Effect of Nb–Zr–N Alloying Layer on Surface Mechanical Properties and Biocompatibility of Medical 316L Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Structures
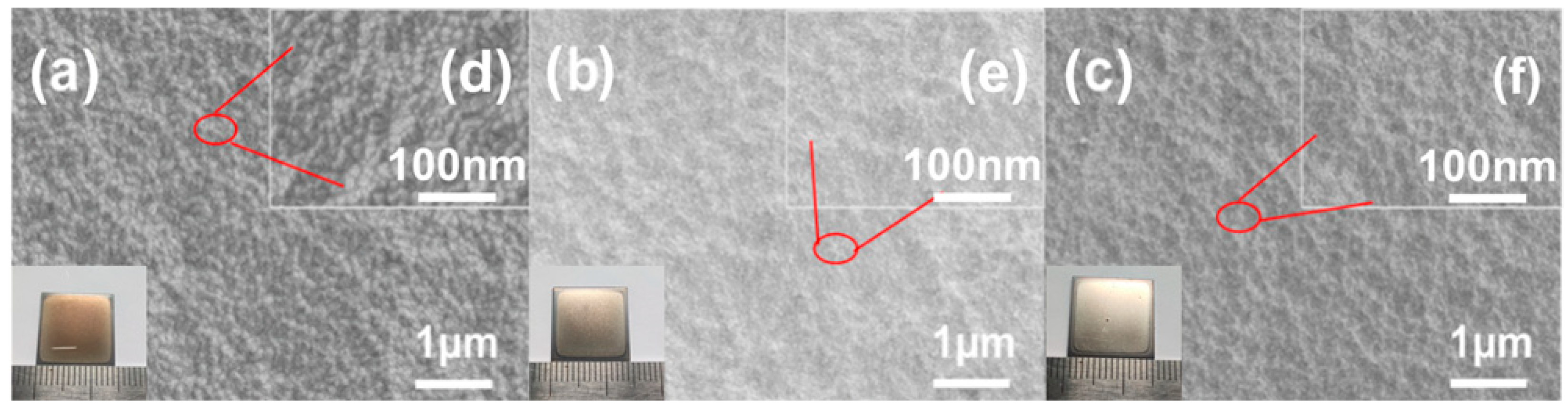
2.2. Phase Analysis and Surface Morphology
2.3. Mechanical Property
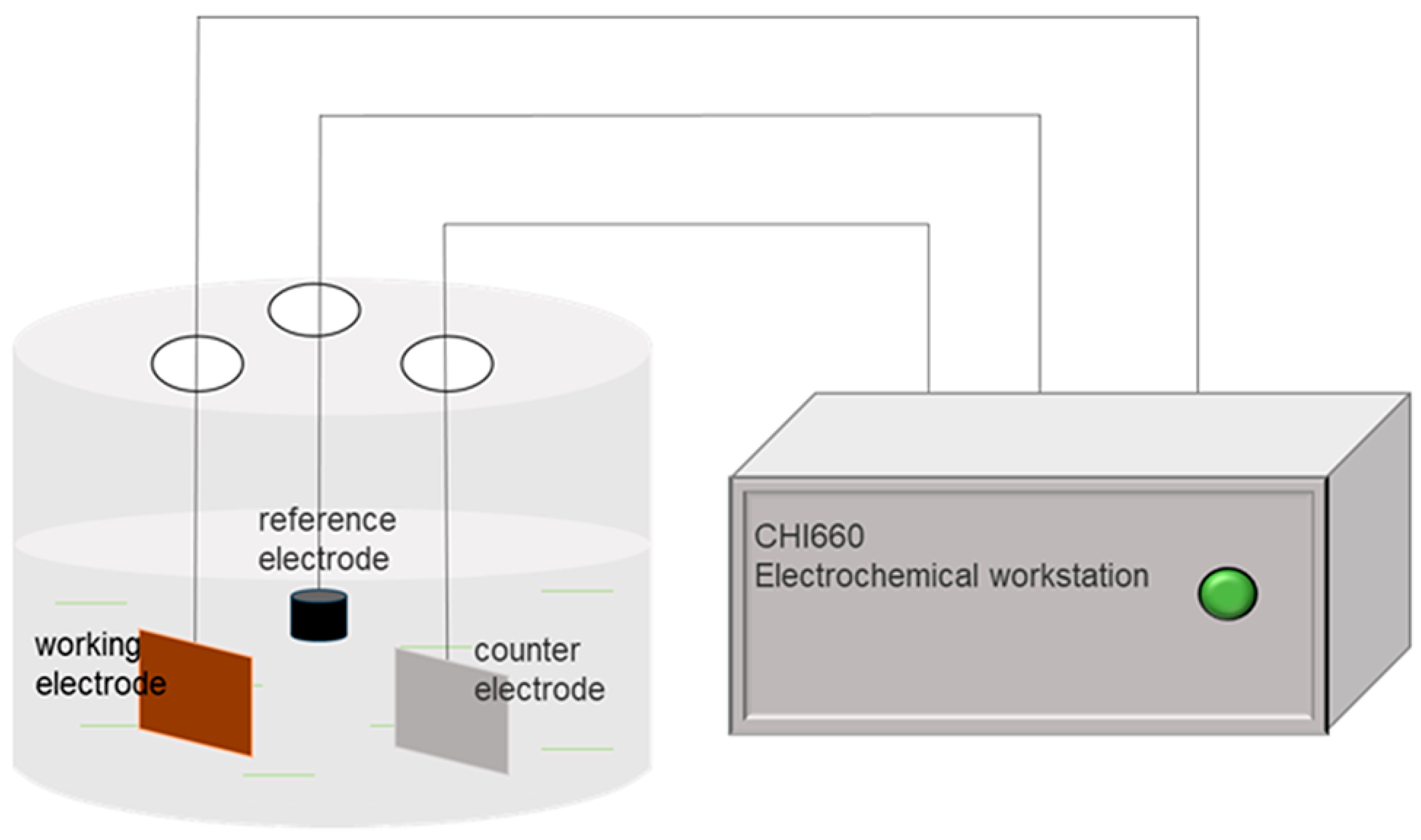
2.4. Electrochemical Measurement
2.5. Biocompatibility Test
3. Results and Discussion
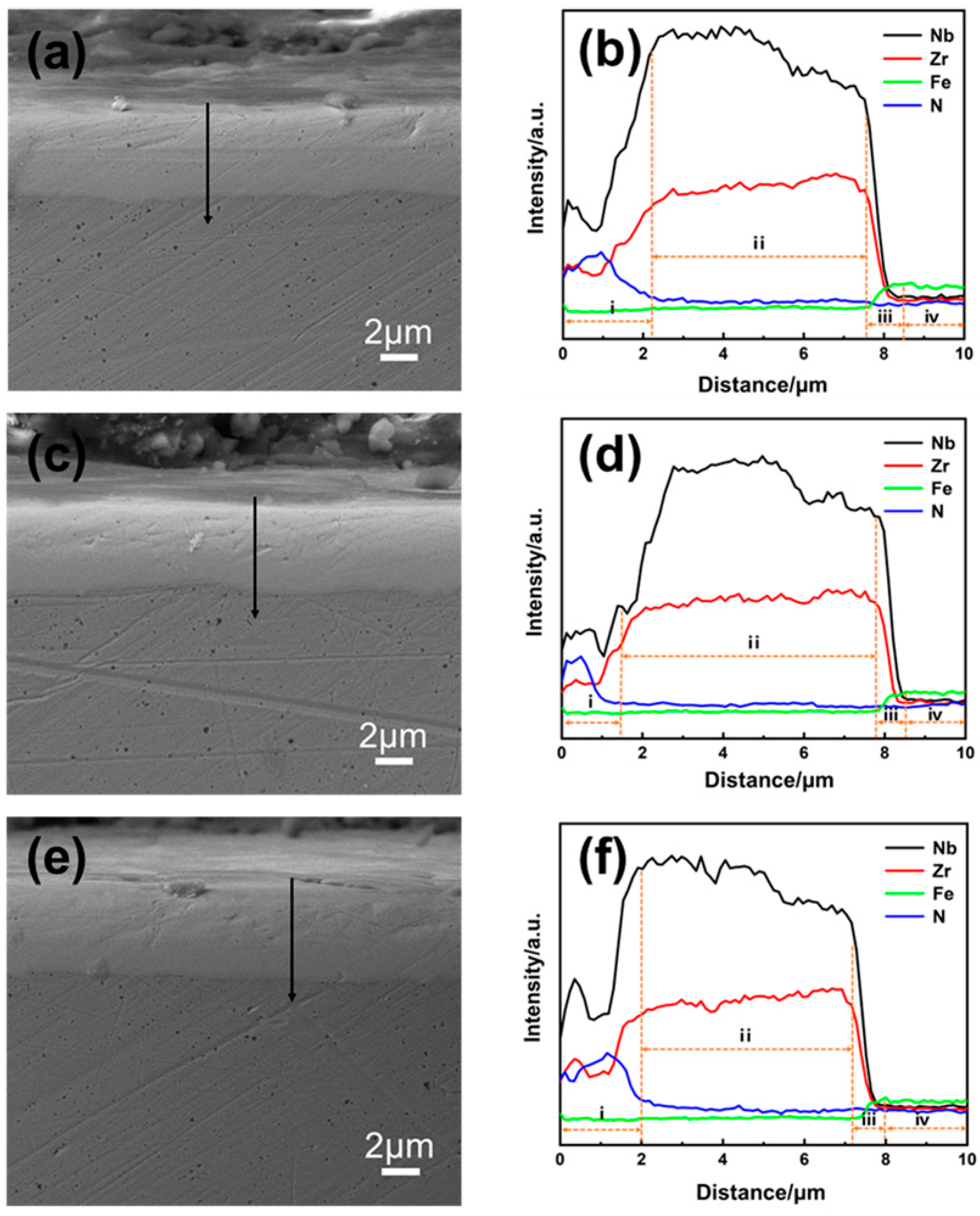
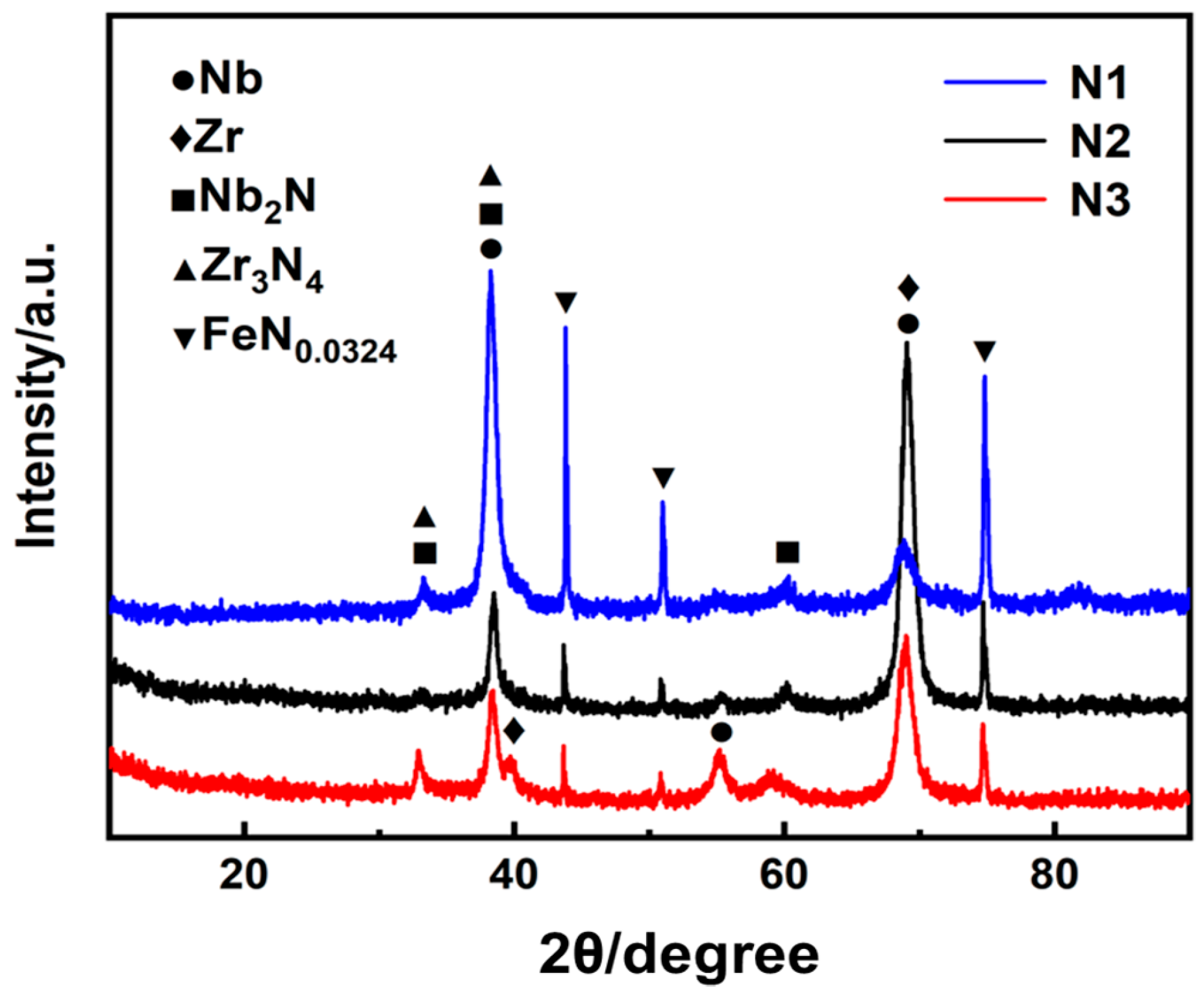
3.1. Structure Characterize
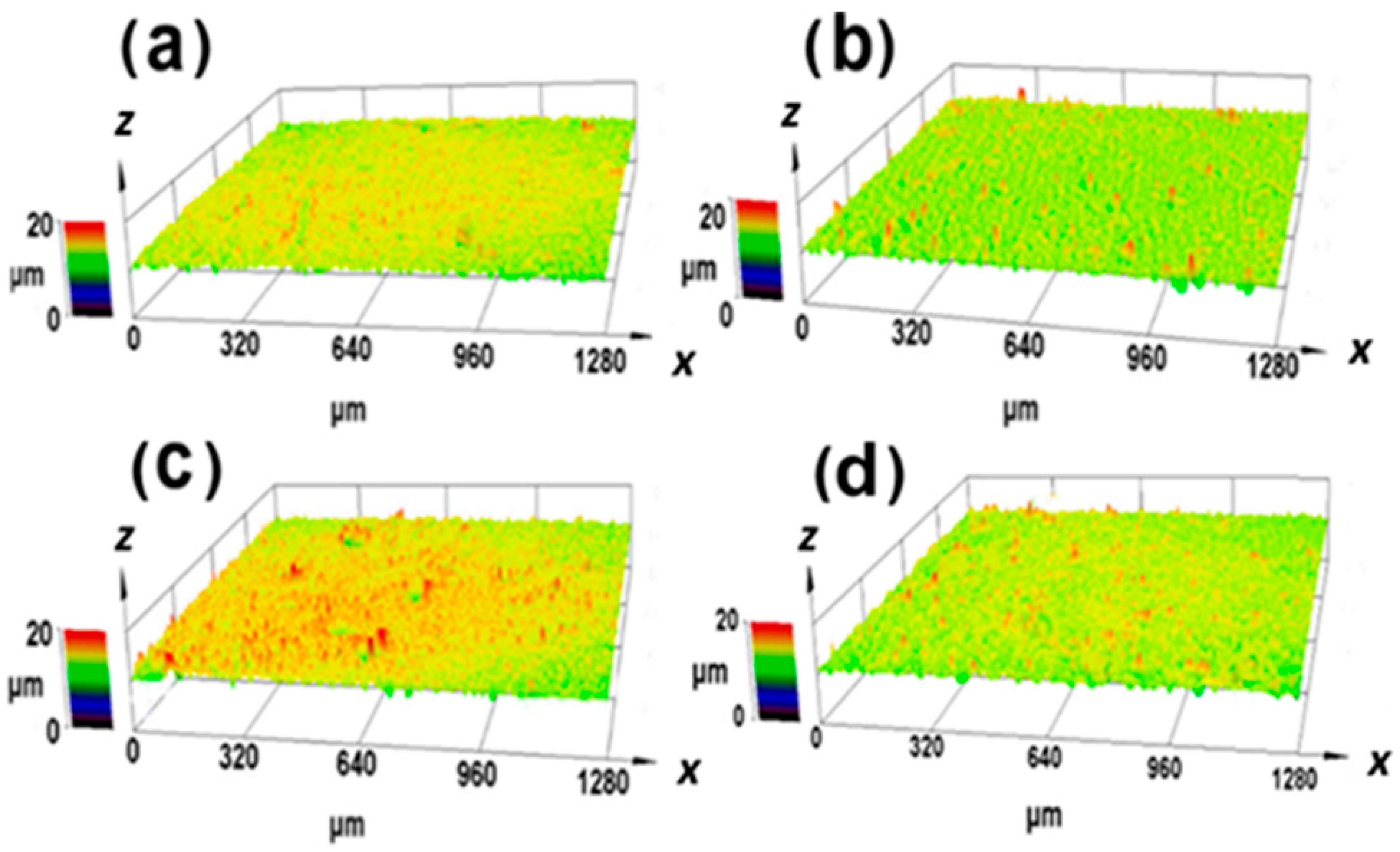
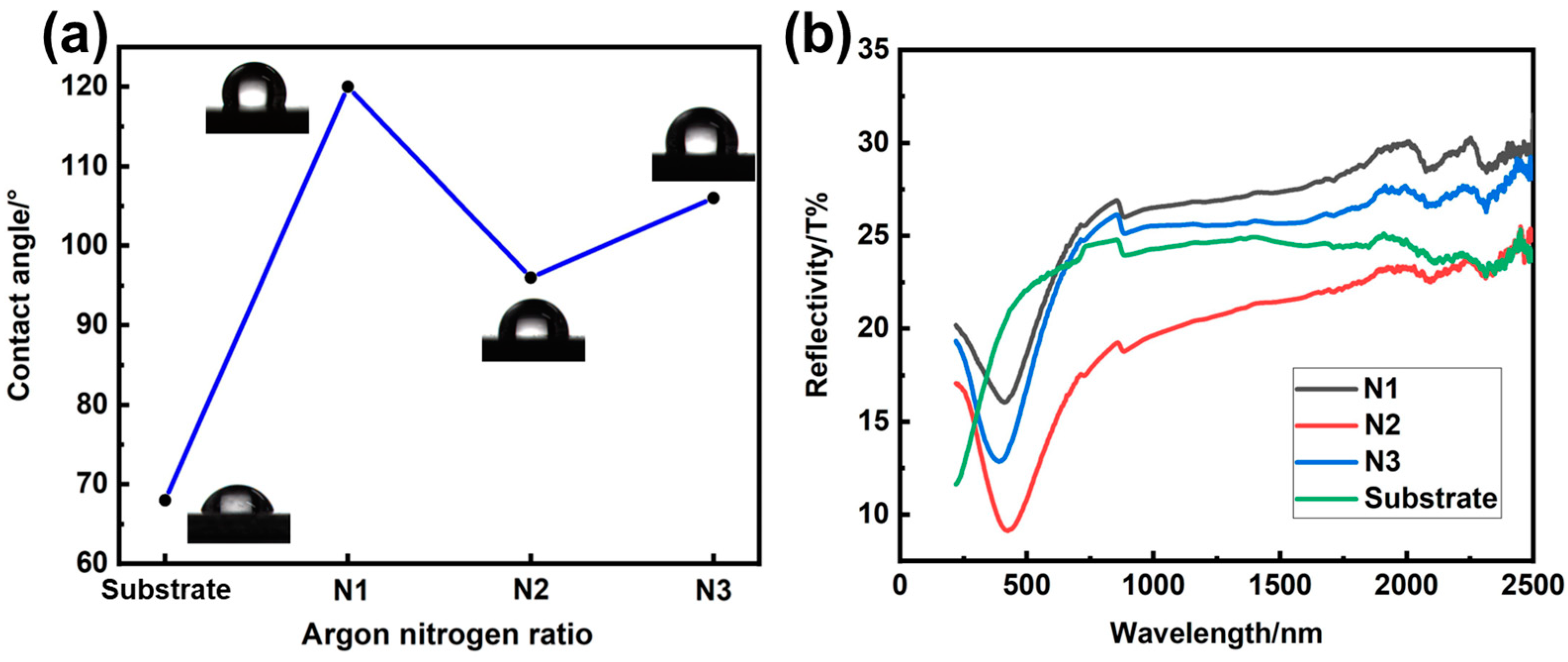
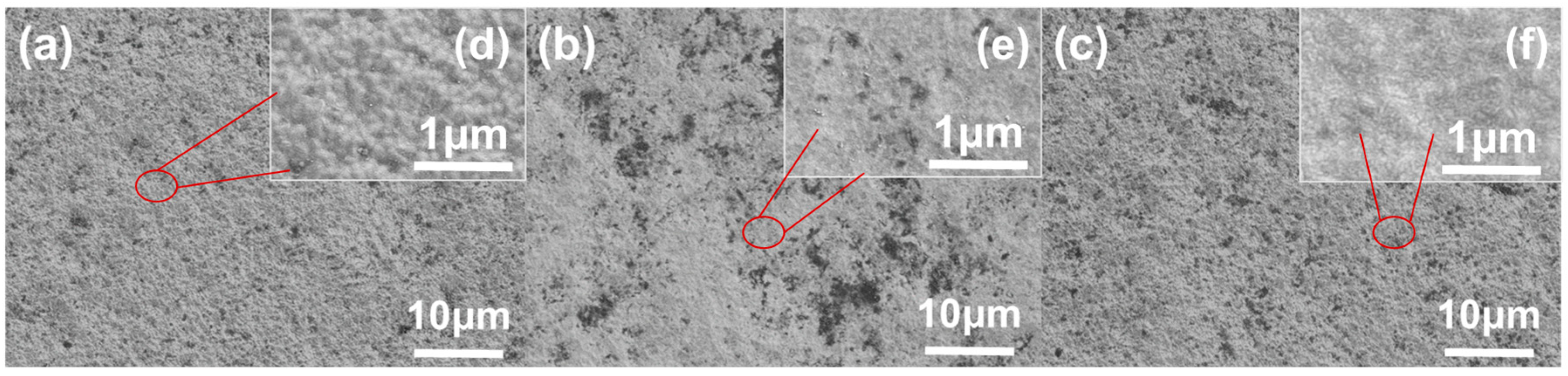
3.2. Research of Surface State
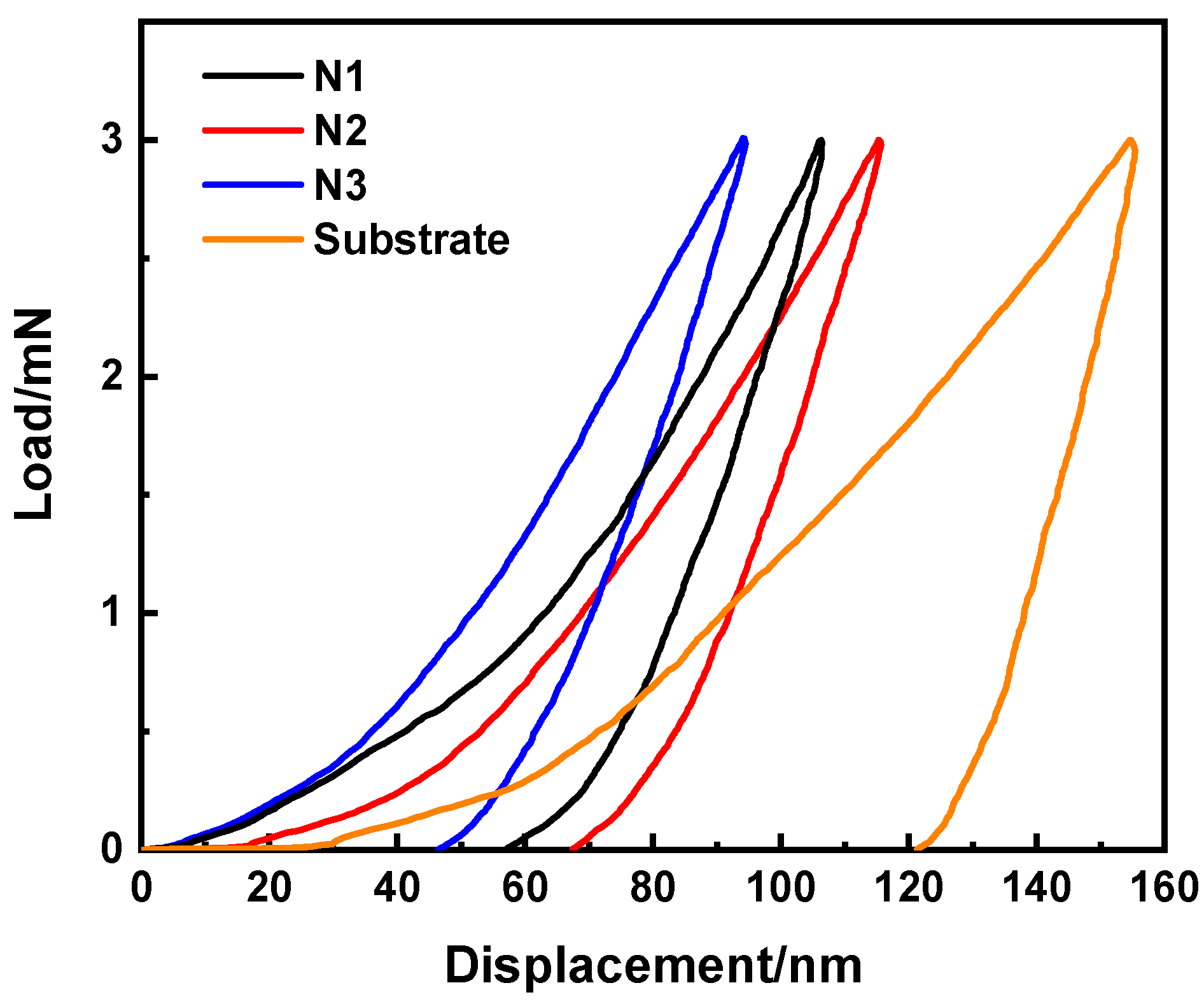
3.3. Research of Mechanical Performance
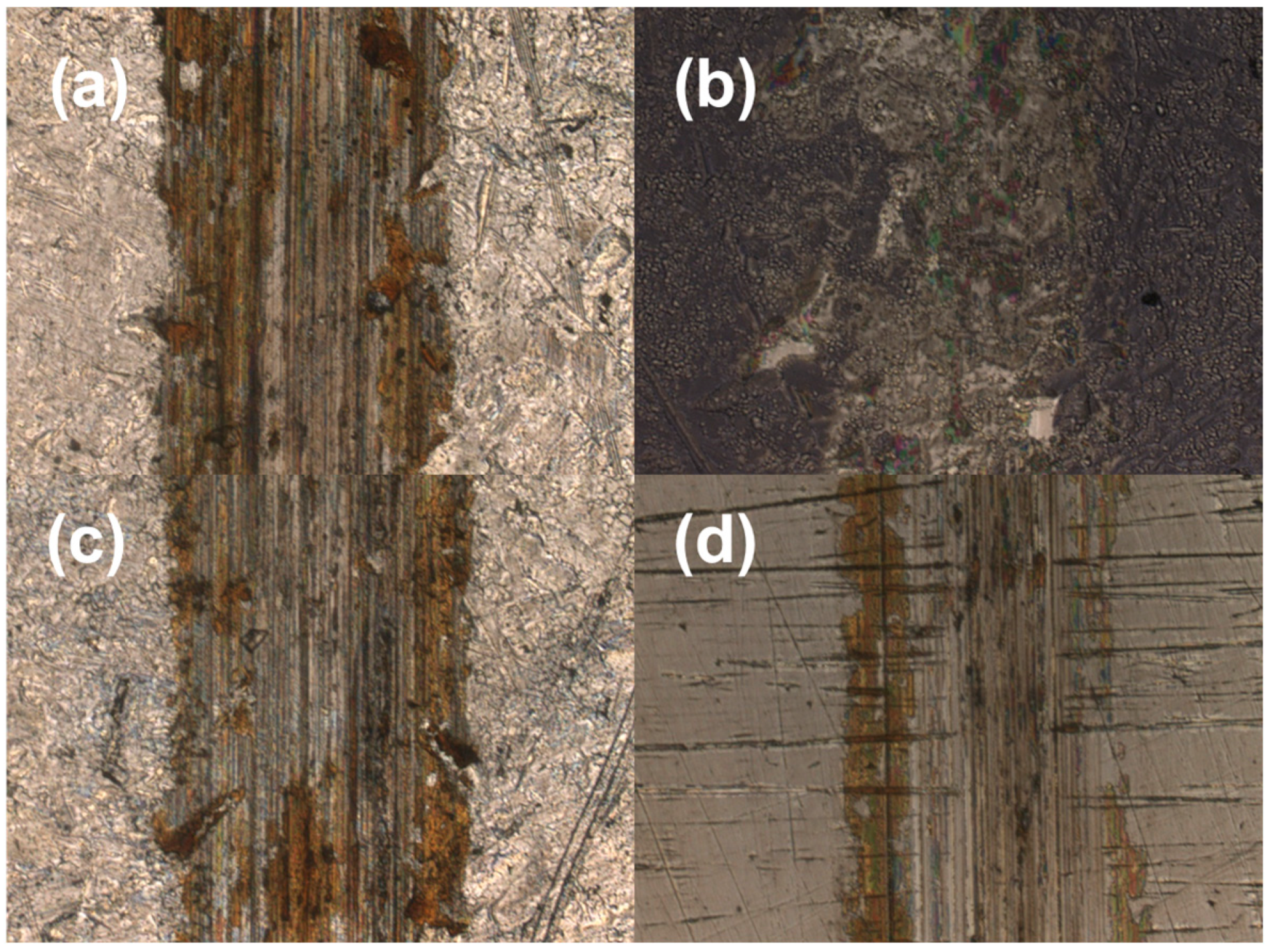
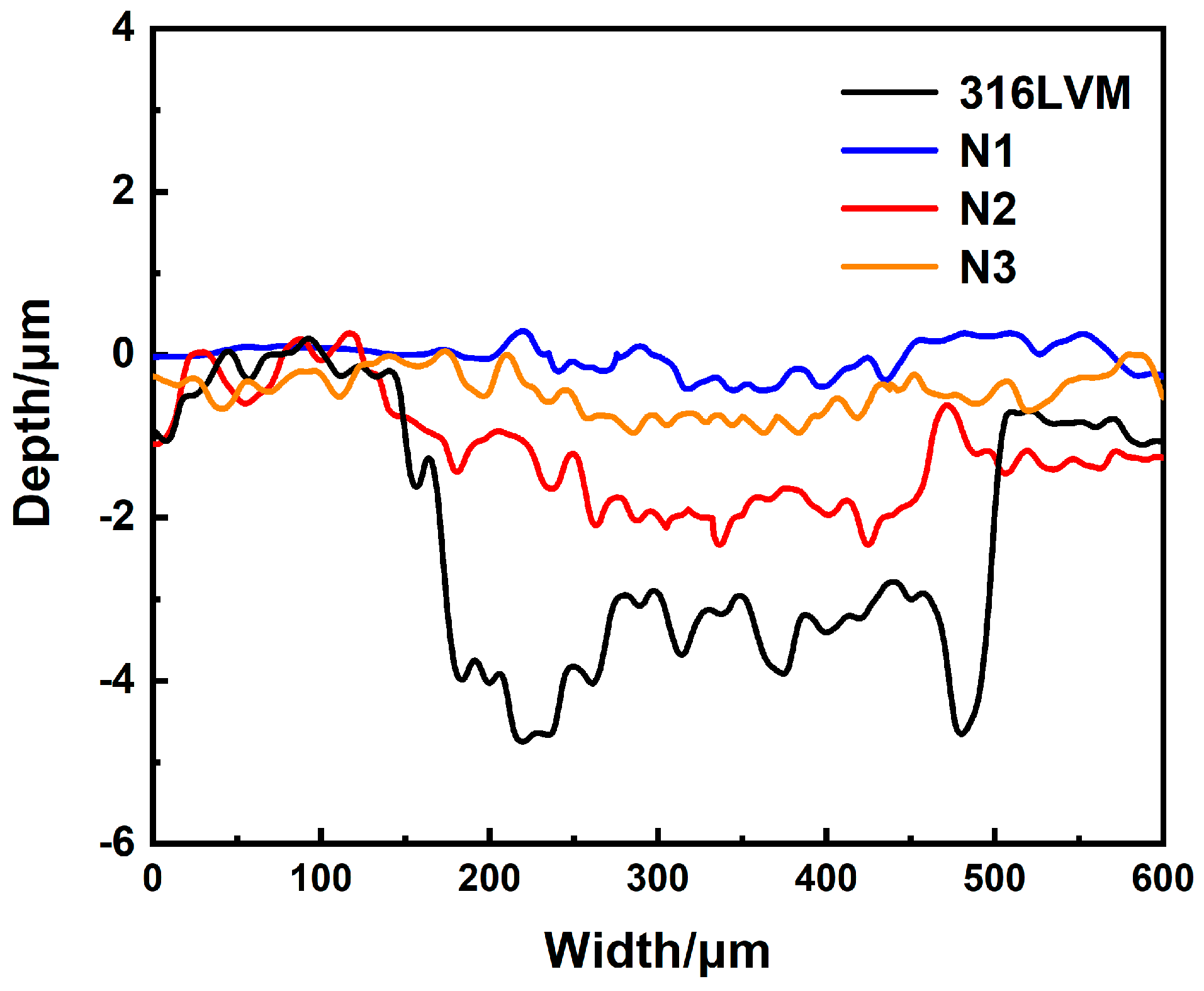
3.4. Research of Friction Properties
3.5. Research of Anti-Corrosion Performance
3.5.1. Dynamic Corrosion Analysis
3.5.2. Static Corrosion Analysis
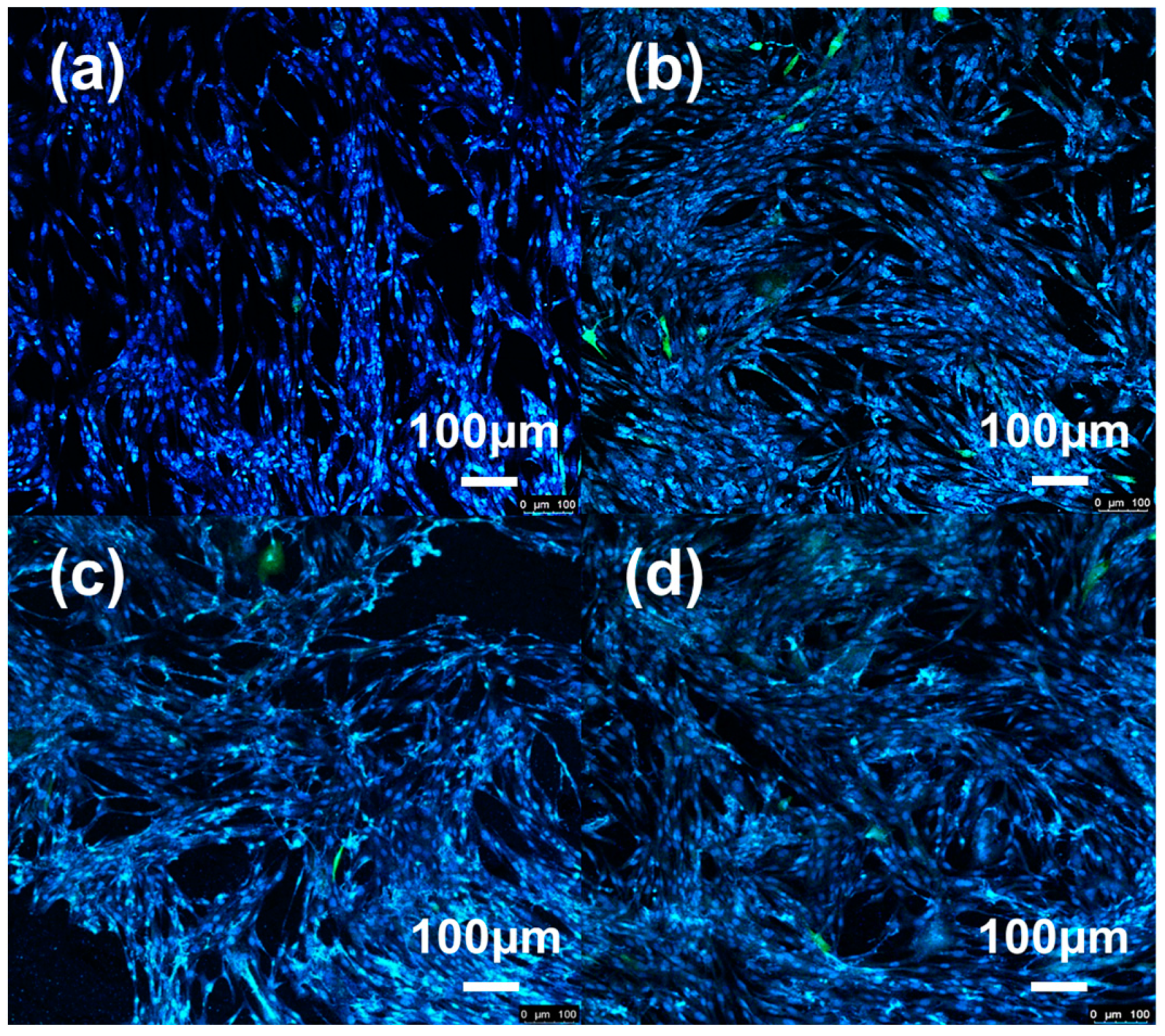
3.6. Research of Biocompatibility
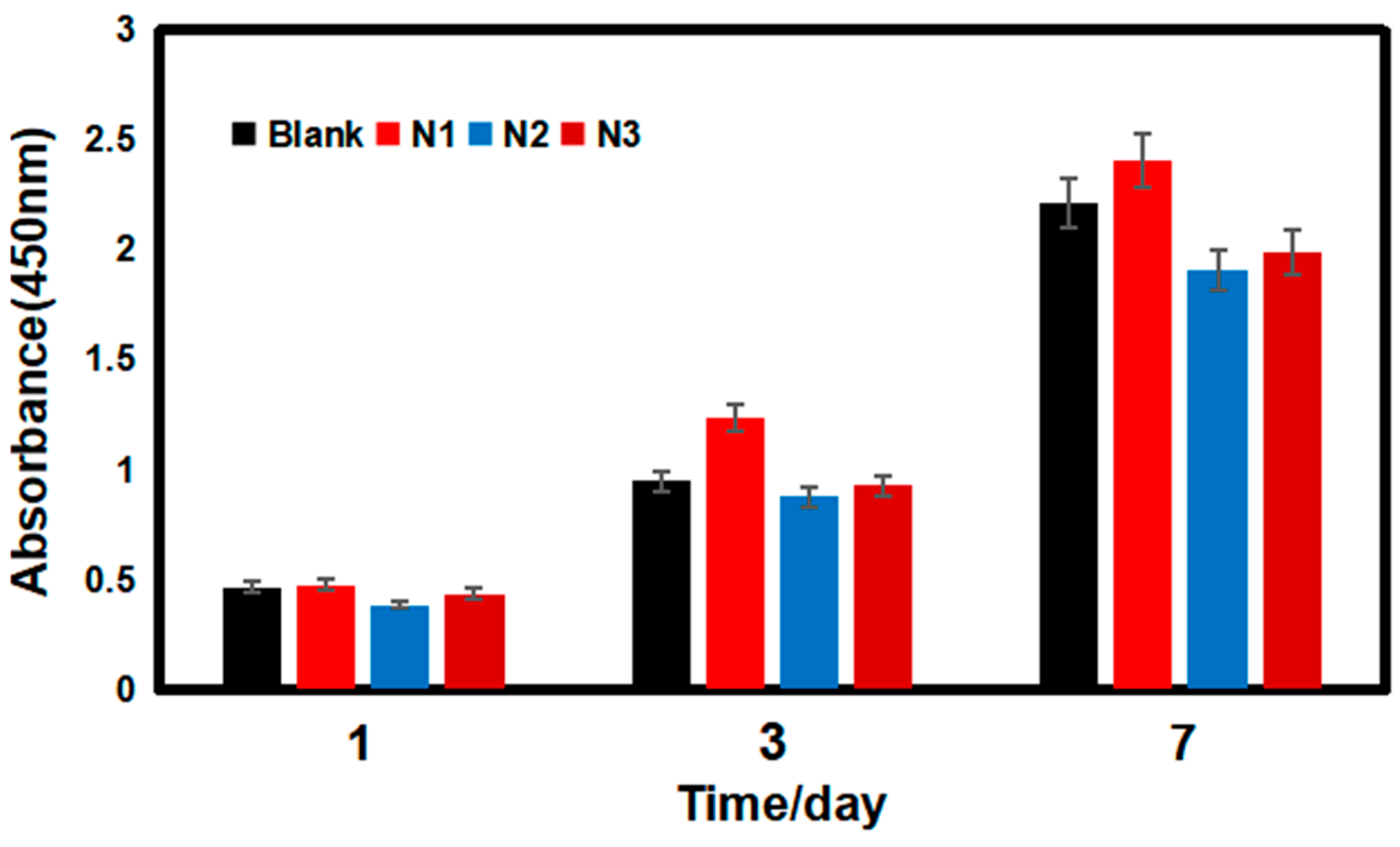
3.6.1. Cell Activity Analysis
3.6.2. Cell Morphology Studies
4. Conclusions
- (1)
- The argon-to-nitrogen ratio in the ion nitriding process affects the surface structure and properties of the alloying layers, among which the N1 sample shows uniform nanoscale cytosolic structure with spheres of 100 nm in diameter with 1:1 argon-to-nitrogen ratio;
- (2)
- The surface hardness of the Nb–Zr–N alloying layer is significantly increased, and the frictional properties are enhanced due to the Nb2N and Zr3N4 formed on the surface of alloying layers with the combination of nanostructures;
- (3)
- The corrosion resistance of Nb–Zr–N alloying layer is significantly higher than that of medical 316LVM, and the passivation film formed by the oxidation of Nb and Zr can hinder the ion invasion, which plays a better protective role in the substrate;
- (4)
- The Nb–Zr–N alloying layer promotes the adsorption, proliferation, and differentiation of cells to a certain extent and has no biological toxicity compared with the blank control group, and its good biocompatibility is of great potential as a medical implant material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.R.; Liu, W.C.; Wang, H.Y.; Li, V.L.; Chen, Y.H.; Wang, A.N.; Wu, C.J.; Li, Y.Q.; Zhao, G.; Lin, C.P.; et al. Polyelectrolyte multilayer composite coating on 316L stainless steel for controlled release of dual growth factors accelerating restoration of bone defects. Mat. Sci. Eng. C-Mater. 2021, 126, 112187. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of nano HA-SiC coating on AISI 316L medical grade stainless steel via electrophoretic deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Ur Rehman, M.A. Ag-Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Wang, N.; Meenashisundaram, G.K.; Chang, S.; Fuh, J.Y.H.; Dheen, T.; Kumar, A.S. A comparative investigation on the mechanical properties and cytotoxicity of Cubic, Octet, and TPMS gyroid structures fabricated by selective laser melting of stainless steel 316L. J. Mech. Behav. Biomed. 2022, 129, 105151. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Afshar, A. In vitro study: Bond strength, electrochemical and biocompatibility evaluations of TiO2/Al2O3 reinforced hydroxyapatite sol-gel coatings on 316L SS. Surf. Coat. Technol. 2021, 405, 126594. [Google Scholar] [CrossRef]
- Kedia, S.; Bonagani, S.K.; Majumdar, A.G.; Kain, V.; Subramanian, M.; Maiti, N.; Nilaya, J.P. Nanosecond laser surface texturing of type 316L stainless steel for contact guidance of bone cells and superior corrosion resistance. Colloid Interface Sci. 2021, 42, 100419. [Google Scholar] [CrossRef]
- Li, H.F.; Huang, J.Y.; Lin, G.C.; Wang, P.Y. Recent advances in tribological and wear properties of biomedical metallic materials. Rare Met. 2021, 40, 3091–3106. [Google Scholar] [CrossRef]
- Moghadasi, K.; Isa, M.S.M.; Ariffin, M.A.; Jamil, M.Z.M.; Raja, S.; Wu, B.; Yamani, M.; Bin Muhamad, M.R.; Yusof, F.; Jamaludin, M.F.; et al. A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J. Mater. Res. Technol. 2022, 17, 1054–1121. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Mohan, B.; Kathiresan, S.; Anbuchezhiyan, G. Effect of process parameters on machinability, hemocompatibility and surface integrity of SS 316L using R-MRAFF. J. Mater. Res. Technol. 2021, 15, 2658–2672. [Google Scholar] [CrossRef]
- Gawad, S.A.; Nasr, A.; Fekry, A.M.; Filippov, L.O. Electrochemical and hydrogen evolution behaviour of a novel nano-cobalt/nano-chitosan composite coating on a surgical 316L stainless steel alloy as an implant. Int. J. Hydrogen Energy 2021, 46, 18233–18241. [Google Scholar] [CrossRef]
- Parau, A.C.; Juravlea, G.A.; Raczkowska, J.; Vitelaru, C.; Dinu, M.; Awsiuk, K.; Vranceanu, D.M.; Ungureanu, E.; Cotrut, C.M.; Vladescu, A. Comparison of 316L and Ti6Al4V biomaterial coated by ZrCu-based thin films metallic glasses: Structure, morphology, wettability, protein adsorption, corrosion resistance, biomineralization. Appl. Surf. Sci. 2023, 612, 155800. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.J.; Gadd, G.M.; Zhao, Q. A sol-gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface functionalized bioceramics coated on metallic implants for biomedical and anticorrosion performance—A review. J. Mater. Chem. B 2021, 9, 9433–9460. [Google Scholar] [CrossRef]
- Liao, T.Y.; Biesiekierski, A.; Berndt, C.C.; King, P.C.; Ivanova, E.P.; Thissen, H.; Kingshott, P. Multifunctional cold spray coatings for biological and biomedical applications: A review. Prog. Surf. Sci. 2022, 97, 100654. [Google Scholar] [CrossRef]
- Shah, R.; Gashi, B.; Hoque, S.; Marian, M.; Rosenkranz, A. Enhancing mechanical and biomedical properties of protheses—Surface and material design. Surf. Interfaces 2021, 27, 101498. [Google Scholar] [CrossRef]
- Hernandez Navarro, C.; Flores Martinez, M.; Camps Carvajal, E.E.; Rivera Resendiz, L.P.; Garcia Bustos, E.D. Analysis of the wear behavior of multilayer coatings of TaZrN/TaZr produced by magnetron sputtering on AISI-316L stainless steel. Int. J. Adv. Manuf. Technol. 2021, 117, 1565–1573. [Google Scholar] [CrossRef]
- Resnik, M.; Bencina, M.; Levicnik, E.; Rawat, N.; Iglic, A.; Junkar, I. Strategies for improving antimicrobial properties of stainless steel. Materials 2020, 13, 2944. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, H.Y.; Xiao, C.L.; Dong, J.; Ren, J.; Peng, Z. Study on corrosion resistance and biological properties of the double glow plasma Nb-Zr biological implantation alloying layers. Coatings 2022, 12, 942. [Google Scholar] [CrossRef]
- Tekdir, H.; Yetim, T.; Yetim, A.F. Corrosion properties of ceramic-based TiO2 films on plasma oxidized Ti6Al4V/316L layered implant structured manufactured by selective laser melting. J. Bionic. Eng. 2021, 18, 944–957. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Design approaches and challenges for biodegradable bone implants: A review. Expert Rev. Med. Devices 2021, 18, 629–647. [Google Scholar] [CrossRef]
- Anju, S.; Mohanan, P.V. Biomedical applications of transition metal dichalcogenides (TMDCs). Synth. Met. 2021, 271, 116610. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Keshavarz, M.K.; Babaei, K. Surface characterization of bioceramic coatings on Zr and its alloys using plasma electrolytic oxidation (PEO): A review. Surf. Interfaces 2021, 25, 101283. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Nakai, M.; Tsutsumi, H.; Kondo, Y.; Hattori, T.; Fukui, H. Mechanical properties and biocompatibilities of Zr-Nb system alloys with different Nb contents for biomedical applications. J. Jpn. Inst. Met. 2011, 75, 445–451. [Google Scholar] [CrossRef][Green Version]
- Zhou, F.Y.; Wang, B.L.; Qiu, K.J.; Lin, W.J.; Li, L.; Wang, Y.B.; Nie, F.L.; Zheng, Y.F. Microstructure, corrosion behavior and cytotoxicity of Zr-Nb alloys for biomedical application. Mat. Sci. Eng. C-Mater. 2012, 32, 851–857. [Google Scholar] [CrossRef]
- Lou, B.S.; Lin, T.Y.; Chen, W.T.; Lee, J.W. Corrosion property and biocompatibility evaluation of Fe-Zr-Nb thin film metallic glasses. Thin Solid Films 2019, 691, 137615. [Google Scholar] [CrossRef]
- Gulsoy, H.O.; Pazarlioglu, S.; Gulsoy, N.; Gundede, B.; Mutlu, O. Effect of Zr, Nb and Ti addition on injection molded 316L stainless steel for bio-applications: Mechanical, electrochemical and biocompatibility properties. J. Mwch. Behav. Biomed. 2015, 51, 215–224. [Google Scholar] [CrossRef]
- Zhang, T.; Ou, P.; Ruan, J.; Yang, H. Nb-Ti-Zr alloys for orthopedic implants. J. Biomater. Appl. 2021, 35, 1284–1293. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb addition on the stability and biological corrosion resistance of Ti-Zr alloy passivation films. Corros. Sci. 2020, 170, 108696. [Google Scholar] [CrossRef]
- Li, L.; Yao, S.L.; Zhao, X.L.; Yang, J.J.; Wang, Y.X.; Wang, L.N. Fabrication and properties of anodic oxide nanotubular arrays on Zr-17Nb alloy. Acta Metall. Sin. 2019, 55, 1008–1018. [Google Scholar] [CrossRef]
- Fontoura, C.P.; Bertele, P.L.; Rodrigues, M.M.; Dotta Maddalozzo, A.E.; Frassini, R.; Celi Garcia, C.S.; Martins, S.T.; Crespo, J.d.S.; Figueroa, C.A.; Roesch-Ely, M.; et al. Comparative Study of Physicochemical Properties and Biocompatibility (L929 and MG63 Cells) of TiN Coatings Obtained by Plasma Nitriding and Thin Film Deposition. ACS Biomater. Sci. Eng. 2021, 7, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.E.B.; Queiroz Neto, M.F.; Braz, J.K.F.S.; Aires, M.d.M.; Silva Farias, N.B.; Barboza, C.A.G.; Cavalcanti Junior, G.B.; Rocha, H.A.O.; Alves Junior, C. Effect of plasma-nitrided titanium surfaces on the differentiation of pre-osteoblastic cells. Artif. Organs 2019, 43, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huo, D.; Zhang, J.; Lin, T.; Zhang, J.; Tan, S.; Yang, L. Fabrication of graphene oxide/copper synergistic antibacterial coating for medical titanium substrate. J. Colloid Interface Sci. 2023, 638, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, C.H.; Fatimi, A. A 20-year patent review and innovation trends on hydrogel-based coatings used for medical device biofabrication. J. Biomater. Sci.-Polym. Ed. 2023, 34, 1255–1273. [Google Scholar] [CrossRef]
- Zia, A.W.; Anestopoulos, I.; Panayiotidis, M.I.; Birkett, M. Soft diamond-like carbon coatings with superior biocompatibility for medical applications. Ceram. Int. 2023, 49, 17203–17211. [Google Scholar] [CrossRef]
- Bracic, M.; Potrc, S.; Finsgar, M.; Gradis, L.; Maver, U.; Budasheva, H.; Korte, D.; Franko, M.; Zemljic, L.F. Amoxicillin doped hyaluronic acid/fucoidan multifunctional coatings for medical grade stainless steel orthopedic implants. Appl. Surf. Sci. 2022, 611, 155621. [Google Scholar] [CrossRef]
- Guan, J.; Jiang, X.; Xiang, Q.; Yang, F.; Liu, J. Corrosion and tribocorrosion behavior of titanium surfaces designed by electromagnetic induction nitriding for biomedical applications. Surf. Coat. Technol. 2021, 409, 126844. [Google Scholar] [CrossRef]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Shanaghi, A.; Souri, A.R.; Saedi, H.; Chu, P.K. Effects of the tantalum intermediate layer on the nanomechanical properties and biocompatibility of nanostructured tantalum/tantalum nitride bilayer coating deposited by magnetron sputtering on the nickel titanium alloy. Appl. Nanosci. 2021, 11, 1867–1880. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Dong, D.; Wang, J.; Zhou, L. Improving wear resistance of Zr-2.5Nb alloy by formation of microtextured nitride layer produced via laser surface texturing/plasma nitriding technology. Surf. Interfaces 2020, 20, 100638. [Google Scholar] [CrossRef]
- Braz, J.K.F.S.; Martins, G.M.; Sabino, V.; Vitoriano, J.O.; Barboza, C.A.G.; Soares, A.K.M.C.; Rocha, H.A.O.; Oliveira, M.F.; Alves Junior, C.; Moura, C.E.B. Plasma nitriding under low temperature improves the endothelial cell biocompatibility of 316L stainless steel. Biotechnol. Lett. 2019, 41, 503–510. [Google Scholar] [CrossRef]
- Perez, E.; Pazos, L.; De las Heras, E.; Parodi, B.; Corengia, P.; Braceras, I. Wear behaviour of UHMWPE against DC-Pulsed plasma nitrided and duplex treated AISI 316L used in hip joint replacements. Plasma Process. Polym. 2009, 6, S75–S80. [Google Scholar] [CrossRef]
- Cubillos, G.I.; Romero, E.; Umana-Perez, A. ZrN-ZrO(x)Ny vs. -ZrO2-ZrOxNy coatings deposited via unbalanced DC magnetron sputtering. Sci. Rep. 2021, 11, 18926. [Google Scholar] [CrossRef]
- Ul-Hamid, A. Synthesis microstructural characterization and nanoindentation of Zr, Zr-nitride and Zr-carbonitride coatings deposited using magnetron sputtering. J. Adv. Res. 2021, 29, 107–119. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, N.M.; Zeng, Q.F.; Zhang, H.X.; Liu, X.P.; Wang, Z.H.; Wu, Y.C. Recent developments in research of double glow plasma surface alloying technology: A brief review. J. Mater. Res. Technol. 2020, 9, 6859–6882. [Google Scholar] [CrossRef]
- Grenadyorov, A.S.; Oskirko, V.O.; Zakharov, A.N.; Goncharenko, I.M.; Semenov, V.A.; Rabotkin, S.V.; Soloyyev, A.A. Hydrogen-free active screen plasma nitriding of AISI 316L stainless steel. Met. Mater. Int. 2022, 29, 1498–1509. [Google Scholar] [CrossRef]
- Qi, Y.; Liang, W.P.; Miao, Q.; Yi, J.W.; Lin, H.; Liu, Y.Y.; Ma, H.R. Role of the nitrogen ratio on mechanical properties and wear resistance of CrN/Fe functionally graded coating produced by double glow plasma alloying. Appl. Surf. Sci. 2022, 585, 152735. [Google Scholar] [CrossRef]
- Sousa, F.A.; da Costa, J.A.P.; de Sousa, R.R.M.; Barbosa, J.C.P.; de Araujo, F.O. Internal coating of pipes using the cathodic cage plasma nitriding technique. Surf. Interfaces 2020, 21, 100691. [Google Scholar] [CrossRef]
- Lou, J.; Ren, B.B.; Zhang, J.; He, H.; Gao, Z.L.; Xu, W. Evaluation of biocompatibility of 316L stainless steels coated with TiN, TiCN, and Ti-DLC films. Coatings 2022, 12, 1073. [Google Scholar] [CrossRef]
- Smyrnova, K.; Sahul, M.; Harsani, M.; Pogrebnjak, A.; Ivashchenko, V.; Beresnev, V.; Stolbovoy, V.; Caplovic, L.; Caplovicova, M.; Vanco, L. Microstructure, mechanical and tribological properties of advanced layered WN/MeN (Me = Zr, Cr, Mo, Nb) nanocomposite coatings. Nanomaterials 2022, 12, 395. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Y.N.; Sauvage, X.; Sha, G.; Lu, K. Grain boundary stability governs hardening and softening in extremely fine nanograined metals. Science 2017, 355, 1292–1296. [Google Scholar] [CrossRef]
- AlMotasem, A.T.; Daghbouj, N.; Sen, H.S.; Mirzaei, S.; Callisti, M.; Polcar, T. Influence of HCP/BCC interface orientation on the tribological behavior of Zr/Nb multilayer during nanoscratch: A combined experimental and atomistic study. Acta Mater. 2023, 249, 118832. [Google Scholar] [CrossRef]
- Dong, W.; Yang, X.; Song, F.; Wu, M.; Zhu, Y.; Wang, Z. Anti-friction and wear resistance analysis of cemented carbide coatings. Int. J. Adv. Manuf. Technol. 2022, 122, 2795–2821. [Google Scholar] [CrossRef]
- Zhai, W.; Bai, L.; Zhou, R.; Fan, X.; Kang, G.; Liu, Y.; Zhou, K. Recent progress on wear-resistant materials: Designs, properties, and applications. Adv. Sci. 2021, 8, 2003739. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. ARAB J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Amirnejad, M.; Rajabi, M.; Jamaati, R. The effect of crystallographic texture as a distinct effective parameter on the biocorrosion performance of Ti6Al4V alloy in PBS solution. Corros. Sci. 2021, 179, 109100. [Google Scholar] [CrossRef]
- Datta, S.; Das, M.; Balla, V.K.; Bodhak, S.; Murugesan, V.K. Mechanical, wear, corrosion and biological properties of arc deposited titanium nitride coatings. Surf. Coat. Technol. 2018, 344, 214–222. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, B.; Lai, W.; Zhang, G.; Zeng, R.; Li, W.; Wang, X. Improved tribological properties, cyto-biocompatibility and anti-inflammatory ability of additive manufactured Ti-6Al-4V alloy through surface texturing and nitriding. Surf. Coat. Technol. 2021, 425, 127686. [Google Scholar] [CrossRef]
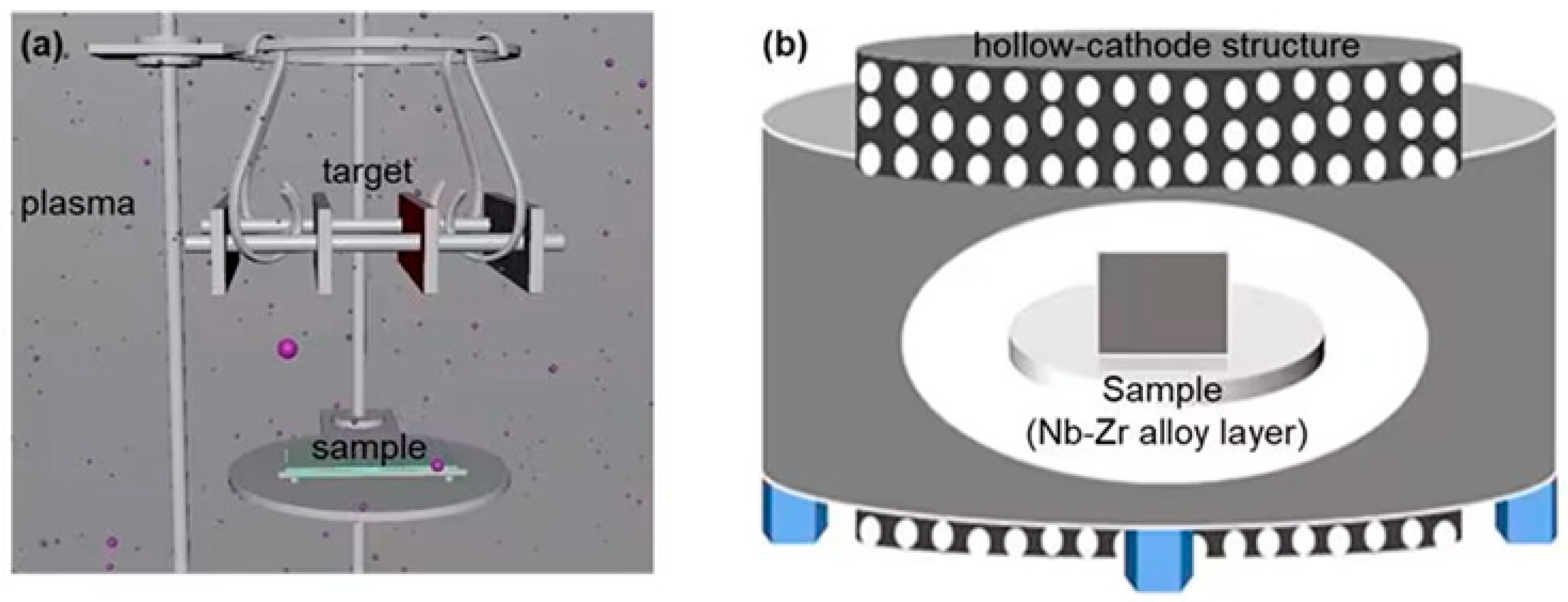


| Samples | Label | Ar/N Ratio | Working Pressure/Pa | Working Hour/h | Working Temperature/°C |
|---|---|---|---|---|---|
| Nitrogen Doped Nb–Zr Alloying layer | N1 | 1:1 | 90 | 4 | 800 |
| N2 | 2:1 | ||||
| N3 | 2:3 |
| Compositions | NaCl | CaCl2 | KCl | Na2CO3 | MgSO4·7H2O | MgCl·6H2O | Na2HPO4·2H2O | KH2PO4 | Glucose |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 8.00 | 0.14 | 0.40 | 0.35 | 0.06 | 0.10 | 0.06 | 0.06 | 1.00 |
| Samples | Ar/N2 Ratio | N/wt.% | Nb/wt.% | Zr/wt.% | Fe/wt.% |
|---|---|---|---|---|---|
| N1 | 1:1 | 9.52 | 87.42 | 1.13 | 1.93 |
| N2 | 2:1 | 6.05 | 90.16 | 1.07 | 2.72 |
| N3 | 2:3 | 12.30 | 84.87 | 1.52 | 1.31 |
| Samples | Hardness H/GPa |
|---|---|
| 316LVM | 5.16 |
| With Nb–Zr Surface | 8.02 |
| N1 | 20.54 |
| N2 | 16.83 |
| N3 | 22.79 |
| Samples | Wear Depth h/10−3 mm | Wear Width b/mm | Wear Volume V/10−5 mm3 | Wear Ratio K/10−6 mm3 N−1 m−1 |
|---|---|---|---|---|
| 316LVM | 4.5 | 0.36 | 540 | 600 |
| N1 | 0.4 | 0.20 | 27 | 30 |
| N2 | 2.1 | 0.32 | 224 | 249 |
| N3 | 1.8 | 0.24 | 144 | 160 |
| Sample | Corrosion Current Icorr/A cm−2 | Corrosion Voltage Ecorr/V | Corrosion Rate (mm/Year) |
|---|---|---|---|
| 316LVM | 6.03 × 10−6 | −3.09 × 10−1 | 7 × 10−2 |
| N1 | 7.10 × 10−8 | −1.98 × 10−1 | 8.4 × 10−4 |
| N2 | 6.46 × 10−7 | −2.69 × 10−1 | 7.6 × 10−3 |
| N3 | 6.87 × 10−7 | −2.22 × 10−1 | 8.1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, R.; Wu, H.; Fan, Z.; Wei, Y.; Yu, L.; Jiang, F. Effect of Nb–Zr–N Alloying Layer on Surface Mechanical Properties and Biocompatibility of Medical 316L Matrix. Coatings 2023, 13, 1346. https://doi.org/10.3390/coatings13081346
Ni R, Wu H, Fan Z, Wei Y, Yu L, Jiang F. Effect of Nb–Zr–N Alloying Layer on Surface Mechanical Properties and Biocompatibility of Medical 316L Matrix. Coatings. 2023; 13(8):1346. https://doi.org/10.3390/coatings13081346
Chicago/Turabian StyleNi, Ruian, Hongyan Wu, Zhehang Fan, Yihan Wei, Linshan Yu, and Fan Jiang. 2023. "Effect of Nb–Zr–N Alloying Layer on Surface Mechanical Properties and Biocompatibility of Medical 316L Matrix" Coatings 13, no. 8: 1346. https://doi.org/10.3390/coatings13081346
APA StyleNi, R., Wu, H., Fan, Z., Wei, Y., Yu, L., & Jiang, F. (2023). Effect of Nb–Zr–N Alloying Layer on Surface Mechanical Properties and Biocompatibility of Medical 316L Matrix. Coatings, 13(8), 1346. https://doi.org/10.3390/coatings13081346






