High-Entropy Composite Coating Based on AlCrFeCoNi as an Anode Material for Li-Ion Batteries
Abstract
:1. Introduction
- An alloy that consists of at least five major metallic elements, each having a concentration of 5%–35%.
- An alloy whose configurational entropy is larger than 1.61*R, where R is the gas constant.
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Coin Cell Preparation
2.4. Electrochemical Characterisation
3. Results and Discussion
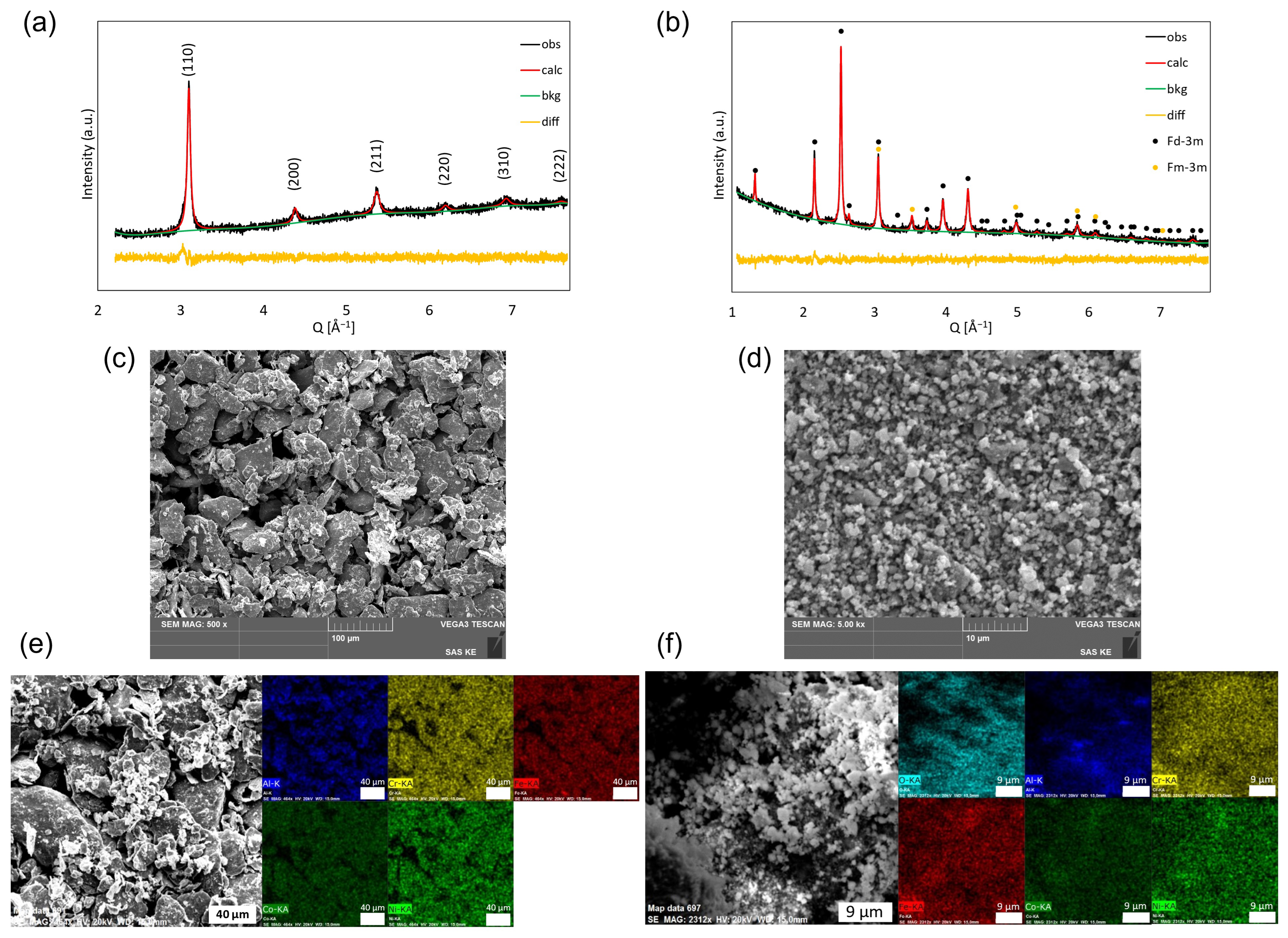
3.1. Material Characterization
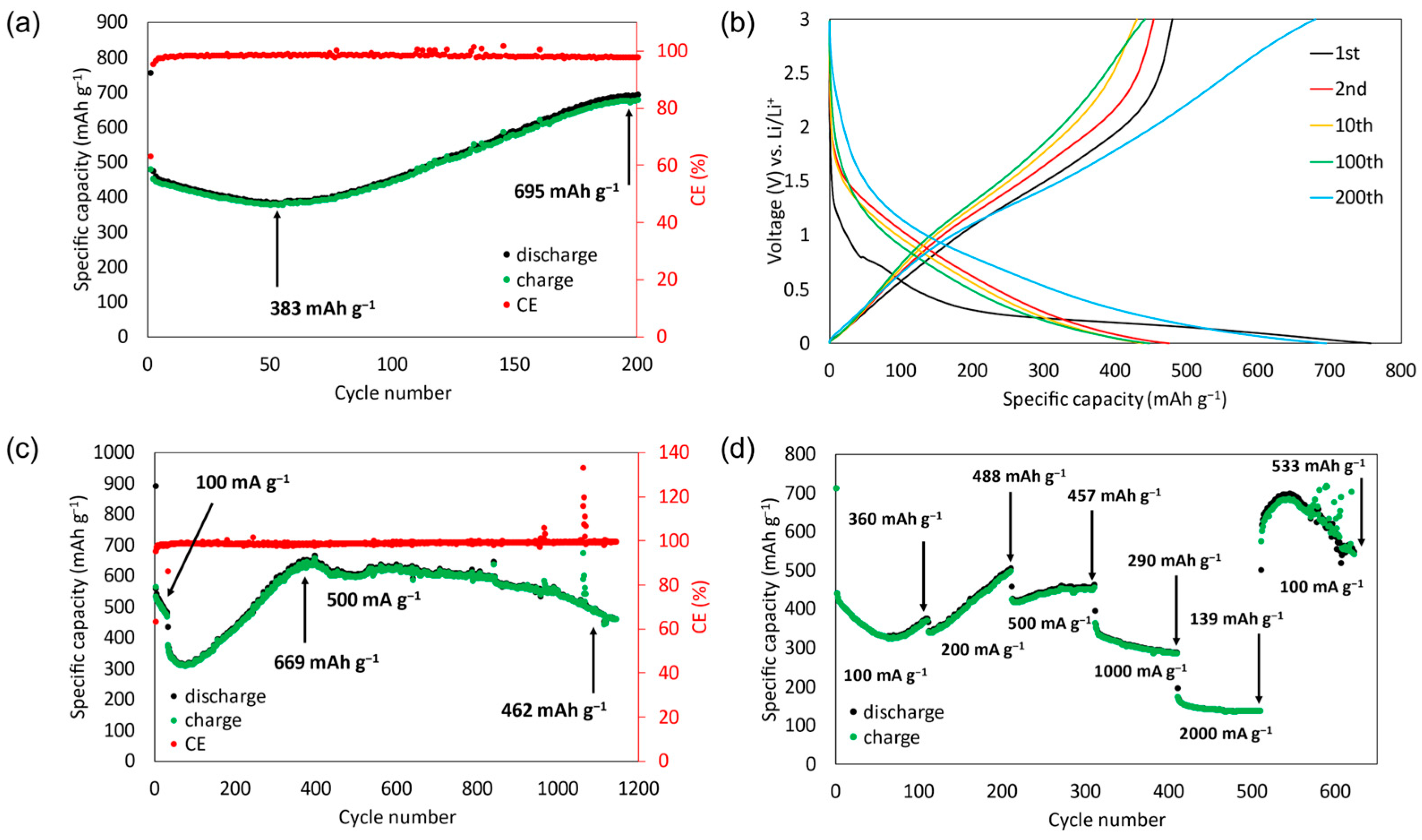
3.2. Electrochemical Characterization
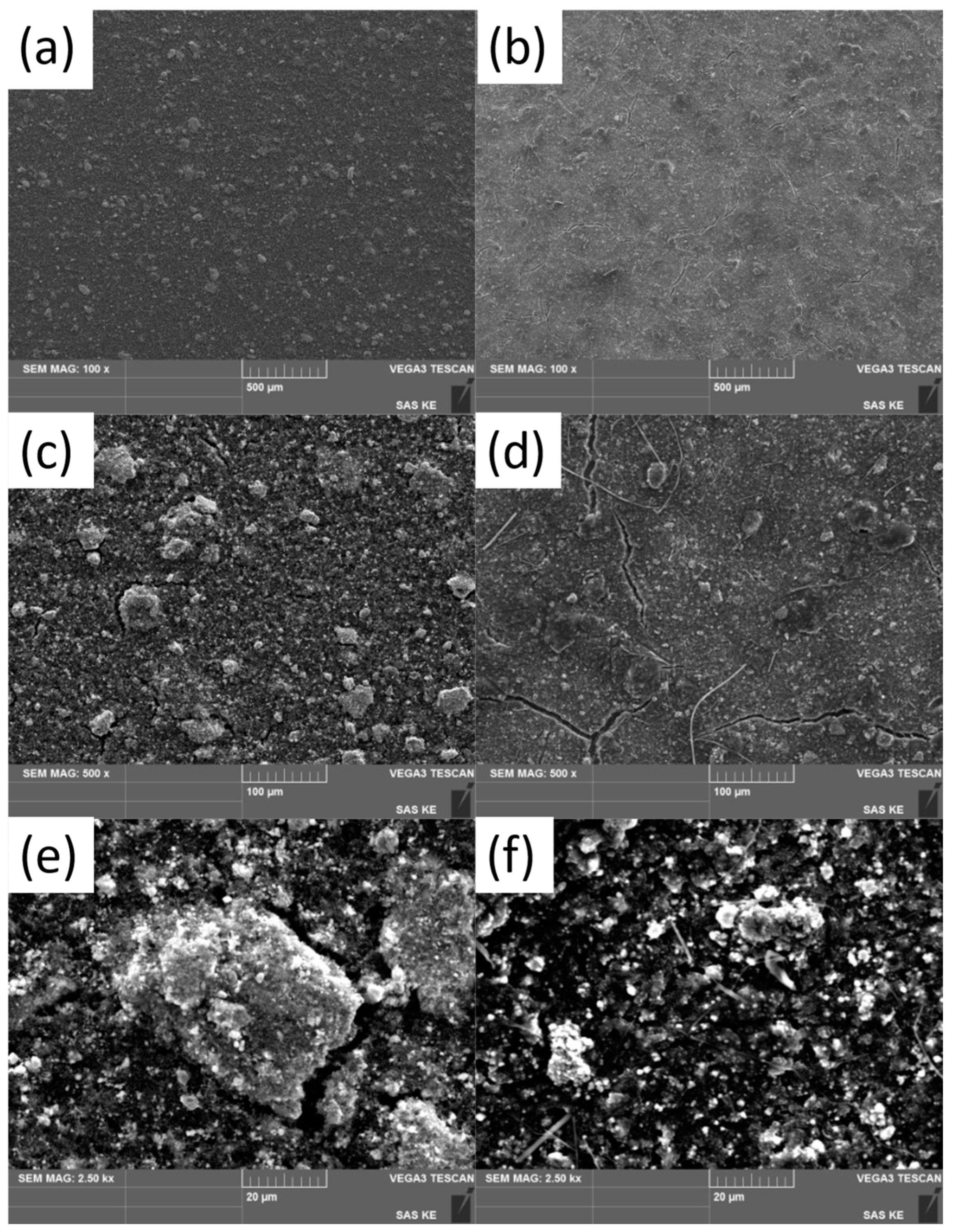
3.3. Electrode Surface Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Zhang, W.J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 1, 13–24. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Wu, F.; Chu, F.; Xue, Z. Lithium-Ion Batteries. In Encyclopedia of Energy Storage; Elsevier: Oxford, UK, 2022; Volume 4, pp. 5–13. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Lai, C. Li-containing alloys beneficial for stabilizing lithium anode: A review. Eng. Rep. 2020, 3, e12339. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902485. [Google Scholar] [CrossRef]
- Duan, C.; Tian, K.; Li, X.; Wang, D.; Sun, H.; Zheng, R.; Wang, Z.; Liu, Y. New spinel high-entropy oxides (FeCoNiCrMnXLi)3O4 (X = Cu, Mg, Zn) as the anode material for lithium-ion batteries. Ceram. Int. 2021, 47, 32025–32032. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Zhang, Y. High-Entropy Materials, 1st ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 12–25. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.; Wei, Z.; Sui, Y.; Shen, B.; Qi, J.; Wei, F.; Zheng, J. High-entropy oxides as advanced anode materials for long-life lithium-ion Batteries. Nano Energy 2022, 95, 106962. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-anionic and -cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Alcoutlabi, M.; Zhang, X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The emergent field of high entropy oxides: Design, prospects, challenges, and opportunities for tailoring material properties. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, N.; Wu, B.; Yang, Z.; Sun, S.; Wang, Y. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium-ion batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.K.; Ting, J.M. High entropy spinel oxide nanoparticles for superior lithiation-delithiation performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Huang, C.-W.; Wu, M.-C.; Patra, J.; Nguyen, T.X.; Chang, M.-T.; Clemens, O.; Ting, J.-M.; Li, J.; Chang, J.-K.; et al. Atomic-scale investigation of Lithiation/Delithiation mechanism in High-entropy spinel oxide with superior electrochemical performance. Chem. Eng. 2021, 420, 129838. [Google Scholar] [CrossRef]
- Wang, X.L.; Jin, E.M.; Sahoo, G.; Jeong, S.M. High-Entropy Metal Oxide (NiMnCrCoFe)3O4 Anode Materials with Controlled Morphology for High-Performance Lithium-Ion Batteries. Batteries 2023, 9, 147. [Google Scholar] [CrossRef]
- Han, B.; Paulauskas, T.; Key, B.; Peebles, C.; Park, J.S.; Klie, R.F.; Vaughey, J.T.; Dogan, F. CS Understanding the Role of Temperature and Cathode Composition on Interface and Bulk: Optimizing Aluminum Oxide Coatings for Li-Ion Cathodes. Appl. Mater. Interfaces 2017, 9, 14769–14778. [Google Scholar] [CrossRef]
- Casino, S.; Heidrich, B.; Makvandi, A.; Beuse, T.; Gallasch, T.; Peterlechner, M.; Wilde, G.; Winter, M.; Niehoff, P. Al2O3 protective coating on silicon thin film electrodes and its effect on the aging mechanism of lithium metal and lithium ion cells. J. Energy Storage 2021, 44, 103479. [Google Scholar] [CrossRef]
- Friesen, A.; Hildebrand, S.; Horsthemke, F.; Börner, M.; Klöpsch, R.; Niehoff, P.; Schappacher, F.M.; Winter, M. Al2O3 coating on anode surface in lithium ion batteries: Impact on low temperature cycling and safety behavior. J. Power Sources 2017, 363, 70–77. [Google Scholar] [CrossRef]
- Lee, J.; Amari, H.; Bahri, M.; Shen, Y.; Xu, C.; Ruff, Z.; Grey, C.P.; Ersen, O.; Aguadero, A.; Browning, N.D.; et al. The Complex Role of Aluminium Contamination in Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries. Batt. Supercaps 2021, 4, 1813–1820. [Google Scholar] [CrossRef]
- Jung, S.; Han, Y.-K. How Do Li Atoms Pass through the Al2O3 Coating Layer during Lithiation in Li-ion Batteries? J. Phys. Chem. Lett. 2013, 4, 2681–2685. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, X.; Lan, X.; Hu, R. A Spinel (FeNiCrMnMgAl)3O4 High Entropy Oxide as a Cycling Stable Anode Material for Li-Ion Batteries. Processes 2022, 10, 49. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Y.; Zhang, F.; Cheng, F.; Cheng, C.; Miao, Y.; Gao, F.; Yu, J. Inactive Al3+-doped La(CoCrFeMnNiAlx)1/(5+x)O3 high-entropy perovskite oxides as high performance supercapacitor electrodes. J. Adv. Ceram. 2022, 11, 742–753. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.; Sun, S.; Wang, Y.; Cui, Y. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J. Alloy. Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Triolo, C.; Wenlei, X.; Petrovičovà, B.; Pinna, N.; Santangelo, S. Evaluation of Entropy-Stabilized (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O Oxides Produced via Solvothermal Method or Electrospinning as Anodes in Lithium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2202892. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2022, 2, 107–123. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Energy Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2004, 34, 210–213. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Ren, M.X.; Fu, H.Z. Microstructure and compressive properties of AlCrFeCoNi high entropy alloy. Mater. Sci. Eng. A 2008, 491, 154–158. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Knuutila, H.K. Evaluating the possibility of high-pressure desorption of CO2 via volatile co-solvent injection. Chem. Eng. Res. Des. 2021, 169, 116–134. [Google Scholar] [CrossRef]
- Fracchia, M.; Manzoli, M.; Anselmi-Tamburini, U.; Ghigna, P. A new eight-cation inverse high entropy spinel with large configurational entropy in both tetrahedral and octahedral sites: Synthesis and cation distribution by X-ray absorption spectroscopy. Scr. Mater. 2020, 188, 26–31. [Google Scholar] [CrossRef]
- Ma, S.; Ding, Q.; Wei, X.; Zhang, Z.; Bei, H. The Effects of Alloying Elements Cr, Al, and Si on Oxidation Behaviors of Ni-Based Superalloys. Materials 2022, 15, 7352. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Kang, H.; Lin, S.; Liu, Y.; Zhang, P.; Qin, W.; Wu, X. Impressive high-temperature oxidation resistance of FeCrNiMnAl high entropy alloy coating on the ferritic/martensitic steel with primordial Al2O3 and Mn3O4 gradient films. J. Alloy. Compd. 2022, 928, 167109. [Google Scholar] [CrossRef]
- Ghadami, F.; Davoudabadi, M.A.; Ghadami, S. Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings 2022, 12, 372. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Yan, S.; Yu, G.; Chen, J.; Yin, F. High temperature oxidation behavior of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings. Mat. Chem. Phys. 2022, 282, 125939. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Chen, Y.; Liu, X.; Zhao, X.; Guo, F.; Xiao, P. Y-Hf co-doped AlCoCrFeNi high-entropy alloy coating with superior oxidation and spallation resistance at 1100 °C. Corr. Sci. 2021, 182, 109267. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Bhatnagar, A. A review on anode materials for lithium/sodim-ion batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Sarkar, A.; Breitung, B.; Hahn, H. High entropy oxides: The role of entropy, enthalpy and synergy. Scr. Mater. 2020, 187, 43–48. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Dippo, O.F.; Vecchio, K.S. A universal configurational entropy metric for high-entropy materials. Scr. Mater. 2021, 201, 113974. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, T.-Y.; Kuo, C.-H.; Lin, C.-C.; Huang, S.-C.; Lin, M.-H.; Wang, C.-C.; Chen, H.-Y. Operando synchrotron transmission X-ray microscopy study on (Mg, Co, Ni, Cu, Zn)O high-entropy oxide anodes for lithium-ion batteries. Mater. Chem. Phys. 2021, 274, 125105. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.; Wei, Z.; Sui, Y.; Shen, B.; Qi, J.; Wei, F.; Meng, Q.; Ren, Y.; et al. High entropy oxides (FeNiCrMnX)3O4 (X = Zn, Mg) as anode materials for lithium ion batteries. Ceram. Int. 2021, 47, 33972–33977. [Google Scholar] [CrossRef]
- Wang, K.; Hua, W.; Huang, X.; Stenzel, D.; Wang, J.; Ding, Z.; Ciu, Y.; Wang, Q.; Ehrenberg, H.; Breitung, B.; et al. Synergy of cations in high entropy oxide lithium-ion battery anode. Nat. Commun. 2023, 14, 1487. [Google Scholar] [CrossRef]
- Fracchia, M.; Callegari, D.; Coduri, M.; Anselmi-Tamburini, U.; Manzoli, M.; Quartarone, E.; Ghigna, P. Electrochemical performance of high and medium entropy oxides for lithium batteries. Front. Energy Res. 2022, 10, 883206. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Li, Y.; Zhang, H.; Jia, Q.; Zhang, S.; Lei, W. High-entropy oxide: A future anode contender for lithium-ion battery. EcoMat 2022, 4, e12261. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Tsai, C.-C.; Patra, J.; Clemens, O.; Chang, J.-K.; Ting, J.-M. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in Lithium-ion batteries. Chem. Eng. J. 2022, 430, 132658. [Google Scholar] [CrossRef]
- Illig, J. Physically Based Impedance Modelling of Lithium-Ion Cells. Ph.D. Thesis, Karlsruher Institut für Technologie, Karlsruhe, Germany, 4 April 2014. [Google Scholar] [CrossRef]
- Illig, J.; Ender, M.; Chrobak, T.; Schmidt, J.P.; Klotz, D.; Ivers-Tiffée, E. Separation of Charge Transfer and Contact Resistance in LiFePO4-Cathodes by Impedance Modeling. J. Electrochem. Soc. 2012, 159, A952. [Google Scholar] [CrossRef]
- Shankar, L.S.; Zalka, D.; Szabó, T.; Székely, E.; Kőrösi, M.; Pászti, Z.; Balázsi, K.; Illés, L.; Czigány, Z.; Kun, R. Supercritical carbon dioxide assisted synthesis of ultra-stable sulfur/carbon composite cathodes for Li–S batteries. Mater. Today Chem. 2022, 26, 101240. [Google Scholar] [CrossRef]
- Landesfeind, J.; Hattendorff, J.; Ehrl, A.; Wall, W.A.; Gasteiger, H.A. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy. J. Electrochem. Soc. 2016, 163, A1373. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Wang, S.-Y.; Kuo, C.-H.; Huang, S.-C.; Lin, M.-H.; Li, C.-H.; Chen, Y.-T.; Wang, C.-C.; Liao, Y.-F.; Lin, C.-C.; et al. In operando synchrotron X-ray studies of a novel spinel (Ni0.2Co0.2Mn0.2Fe0.2Ti0.2)3O4 high-entropy oxide for energy storage applications. J. Mater. Chem. A 2020, 8, 21756–21770. [Google Scholar] [CrossRef]
- Kim, H.; Choi, W.; Yoon, J.; Um, J.H.; Lee, W.; Kim, J.; Cabana, J.; Yoon, W.-S. Exploring Anomalous Charge Storage in Anode Materials for Next-Generation Li Rechargeable Batteries. Chem. Rev. 2020, 120, 6934–6976. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Heubner, C.; Schneider, M.; Michaelis, A. Diffusion-Limited C-Rate: A Fundamental Principle Quantifying the Intrinsic Limits of Li-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902523. [Google Scholar] [CrossRef]
- Minouei, H.; Tsvetkov, N.; Kheradmandfard, M.; Han, J.; Kim, D.-E.; Hong, S.I. Tuning the electrochemical performance of high-entropy oxide nanopowder for anode Li-ion storage via structural tailoring. J. Power Sources 2022, 549, 232041. [Google Scholar] [CrossRef]
- Lingappan, N.; Kong, L.; Pecht, M. The significance of aqueous binders in lithium-ion batteries. Renew. Sust. Energ. Rev. 2021, 147, 111227. [Google Scholar] [CrossRef]
- Zhu, Z.; He, Y.; Hu, H.; Zhang, F. Evolution of Internal Stress in Heterogeneous Electrode Composite during the Drying Process. Energies 2021, 14, 1683. [Google Scholar] [CrossRef]
- Yen, J.-Z.; Yang, Y.-C.; Tuan, H.Y. Interface engineering of high entropy Oxide@Polyaniline heterojunction enables highly stable and excellent lithium ion storage performance. Chem. Eng. J. 2022, 450, 137924. [Google Scholar] [CrossRef]

| HEA | HEM | |||||||
|---|---|---|---|---|---|---|---|---|
| Element | wt.% | σ wt.% | at.% | σ at.% | wt.% | σ wt.% | at.% | σ at.% |
| Oxygen | - | - | - | - | 27.28 | 0.51 | 54.12 | 0.64 |
| Aluminum | 7.15 | 2.95 | 13.69 | 5.51 | 7.93 | 0.08 | 9.33 | 0.15 |
| Chromium | 21.38 | 1.34 | 21.55 | 1.87 | 15.55 | 0.22 | 9.49 | 0.20 |
| Iron | 24.10 | 1.94 | 22.64 | 2.54 | 16.84 | 0.19 | 9.57 | 0.10 |
| Cobalt | 25.26 | 0.61 | 22.44 | 1.20 | 17.10 | 0.31 | 9.21 | 0.22 |
| Nickel | 22.11 | 0.99 | 19.69 | 0.77 | 15.31 | 0.06 | 8.28 | 0.09 |
| HEM before Cycling | HEM after Cycling | |||
|---|---|---|---|---|
| Χ2/|Z| = 2.0341·10−3 | Χ2/|Z| = 6.2658·10−4 | |||
| Estimated Value | Standard Error | Estimated Value | Standard Error | |
| Rs (Ohm) | 3.60 | ±6.6613·10−2 | 4.8253 | ±0.2703 |
| R1 (Ohm) | 147.179 | ±3.0541 | 121.1729 | ±2.9620 |
| Q1 (F) | 1.1875·10−5 | ±3.6114·10−7 | 1.1387∙10−5 | ±3.1665·10−7 |
| n1 | 0.710644 | ±3.3045·10−2 | 0.90256 | ±1.1401·10−3 |
| R2 (Ohm) | 201.06802 | ±2.0223·10−1 | 186.4122 | ±3.6832·10−2 |
| Q2 (F) | 1.95700·10−3 | ±2.2669·10−5 | 6.0293∙10−5 | ±7.6112·10−1 |
| n2 | 0.731745 | ±7.8472·10−3 | 0.7651 | ±9.5216·10−3 |
| R3 (Ohm) | 27.26705 | ±1.8926·10−1 | 44.0167 | ±1.7065·10−2 |
| Q3 (F) | 3.02459∙10−2 | ±3.4817·10−3 | 9.9871∙10−2 | ±1.8089∙10−3 |
| n3 | 0.613330 | ±2.0345·10−2 | 0.6981 | ±3.5001∙10−3 |
| Rion (Ohm) | 172.1767 | ±3.6627·10−1 | 55.6004 | ±1.9780∙10−1 |
| Qs (F) | 1.67485∙10−4 | ±4.0219∙10−5 | 6.3282∙10−4 | ±1.6962∙10−5 |
| γ | 0.91243 | ±5.0066∙10−4 | 0.8006 | ±4.4166∙10−3 |
| Material | Particle Size | Long-Term Cycling | Rate Capability Test-Specific Capacities (mAh g−1) at Current Densities | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| 100 mA g−1 | 200 mA g−1 | 500 mA g−1 | 1000 mA g−1 | 2000 mA g−1 | 100 mA g−1 | ||||
| (CrMnFeCoNi)3O4 | 200–900 nm | 402 mAh g−1 at 500 mA g−1 (300 cycles) | 586 | 478 | 361 | 269 | 180 | 483 | [4] |
| (CrMnFeCoNi)3O4 | 200–500 nm | 693 mAh g−1 at 500 mA g−1 (260 cycles) | 967 | 828 | 740 | 625 | 532 | 837 at 200 mA g−1 | [11] |
| (CrMnFeCoNi)3O4 | 100–200 nm | 750 mAh g−1 at 500 mA g−1 (200 cycles) | 1072 | 979 | 824 | 649 | 500 | - | [16] |
| (MgTiZnCuFe)3O4 | 51–345 nm | 504 mAh g−1 at 100 mA g−1 (300 cycles) | 571 | 460 | 405 | 342 | 268 | 552 | [15] |
| (CrMnFeNiCu)3O4 | 500–800 nm | 512 mAh g−1 at 500 mA g−1 (250 cycles) | 567 | 482 | 400 | 297 | 226 | - | [50] |
| (TiMnFeCoNi)3O4 | micrometer sizes | 536 mAh g−1 at 100 mA g−1 (100 cycles) | 496 | 455 at 250 mA g−1 | 435 | 401 | 343 at 2500 mA g−1 | 590 at 50 mA g−1 | [56] |
| (MgCrMnFeCo)3O4 | 50 nm | 673 mAh g−1 at 1000 mA g−1 (1000 cycles) | 964 | 892 | 787 | 658 | 541 | 594 | [60] |
| (AlCrFeCoNi)3O4 | micrometer sizes | 543 mAh g−1 at 500 mA g−1 (1000 cycles) | 360 | 488 | 457 | 290 | 139 | 533 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csík, D.; Baranová, G.; Džunda, R.; Zalka, D.; Breitung, B.; Hagarová, M.; Saksl, K. High-Entropy Composite Coating Based on AlCrFeCoNi as an Anode Material for Li-Ion Batteries. Coatings 2023, 13, 1219. https://doi.org/10.3390/coatings13071219
Csík D, Baranová G, Džunda R, Zalka D, Breitung B, Hagarová M, Saksl K. High-Entropy Composite Coating Based on AlCrFeCoNi as an Anode Material for Li-Ion Batteries. Coatings. 2023; 13(7):1219. https://doi.org/10.3390/coatings13071219
Chicago/Turabian StyleCsík, Dávid, Gabriela Baranová, Róbert Džunda, Dóra Zalka, Ben Breitung, Mária Hagarová, and Karel Saksl. 2023. "High-Entropy Composite Coating Based on AlCrFeCoNi as an Anode Material for Li-Ion Batteries" Coatings 13, no. 7: 1219. https://doi.org/10.3390/coatings13071219







