Evolutions of the Microstructure and Properties of the (CrMoNbTaZr)NX Films Prepared by Reactive Magnetron Sputtering: Effects of Stoichiometry and Crystallinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Microstructure Characterization
2.3. Properties’ Characterization
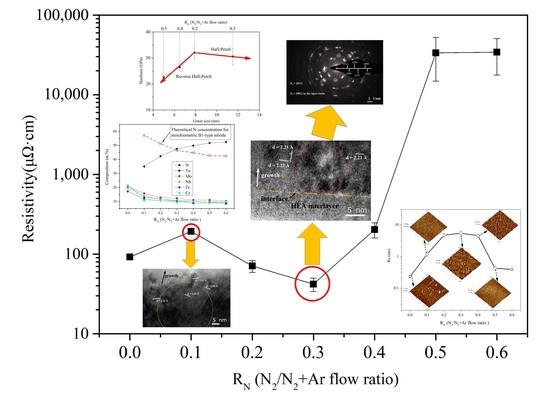
3. Results and Discussion
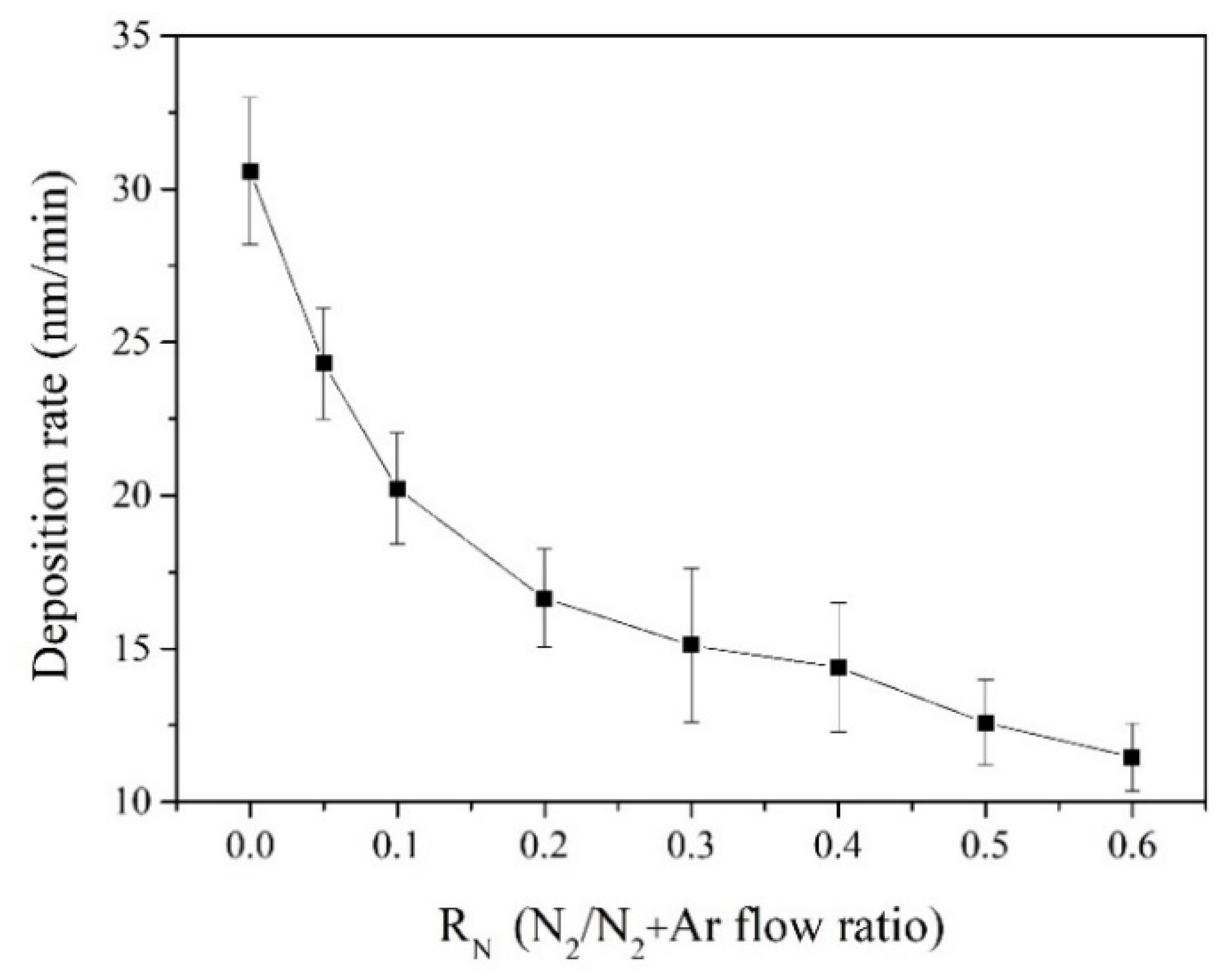
3.1. Chemical Composition and Deposition Rate
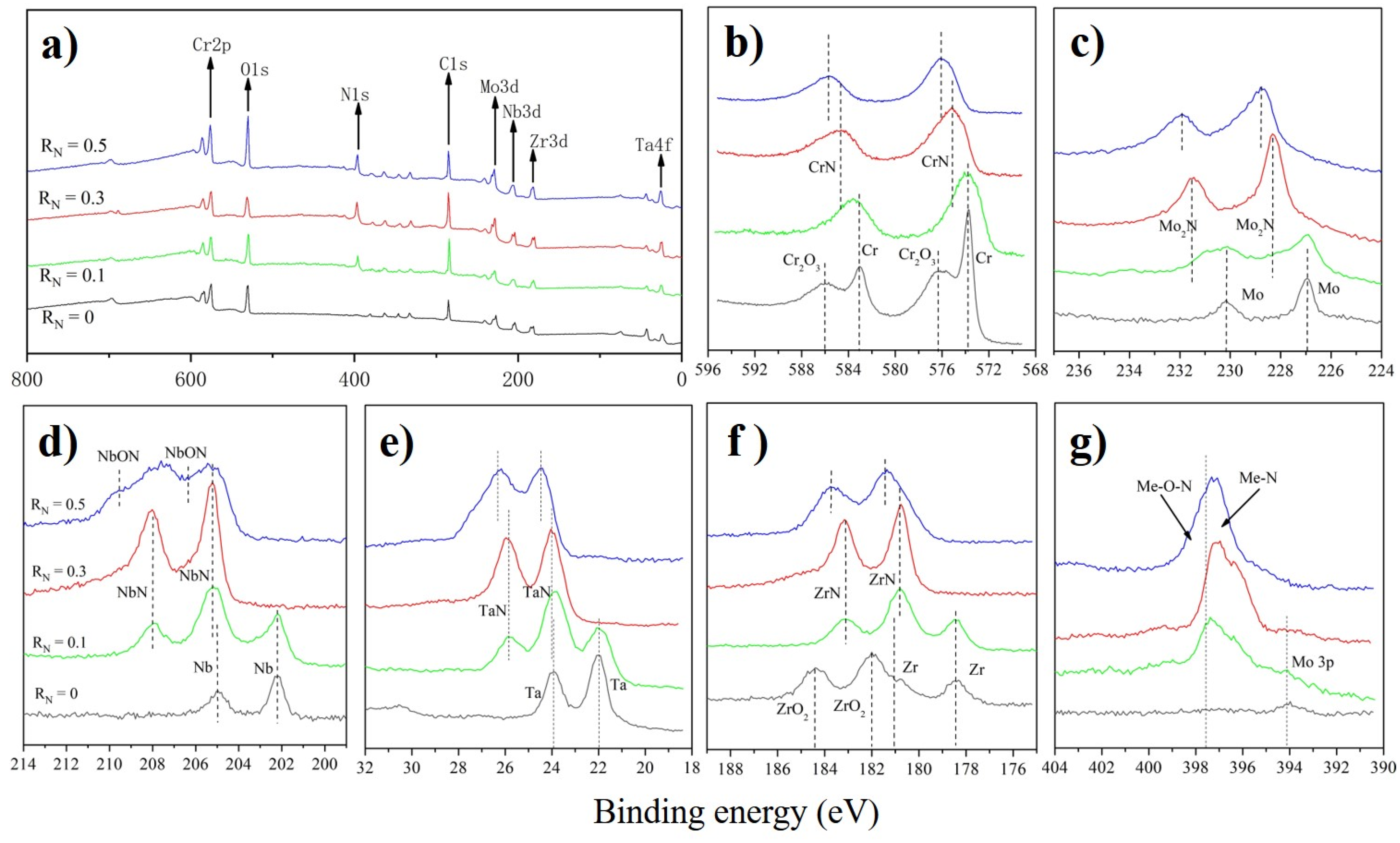
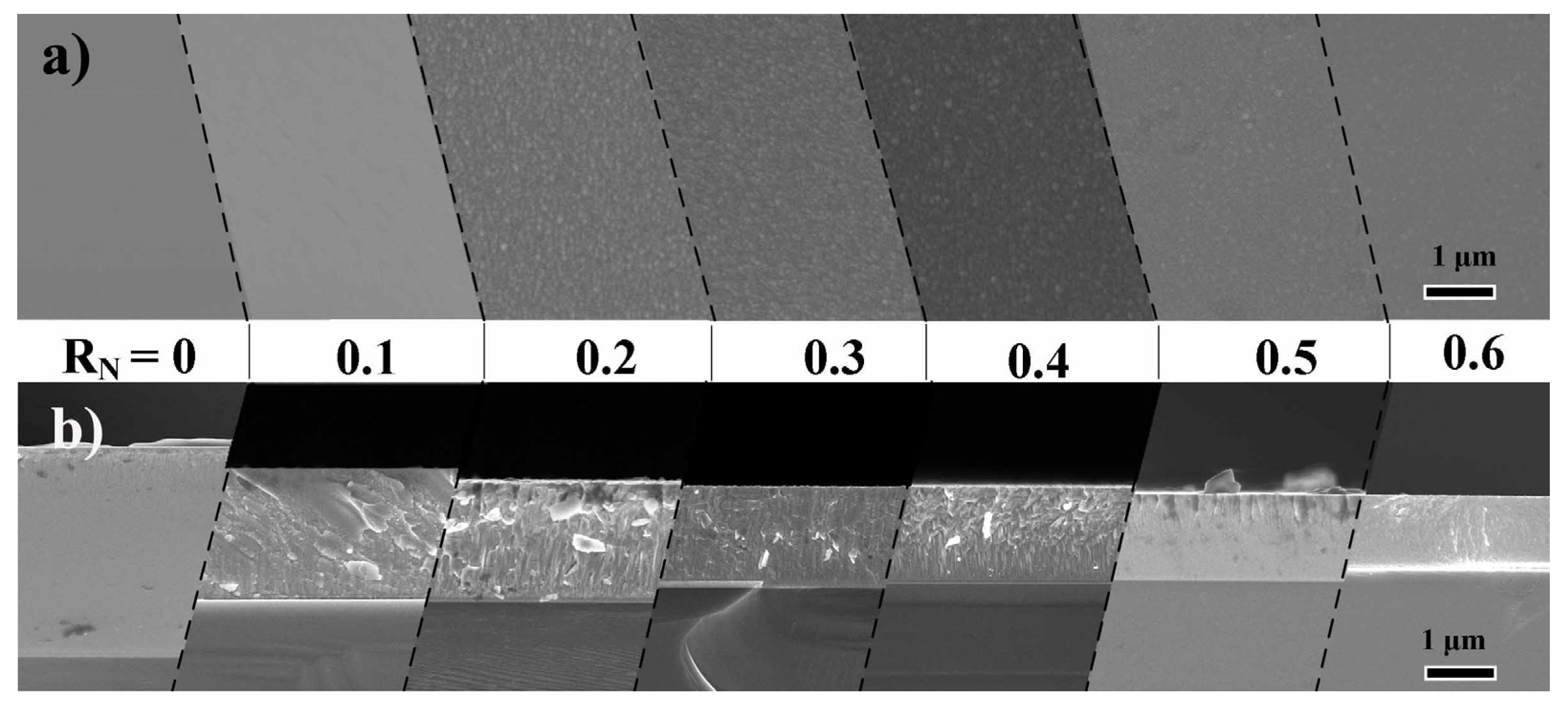
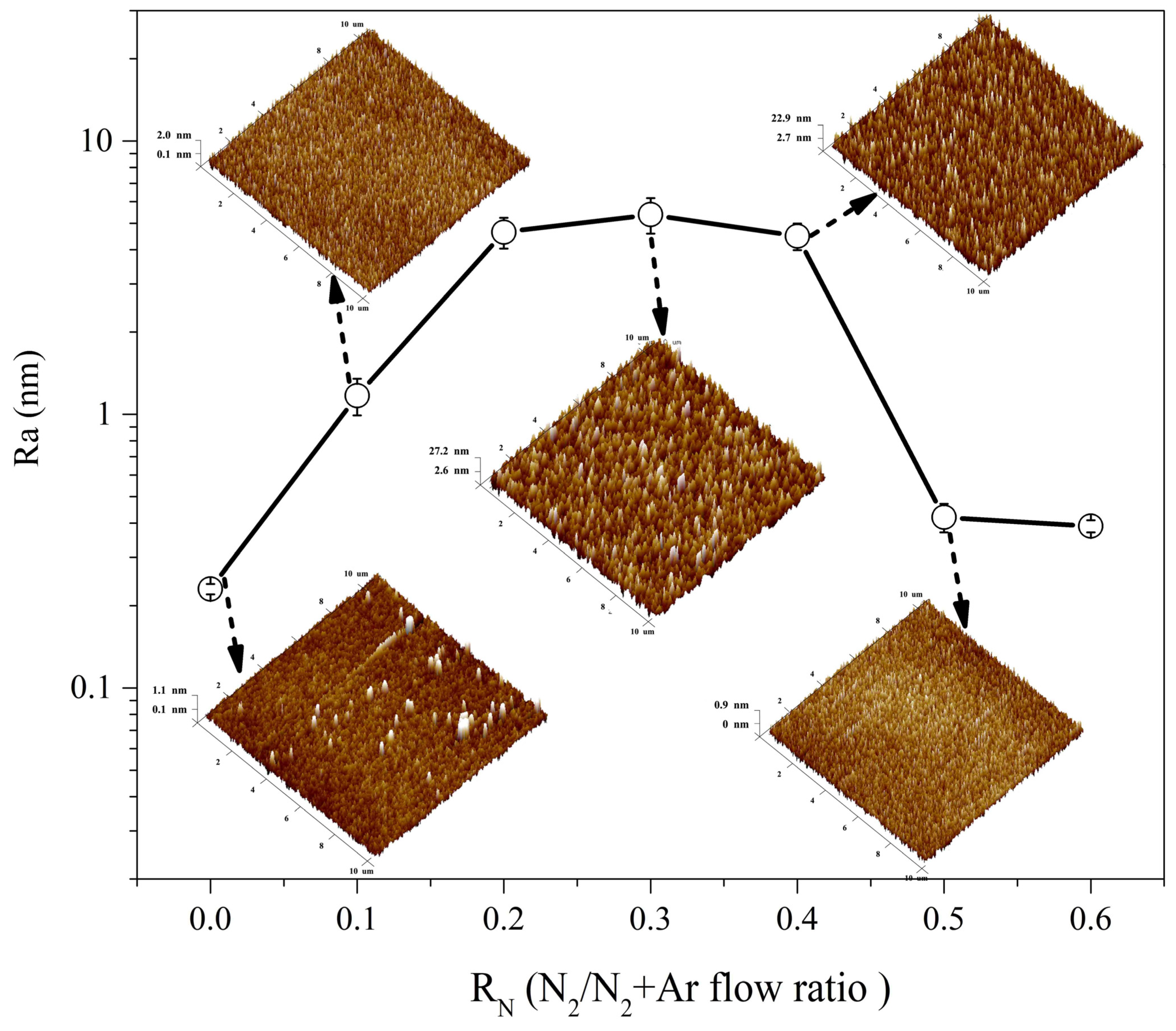
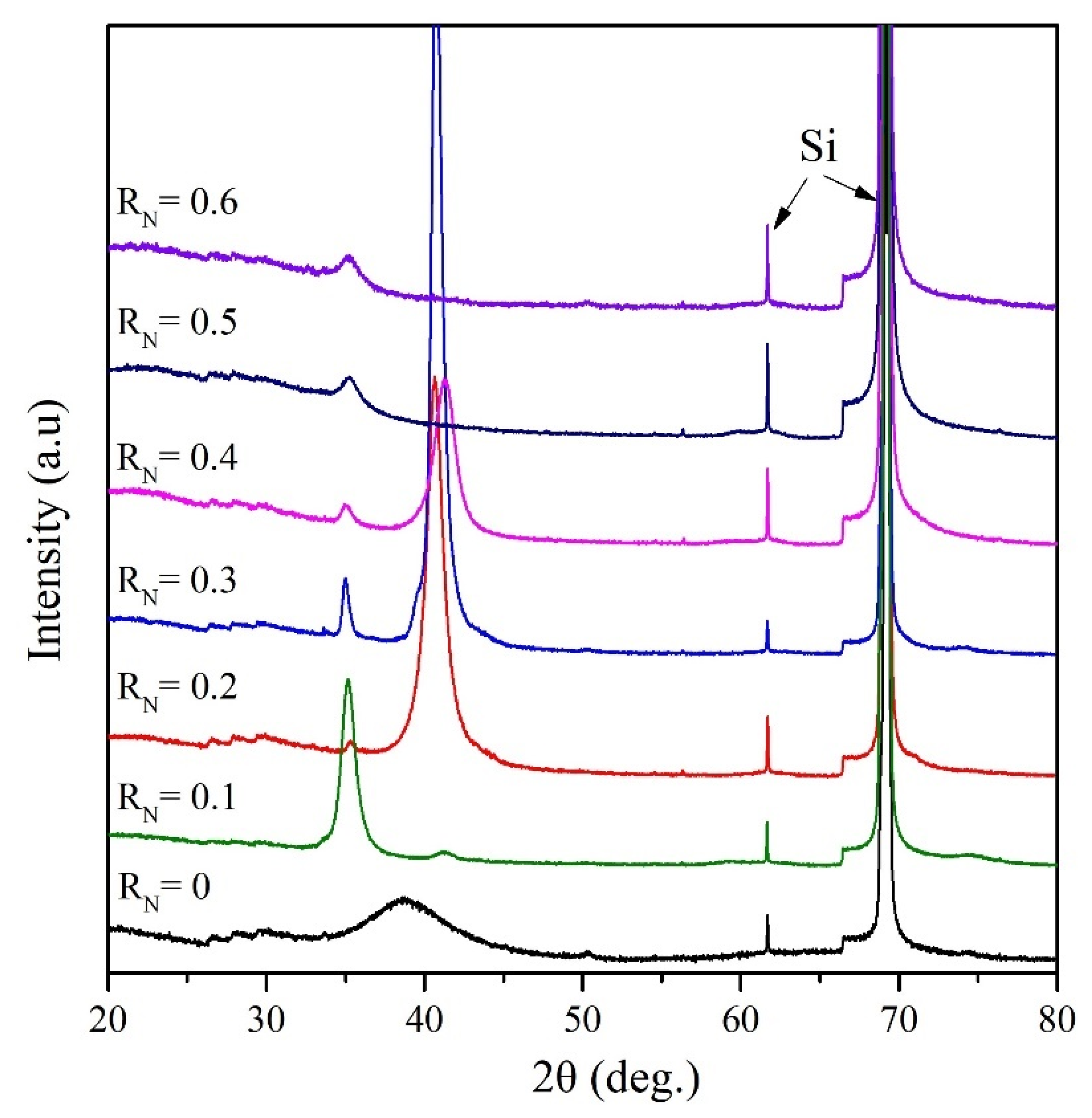
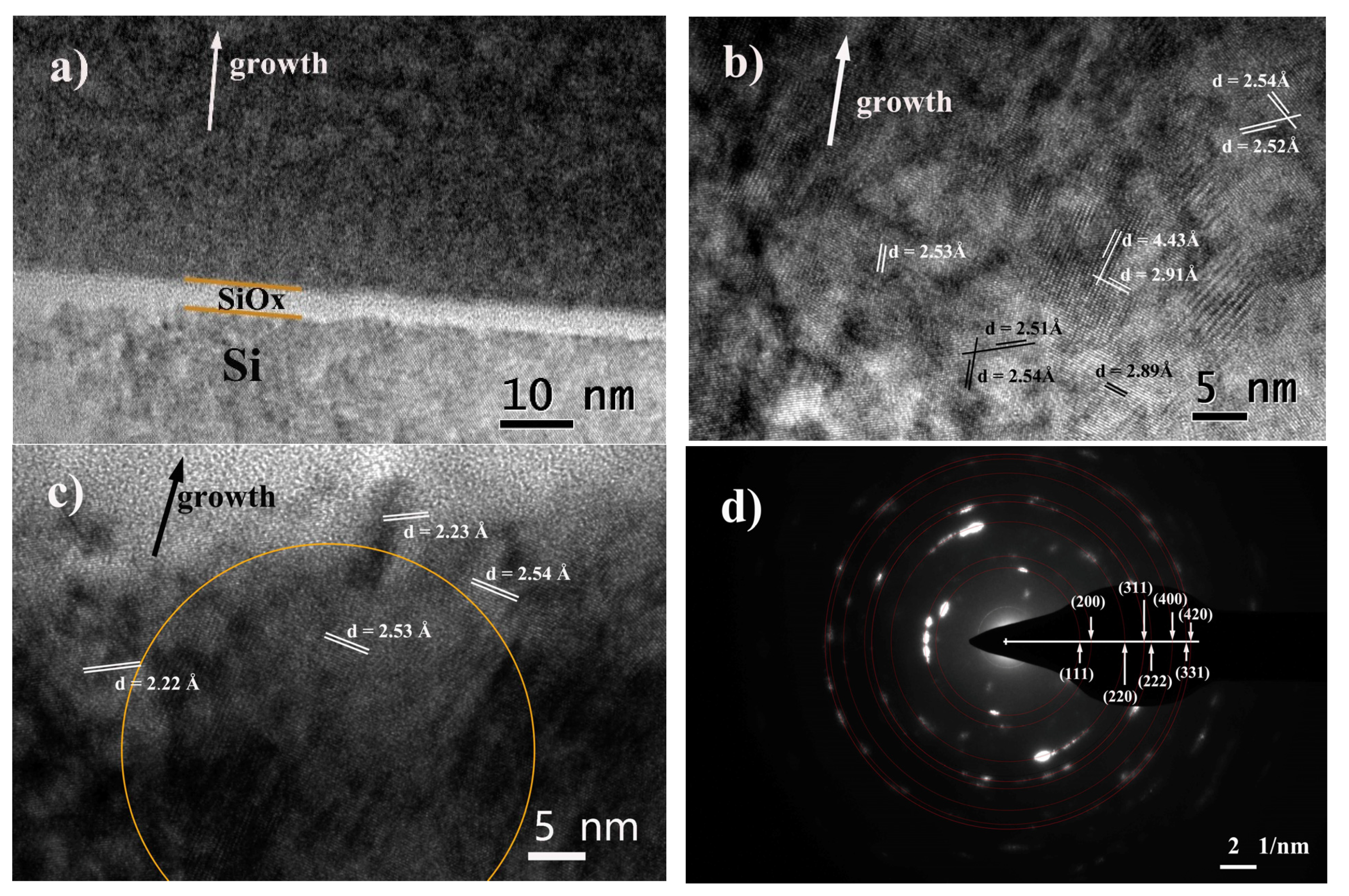
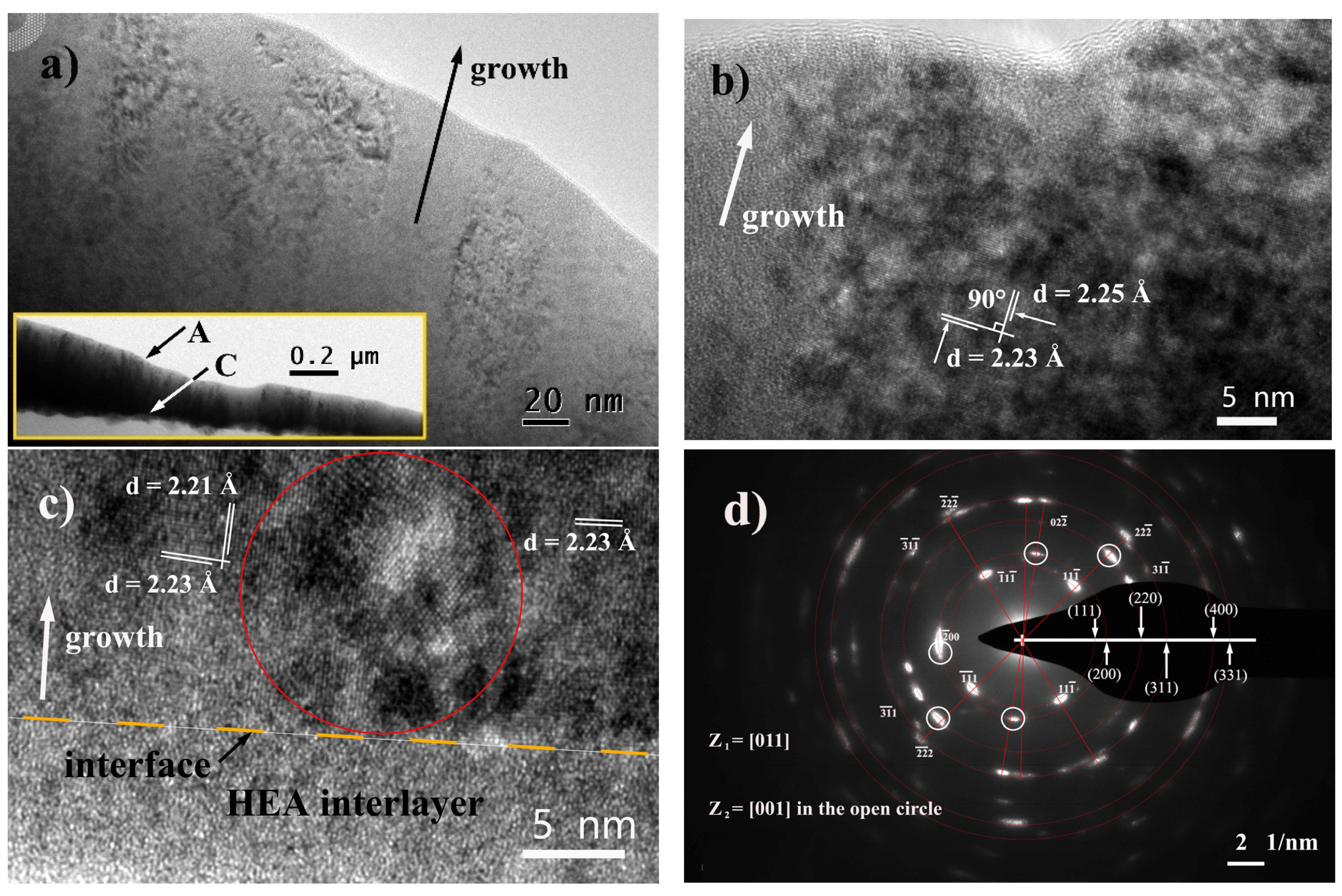
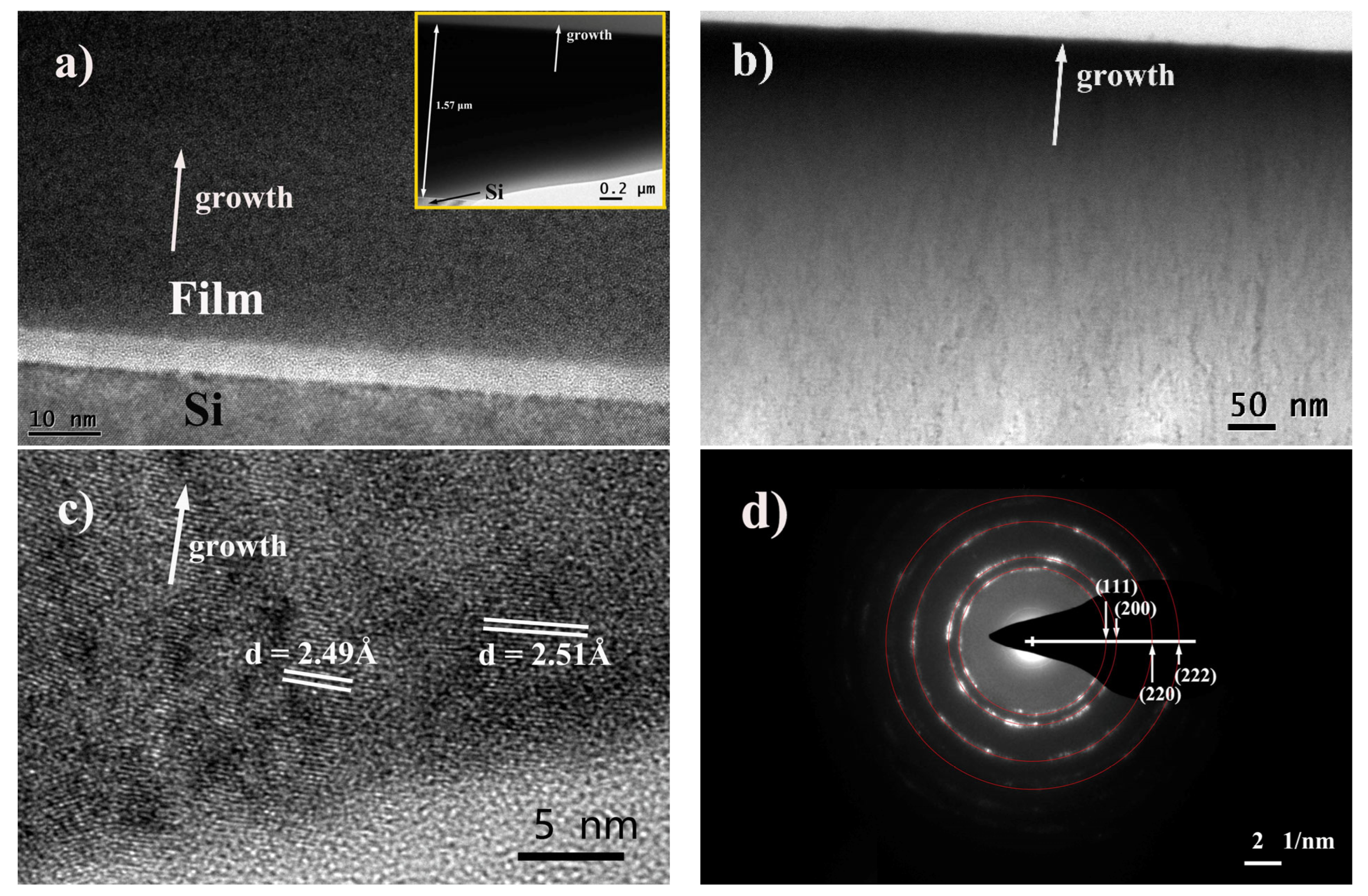
3.2. Microstructure and Crystallography
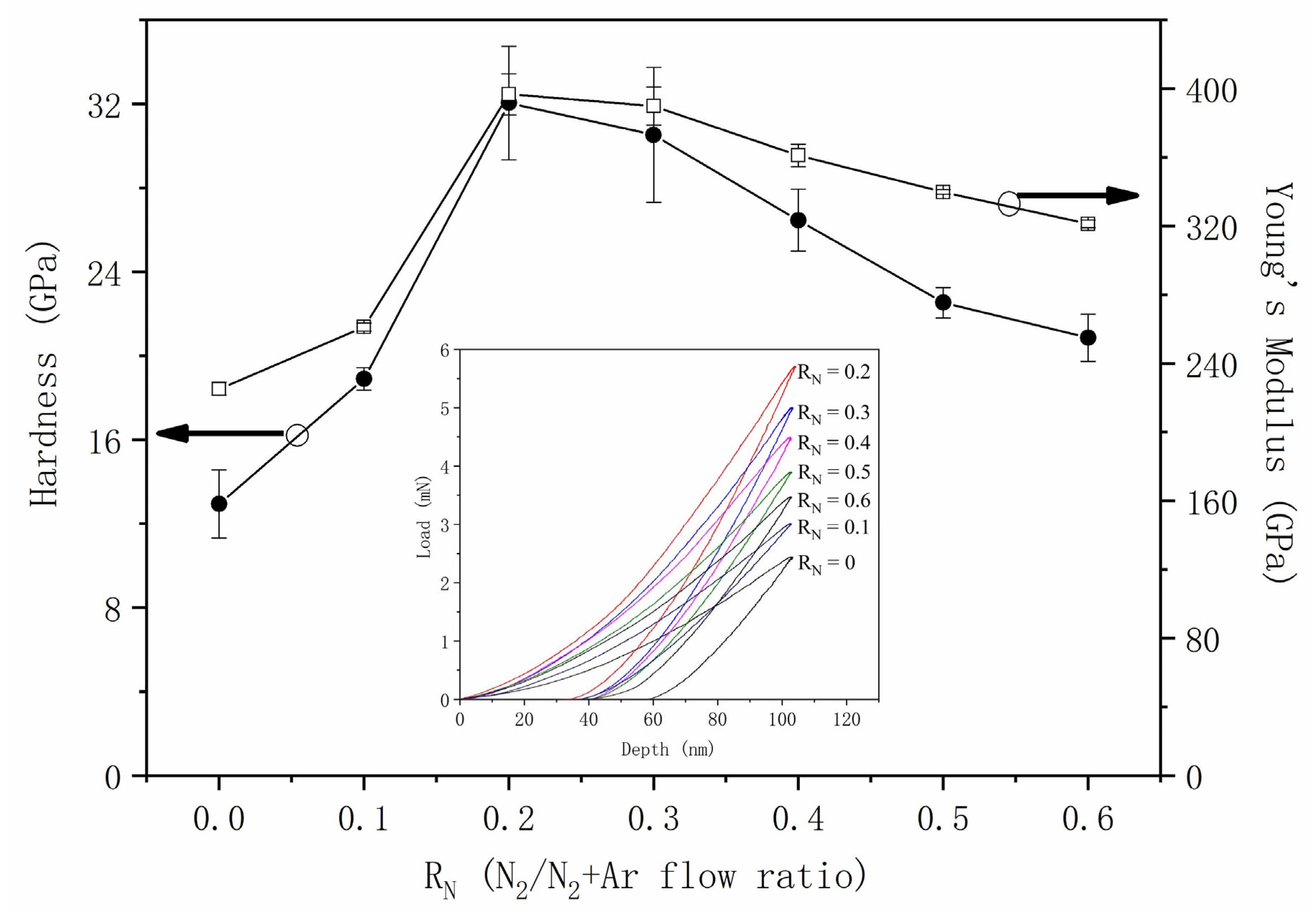
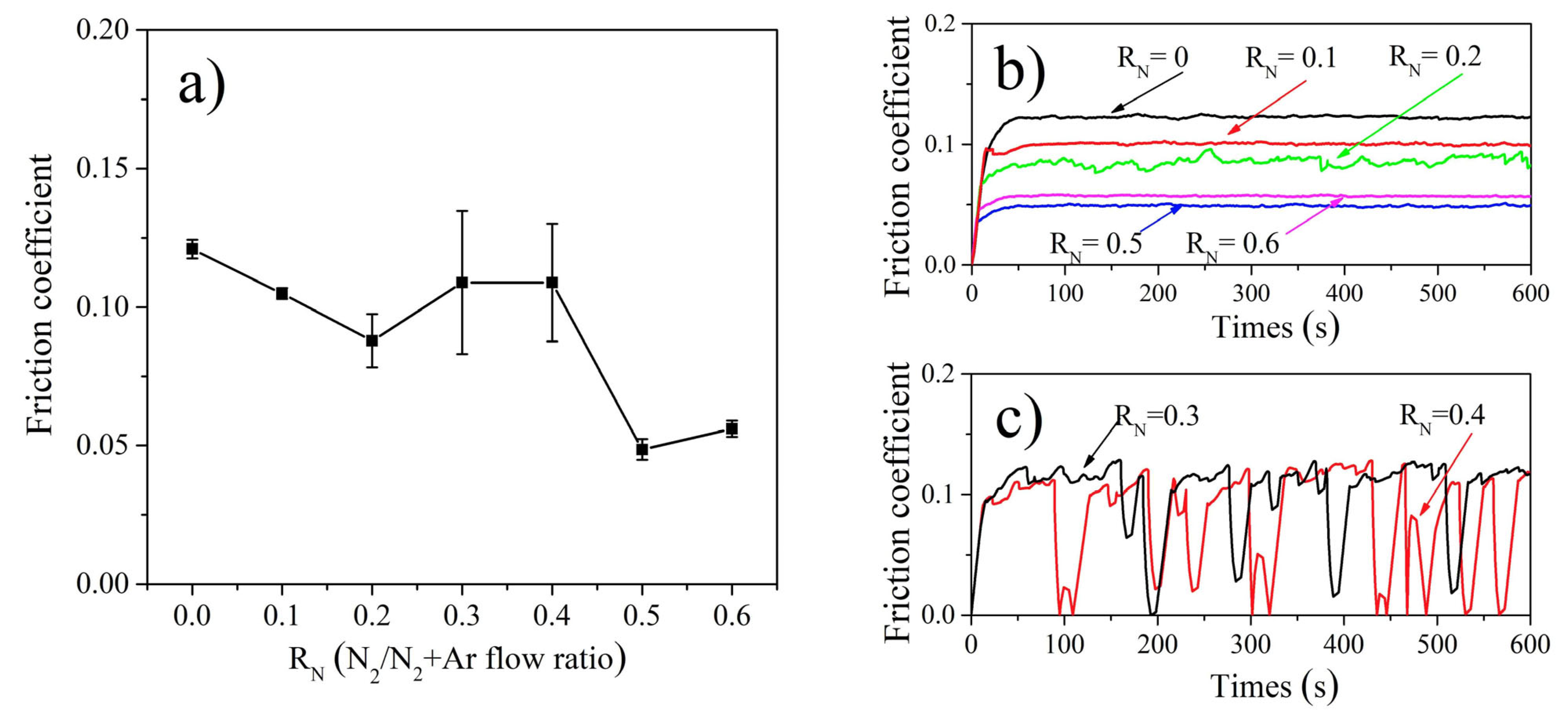
3.3. Mechanical and Electrical Properties
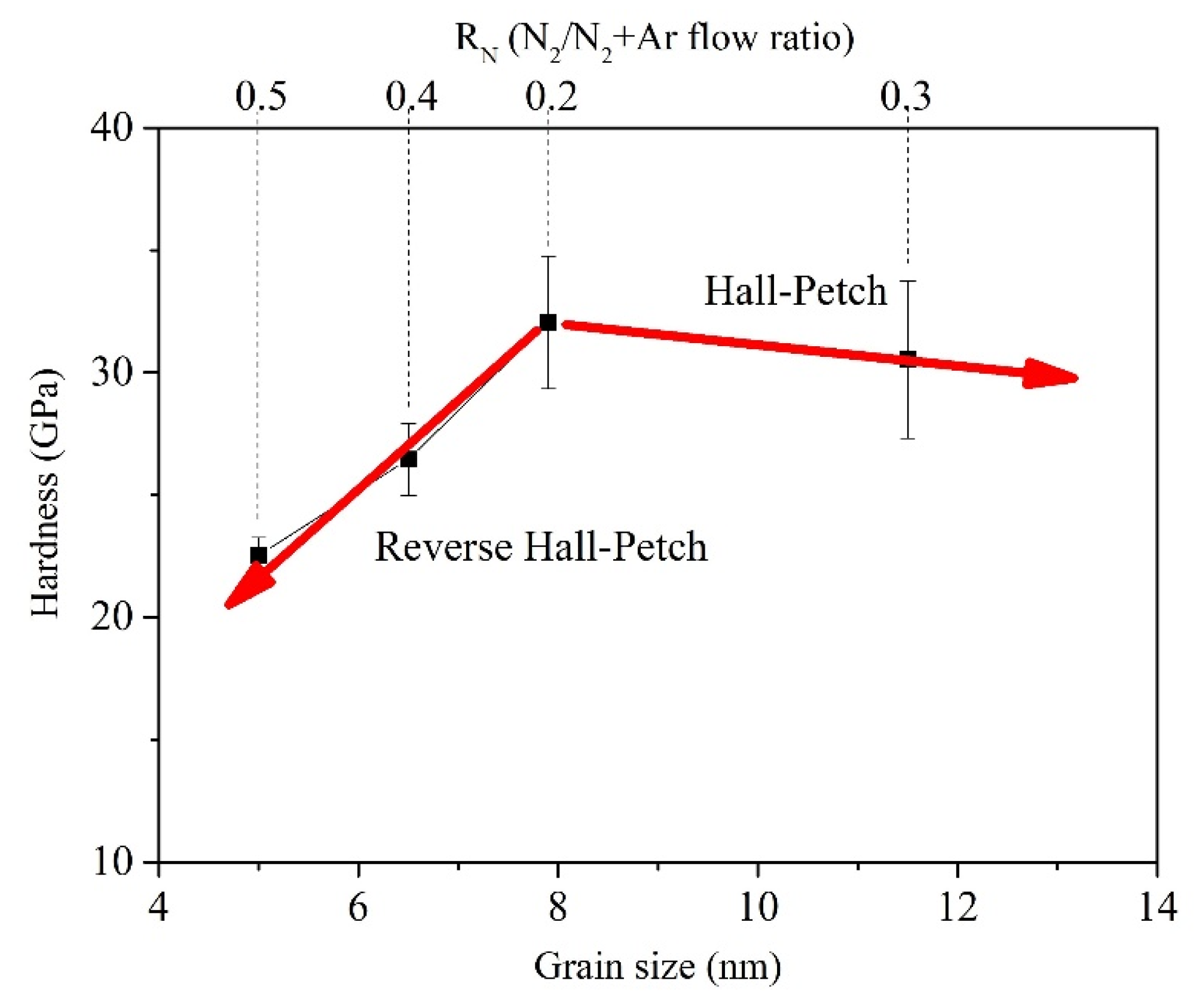
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.S.; Yadav, S.; Biswas, K.; Basu, B. High-entropy alloys and metallic nanocomposites: Processing challenges, microstructure development and property enhancement. Mater. Sci. Eng. R 2018, 131, 1–42. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gao, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C. A promising new class of irradiation tolerant materials:Ti2ZrHfV0.5Mo0.2 high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 369–373. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of NbMoTaW and VNbMoTaW refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thinaharan, C.; Ningshen, S.; Mallika, C.; Kamachi Mudali, U. Corrosion behavior and surface film characterization of TaNbHfZrTi high entropy alloy in aggressive nitric acid medium. Intermetallics 2017, 89, 123–132. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, X.; Cai, H.; Ma, S.; Xu, Z.; Ali, T. Microstructure, mechanical and physical properties of FeCoNiAlMnW high-entropy films deposited by magnetron sputtering. Appl. Surf. Sci. 2020, 507, 145131. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Gili, F.; Mangherini, D.; Battezzati, L. Development of a new high entropy alloy for wear resistance: FeCoCrNiW0.3 and FeCoCrNiW0.3 + 5at.% of C. Mater. Des. 2016, 115, 247–254. [Google Scholar] [CrossRef]
- Kao, W.H.; Su, Y.L.; Horng, J.H.; Wu, C.M. Structure, mechanical properties and thermal stability of nitrogen-doped TaNbSiZrCr high entropy alloy coatings and their application to glass moulding and micro-drills. Surf. Coat. Technol. 2020, 405, 126539. [Google Scholar] [CrossRef]
- Lai, C.; Tsai, M.; Lin, S.; Yeh, J. Influence of substrate temperature on structure and mechanical, properties of multi-element (AlCrTaTiZr)N coatings. Surf. Coat. Technol. 2007, 201, 6993–6998. [Google Scholar] [CrossRef]
- Feng, X.; Tang, G.; Sun, M.; Ma, X.; Wang, L. Chemical state and phase structure of (TaNbTiW)N films prepared by combined magnetron sputtering and PBII. Appl. Surf. Sci. 2013, 280, 388–393. [Google Scholar] [CrossRef]
- von Fieandt, K.; Paschalidou, E.M.; Srinath, A.; Soucek, P.; Lewin, E. Multi-component (Al,Cr,Nb,Y,Zr)N thin films by reactive magnetron sputter deposition for increased hardness and corrosion resistance. Thin Solid Films 2019, 693, 137685. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.B.; Hou, C.; Li, J.C.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar] [CrossRef]
- Strzelecki, G.W.; Nowakowska-Langier, K.; Mulewska, K.; Zieliński, M.; Kosińska, A.; Okrasa, S.; Wilczopolska, M.; Chodun, R.; Wicher, B.; Mirowski, R.; et al. Multi-component low and high entropy metallic coatings synthesized by pulsed magnetron sputtering. Surf. Coat. Technol. 2022, 446, 128802. [Google Scholar] [CrossRef]
- Sheng, W.; Yang, X.; Wang, C.; Zhan, Y. Nano-Crystallization of High-Entropy Amorphous NbTiAlSiWxNy Films Prepared by Magnetron Sputtering. Entropy 2016, 18, 226. [Google Scholar] [CrossRef]
- Sha, C.; Zhou, Z.; Xie, Z.; Munroe, P. FeMnNiCoCr-based high entropy alloy coatings: Effect of nitrogen additions on microstructural development, mechanical properties and tribological performance. Appl. Surf. Sci. 2019, 507, 145101. [Google Scholar] [CrossRef]
- Tsai, D.; Huang, Y.; Lin, S.; Liang, S.; Shieu, F. Effect of nitrogen flow ratios on the structure and mechanical properties of (TiVCrZrY)N coatings prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2010, 257, 1361–1367. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Xia, Y. Synthesis and characterization of super-hard AlCrTiVZr high-entropy alloy nitride films deposited by HiPIMS. Appl. Surf. Sci. 2020, 523, 146529. [Google Scholar] [CrossRef]
- von Fieandt, K.; Riekehr, L.; Osinger, B.; Fritze, S.; Lewin, E. Influence of N content on structure and mechanical properties of multi-component Al-Cr-Nb-Y-Zr based thin films by reactive magnetron sputtering. Surf. Coat. Technol. 2020, 389, 125614. [Google Scholar] [CrossRef]
- Sun, X.; Lv, S.; Li, Y.; Huang, C.; Luo, J. Influence of TaN films deposited using different N2 flow rates on the properties of Ta and Cu films in advanced 3D NAND memory. Mater. Sci. Semicon. Proc. 2020, 115, 105120. [Google Scholar] [CrossRef]
- Tsai, M.H.; Wang, C.W.; Lai, C.H.; Yeh, J.W.; Gan, J.Y. Thermally stable amorphous (AlMoNbSiTaTiVZr)50N50 nitride film as diffusion barrier in copper metallization. Appl. Phys. Lett. 2008, 92, 052109. [Google Scholar] [CrossRef]
- An, B.; Kwon, Y.; Oh, J.; Lee, C.; Choi, S.; Kim, H.; Lee, M.; Pae, S.; Yang, C. Characteristics of an Amorphous Carbon Layer as a Diffusion Barrier for an Advanced Copper Interconnect. ACS Appl. Mater. Inter. 2020, 12, 3104–3113. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Films 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Cheng, Y.; Liaw, P.K. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Sci. Rep. 2013, 3, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface stability, mechanical and corrosion properties of AlCrMoNbZr/(AlCrMoNbZr)N high-entropy alloy multilayer coatings under helium ion irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Yth, A.; Cht, A.; Sjla, B.; Jwya, B.; Syca, B. Thermodynamic route for self-forming 1.5 nm V-Nb-Mo-Ta-W high-entropy alloy barrier layer: Roles of enthalpy and mixing entropy. Acta Mater. 2020, 199, 107–115. [Google Scholar] [CrossRef]
- Chang, S.Y.; Chen, D.S. (AlCrTaTiZr)N/(AlCrTaTiZr)N0.7 bilayer structure of high resistance to the interdiffusion of Cu and Si at 900 °C. Mater. Chem. Phys. 2011, 125, 5–8. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, C.E.; Chiang, S.C.; Huang, Y.C. 4-nm thick multilayer structure of multi-component (AlCrRuTaTiZr)Nx as robust diffusion barrier for Cu interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Ye, Y.F.; Liu, X.D.; Wang, S.; Liu, C.T.; Yang, Y.J.I. The general effect of atomic size misfit on glass formation in conventional and high-entropy alloys. Intermetallics 2016, 78, 30–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Wang, S.P.; Xu, J. (TiZrNbTa)-Mo high-entropy alloys: Dependence of microstructure and mechanical properties on Mo concentration and modeling of solid solution strengthening. Intermetallics 2018, 95, 59–72. [Google Scholar] [CrossRef]
- Xia, A.; Dedoncker, R.; Glushko, O.; Cordill, M.J.; Franz, R. Influence of the nitrogen content on the structure and properties of MoNbTaVW high entropy alloy thin films. J. Alloys Compd. 2021, 850, 156740. [Google Scholar] [CrossRef]
- Shu, R.; Paschalidou, E.-M.; Rao, S.G.; Lu, J.; Greczynski, G.; Lewin, E.; Nyholm, L.; le Febvrier, A.; Eklund, P. Microstructure and mechanical, electrical, and electrochemical properties of sputter-deposited multicomponent (TiNbZrTa)Nx coatings. Surf. Coat. Technol. 2020, 389, 125651. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, K.; Zheng, Y.; Zhou, H.; Wan, Z. Chemical state, structure and mechanical properties of multi-element (CrTaNbMoV)Nx films by reactive magnetron sputtering. Mater. Chem. Phys. 2020, 239, 121991. [Google Scholar] [CrossRef]
- Hong, C.; He, P.; Tian, J.; Chang, F.; Wu, J.; Zhang, P.; Dai, P. On the microstructure and mechanical properties of CrNx/Ag multilayer films prepared by magnetron sputtering. Materials 2020, 13, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Shen, Z.; Liu, Z. Structure and mechanical properties of multi-element (AlCrMnMoNiZr)Nx coatings by reactive magnetron sputtering. J. Alloy. Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and mechanical properties of multi-element (AlCrMoTaTiZr)Nx coatings by reactive magnetron sputtering. Thin Solid Films 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Stampfl, C.; Freeman, A.J. Stable and metastable structures of the multiphase tantalum nitride system. Phys. Rev. B 2005, 71, 024111. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Yang, C.B.; Sung, C.C.; Hsu, C.Y. Structure and tribological behavior of (AlCrNbSiTiV)N film deposited using direct current magnetron sputtering and high power impulse magnetron sputtering. Thin Solid Films 2018, 668, 63–68. [Google Scholar] [CrossRef]
- Dedoncker, R.; Djemia, P.; Radnóczi, G.; Tétard, F.; Belliard, L.; Abadias, G.; Martin, N.; Depla, D. Reactive sputter deposition of CoCrCuFeNi in nitrogen/argon mixtures. J. Alloy. Compd. 2018, 769, 881–888. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu oxidation mechanism on Cu-Zr(O)N coatings: Role on functional properties. Appl. Surf. Sci. 2021, 555, 149704. [Google Scholar] [CrossRef]
- Cui, P.; Li, W.; Liu, P.; Zhang, K.; Ma, F.; Chen, X.; Feng, R.; Liaw, P.K. Effects of nitrogen content on microstructures and mechanical properties of (AlCrTiZrHf)N high-entropy alloy nitride films. J. Alloy. Compd. 2020, 834, 155063. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Roh, A.; Son, M.; Ham, M.H.; Kim, J.H.; Choi, H. Mechanical and electrical properties of NbMoTaW refractory high-entropy alloy thin films. Int. J. Refract. Met. Hard Mater. 2019, 80, 286–291. [Google Scholar] [CrossRef]
- Han, L.; Jeurgens, L.; Cancellieri, C.; Wang, J.; Xu, Y.; Huang, Y.; Liu, Y.; Wang, Z. Anomalous texture development induced by grain yielding anisotropy in Ni and Ni-Mo alloys. Acta Mater. 2020, 200, 857–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy Design and Properties Optimization of High-Entropy Alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Dirras, G.; Lilensten, L.; Djemia, P.; Laurent-Brocq, M.; Tingaud, D.; Couzinié, J.P.; Perrière, L.; Chauveau, T.; Guillot, I. Elastic and plastic properties of as-cast equimolar TiHfZrTaNb high-entropy alloy. Mater. Sci. Eng. A 2016, 654, 30–38. [Google Scholar] [CrossRef]
- Tunes, M.A.; Vishnyakov, V.M. Microstructural origins of the high mechanical damage tolerance of NbTaMoW refractory high-entropy alloy thin films. Mater. Des. 2019, 170, 107692. [Google Scholar] [CrossRef]
- Liao, W.; Lan, S.; Gao, L.; Zhang, H.; Xu, S.; Song, J.; Wang, X.; Lu, Y. Nanocrystalline high-entropy alloy (CoCrFeNiAl0.3) thin-film coating by magnetron sputtering. Thin Solid Films 2017, 638, 383–388. [Google Scholar] [CrossRef]
- Shu, R.; Paschalidou, E.M.; Rao, S.G.; Bakhit, B.; Boyd, R.; Moro, M.V.; Primetzhofer, D.; Greczynski, G.; Nyholm, L.; le Febvrier, A.; et al. Effect of nitrogen content on microstructure and corrosion resistance of sputter-deposited multicomponent (TiNbZrTa)Nx films. Surf. Coat. Technol. 2020, 404, 126485. [Google Scholar] [CrossRef]
- Ren, B.; Yan, S.Q.; Zhao, R.F.; Liu, Z.X. Structure and properties of (AlCrMoNiTi)Nx and (AlCrMoZrTi)Nx films by reactive RF sputtering. Surf. Coat. Technol. 2013, 235, 764–772. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall–Petch relationship in nanocrystalline materials. Prog. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and wear behavior of a single-phase equiatomic TiZrHfNb high-entropy alloy studied using a nanoscratch technique. Acta Mater. 2018, 147, 78–89. [Google Scholar] [CrossRef]
- Misra, A.; Huang, S. Effect of loading induced anisotropy on the shear behavior of rough interfaces. Tribol. Int. 2011, 44, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.K.; Yeh, J.W.J.S.; Technology, C. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Fang, S.; Wang, C.; Li, C.L.; Luan, J.H.; Hsueh, C.H. Microstructures and mechanical properties of CoCrFeMnNiVx high entropy alloy films. J. Alloys Compd. 2020, 820, 153388. [Google Scholar] [CrossRef]
- Khan, N.A.; Akhavan, B.; Zhou, H.; Chang, L.; Wang, Y.; Sun, L.; Bilek, M.M.; Liu, Z. High entropy alloy thin films of AlCoCrCu0.5FeNi with controlled microstructure. Appl. Surf. Sci. 2019, 495, 143560. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, K.; Zheng, Y.; Zhou, H.; Wan, Z. Effect of Zr content on structure and mechanical properties of (CrTaNbMoV)Zrx high-entropy alloy films. Nucl. Instrum. Methods Phys. Res. 2019, 457, 56–62. [Google Scholar] [CrossRef]
- Ye, Y.F.; Liu, C.T.; Yang, Y. A geometric model for intrinsic residual strain and phase stability in high entropy alloys. Acta Mater. 2015, 94, 152–161. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Madan, A.; Barnett, S.A.; Misra, A.; Kung, H.; Nastasi, M. Structure, stability, and mechanical properties of epitaxial W/NbN superlattices. J. Vac. Sci. Technol. A 2001, 19, 952–957. [Google Scholar] [CrossRef]
- Takano, I.; Isobe, S.; Sasaki, T.A.; Baba, Y. Nitrogenation of various transition metals by N2+-ion implantation. Appl. Surf. Sci. 1989, 37, 25–32. [Google Scholar] [CrossRef]
- Qi, Z.B.; Sun, P.; Zhu, F.P.; Wang, Z.C.; Peng, D.L.; Wu, C.H. The inverse Hall–Petch effect in nanocrystalline ZrN coatings. Surf. Coat. Technol. 2011, 205, 3692–3697. [Google Scholar] [CrossRef]
- Schiotz, J.; Tolla, F.; Jacobsen, K.W. Softening of nanocrystalline metals at very small grain sizes. Nature 1998, 391, 561–563. [Google Scholar] [CrossRef]
- Anitha, V.P.; Major, S.; Chandrashekharam, D.; Bhatnagar, M. Deposition of molybdenum nitride thin films by r.f. reactive magnetron sputtering. Surf. Coat. Technol. 1996, 79, 50–54. [Google Scholar] [CrossRef]
- Wang, Z.; Kawakami, A.; Uzawa, Y.; Komiyama, B. Superconducting properties and crystal structures of single-crystal niobium nitride thin films deposited at ambient substrate temperature. J. Appl. Phys. 1996, 79, 7837–7842. [Google Scholar] [CrossRef]
- Subramanian, B.; Prabakaran, K.; Jayachandran, M. Influence of nitrogen flow rates on materials properties of CrNx films grown by reactive magnetron sputtering. Bull. Mater. Sci. 2012, 35, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Zahid, F.; Timoshevskii, V.; Xia, K.; Gall, D.; Guo, H. Resistivity of thin Cu films with surface roughness. Phys. Rev. B 2009, 79, 155406. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.H.; Weng, C.H.; Lai, C.H.; Lin, S.J. Study on adhesion and wear resistance of multi-element (AlCrTaTiZr)N coatings. Thin Solid Films 2009, 517, 4989–4993. [Google Scholar] [CrossRef]



| Process | Target Power (W) | N2 Flow (sccm) | Bias (V) | Deposition Time (Min) | |
|---|---|---|---|---|---|
| Cr | HEA | ||||
| Target cleaning | 30 | 120 | 0 | float | 10 |
| Adhesion layer | 30 | 120 | RN a | −600 to −120 b | 5 |
| Top layer | 30 | 120 | RN a | −120 | 120 |
| Δ (%) | ΔHmix (kJ/mol) | ΔSmix (mol·K) | VEC | Δχ | Ω |
|---|---|---|---|---|---|
| 7.34 | −5.07 | 13.29 J | 5.27 | 0.35 | 7.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, Y.; Zhang, X.; Lin, Z.; Liu, D.; Hong, C.; Dai, P. Evolutions of the Microstructure and Properties of the (CrMoNbTaZr)NX Films Prepared by Reactive Magnetron Sputtering: Effects of Stoichiometry and Crystallinity. Coatings 2023, 13, 1424. https://doi.org/10.3390/coatings13081424
Wang X, Zhang Y, Zhang X, Lin Z, Liu D, Hong C, Dai P. Evolutions of the Microstructure and Properties of the (CrMoNbTaZr)NX Films Prepared by Reactive Magnetron Sputtering: Effects of Stoichiometry and Crystallinity. Coatings. 2023; 13(8):1424. https://doi.org/10.3390/coatings13081424
Chicago/Turabian StyleWang, Xiang, Yanhong Zhang, Xin Zhang, Zhihe Lin, Dongguang Liu, Chunfu Hong, and Pinqiang Dai. 2023. "Evolutions of the Microstructure and Properties of the (CrMoNbTaZr)NX Films Prepared by Reactive Magnetron Sputtering: Effects of Stoichiometry and Crystallinity" Coatings 13, no. 8: 1424. https://doi.org/10.3390/coatings13081424
APA StyleWang, X., Zhang, Y., Zhang, X., Lin, Z., Liu, D., Hong, C., & Dai, P. (2023). Evolutions of the Microstructure and Properties of the (CrMoNbTaZr)NX Films Prepared by Reactive Magnetron Sputtering: Effects of Stoichiometry and Crystallinity. Coatings, 13(8), 1424. https://doi.org/10.3390/coatings13081424