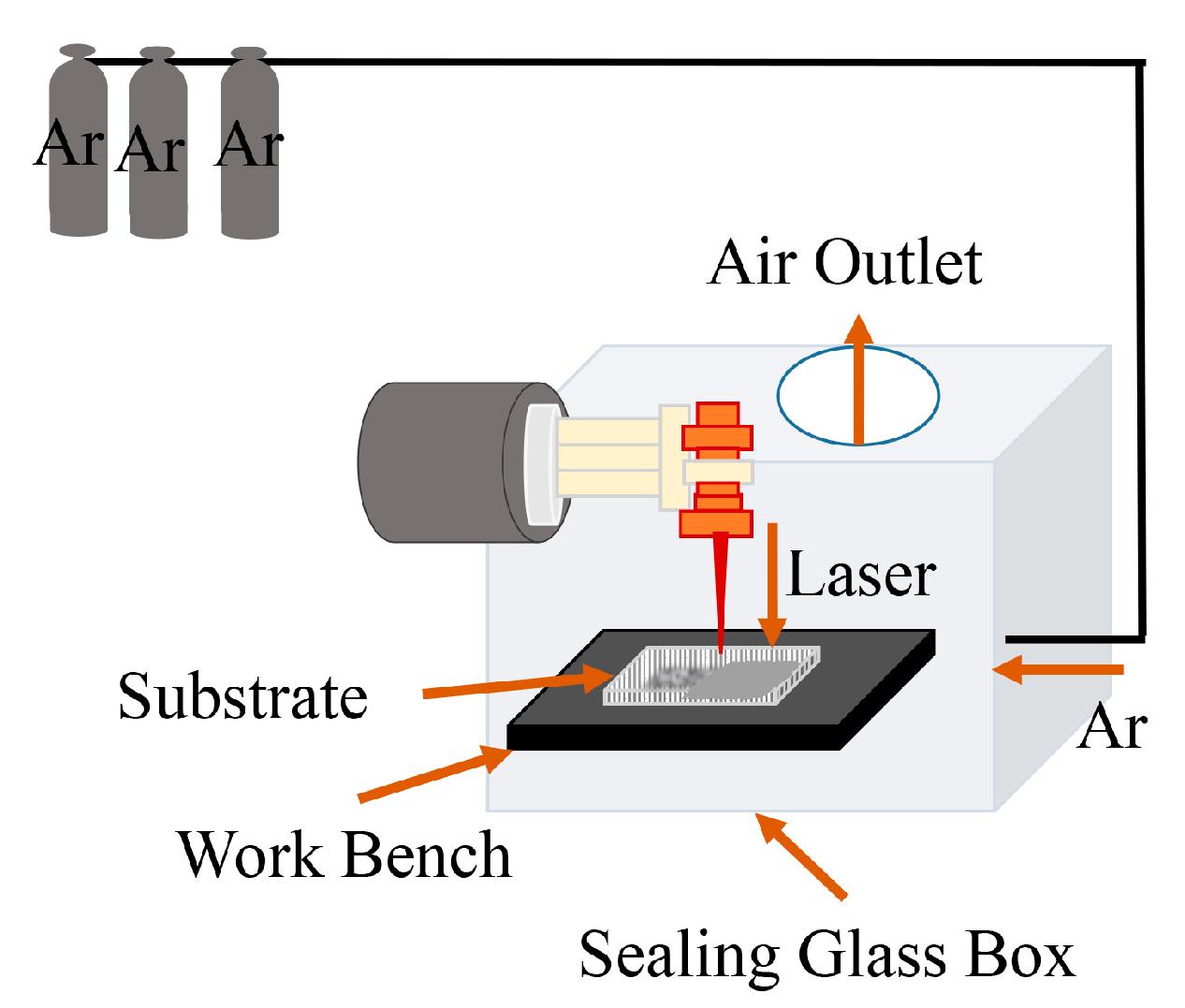
2.1. Experimental Materials and Design
2.2. Microstructure and Mechanical Properties Analysis
2.3. Electrochemical Test
2.4. Biocompatibility
3. Results
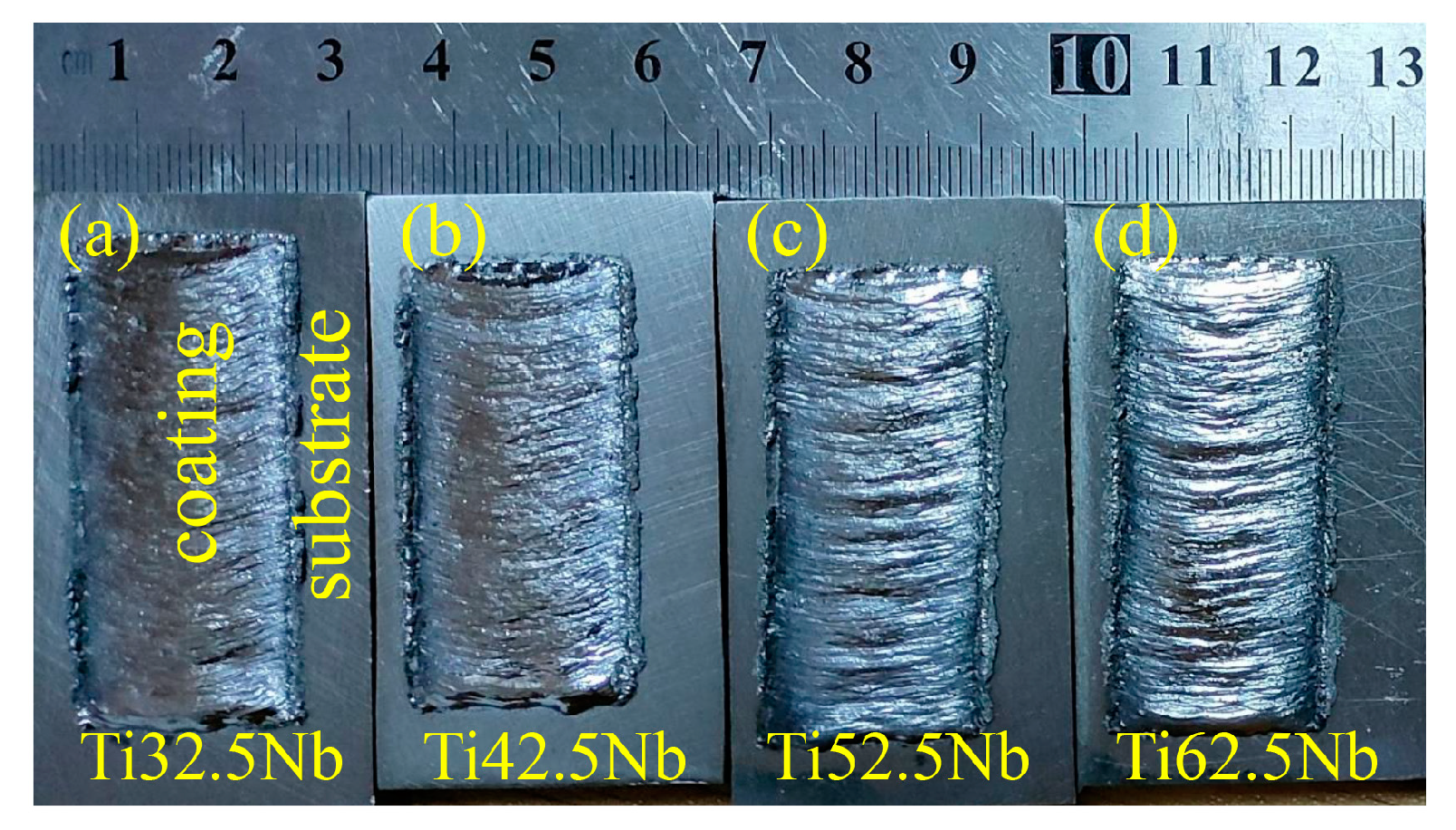
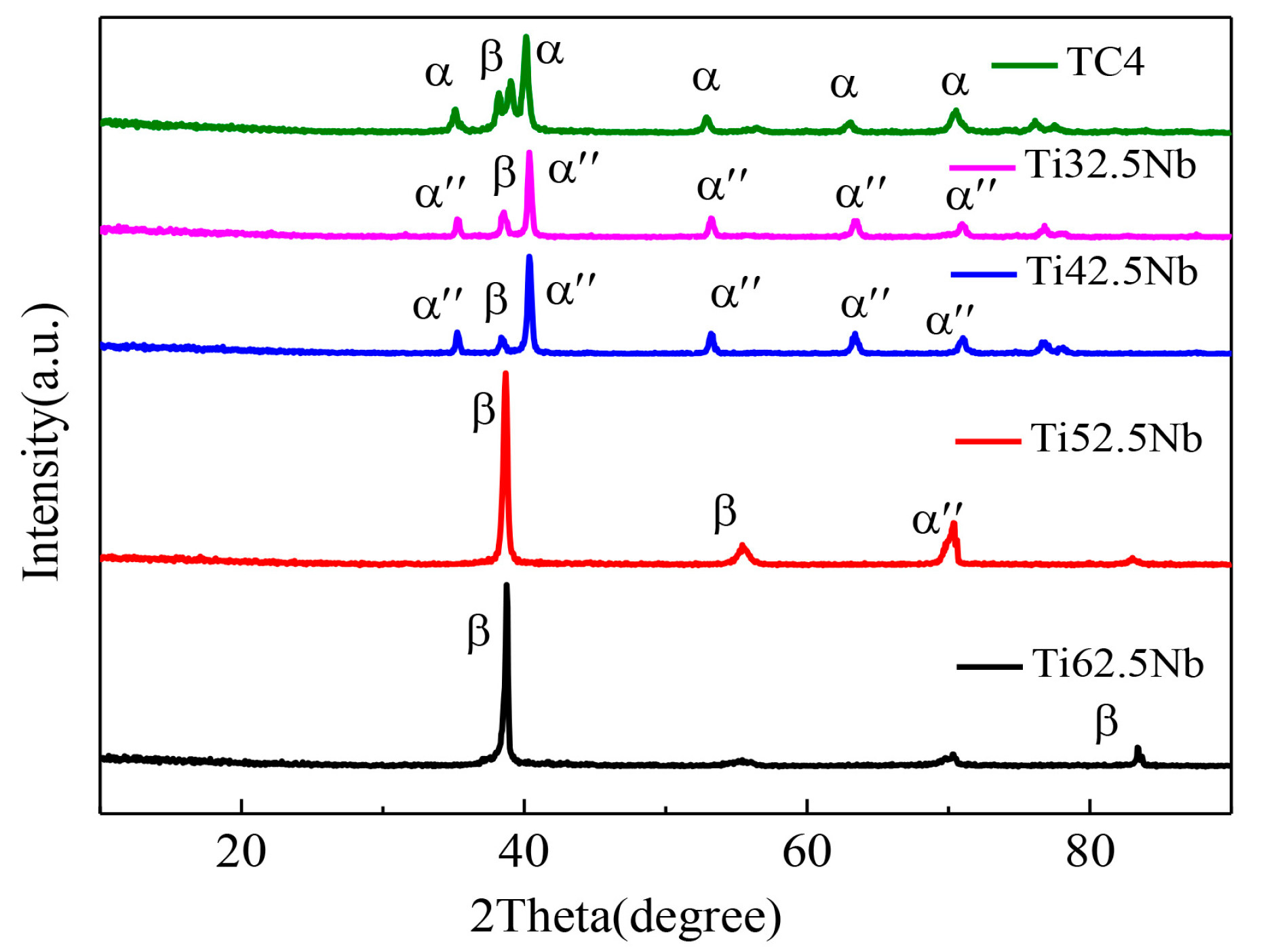
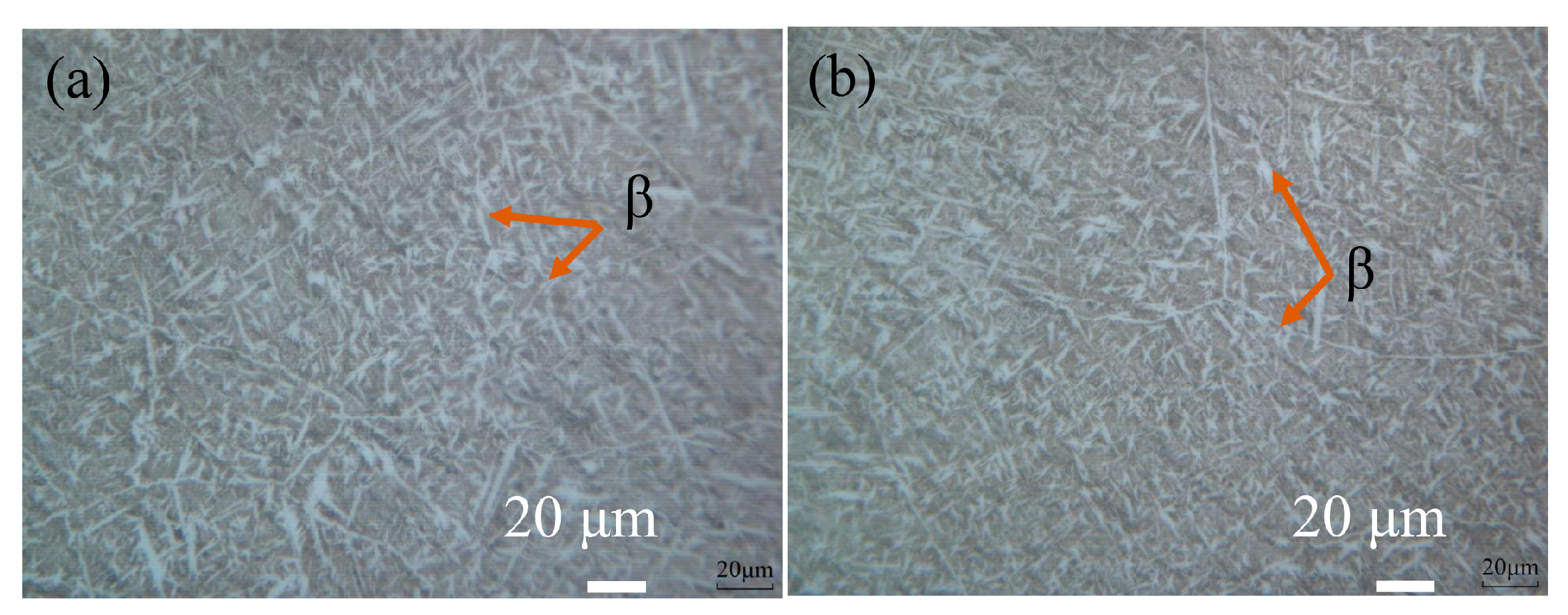
3.1. Phase Analysis and Microstructure
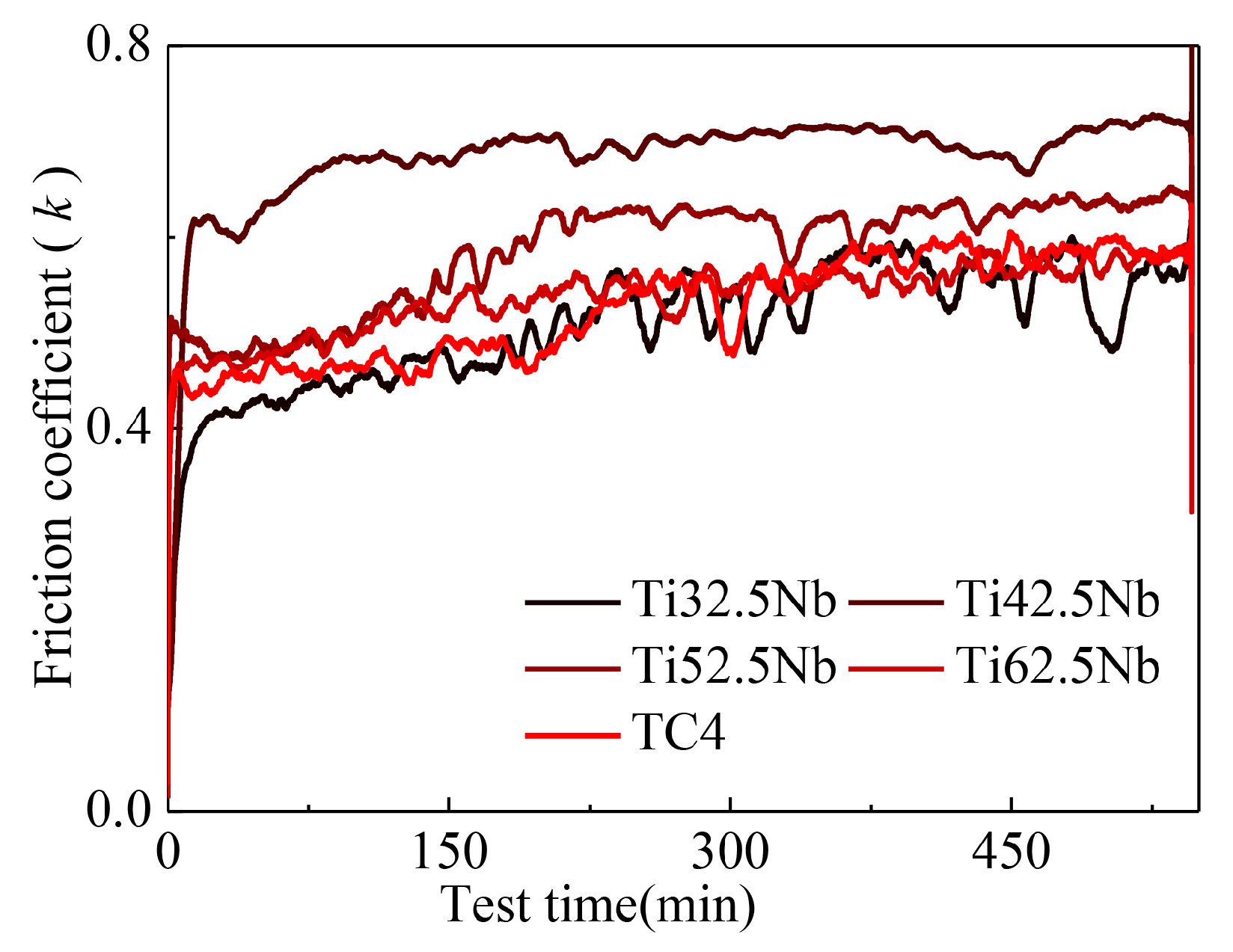
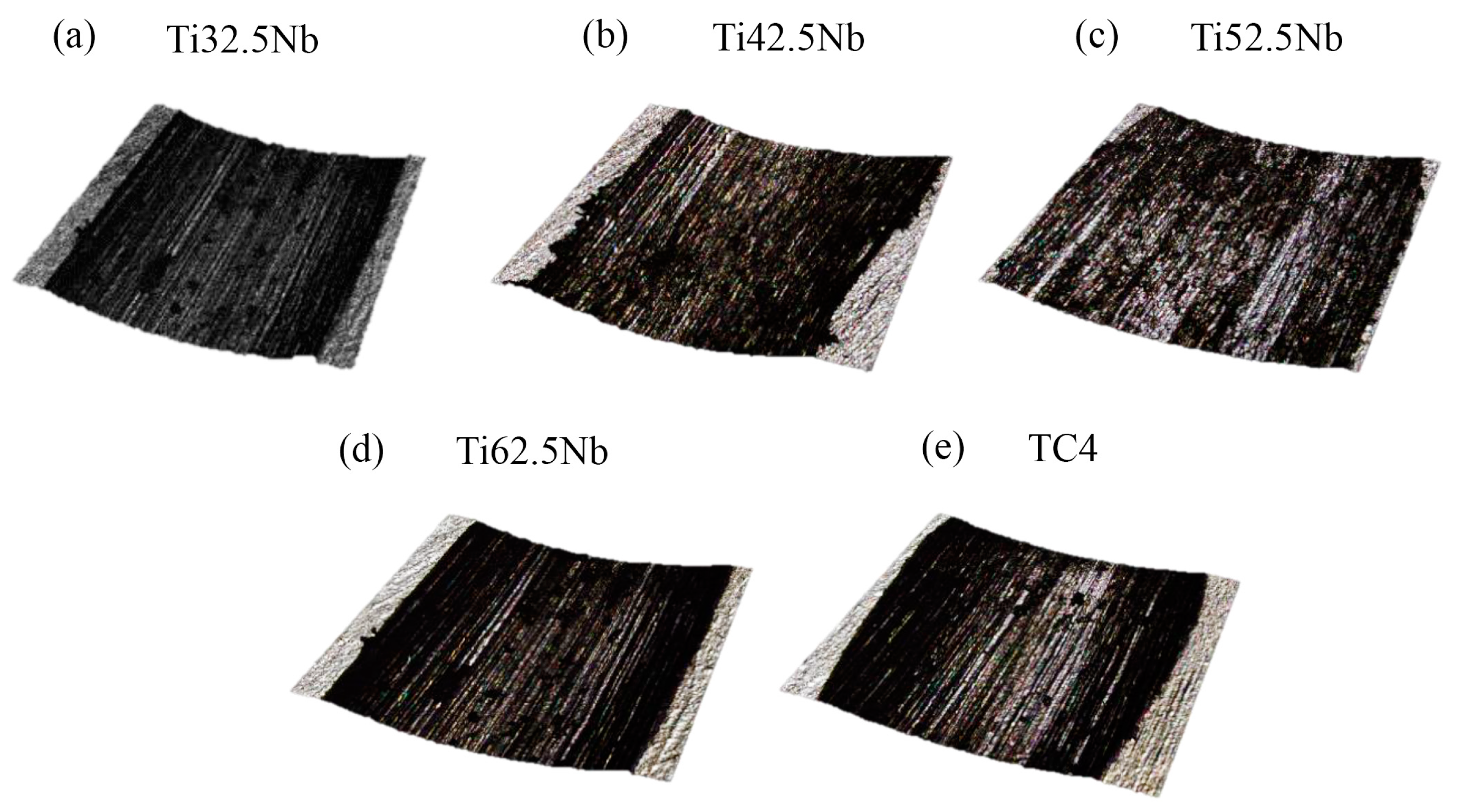
3.2. Microhardness and Wear Resistance
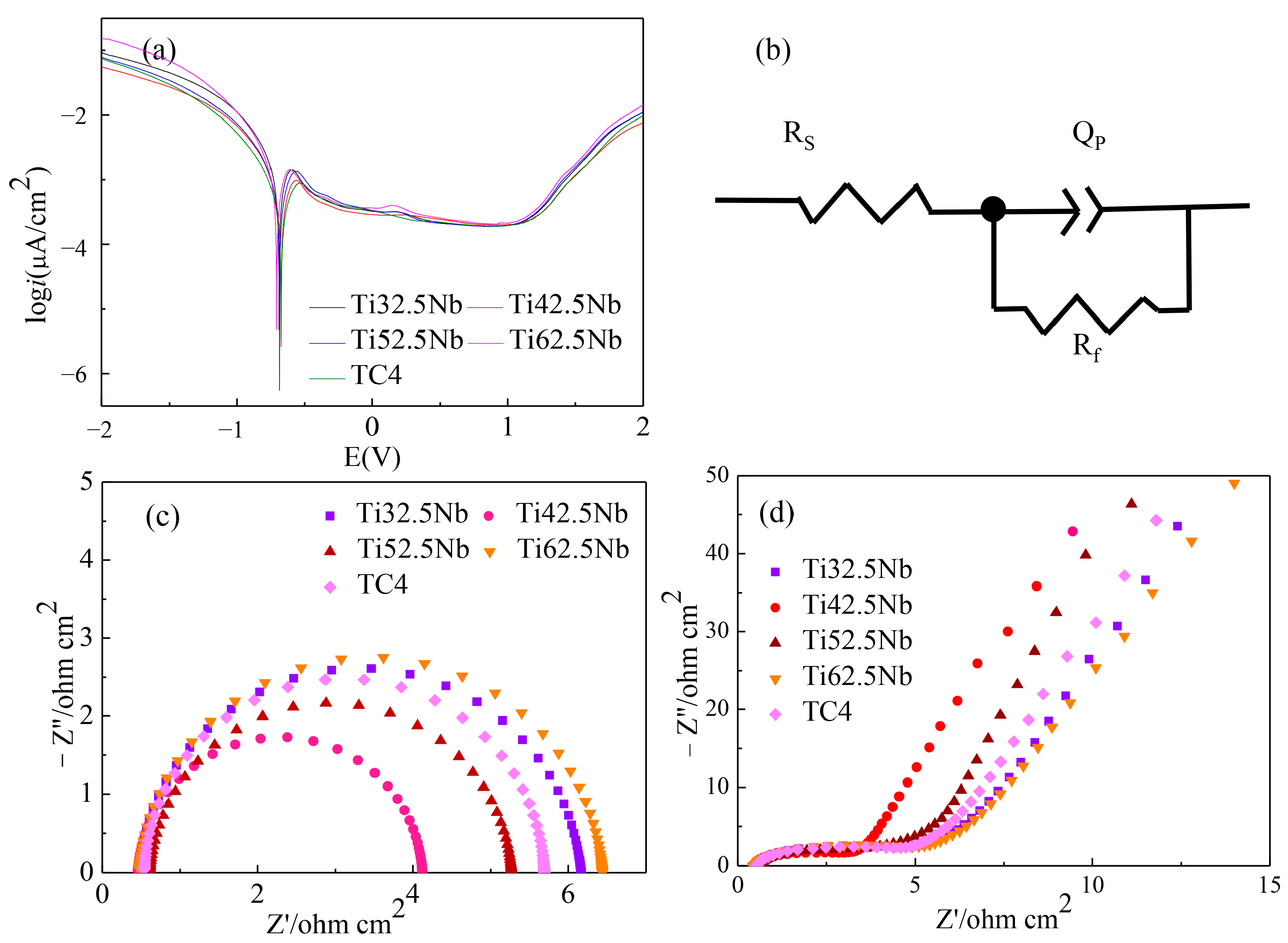
3.3. Corrosion Resistance Analysis
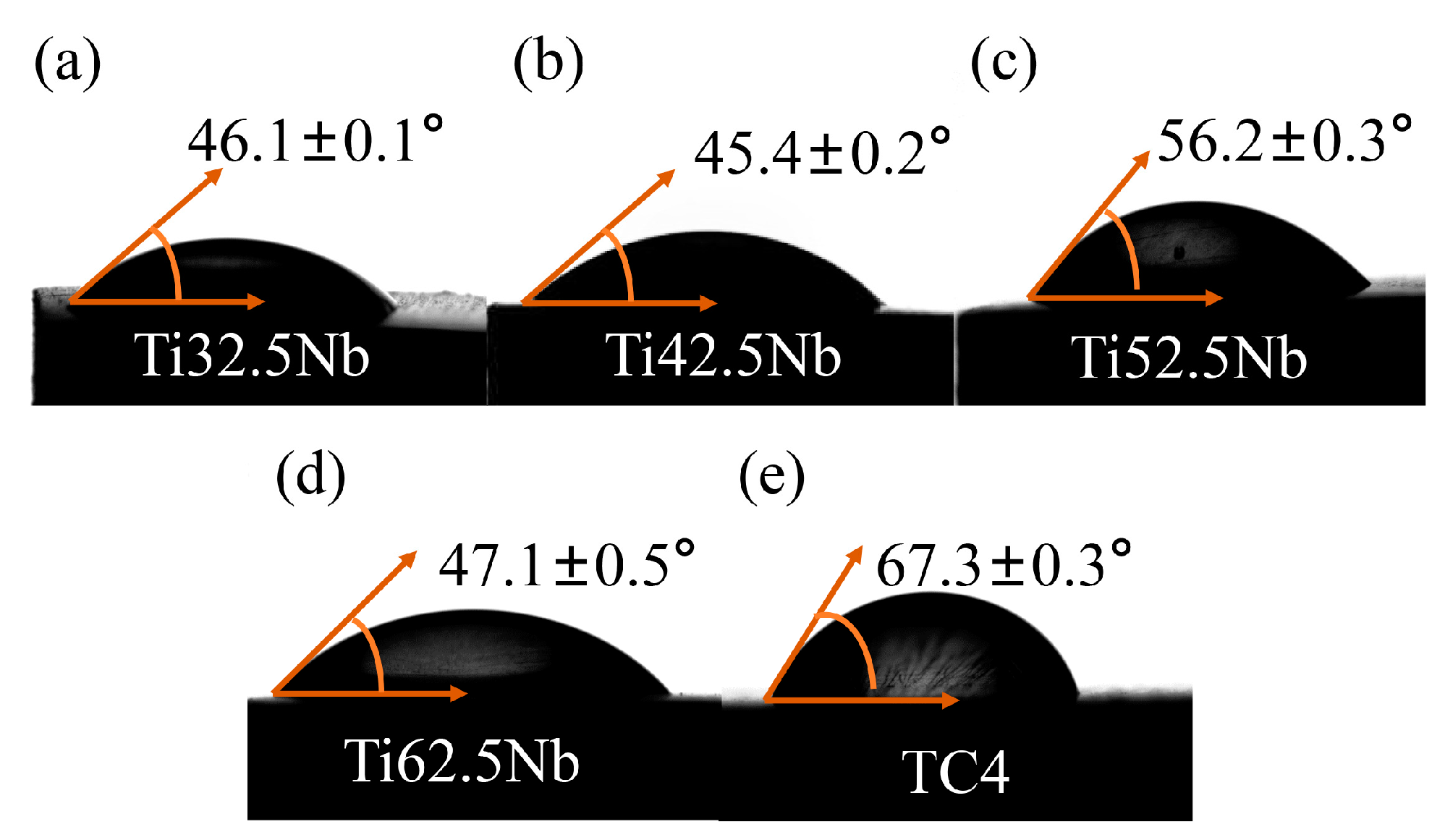
3.4. In Vitro Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pesode, P.; Barve, S. Surface Modification of Titanium and Titanium Alloy by Plasma Electrolytic Oxidation Process for Biomedical Applications: A Review. Mater. Today Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.B.; Donato, T.A.G.; Arana-Chavez, V.E.; Buzalaf, M.A.R.; Grandini, C.R. The Effect of the Solute on the Structure, Selected Mechanical Properties, and Biocompatibility of Ti–Zr System Alloys for Dental Applications. Mater. Sci. Eng. C 2014, 34, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Gialanella, S.; Malandruccolo, A. Titanium and Titanium Alloys. In Aerospace Alloys; Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–189. [Google Scholar]
- Li, S.J.; Cui, T.C.; Hao, Y.L.; Yang, R. Fatigue Properties of a Metastable β-Type Titanium Alloy with Reversible Phase Transformation. Acta Biomater. 2008, 4, 305–317. [Google Scholar] [CrossRef]
- Kolli, R.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xue, X.; Gao, H.; Luo, W.; Wang, K.; Li, S.; Liu, X.; Du, Y. Investigation of High-Temperature Constitutive Behavior of Ti555211 Titanium Alloy Subjected to Plastic Deformation in the Different Phase Regions. Metals 2022, 12, 1562. [Google Scholar] [CrossRef]
- Shui, J.; Chen, S.L.; Xia, T.T.; Liu, H.; Yang, G.J.; Niu, J.; Gong, W.Q. Effect of Non-Equilibrium Solid State Phase Transformation on Welding Temperature Field during Keyhole Mode Laser Welding of Ti6Al4V Alloy. Opt. Laser Technol. 2022, 145, 107461. [Google Scholar] [CrossRef]
- Pang, E.L.; Hildyard, E.M.; Connor, L.D.; Pickering, E.J.; Jones, N.G. The Effect of Quench Rate on the β-A″ Martensitic Transformation in Ti–Nb Alloys. Mater. Sci. Eng. A 2021, 817, 141240. [Google Scholar] [CrossRef]
- Thoemmes, A.; Bataev, I.A.; Lazurenko, D.V.; Ruktuev, A.A.; Ivanov, I.V.; Afonso, C.R.M.; Stark, A.; Jorge, A.M., Jr. Microstructure and Lattice Parameters of Suction-Cast Ti–Nb Alloys in a Wide Range of Nb Concentrations. Mater. Sci. Eng. A 2021, 818, 141378. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Cheng, J.; Tsoi, J.K.H.; Chen, J. Mechanical and Biological Properties of Ti–(0–25 Wt. %) Nb Alloys for Biomedical Implants Application. Regen. Biomater. 2020, 7, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Bahl, S.; Suwas, S.; Chatterjee, K. Comprehensive Review on Alloy Design, Processing, and Performance of β Titanium Alloys as Biomedical Materials. Int. Mater. Rev. 2021, 66, 114–139. [Google Scholar] [CrossRef]
- Zhao, G.-H.; Liang, X.Z.; Xu, X.; Gamża, M.B.; Mao, H.; Louzguine-Luzgin, D.V.; Rivera-Díaz-del-Castillo, P.E.J. Alloy Design by Tailoring Phase Stability in Commercial Ti Alloys. Mater. Sci. Eng. A 2021, 815, 141229. [Google Scholar] [CrossRef]
- Godley, R.; Starosvetsky, D.; Gotman, I. Corrosion Behavior of a Low Modulus β-Ti-45%Nb Alloy for Use in Medical Implants. J. Mater. Sci. Mater. Med. 2006, 17, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Jouzeau, J.Y.; Mainard, D.; Payan, E.; Netter, P.; Rie, K.T.; Stucky, T.; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5Fe Alloys According to Three Surface Treatments, Using Human Fibroblasts and Osteoblasts. Biomaterials 1996, 17, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.J.; Wang, Y.B.; Cheng, Y.; Deng, F.; Zheng, Y.F.; Wei, S.C. Comparative Study on the Corrosion Behavior of Ti–Nb and TMA Alloys for Dental Application in Various Artificial Solutions. Mater. Sci. Eng. C 2011, 31, 702–711. [Google Scholar] [CrossRef]
- Cremasco, A.; Messias, A.D.; Esposito, A.R.; Duek, E.A.d.R.; Caram, R. Effects of Alloying Elements on the Cytotoxic Response of Titanium Alloys. Mater. Sci. Eng. C 2011, 31, 833–839. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Corrosion Behaviour of Ti–6Al–7Nb and Ti–6Al–4V ELI Alloys in the Simulated Body Fluid Solution by Electrochemical Impedance Spectroscopy. Electrochim. Acta 2006, 52, 839–846. [Google Scholar] [CrossRef]
- Watanabe, I.; Wataha, J.C.; Lockwood, P.E.; Shimizu, H.; Cai, Z.; Okabe, T. Cytotoxicity of Commercial and Novel Binary Titanium Alloys with and without a Surface-Reaction Layer: Cytotoxicity of Titanium Alloys. J. Oral Rehabil. 2004, 31, 185–189. [Google Scholar] [CrossRef]
- Choubey, A.; Balasubramaniam, R.; Basu, B. Effect of Replacement of V by Nb and Fe on the Electrochemical and Corrosion Behavior of Ti–6Al–4V in Simulated Physiological Environment. J. Alloys Compd. 2004, 381, 288–294. [Google Scholar] [CrossRef]
- Iijima, D. Wear Properties of Ti and Ti–6Al–7Nb Castings for Dental Prostheses. Biomaterials 2003, 24, 1519–1524. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinomi, M. Recent Research and Development in Titanium Alloys for Biomedical Applications and Healthcare Goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in Metals and Alloys for Joint Replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium Alloys in Total Joint Replacement—A Materials Science Perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, Mechanical Behavior and Biocompatibility of Powder Metallurgy Nb-Ti-Ta Alloys as Biomedical Material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef]
- Kushwaha, M.; Pan, X.; Holloway, J.A.; Denry, I.L. Differentiation of Human Mesenchymal Stem Cells on Niobium-Doped Fluorapatite Glass-Ceramics. Dent. Mater. 2012, 28, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Deng, Y.; Zheng, Y.; Li, Y.; Zhang, R.; Lv, Y.; Zhao, Q.; Wei, S. Characterization, Corrosion Behavior, Cellular Response and in Vivo Bone Tissue Compatibility of Titanium–Niobium Alloy with Low Young’s Modulus. Mater. Sci. Eng. C 2016, 59, 565–576. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Geetha, M.; Siddiquee, A.N. Microstructure, Mechanical Properties and Electrochemical Behavior of a Novel Biomedical Titanium Alloy Subjected to Thermo-Mechanical Processing Including Aging. J. Alloys Compd. 2015, 634, 272–280. [Google Scholar] [CrossRef]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-Alloys for Long-Term Implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Ribeiro, A.L.R.; Junior, R.C.; Cardoso, F.F.; Filho, R.B.F.; Vaz, L.G. Mechanical, Physical, and Chemical Characterization of Ti–35Nb–5Zr and Ti–35Nb–10Zr Casting Alloys. J. Mater. Sci. Mater. Med. 2009, 20, 1629–1636. [Google Scholar] [CrossRef]
- Li, G.J.; Li, J.; Luo, X. Effects of High Temperature Treatment on Microstructure and Mechanical Properties of Laser-Clad NiCrBSi/WC Coatings on Titanium Alloy Substrate. Mater. Charact. 2014, 98, 83–92. [Google Scholar] [CrossRef]
- Shang, X.; Bo, S.; Guo, Y.; Liu, Q. ZrC Reinforced Refractory-High-Entropy-Alloy Coatings: Compositional Design, Synthesis, Interstitials, and Microstructure Evolution Effects on Wear, Corrosion and Oxidation Behaviors+. Appl. Surf. Sci. 2021, 564, 150466. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Smugeresky, J.E.; Schoenung, J.M.; Lavernia, E.J. Thermal Behavior and Microstructure Evolution during Laser Deposition with Laser-Engineered Net Shaping: Part II. Experimental Investigation and Discussion. Metall. Mater. Trans. A 2008, 39, 2237–2245. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E. Selective Laser Melting of Titanium Alloy with 50 Wt% Tantalum: Microstructure and Mechanical Properties. J. Alloys Compd. 2016, 660, 461–470. [Google Scholar] [CrossRef]
- De Mello, M.G.; Dainese, B.P.; Caram, R.; Cremasco, A. Influence of Heating Rate and Aging Temperature on Omega and Alpha Phase Precipitation in Ti 35Nb Alloy. Mater. Charact. 2018, 145, 268–276. [Google Scholar] [CrossRef]
- Han, M.-K.; Kim, J.-Y.; Hwang, M.-J.; Song, H.-J.; Park, Y.-J. Effect of Nb on the Microstructure, Mechanical Properties, Corrosion Behavior, and Cytotoxicity of Ti-Nb Alloys. Materials 2015, 8, 5986–6003. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Kumar, P.; Yeong, W.Y.; Narayan, R.L.; Ramamurty, U. Fracture Behavior of Laser Powder Bed Fusion Fabricated Ti41Nb via In-Situ Alloying. Acta Mater. 2022, 225, 117593. [Google Scholar] [CrossRef]
- Gupta, A.; Khatirkar, R.; Singh, J. A Review of Microstructure and Texture Evolution during Plastic Deformation and Heat Treatment of β-Ti Alloys. J. Alloys Compd. 2022, 899, 163242. [Google Scholar] [CrossRef]
- Bahador, A.; Saud, S.N.; Hamzah, E.; Abubakar, T.; Yusof, F.; Ibrahim, M.K. Nd:YAG Laser Welding of Ti-27 at.% Nb Shape Memory Alloys. Weld World 2016, 60, 1133–1139. [Google Scholar] [CrossRef]
- Doraiswamy, D.; Ankem, S. The Effect of Grain Size and Stability on Ambient Temperature Tensile and Creep Deformation in Metastable Beta Titanium Alloys. Acta Mater. 2003, 51, 1607–1619. [Google Scholar] [CrossRef]
- Abe, F.; Osakada, K.; Shiomi, M.; Uematsu, K.; Matsumoto, M. The Manufacturing of Hard Tools from Metallic Powders by Selective Laser Melting. J. Mater. Process. Technol. 2001, 111, 210–213. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.C.; Wei, M.; Su, H.X.; Wu, W.; Lin, J.G. Effects of the Zr and Mo Contents on the Electrochemical Corrosion Behavior of Ti-22Nb Alloy: Effects of the Zr and Mo Contents on Corrosion of Ti-22Nb Alloy. Mater. Corros. 2013, 64, 402–407. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, J.; Li, G.; Wang, P.; Wang, P.; Li, F.; Zhang, B.; Chi, H. Corrosion Inhibition Efficiency of Compound Nitrite with D-Sodium Gluconate on Carbon Steel in Simulated Concrete Pore Solution. Constr. Build. Mater. 2021, 288, 123101. [Google Scholar] [CrossRef]
- He, X.; Song, R.G.; Kong, D.J. Microstructure and Corrosion Behaviours of Composite Coatings on S355 Offshore Steel Prepared by Laser Cladding Combined with Micro-Arc Oxidation. Appl. Surf. Sci. 2019, 497, 143703. [Google Scholar] [CrossRef]
- Ju, S.; Kang, H.; Jun, J.; Son, S.; Park, J.; Kim, W.; Lee, H. Periodic Micropillar-Patterned FTO/BiVO 4 with Superior Light Absorption and Separation Efficiency for Efficient PEC Performance. Small 2021, 17, 2006558. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, M.C.; Saldaña, L.; Vallés, G.; González-Carrasco, J.L.; González-Cabrero, J.; Martínez, M.E.; Gil-Garay, E.; Munuera, L. In Vitro Corrosion Behaviour and Osteoblast Response of Thermally Oxidised Ti6Al4V Alloy. Biomaterials 2003, 24, 19–26. [Google Scholar] [CrossRef]
- Qin, P.; Chen, L.Y.; Zhao, C.H.; Liu, Y.J.; Cao, C.D.; Sun, H. Synthesis and Characterization of nickel free titanium–hydroxyapatite, L.C. Corrosion Behavior and Mechanism of Selective Laser Melted Ti35Nb Alloy Produced Using Pre-Alloyed and Mixed Powder in Hank’s Solution. Corros. Sci. 2021, 189, 109609. [Google Scholar] [CrossRef]
- Schwartz, Z.; Boyan, B.D. Underlying Mechanisms at the Bone-Biomaterial Interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, J.H. Hydrophilization of Synthetic Biodegradable Polymer Scaffolds for Improved Cell/Tissue Compatibility. Biomed. Mater. 2013, 8, 014101. [Google Scholar] [CrossRef]
- Sousa, S.R.; Lamghari, M.; Sampaio, P.; Moradas-Ferreira, P.; Barbosa, M.A. Osteoblast Adhesion and Morphology on TiO2 Depends on the Competitive Preadsorption of Albumin and Fibronectin. J. Biomed. Mater. Res. Part A 2008, 84, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Kawai, I.; Kuroda, K.; Ichino, R.; Okido, M.; Seki, A. Osteoconductivity of Anodized Titanium with Controlled Micron-Level Surface Roughness. Mater. Trans. 2011, 52, 1650–1654. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, D.; Kawai, I.; Kuroda, K.; Ichino, R.; Okido, M.; Seki, A. Osteoconductivity and Hydrophilicity of TiO2 Coatings on Ti Substrates Prepared by Different Oxidizing Processes. Bioinorg. Chem. Appl. 2012, 2012, 495218. [Google Scholar] [CrossRef] [PubMed] [Green Version]



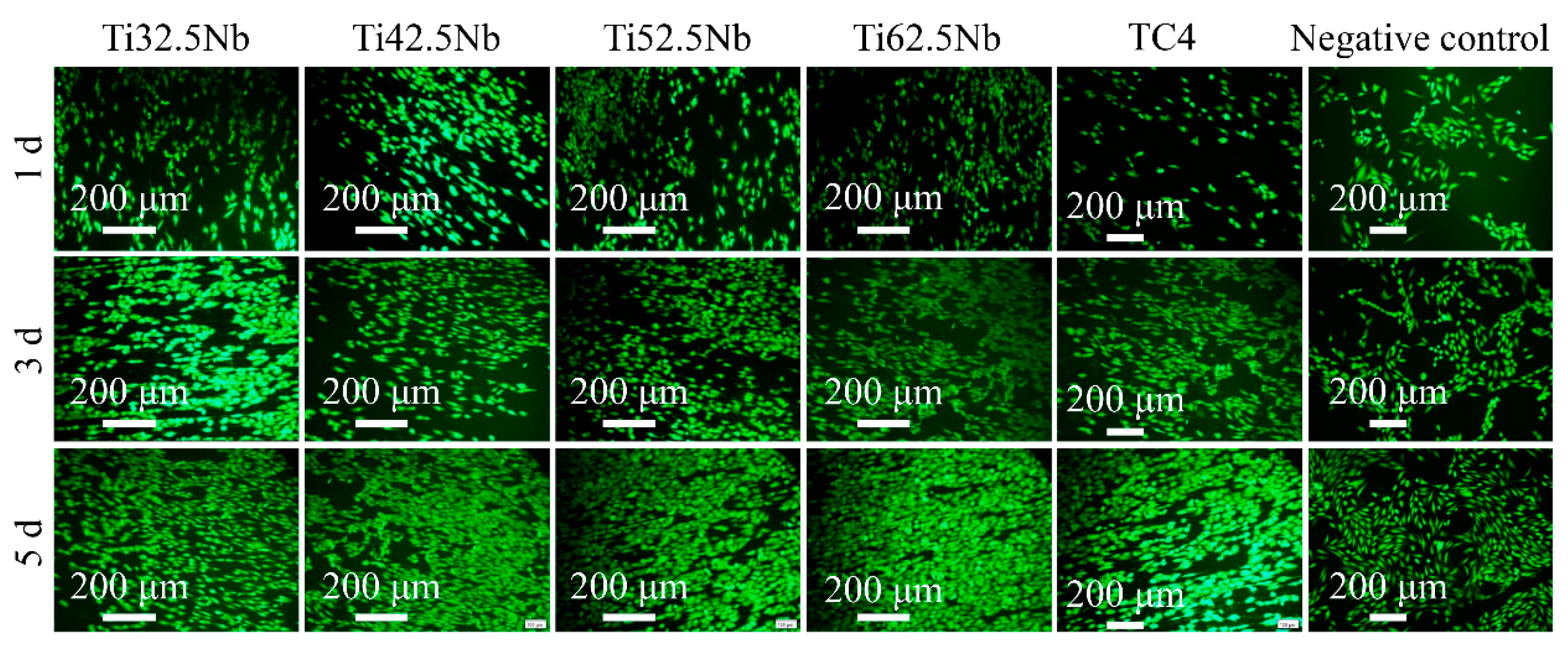
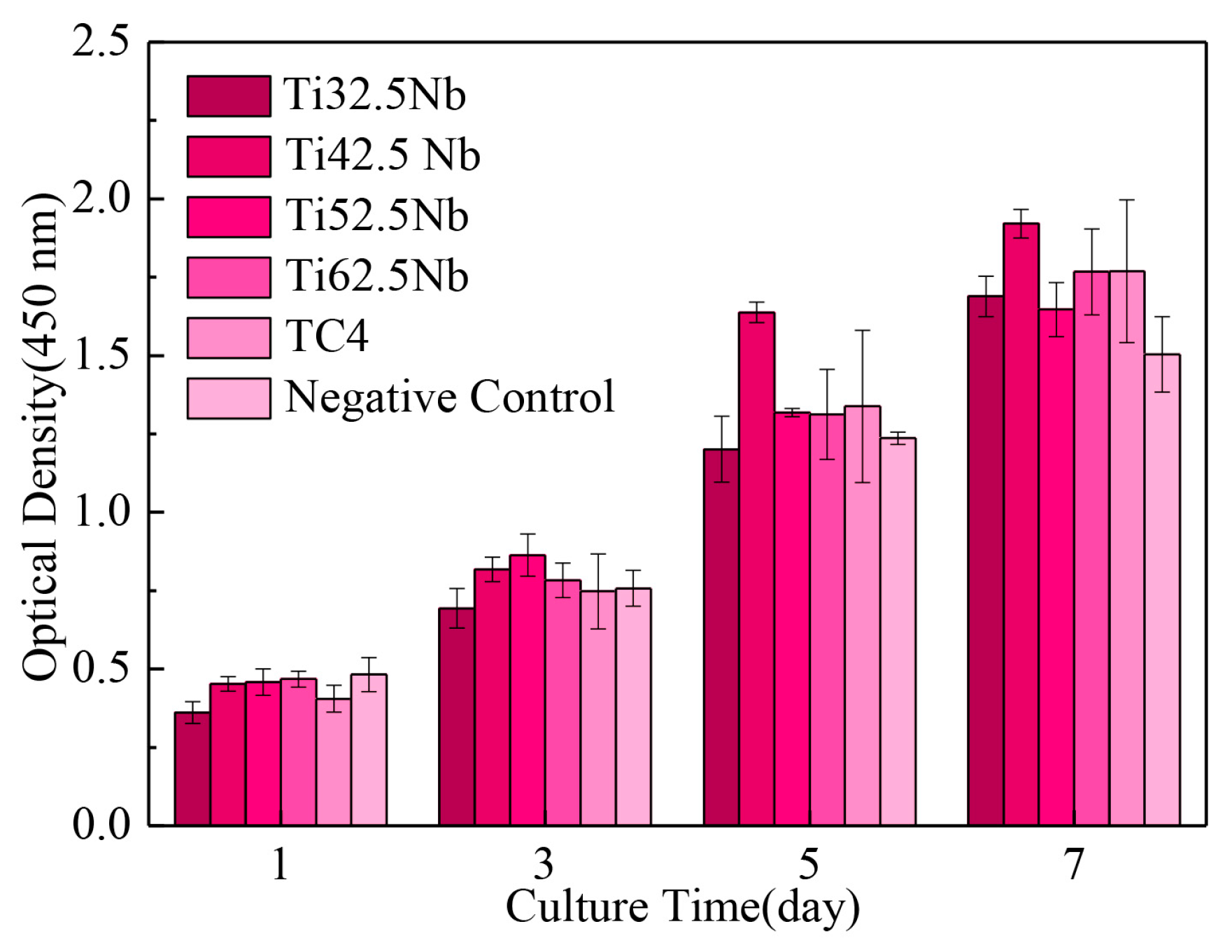
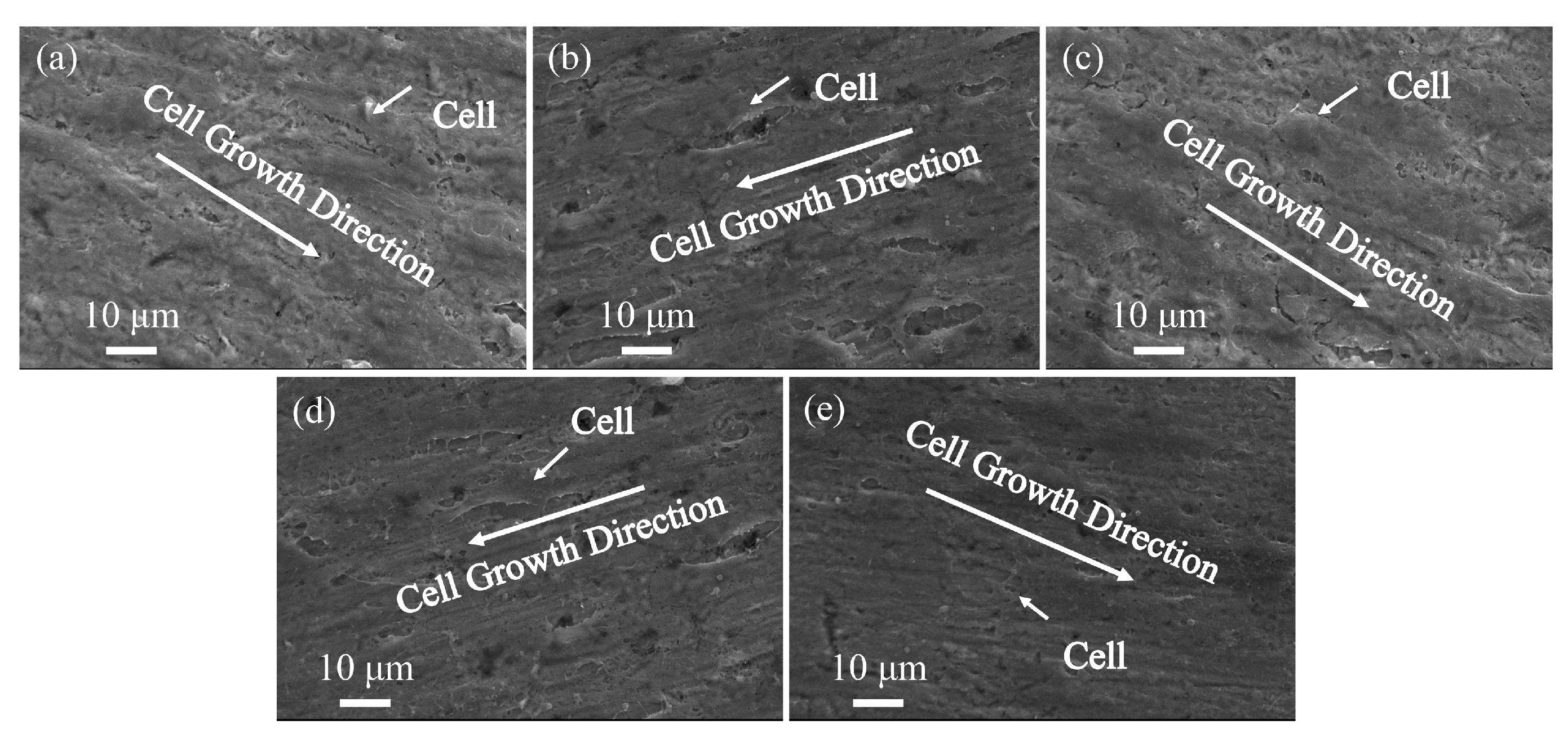
| Elements | Ti | Al | V | Fe | C | N | H | O |
|---|---|---|---|---|---|---|---|---|
| Wt.% | Balance | 6.05 | 3.90 | 0.15 | 0.02 | 0.006 | 0.004 | 0.12 |
| Element | Purity (%) | Density (g/cm3) | Melting Point (°C) |
|---|---|---|---|
| Ti | >99.9 | 4.51 | 1660 |
| Nb | >99.9 | 8.57 | 2468 |
| Order | Reagent | Amount (/1000 mL) | Purity (%) | Molecular Weight |
|---|---|---|---|---|
| 1 | NaCl | 8.035 g | 99.5 | 58.4430 |
| 2 | NaHCO3 | 0.355 g | 99.5 | 84.0068 |
| 3 | KCl | 0.225 g | 99.5 | 74.5515 |
| 4 | K2HPO4·3H2O | 0.231 g | 99.0 | 228.2220 |
| 5 | MgCl2·6H2O | 0.311 g | 98.0 | 203.3034 |
| 6 | 1.0 M-HCl | 39 mL | - | - |
| 7 | CaCl2 | 0.292 g | 95.0 | 110.9848 |
| 8 | Na2SO4 | 0.072 g | 99.0 | 142.0428 |
| 9 | Tris | 6.118 g | 99.0 | 121.1356 |
| 10 | 1.0 M-HCl | 0-5 mL | - | - |
| Alloy | Ti32.5Nb | Ti42.5Nb | Ti52.5Nb | Ti62.5Nb | TC4 |
|---|---|---|---|---|---|
| k | 0.507 | 0.684 | 0.587 | 0.537 | 0.526 |
| Alloy | Ecorr (± SD) (V) | Icorr (± SD) (μA/cm3) |
|---|---|---|
| Ti32.5Nb | −0.69 ± 0.02 | (1.632 ± 0.036) × 10−3 |
| Ti42.5Nb | −0.675 ± 0.01 | (6.282 ± 0.028) × 10−4 |
| Ti52.5Nb | −0.686 ± 0.02 | (7.768 ± 0.013) × 10−4 |
| Ti62.5Nb | −0.707 ± 0.03 | (1.293 ± 0.057) × 10−3 |
| TC4 | −0.675 ± 0.03 | (4.579 ± 0.032) × 10−4 |
| Alloy | Rs (Ω cm2) | Cf (F cm−2) | QPE | n | Rf (Ω cm2) |
|---|---|---|---|---|---|
| Ti32.5Nb | 0.463 | 2.504 × 10−7 | 5.187 × 10−6 | 0.944 | 6.073 |
| Ti42.5Nb | 0.457 | 2.192 × 10−7 | 6.953 × 10−6 | 0.963 | 4.067 |
| Ti52.5Nb | 0.609 | 1.385 × 10−7 | 4.709 × 10−8 | 0.953 | 5.264 |
| Ti62.5Nb | 0.476 | 6.901 × 10−8 | 5.001 × 10−8 | 0.950 | 6.196 |
| TC4 | 0.538 | 2.210 × 10−7 | 3.854 × 10−6 | 0.975 | 5.651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Xu, P. Effect of Nb Content on Phase Transformation and Comprehensive Properties of TiNb Alloy Coating. Coatings 2023, 13, 1186. https://doi.org/10.3390/coatings13071186
Zheng Y, Xu P. Effect of Nb Content on Phase Transformation and Comprehensive Properties of TiNb Alloy Coating. Coatings. 2023; 13(7):1186. https://doi.org/10.3390/coatings13071186
Chicago/Turabian StyleZheng, Yu, and Peng Xu. 2023. "Effect of Nb Content on Phase Transformation and Comprehensive Properties of TiNb Alloy Coating" Coatings 13, no. 7: 1186. https://doi.org/10.3390/coatings13071186
APA StyleZheng, Y., & Xu, P. (2023). Effect of Nb Content on Phase Transformation and Comprehensive Properties of TiNb Alloy Coating. Coatings, 13(7), 1186. https://doi.org/10.3390/coatings13071186





