Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Hydrothermal Carbonization
2.3. Activation after HTC
2.4. Characterizations
2.5. Adsorption Studies
2.6. Reusability and Recovery of Adsorbent
3. Results and Discussions
3.1. Formation and Morphologies of Carbon Microspheres
3.2. Surface Textural Properties of Carbon Microspheres
3.3. Adsorption Studies
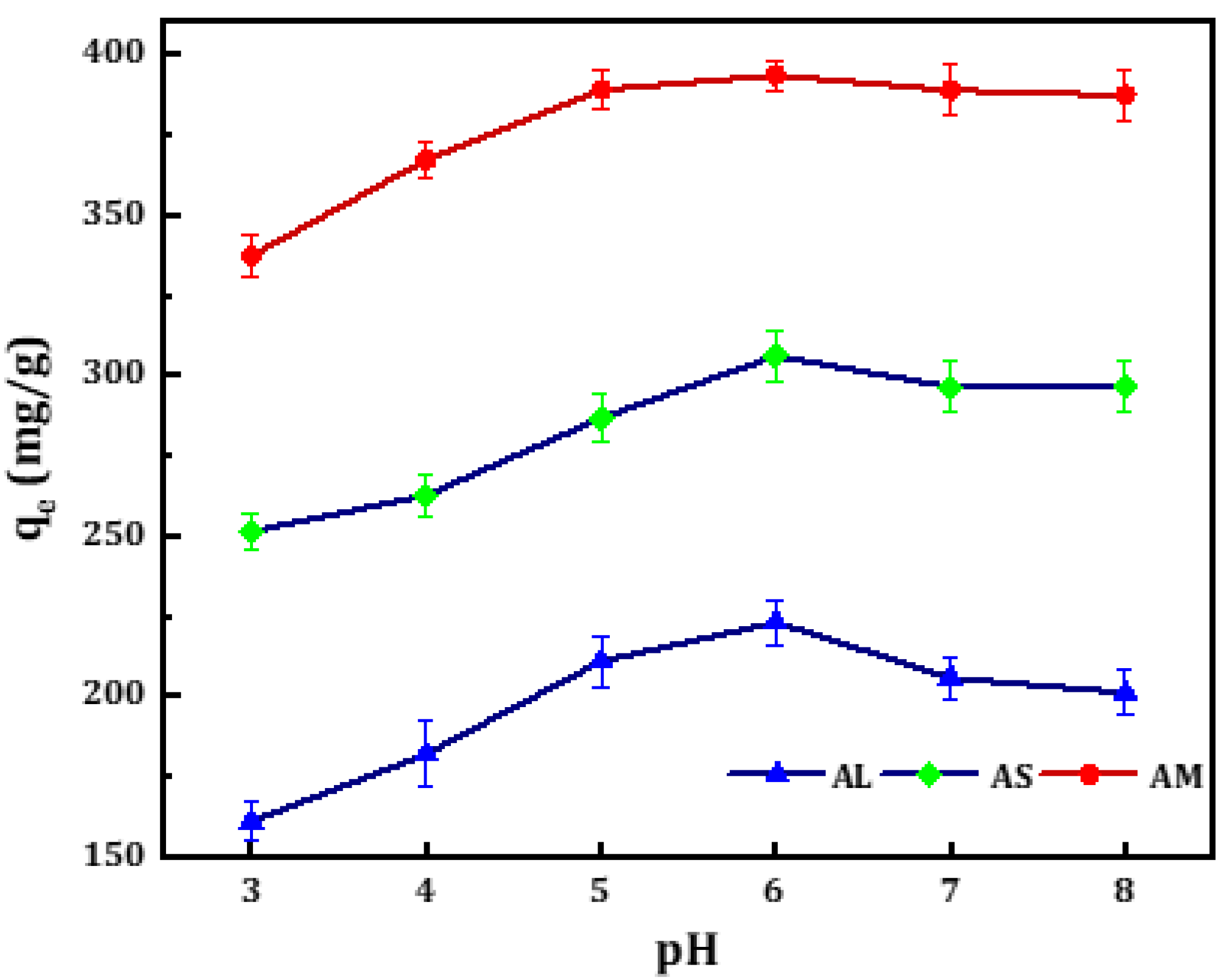
3.3.1. Effect of pH
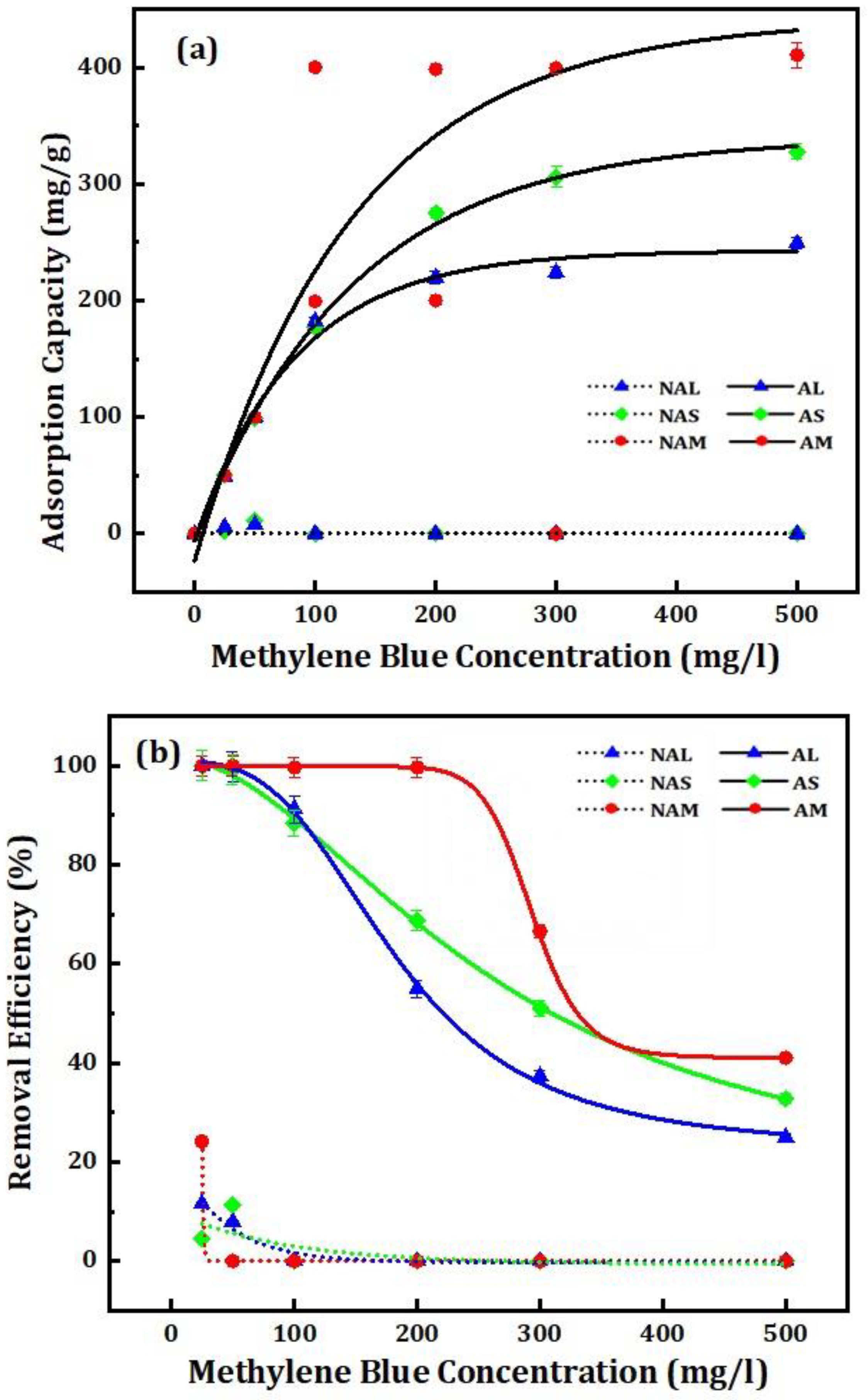
3.3.2. The Influence of Initial Concentration and Contact Time
3.3.3. Adsorption Isotherm
Langmuir Isotherm
Freundlich Isotherm
Temkin Isotherm
3.3.4. Adsorption Kinetics
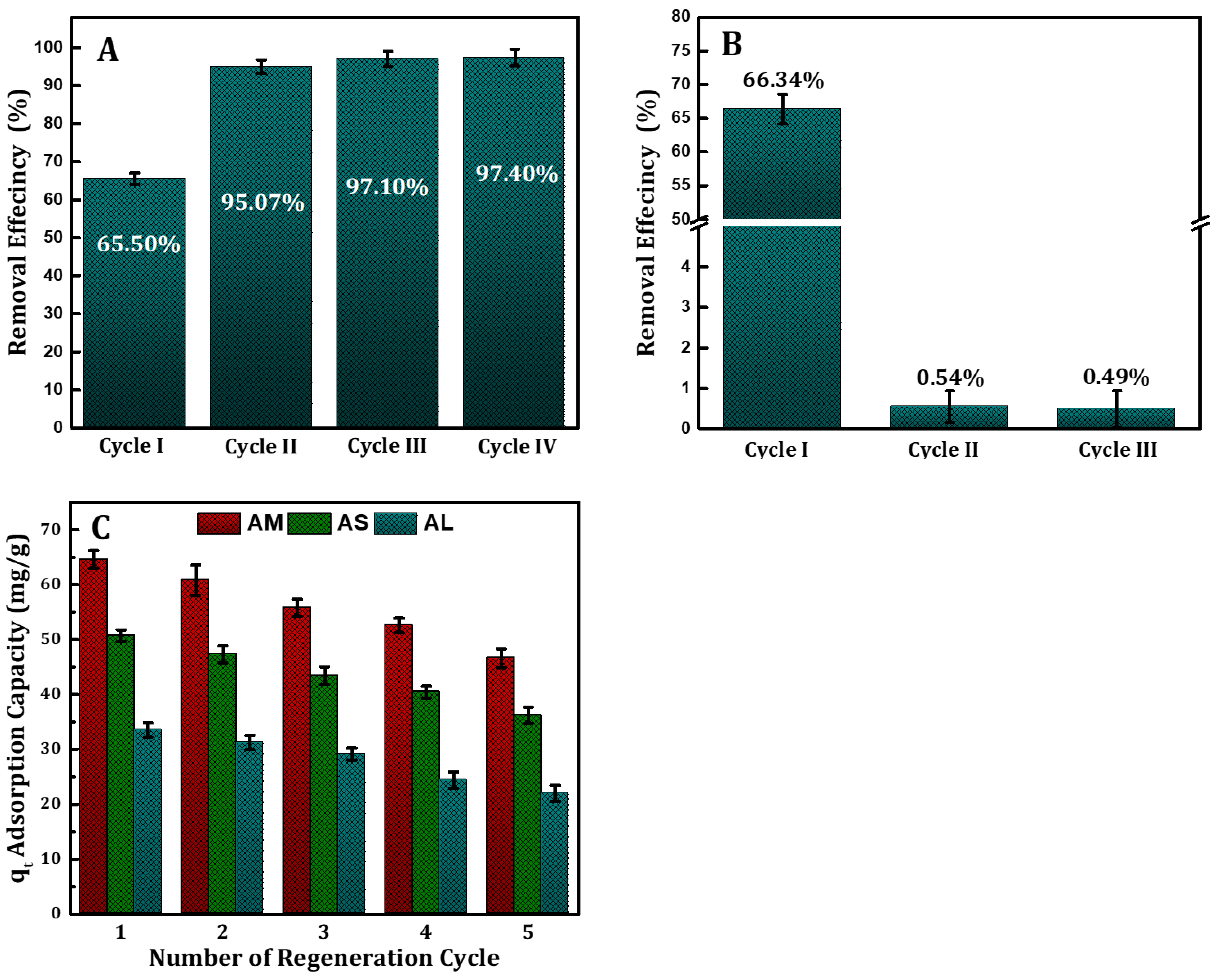
3.4. Multistage Extraction, Reusability, and Recovery Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryu, J.; Suh, Y.W.; Suh, D.J.; Ahn, D.J. Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon 2010, 48, 1990–1998. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.-M. Carboxylate-rich carbonaceous materials via one-step hydrothermal carbonization of glucose in the presence of acrylic acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Al-Awadi, A.S.; El-Harbawi, M.; Algarawi, A.; Alalawi, A.; Alrashed, M.M.; Yin, C.Y. Synthesis of carbon microspheres via hydrothermal carbonization of Sabal palms (Sabal palmetto) biomass for adsorption of methylene blue. Biomass Conv. Bioref. 2022, 1–11. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Liew, R.K.; Mahari, W.A.W.; Peng, W.; Sonne, C.; Kong, S.H.; Tabatabaei, M.; Aghbashlo, M.; Park, Y.K.; Lam, S.S. Production of value-added hydrochar from single-mode microwave hydrothermal carbonization of oil palm waste for de-chlorination of domestic water. Sci. Total Environ. 2022, 833, 154968. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, Q.M.; Zhao, X.R. The hydrochar characters of municipal sewage sludge under different hydrothermal temperatures and durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wan, Z.; Jing, F.; Li, Z.; Chen, J.; Tsang, D.C. Tailored design of food waste hydrochar for efficient adsorption and catalytic degradation of refractory organic contaminant. J. Clean. Prod. 2021, 310, 127482. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Pak, S.; Ahn, J.; Kim, H. Synthesis of Saccharide-based Hydrochar with Macroporous Structure for Effective Organic Pollutant Removal. Fibers Polym. 2022, 23, 1789–1796. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Jabeen, S.; Gao, X.; Hayashi, J.I.; Altarawneh, M.; Dlugogorski, B.Z. Effects of product recovery methods on the yields and properties of hydrochars from hydrothermal carbonization of algal biomass. Fuel 2023, 332, 126029. [Google Scholar] [CrossRef]
- El-Harbawi, M.; Alhawtali, S.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Alzobidi, A.; Yin, C.Y. Synthesis of Carbon Microspheres from Inedible Crystallized Date Palm Molasses: Influence of Temperature and Reaction Time. Materials 2023, 16, 1672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Guo, J.Z.; Wu, C.; Huan, W.W.; Chen, L.; Li, B. Enhanced removal of Cr (VI) by cation functionalized bamboo hydrochar. Bioresour. Technol. 2022, 347, 126703. [Google Scholar] [CrossRef]
- Kamarudzaman, A.N.; Adan, S.N.A.C.; Hassan, Z.; Wahab, M.A.; Makhtar, S.M.Z.; Seman, N.A.A.; Jalil, M.F.A.; Handayani, D.; Syafiuddin, A. Biosorption of Copper (II) and Iron (II) using Spent Mushroom Compost as Biosorbent. Biointerface Res. Appl. Chem. 2022, 12, 7775–7786. [Google Scholar]
- Salami, B.A.; Oyehan, T.A.; Gambo, Y.; Badmus, S.O.; Tanimu, G.; Adamu, S.; Lateef, S.A.; Saleh, T.A. Technological trends in nanosilica synthesis and utilization in advanced treatment of water and wastewater. Environ. Sci. Pollut. Res. 2022, 29, 42560–42600. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Eccles, H. Treatment of metal-contaminated wastes: Why select a biological process? Trends Biotechnol. 1999, 17, 462–465. [Google Scholar] [CrossRef]
- Da’ana, D.A.; Zouari, N.; Ashfaq, M.Y.; Abu-Dieyeh, M.; Khraisheh, M.; Hijji, Y.M.; Al-Ghouti, M.A. Removal of toxic elements and microbial contaminants from groundwater using low-cost treatment options. Curr. Pollut. Rep. 2021, 7, 300–324. [Google Scholar] [CrossRef]
- Ali, A.M.; Shahbaz, M.; Shahzad, K.; Inayat, M.; Naqvi, S.; Al-Zahrani, A.A.; Rashid, M.I.; Rehan, M.; Mahpudz, A.B. Polygeneration syngas and power from date palm waste steam gasification through an Aspen Plus process modeling. Fuel 2023, 332, 126120. [Google Scholar] [CrossRef]
- Faiad, A.; Alsmari, M.; Ahmed, M.M.; Bouazizi, M.L.; Alzahrani, B.; Alrobei, H. Date palm tree waste recycling: Treatment and processing for potential engineering applications. Sustainability 2022, 14, 1134. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.M.; Sulaiman, O. Optimized preparation for large surface area activated carbon from date (Phoenix dactylifera L.) stone biomass. Biomass Bioenergy 2014, 61, 167–178. [Google Scholar] [CrossRef]
- Sabzevari, A.; Kabiri, K. Converting date seed biomass into highly absorbing hydrogel. Iran. Polym. J. 2016, 25, 597–606. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Jamil, F.; Ala’a, H.; Baawain, M.; Al-Haj, L.; Al-Hinai, M.; Al-Abri, M.; Rafiq, S. Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Convers. Manag. 2017, 135, 236–243. [Google Scholar] [CrossRef]
- Elnajjar, E.; Syam, M.M.; Al-Omari, S.A.B. Experimental investigations of bio-syngas production using microwave pyrolysis of UAE’S palm date seed pits. Fuel 2021, 303, 121348. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Długoński, A.; Bernat, P.; Długoński, J.; Jasińska, A. Environmental and molecular approach to dye industry waste degradation by the ascomycete fungus Nectriella pironii. Sci. Rep. 2021, 11, 23829. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X.; Wang, H.; Tsend, N. Hydrochars from bamboo sawdust through acid assisted and two-stage hydrothermal carbonization for removal of two organics from aqueous solution. Bioresour. Technol. 2018, 261, 257–264. [Google Scholar] [CrossRef]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, K.; Hang, F.; Zhang, Z.; Chen, P.; Wei, L.; Xie, C. Efficient removal of methylene blue by activated hydrochar prepared by hydrothermal carbonization and NaOH activation of sugarcane bagasse and phosphoric acid. RSC Adv. 2022, 12, 1885–1896. [Google Scholar] [CrossRef]
- Lotfiman, S.; Awang Biak, D.R.; Ti, T.B.; Kamarudin, S.; Nikbin, S. Influence of date syrup as a carbon source on bacterial cellulose production by Acetobacter xylinum 0416. Adv. Polym. Technol. 2018, 37, 1085–1091. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- Nasser, R.A.; Salem, M.Z.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical analysis of different parts of date palm (Phoenix dactylifera L.) using ultimate, proximate and thermo-gravimetric techniques for energy production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Lillo-Ro’denas, M.A.; Salinas-Martı’nez de Lecea, C.; Linares-Solano, A. Hydrothermal and conventional H3PO4 activation of two natural bio-fibers. Carbon 2012, 50, 3158–3169. [Google Scholar] [CrossRef]
- Romero-Anaya, A.J.; Ouzzine, M.; Lillo-Rodenas, M.A.; Linares-Solano, A. Spherical carbons: Synthesis, characterization and activation processes. Carbon 2014, 68, 296–307. [Google Scholar] [CrossRef]
- Boehm, H.-P. Chemical identification of surface groups. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, UK, 1966; pp. 179–274. [Google Scholar]
- Boehm, H.-P. Surface chemical characterization of carbons from adsorption studies. In Adsorption by Carbons; Elsevier: Amsterdam, The Netherlands, 2008; pp. 301–327. [Google Scholar]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: An application in methylene blue removal by RSM optimization. Int. J. Phytoremediat. 2021, 24, 1–13. [Google Scholar] [CrossRef]
- Everett, D.H. Manual of symbols and terminology for physicochemical quantities and units, appendix II: Definitions, terminology and symbols in colloid and surface chemistry. Pure Appl. Chem. 1971, 31, 577–638. [Google Scholar] [CrossRef]
- De Souza Macedo, J.; da Costa Júnior, N.B.; Almeida, L.E.; da Silva Vieira, E.F.; Cestari, A.R.; de Fátima Gimenez, I.; Villarreal Carreno, N.L.; Barreto, L.S. Kinetic and calorimetric study of the adsorption of dyes on mesoporous activated carbon prepared from coconut coir dust. J. Colloid Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of Methylene Blue from Aqueous Solution by Bone Char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Morimoto, M.; Takanohashi, T. Production of carbonaceous microspheres from wood sawdust by a novel hydrothermal carbonization and extraction method. RSC Adv. 2017, 7, 42123–42128. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; El-Subruiti, G.M.; Ali, B.M.; Abd El-Latif, M.M.; Omer, A.M. Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. J. Porous Mater. 2023, 30, 607–618. [Google Scholar] [CrossRef]
- Basha, K.; El-Monaem, A.; Eman, M.; Khalifa, R.E.; Omer, A.M.; Eltaweil, A.S. Sulfonated graphene oxide impregnated cellulose acetate floated beads for adsorption of methylene blue dye: Optimization using response surface methodology. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Hurairah, S.N.; Lajis, N.M.; Halim, A.A. Methylene blue removal from aqueous solution by adsorption on Archidendron jiringa seed shells. J. Geosci. Environ. Prot. 2020, 8, 128–143. [Google Scholar] [CrossRef]
- Bharathi, K.S.; Ramesh, S.T. Removal of dyes using agricultural waste as low-cost adsorbents: A review. Appl. Water Sci. 2013, 3, 773–790. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Fytianos, K.; Voudrias, E.; Kokkalis, E. Sorption-desorption Behaviour of 2, 4-dichlorophenol by Marine Sediments. Chemosphere 2000, 40, 3–6. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and kinetics of processes on nonhomogeneous surfaces and at interaction between adsorbed molecules. Zh. Fiz. Khim. 1941, 15, 296–332. [Google Scholar]
- Sun, Z.; Liu, Y.; Srinivasakannan, C. Green Preparation and Environmental Application of Porous Carbon Microspheres. ChemistrySelect 2020, 5, 9308–9312. [Google Scholar] [CrossRef]
- Salimi, M.; Balou, S.; Kohansal, K.; Babaei, K.; Tavasoli, A.; Andache, M. Optimizing the preparation of meso-and microporous canola stalk-derived hydrothermal carbon via response surface methodology for methylene blue removal. Energy Fuels 2017, 31, 12327–12338. [Google Scholar] [CrossRef]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage sludge hydrochar: An option for removal of methylene blue from wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I. Novel oxone treated hydrochar for the removal of Pb (II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of methylene blue and Cd (II) onto maleylated modified hydrochar from water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef]
- Zou, W.; Li, K.; Bai, H.; Shi, X.; Han, R. Enhanced cationic dyes removal from aqueous solution by oxalic acid modified rice husk. J. Chem. Eng. Data 2011, 56, 1882–1891. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Borja, R. Methylene blue number as useful indicator to evaluate the adsorptive capacity of granular activated carbon in batch mode: Influence of adsorbate/adsorbent mass ratio and particle size. J. Hazard. Mater. 2009, 165, 291–299. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, F.G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, F.G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
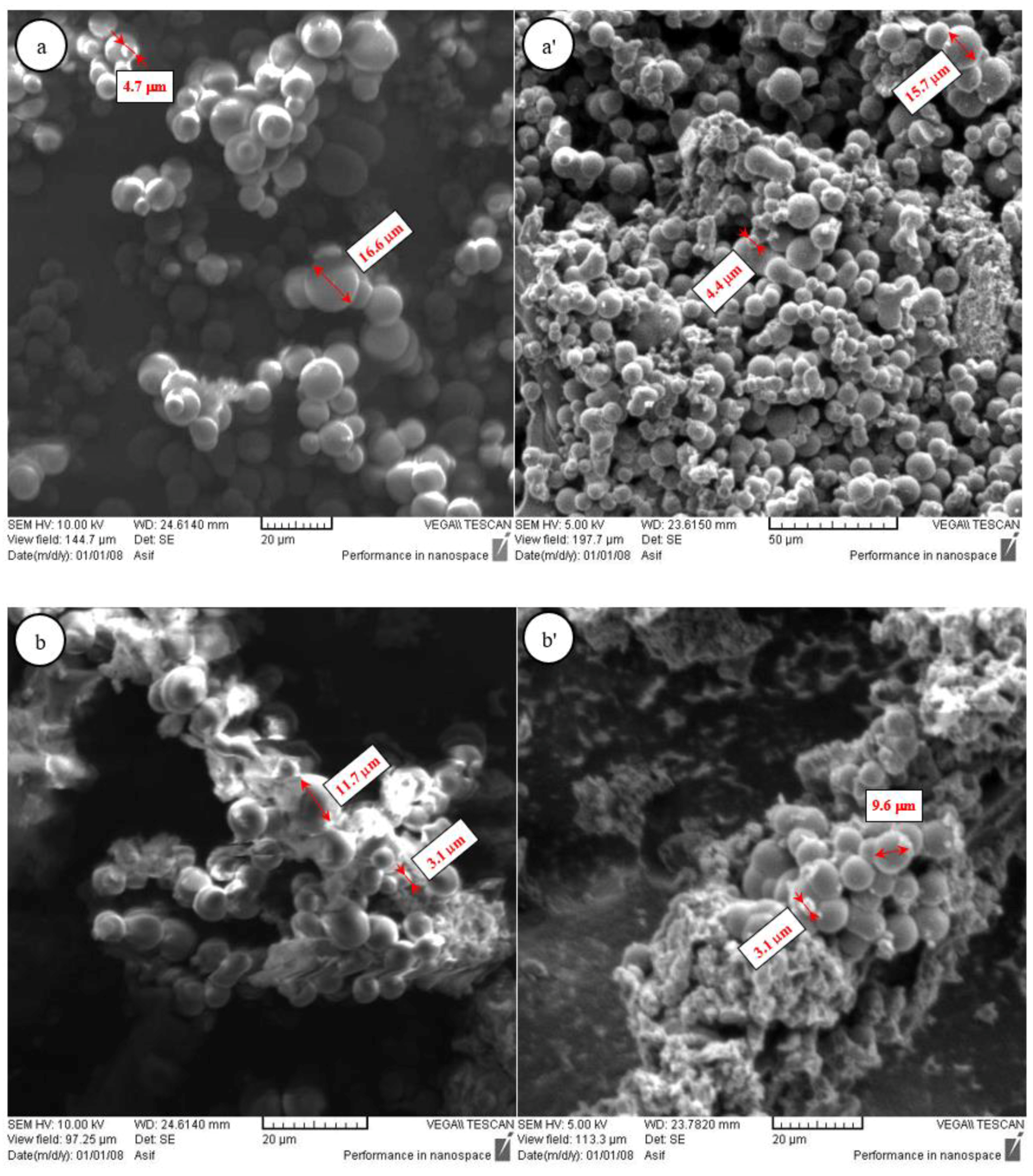

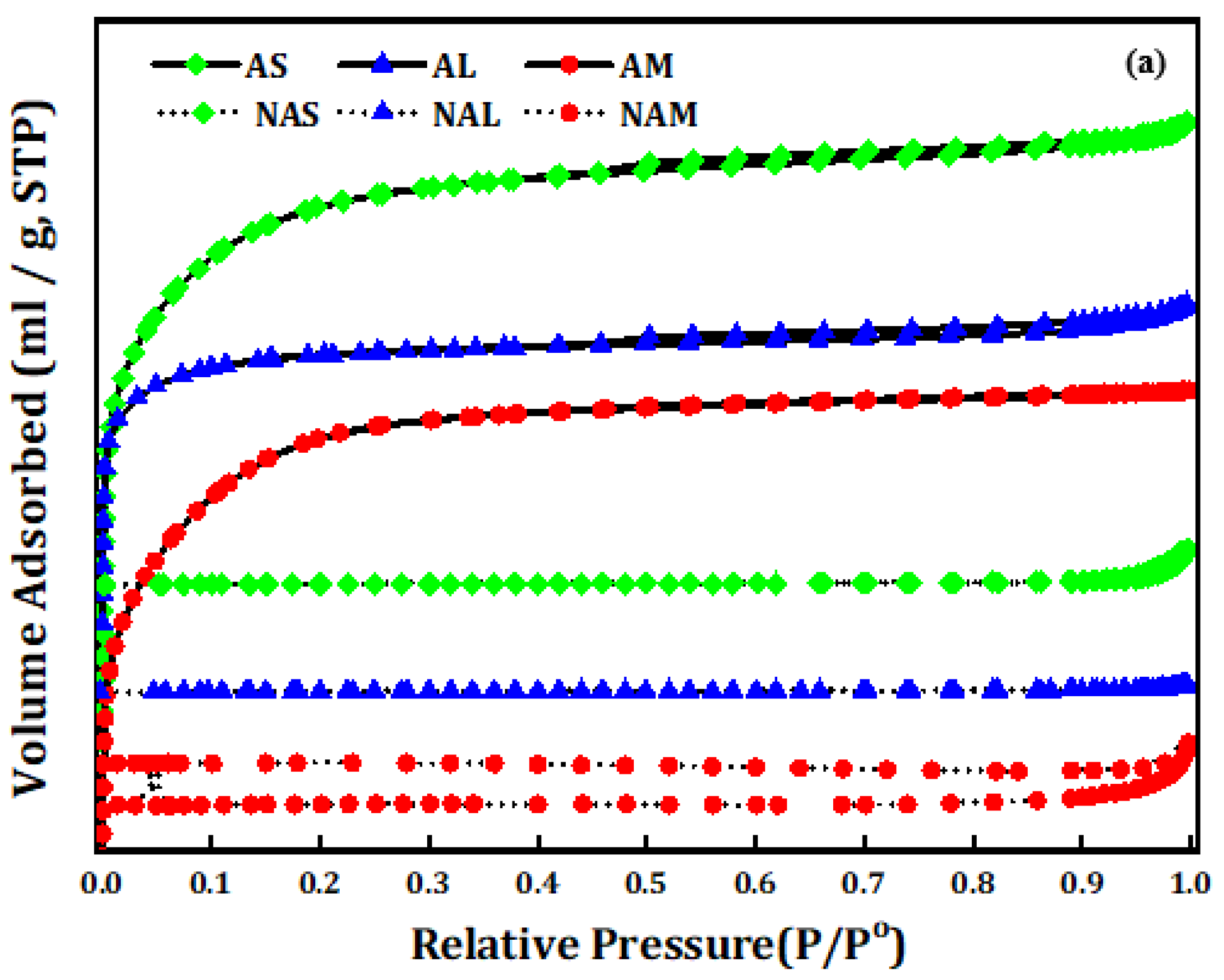
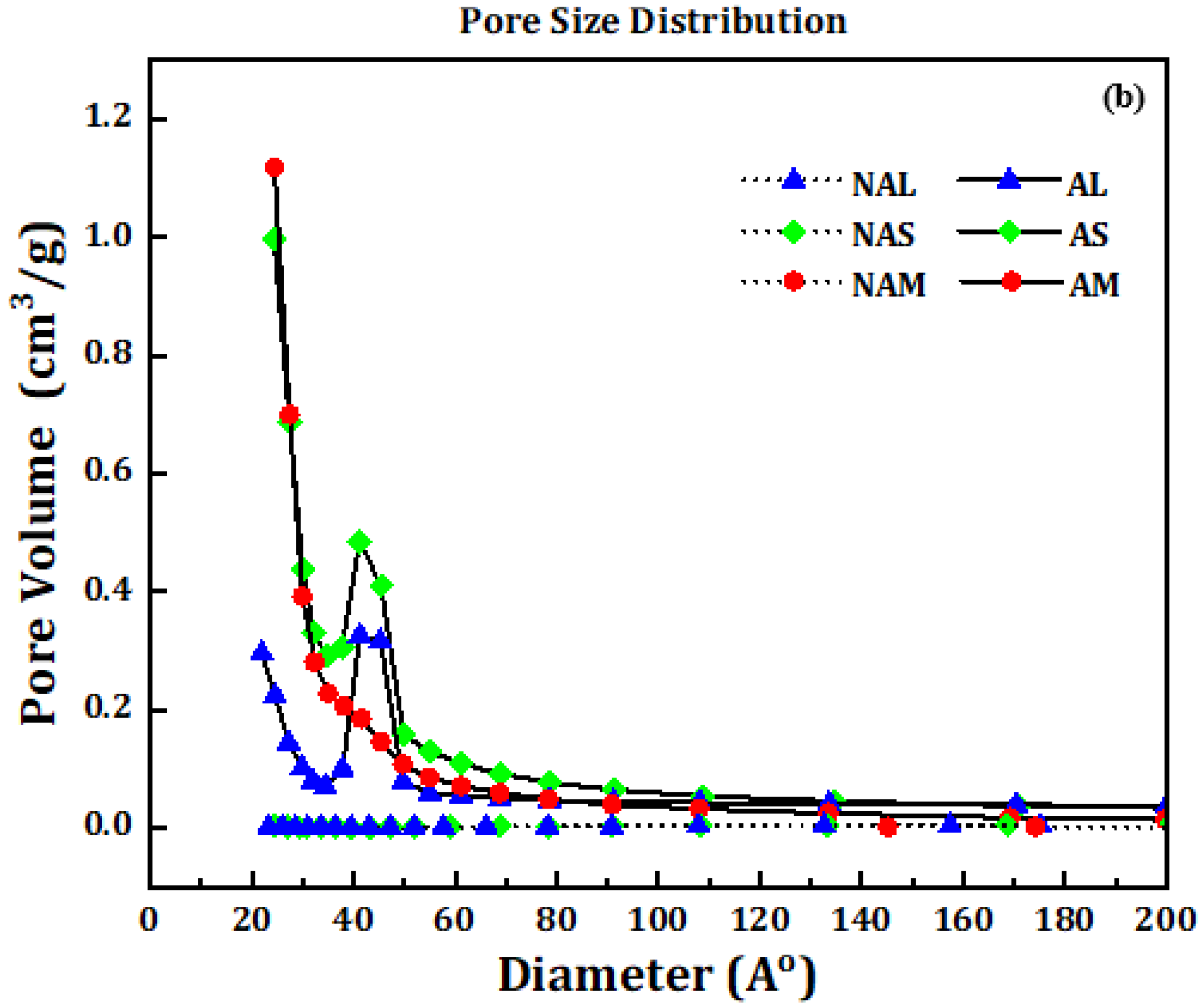

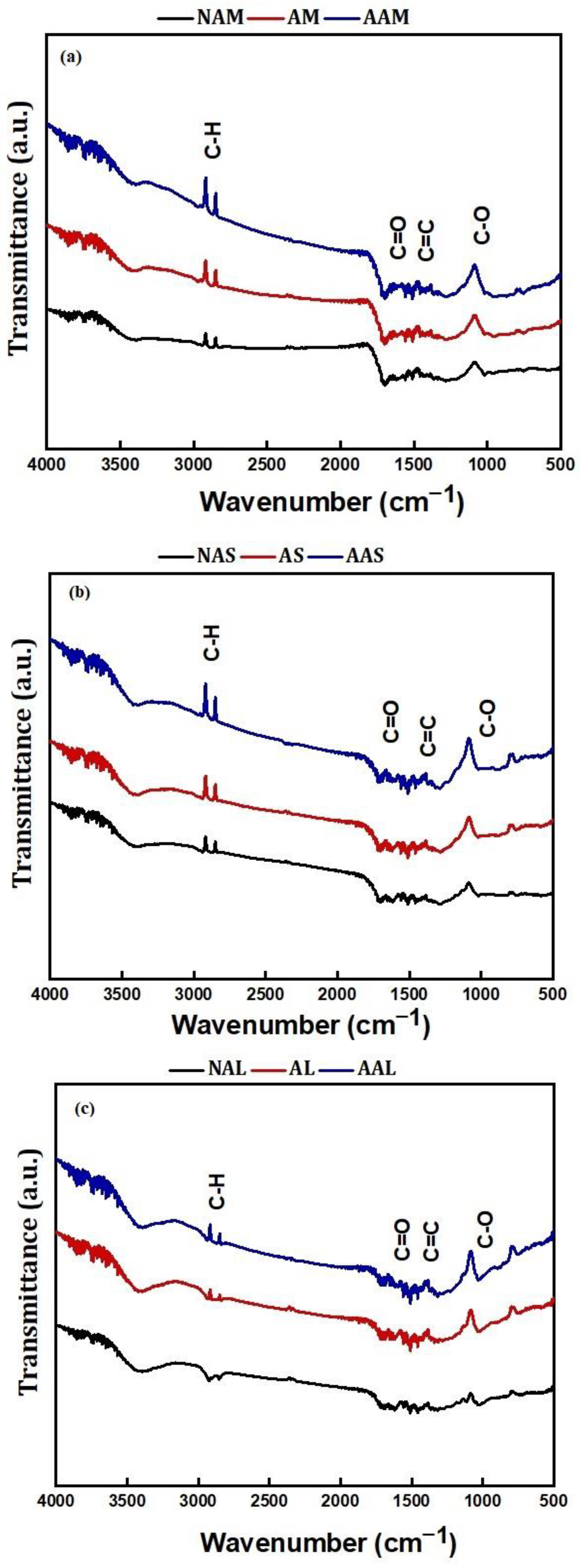
| CM Samples | Before Activation | ||
|---|---|---|---|
| BET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | |
| Non-activated seeds (NAS) | 5.02 | 0.041 | 30.44 |
| Non-activated leaflets (NAL) | 2.21 | 0.0086 | 18.04 |
| Non-activated molasses (NAM) | 0.72 | 0.0033 | 36.66 |
| Activated seeds (AS) | 1584 | 0.47 | 2.52 |
| Activated leaflets (AL) | 808 | 0.156 | 3.33 |
| Activated molasses (AM) | 1543 | 0.48 | 2.22 |
| CM Sample | Chemical Composition | O/C (Atomic) | H/C (Atomic) | ||||
|---|---|---|---|---|---|---|---|
| C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | |||
| NAS | 65.53 ± 0.58 | 4.86 ± 0.09 | 12.65 ± 0.14 | 0.69 ± 0.005 | 16.27 ± 0.16 | 0.19 | 0.89 |
| NAL | 58.99 ± 0.53 | 6.58 ± 0.13 | 21.65 ± 0.24 | 0.92 ± 0.007 | 11.86 ± 0.13 | 0.15 | 1.34 |
| NAM | 67.80 ± 0.60 | 4.81 ± 0.09 | 14.78 ± 0.16 | 0.62 ± 0.005 | 11.99 ± 0.14 | 0.13 | 0.85 |
| AS | 77.48 ± 0.69 | 2.96 ± 0.06 | 11.42 ± 0.12 | 0.36 ± 0.003 | 7.78 ± 0.12 | 0.08 | 0.46 |
| AL | 70.12 ± 0.63 | 2.57 ± 0.05 | 25.35 ± 0.27 | 0.72 ± 0.006 | 1.24 ± 0.01 | 0.01 | 0.44 |
| AM | 49.41 ± 0.44 | 3.57 ± 0.07 | 8.29 ± 0.09 | 0.49 ± 0.004 | 38.24 ± 0.31 | 0.58 | 0.87 |
| CM Samples | Langmuir Constants | Freundlich Constants | Temkin Constants | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qmax | KL | R2 | 1/n | KF | R2 | B | A | R2 | |
| AS | 322.54 | 0.124 | 0.903 | 0.178 | 122.10 | 0.967 | 35.76 | 27.81 | 0.964 |
| AL | 219.71 | 11.55 | 0.919 | 0.098 | 138.90 | 0.953 | 16.69 | 57.18 × 102 | 0.955 |
| AM | 414.63 | 4.54 | 0.880 | 0.054 | 306.20 | 0.842 | 19.59 | 62.16 × 105 | 0.844 |
| Adsorbent | qmax, (mg/g) | Reference |
|---|---|---|
| Coconut shell | 200.01 | [31] |
| Peanut shell | 1368 | [56] |
| Canola stalk | 93.4 | [57] |
| Bamboo | 655.76 | [30] |
| Sewage sludge | 52.56 | [58] |
| Pine wood | 86.7 | [59] |
| Bamboo | 1155.57 | [60] |
| Rice husk | 53.21 | [61] |
| Coffee husk | 418.78 | [32] |
| Filtrasorb 400 | 295 ± 3 | [62] |
| Norit | 276 ± 3 | [62] |
| Picacarb | 248 ± 2 | [62] |
| Palm date molasses-activated hydrochar (AM) | 414.63 | This work |
| Palm date seed-activated hydrochar (AS) | 322.54 | This work |
| Palm date leaflet-activated hydrochar (AL) | 219.71 | This work |
| CM Samples | qexp | qtheo. | K | R2 |
|---|---|---|---|---|
| AS | 275.1 ± 4.7 | 270.78 ± 2.3 | 0.0012 | 0.994 |
| AL | 219.6 ± 5.6 | 223.77 ± 3.7 | 2.5901 | 0.985 |
| AM | 398.5 ± 0.065 | 399.62 ± 0.8 | 0.0014 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhawtali, S.; El-Harbawi, M.; Al-Awadi, A.S.; El Blidi, L.; Alrashed, M.M.; Yin, C.-Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings 2023, 13, 1287. https://doi.org/10.3390/coatings13071287
Alhawtali S, El-Harbawi M, Al-Awadi AS, El Blidi L, Alrashed MM, Yin C-Y. Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings. 2023; 13(7):1287. https://doi.org/10.3390/coatings13071287
Chicago/Turabian StyleAlhawtali, Saeed, Mohanad El-Harbawi, Abdulrhman S. Al-Awadi, Lahssen El Blidi, Maher M. Alrashed, and Chun-Yang Yin. 2023. "Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes" Coatings 13, no. 7: 1287. https://doi.org/10.3390/coatings13071287
APA StyleAlhawtali, S., El-Harbawi, M., Al-Awadi, A. S., El Blidi, L., Alrashed, M. M., & Yin, C.-Y. (2023). Enhanced Adsorption of Methylene Blue Using Phosphoric Acid-Activated Hydrothermal Carbon Microspheres Synthesized from a Variety of Palm-Based Biowastes. Coatings, 13(7), 1287. https://doi.org/10.3390/coatings13071287






