UV-Curable Fluorocarbon Polyurethane Coatings for Marble Kitchen Countertops
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Synthesis of HEA-HDI-TEOH-6
2.3. Preparation of Light-Curable Fluorocarbon Polyurethane Coatings
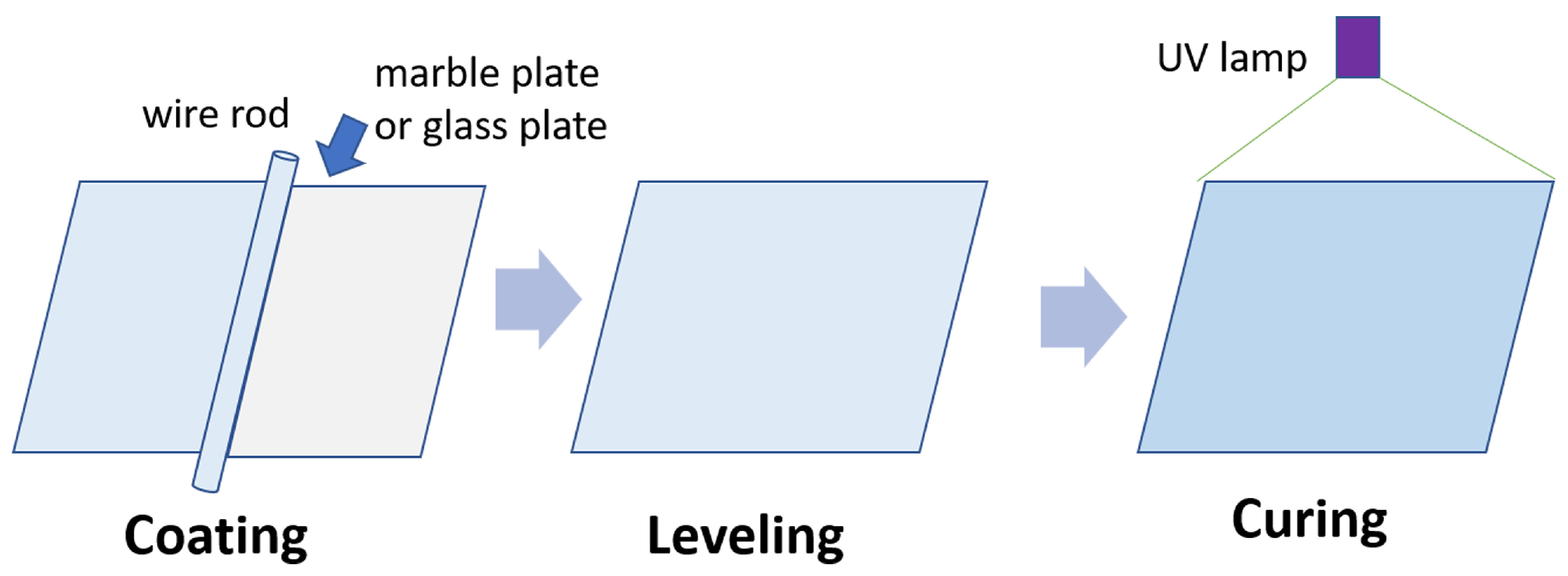
2.4. Preparation of UV-Curable Fluorocarbon Polyurethane Coating Film
2.5. Characterization
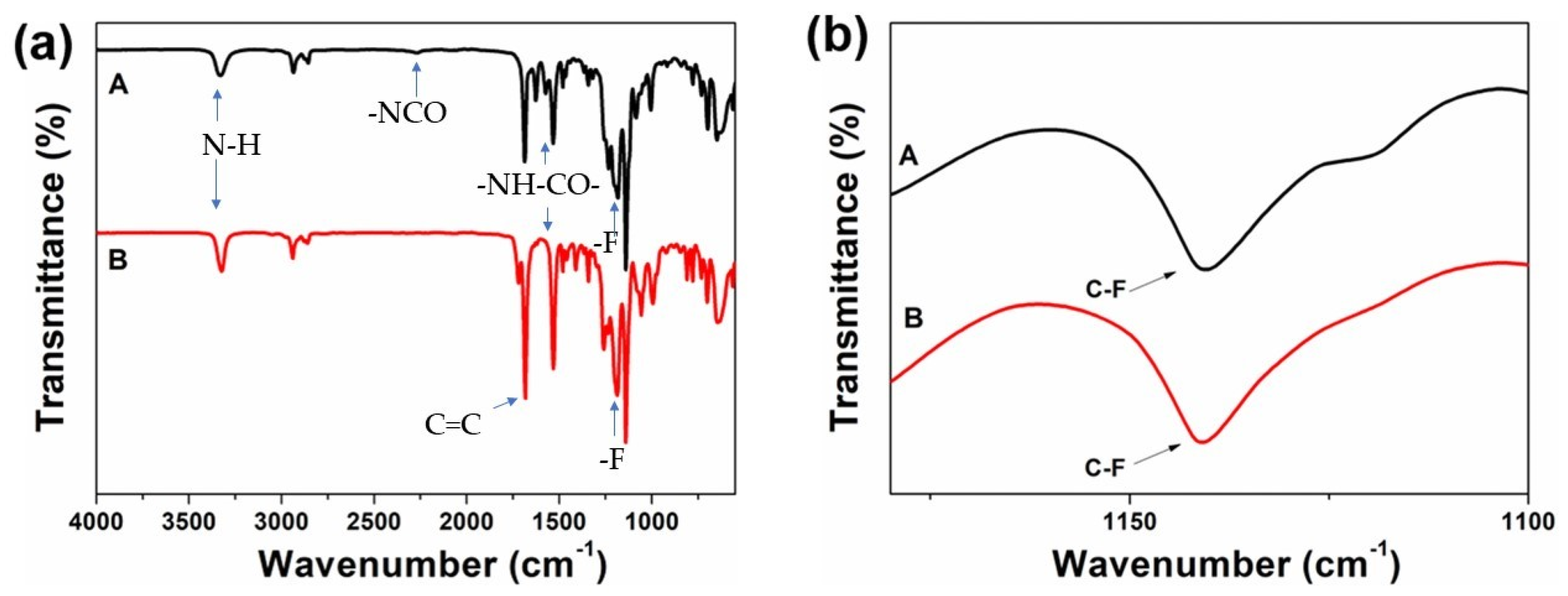
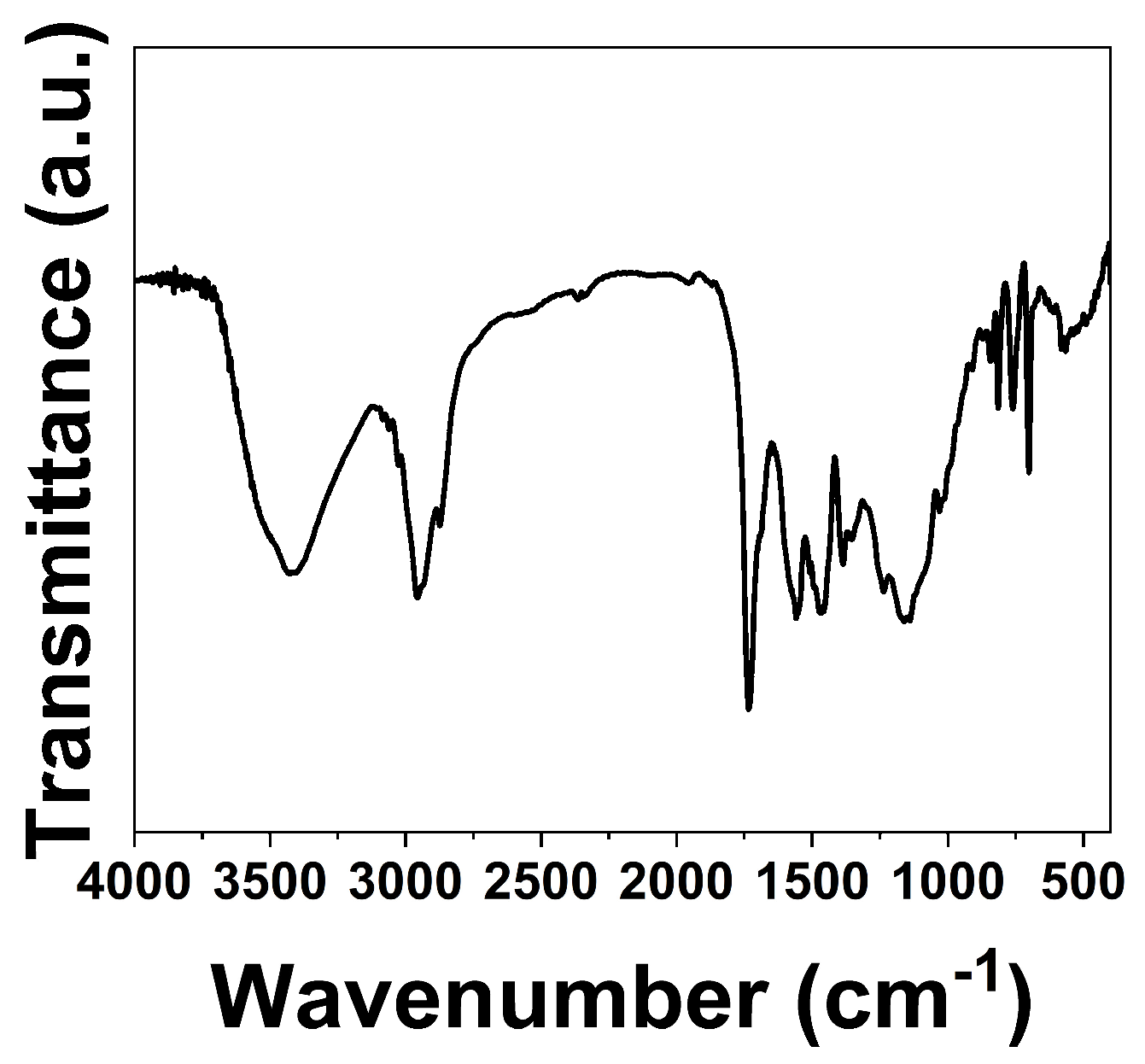
2.5.1. Fourier Transform Infrared Spectroscopy (FT−IR)
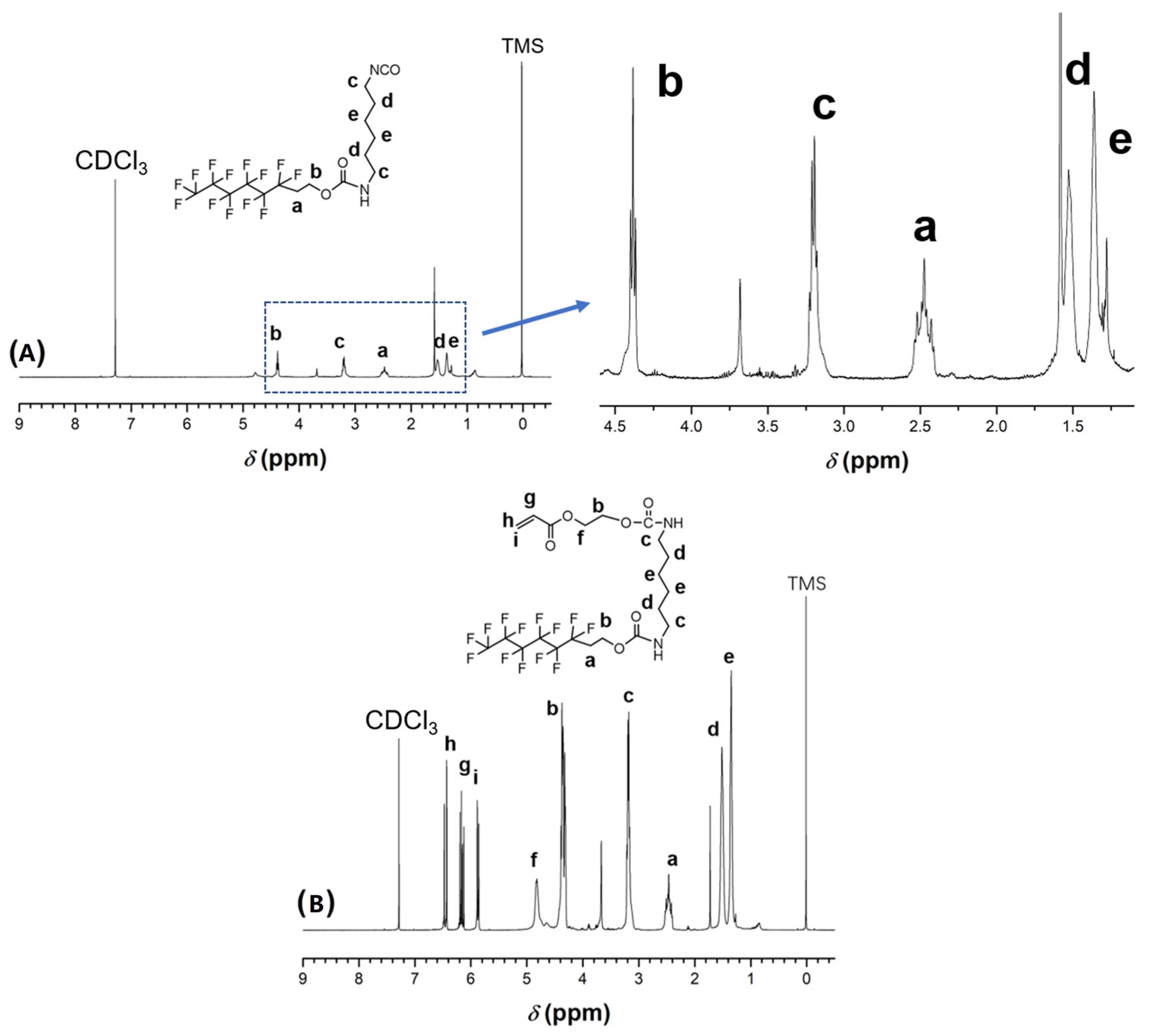
2.5.2. Nuclear Magnetic Resonance (NMR)
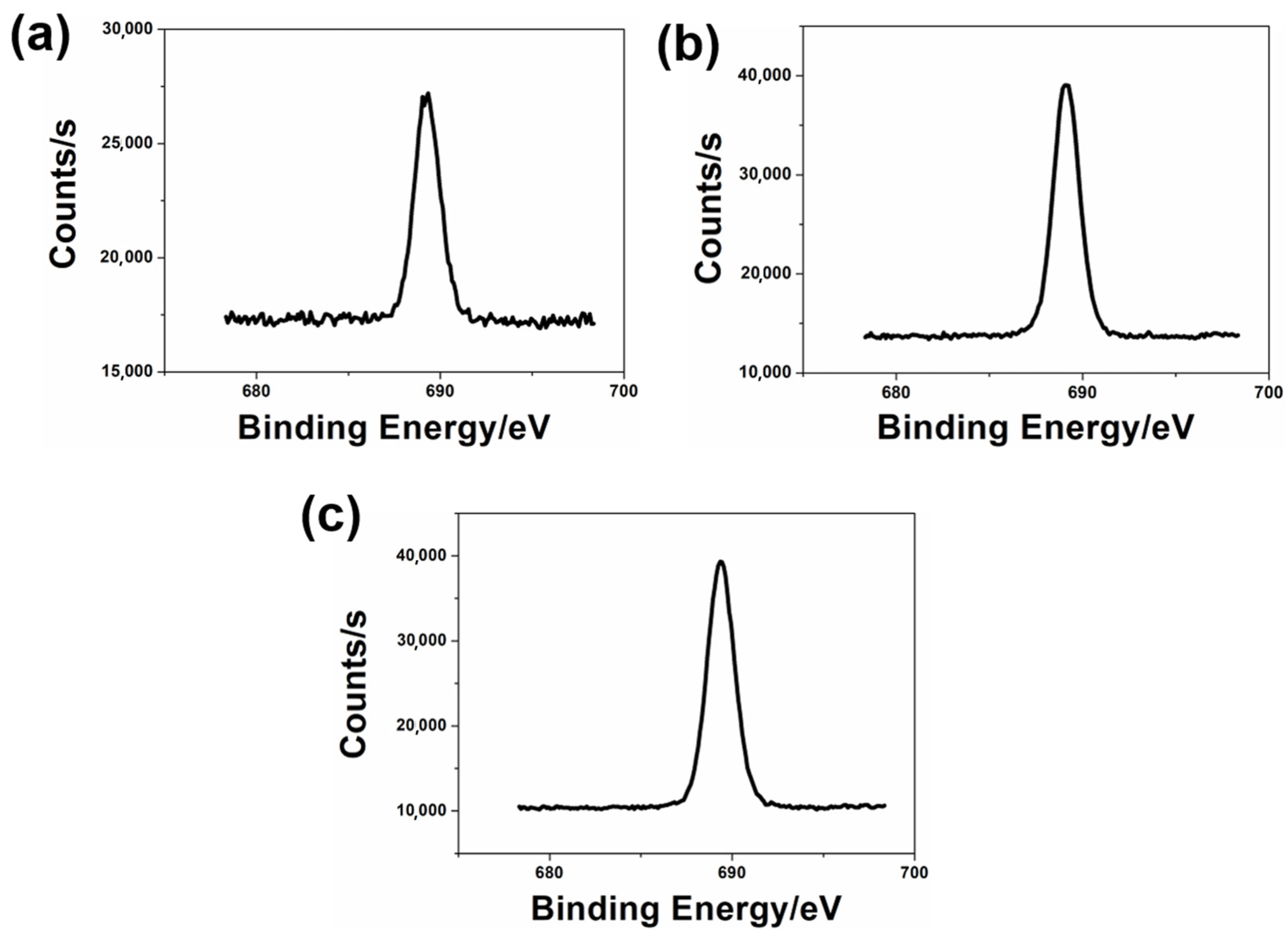
2.5.3. X-ray Photoelectron Spectroscopy (XPS)
2.5.4. Scanning Electron Microscope (SEM)
2.5.5. Coating Film Basic Performance
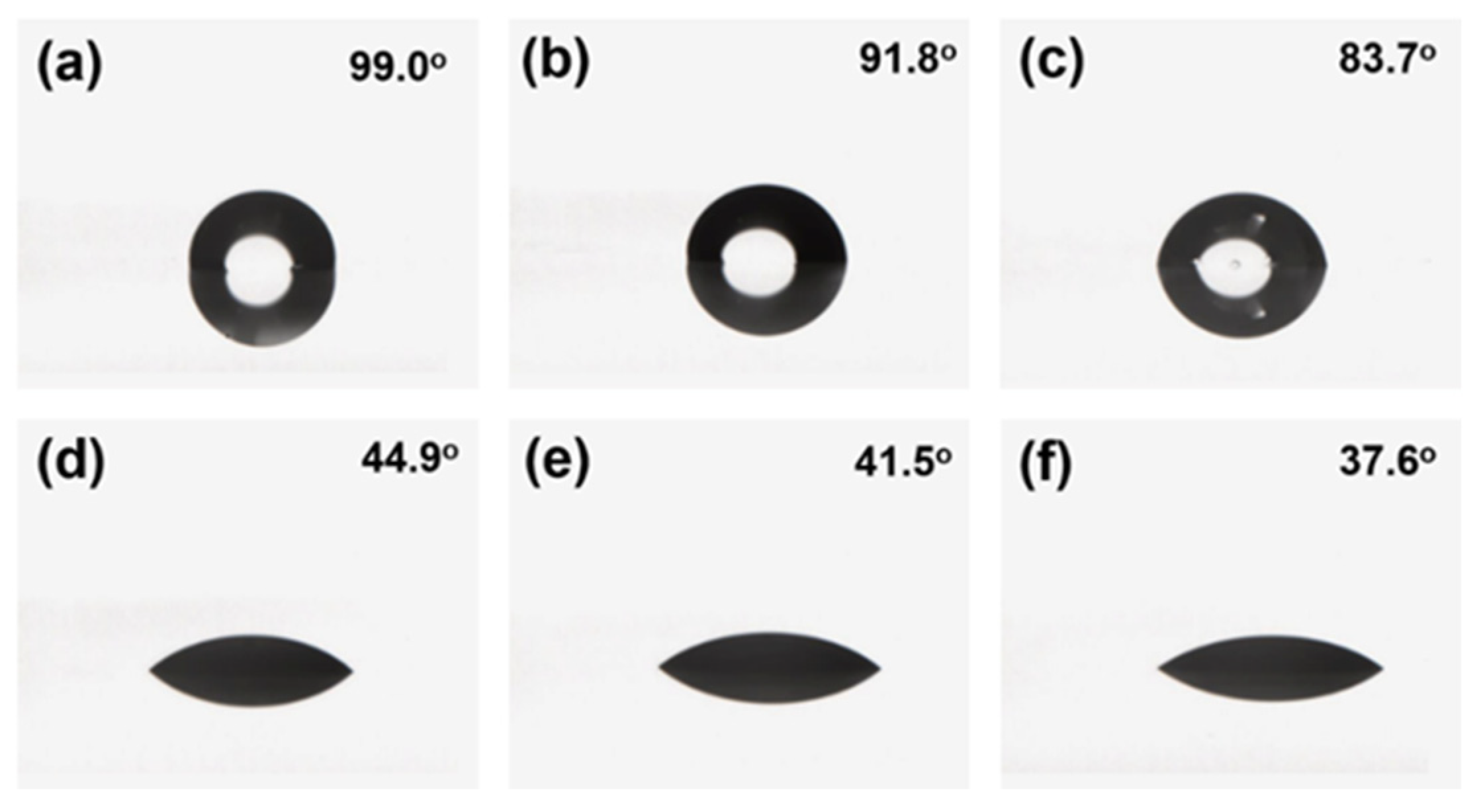
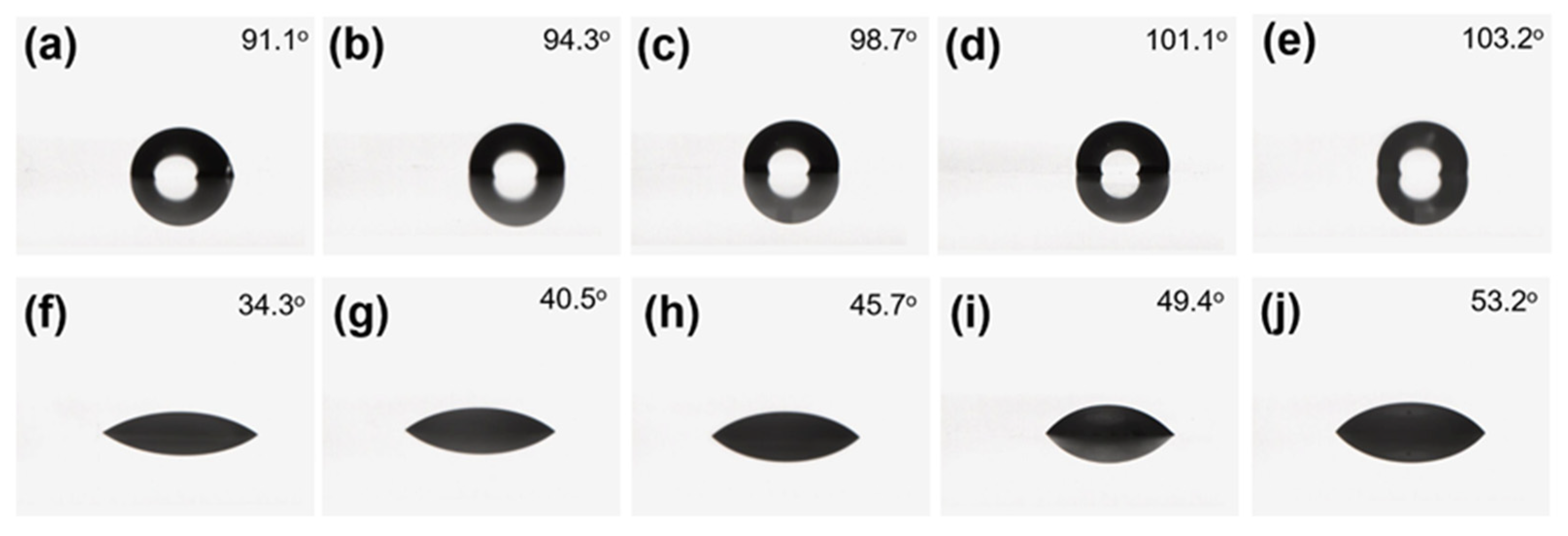
2.5.6. Contact Angle Test
2.5.7. Stain Resistance Test
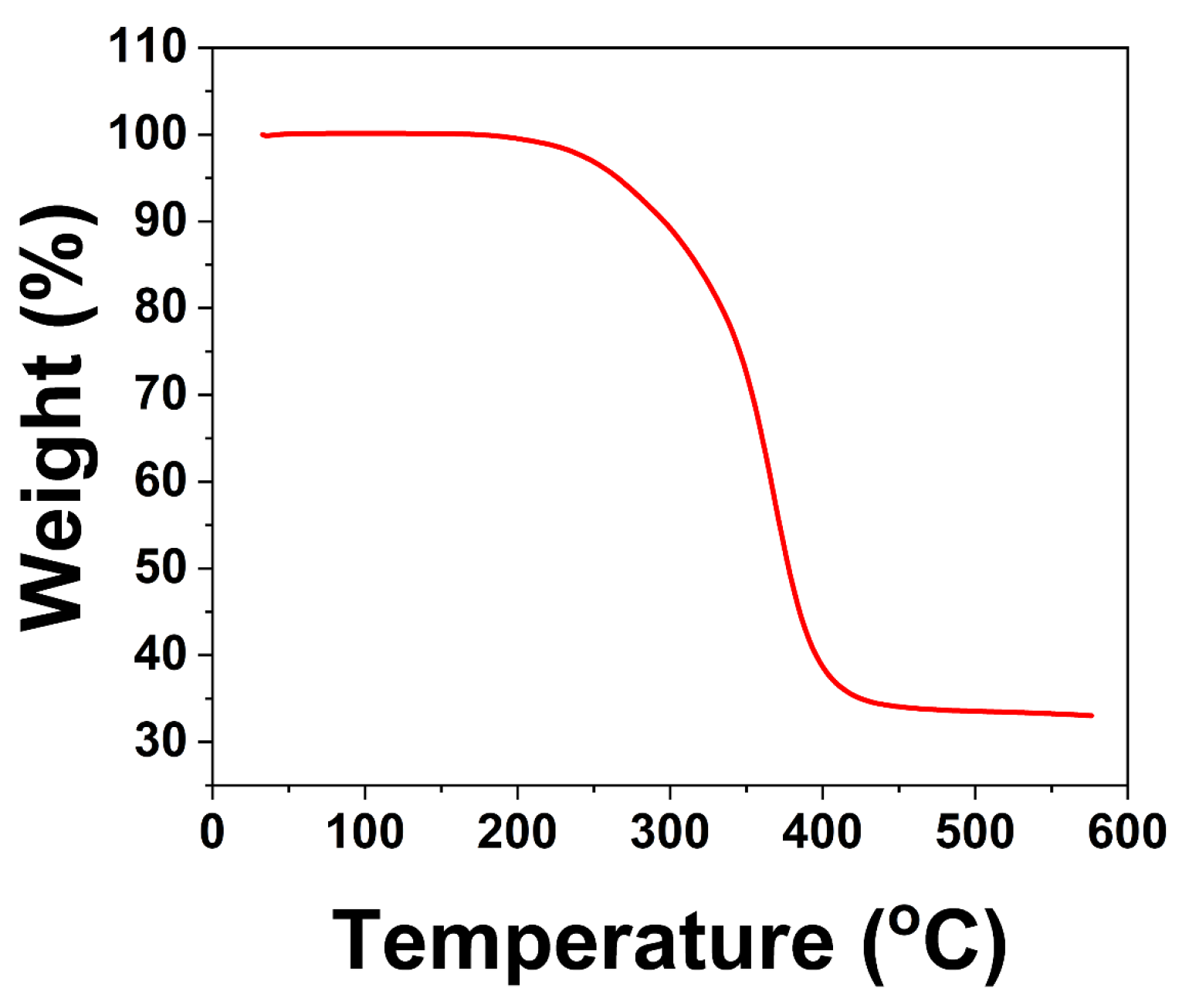
2.5.8. TGA Test
2.5.9. DSC Test
3. Results and Discussion
3.1. Synthesis of HDI-TEOH-6 and HEA-HDI-TEOH-6
3.2. Basic Performance of Coating Films
3.3. The Structure and Morphologies of the Coating Films
3.4. Hydrophobic and Oleophobic Properties of Coating Film after Abrasion with Pasteur Cloth
3.5. Hydrophobic and Oleophobic Properties of Coating Film after Acid and Alkaline Immersion
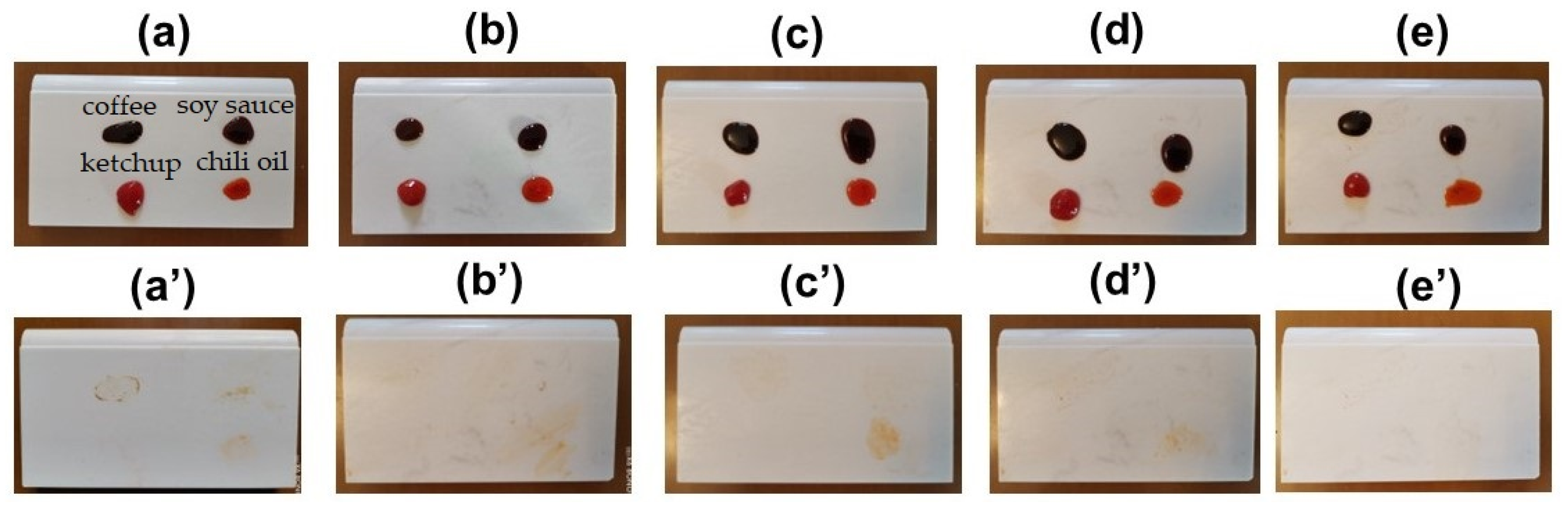
3.6. Stain Resistance of UV-Curable Fluorocarbon Polyurethane Coating Film
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bayer, I.S.; Megaridis, C.M.; Zhang, J.; Gamota, D.; Biswas, A. Analysis and surface energy estimation of various model polymeric surfaces using contact angle hysteresis. J. Adhes. Sci. Technol. 2007, 21, 1439–1467. [Google Scholar] [CrossRef]
- Jiang, L.; Feng, L. Bionic Intelligent Nano-Interface Materials; Beijing Chemical Industry Press: Beijing, China, 2007; Volume 1, pp. 3–175. [Google Scholar]
- Bai, X.; Chen, F. Progress in the preparation of superhydrophobic surfaces by femtosecond laser. J. Optics 2021, 1, 2–10. [Google Scholar]
- Zhang, X.; Chen, H.; Pi, P.; Wen, X.; Cheng, J.; Yang, Z. Study on the crystallization of fluorinated acrylate copolymers and surface wetting properties of coated films. J. Chem. Eng. High Schools 2012, 1, 3–5. [Google Scholar]
- Hu, F. Material Surfaces and Interfaces; East China University of Science and Technology Press: Shanghai, China, 2008; Volume 2, pp. 2–6. [Google Scholar]
- Fan, Z.; Wei, Z.; Tian, D.; Xiao, C.; Sun, X.; Chen, C.; Liu, W. New advances in the preparation, application and theory of superhydrophobic material surfaces. Polym. Bull. 2005, 11, 105–110. [Google Scholar]
- Teng, X.R. Surface Physical Chemistry. In Physical Chemistry of Surfaces; Beijing Chemical Industry Press: Beijing, China, 2009; Volume 3, pp. 4–37. [Google Scholar]
- Sheng, M.; Deng, G. New Polyurethane Coating Production Technology and Application; Guangdong Science and Technology Publishing House: Guangzhou, China, 2001; Volume 1, pp. 3–6. [Google Scholar]
- Park, Y.M.; Gang, M.; Seo, Y.H.; Kim, B.H. Artificial petal surface based on hierarchical micro- and nanostructures. Thin Solid Films 2011, 520, 362–367. [Google Scholar] [CrossRef]
- Lee, W.; Jin, M.K.; Yoo, W.C.; Lee, J.K. Nano-structure of a polymeric substrate with well-defined nanometer-scale topography and tailored surface wettability. Langmuir 2004, 20, 7665–7669. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.J.; Chen, S.C.; Lin, J.F. Adhesion Forces and Contact Angles of Water Strider Legs. Langmuir 2008, 25, 1526–1528. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Bormashenko, E. Physics of solid–liquid interfaces: From the Young equation to the superhydrophobicity. Low Temp. Phys. 2016, 42, 622–635. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef]
- Jisr, R.M.; Rmaile, H.H.; Schlenoff, J.B. Hydrophobic and ultrahydrophobic multilayer thin films from perfluorinated polyelectrolytes. Angew. Chem. Int. Ed. 2004, 44, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Extrand, C.W. Contact Angles and Their Hysteresis as a Measure of Liquid−Solid Adhesion. Langmuir 2004, 20, 4017–4021. [Google Scholar] [CrossRef] [PubMed]
- Du, Q. Rough ideas on wetting. Phys. A Stat. Mech. Its Appl. 2002, 313, 32–46. [Google Scholar]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165–170. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, Y.; Zhai, J.; Jiang, L. Bioinspired Super-antiwetting Interfaces with Special Liquid−Solid Adhesion. Acc. Chem. Res. 2010, 43, 368–377. [Google Scholar] [CrossRef]
- Chaudhary, A.; Barshilia, H.C. Nanometric Multiscale Rough CuO/Cu(OH)2 Superhydrophobic Surfaces Prepared by a Facile One-Step Solution-Immersion Process: Transition to Superhydrophilicity with Oxygen Plasma Treatment. J. Phys. Chem. C 2011, 115, 18213–18220. [Google Scholar] [CrossRef]
- Gao, N.; Yan, Y. Modeling Superhydrophobic Contact Angles and Wetting Transition. J. Bionic Eng. 2009, 6, 335–340. [Google Scholar] [CrossRef]
- Jopp, J.; Grüll, H.; Yerushalmi-Rozen, R. Wetting Behavior of Water Droplets on Hydrophobic Microtextures of Comparable Size. Langmuir 2004, 20, 10015–10019. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Bhushan, B. Wetting transition of water droplets on superhydrophobic patterned surfaces. Scr. Mater. 2007, 57, 1057–1060. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Biomimetic Superhydrophobic Surfaces: Multiscale Approach. Nano Lett. 2007, 7, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y. Preparation and Tissue Property Analysis of Hydrophobic Fluorinated Polyurethane Coatings; North China University of Water Resources and Hydropower: Zhengzhou, China, 2018; Volume 3, pp. 4–7. [Google Scholar]
- Wang, L.-F. Studies on fluorinated polyurethanes by X-ray diffraction and density functional theory calculations with periodic boundary conditions. Polymer 2007, 48, 7414–7418. [Google Scholar] [CrossRef]
- Turri, S.; Levi, M.; Trombetta, T. Waterborne anionomeric polyurethane-ureas from functionalized fluoropolyethers. J. Appl. Polym. Sci. 2004, 93, 136–144. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, F.; Xu, B.; Xu, J.; Jiang, Y.; Yang, D.; Li, P. Preparation mechanical properties and surface morphologies of waterborne fluorinated polyurethane-acylate. Prog. Org. Coat. 2013, 76, 876–883. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Fang, G. A new model for thermodynamic analysis on wetting behavior of superhydrophobic surfaces. Appl. Surf. Sci. 2012, 258, 2707–2716. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Zhang, X.; Miao, F.; Li, T.; Liu, H. Determination of the second step microstructure for superhydrophobic surfaces. Surf. Interface Anal. 2013, 5, 919–929. [Google Scholar] [CrossRef]
- Akram, R.M.; Kooij, E.S.; van Silfhout, A.; Poelsema, B. Superhydrophobic surfaces by anomalous fluoroalkylsilane self-assembly on silica nanosphere arrays. Langmuir 2010, 26, 12962–12972. [Google Scholar] [CrossRef]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Bormashenko, Y.; Erlich, M. Vibration-induced Cassie-Wenzel wetting transition on rough surfaces. Appl. Phys. Lett. 2007, 90, 201917. [Google Scholar] [CrossRef]
- Krupenkin, T.N.; Taylor, J.A.; Schneider, T.M.; Yang, S. From Rolling Ball to Complete Wetting: The Dynamic Tuning of Liquids on Nanostructured Surfaces. Langmuir 2004, 20, 3824–3827. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Starov, V. Impact of surface forces on wetting of hierarchical surfaces and contact angle hysteresis. Colloid Polym. Sci. 2013, 291, 343–346. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Cansoy, C.E. Range of Applicability of the Wenzel and Cassie−Baxter Equations for Superhydrophobic Surfaces. Langmuir 2009, 25, 14135–14145. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Godeau, G.; Darmanin, T.; Guittard, F. Ante versus post-functionalization to control surface structures with superhydrophobic and superoleophobic properties. RSC Adv. 2015, 5, 63945–63951. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Marmur, A. From Hygrophilic to Superhygrophobic: Theoretical Conditions for Making High-Contact-Angle Surfaces from Low-Contact-Angle Materials. Langmuir 2008, 24, 7573–7579. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-Eye Inspired Superhydrophobic Anti-Fogging Inorganic Nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Min, Q. Numerical investigations of the spreading and retraction dynamics of viscous droplets impact on solid surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125649. [Google Scholar] [CrossRef]
- Yang, C.; Hao, P.; He, F. Properties of droplet motion on microstructured superhydrophobic surfaces. Sci. Bull. 2009, 54, 436–440. [Google Scholar]
- Cai, D.; Wang, Q.; Zhao, T.; Liu, H.; Jiang, L. Progress in the construction of anisotropic micro- and nano-graded structured surfaces with special infiltration properties. Chem. Bull. 2014, 8, 4–6. [Google Scholar]
- Wang, J.; Wang, K.; Zheng, Y.; Jiang, L. Relationship between nanostructures and wettability on the surface of lotus leaves. J. Chem. High. Educ. 2010, 8, 3–8. [Google Scholar]
- Gao, X.; Lei, J. New advances in the study of natural superhydrophobic biological surfaces. Physics 2006, 7, 2–8. [Google Scholar]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, G.; Dang, H. Research progress of superhydrophobic transparent coatings. J. Henan Univ. Nat. Sci. Ed. 2004, 4, 6–9. [Google Scholar]
- Gauthier, A.; Symon, S.; Clanet, C.; Quere, D. Water impacting on superhydrophobic macro textures. Nat. Commun. 2015, 6, 8001. [Google Scholar] [CrossRef] [Green Version]
- Field, D.E.; Griffith, J.R. Fluorinated naval coating. Ind. Eng. Chem. Prod. Res. 1975, 14, 52. [Google Scholar]
- Di Gianni, A.; Bongiovanni, R.; Priola, A.; Turri, S. UV-cured fluorinated coatings for plastics: Effect of the photoinitiator and of the substrate filler on adhesion. Int. J. Adhes. Adhes. 2004, 24, 513–518. [Google Scholar] [CrossRef]
- Dong, J. Preparation of UV-Curable Fluorosilicon Low Surface Energy Coatings and Its Surface Behavior; Nanchang University of Aeronautics: Nanchang, China, 2017; pp. 20–35. [Google Scholar]
- Li, G.T.; Peng, Y.; Fu, L.; Wu, Y. Preparation and properties of waterborne UV-curable fluorinated polyurethane resins. Chem. New Mater. 2015, 7, 5–9. [Google Scholar]
- Zhang, Y. Development of UV-Curable Fluorine-Containing Coatings; Jinan University: Guangzhou, China, 2016; pp. 30–42. [Google Scholar]
- Christian, D. The use of UV irradiation in polymerization. Polym. Int. 1999, 3, 11–16. [Google Scholar]
- Kaewpirom, S.; Kunwong, D. Curing behavior and cured film performance of easy-to-clean UV-curable coatings based on hybrid urethane acrylate oligomers. J. Polym. Res. 2012, 10, 5–7. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- GB/T 6739-2006; Determination of Paint Film Hardness by Pencil Method for Color Paint and Varnish. Standardization Administration of China: Beijing, China, 2006.
- GB/T 1720-1989; Determination of Paint Adhesion by Pencil Method. Standardization Administration of China: Beijing, China, 1989.
- GB/T 1733-1993; Determination of Water Resistance of Paint Film. Standardization Administration of China: Beijing, China, 1993.
- GB/T 9754-2007; Glossiness Test Standard for Color Paint and Varnish. Standardization Administration of China: Beijing, China, 2007.
- Yu, F.Y.; Gao, J.; Liu, C.P.; Chen, Y.K.; Zhong, G.; Hodges, C.; Chen, M.F.; Zhang, H.G. Preparation and UV aging of nano–SiO2/fluorinated polyacrylate polyurethane hydrophobic composite coating. Prog. Org. Coat. 2020, 141, 105556. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, W.J.; Wang, X.J.; Mu, Y.P. Effect of average functionality on properties of UV-curable waterborne polyurethane-acrylate. Prog. Org. Coat. 2010, 3, 201–207. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zhang, X.Y.; Dai, J.B.; Li, W.H. A new UV curable waterborne polyurethane: Effect of C=C content on the film properties. Prog. Org. Coat. 2006, 55, 291–295. [Google Scholar] [CrossRef]
- Corcione, C.E.; Striani, R.; Frigione, M. Organic–inorganic UV-cured methacrylic-based hybrids as protective coatings for different substrates. Prog. Org. Coat. 2014, 77, 1117–1125. [Google Scholar] [CrossRef]
- Wei, D.; Ye, D.; Huang, H.; Huanqin, C. Multi-cross-linked UV-curable waterborne polyurethane coatings. Coat. Ind. 2008, 5, 1–5. [Google Scholar]
- Hiroshi, K.; Takakazu, Y. Development of high stain resistant coating: Hydrophilic clear coat which is applicable to wet on wet base coat. JSAE Rev. 2002, 23, 271–277. [Google Scholar]
- Montefusco, F.; Bongiovanni, R.; Sangermano, M.; Priola, A. Fluorinated networks through photo polymerization processes: Synthesis, characterization and properties. Polymer 2004, 45, 4663. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, R.; Ma, G.; Hou, C.; Zhang, H. Preparation and properties of fluorinated oligomer with tertiary amine structure in the UV curable coatings. J. Appl. Polym. Sci. 2017, 134, 28–35. [Google Scholar] [CrossRef]
- Masson, C.; Decker, T.; Jaworek, R. UV-radiation curing of waterbased urethane–acrylate coatings. Prog. Org. Coat. 2000, 39, 20–25. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, Y.-H.; Park, H.; Kim, H.-D. Preparation and properties of UV-curable fluorinated polyurethane acrylates. J. Appl. Polym. Sci. 2014, 131, 67–83. [Google Scholar] [CrossRef]
- Wang, F.; Hu, J.; Tu, W. Study on microstructure of UV-curable polyurethane acrylate films. Prog. Org. Coat. 2008, 62, 245–250. [Google Scholar] [CrossRef]
- Pablo, J.; Peruzzo, P.S.; Anbinder, O.R.; Pardini, J.; Vega, C.; Costa, F.; Galembeck, J. Waterborne polyurethane/acrylate: Comparison of hybrid and blend systems. Prog. Org. Coat. 2011, 72, 429–437. [Google Scholar]
- Liu, C.; Nie, J.; He, Y. High compatible free radical UV-curable fluorine-containing polyacrylic acrylate prepolymer. J. Fluor. Chem. 2015, 173, 47–54. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kim, H.J. Enhanced thermal and surface proper ties of waterborne UV-curable polycarbonate-based polyu rethane (meth)acrylate dispersion by incorporation of polydimethyl siloxane. React. Funct. Polym. 2011, 71, 655–665. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, E.Y.; Kang, Y.S. High solid and high-performance UV cured waterborne polyurethanes. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 58–63. [Google Scholar] [CrossRef]
- Li, G.T.; Zhang, L.; Peng, Y.C. Preparation and properties of aqueous UV-cured fluorinated polyurethane resins. New Chem. Mater. 2015, 7, 57–59. [Google Scholar]
- Bongiovanni, R.; Zeno, E.; Pollicino, A.; Serafini, P.M.; Tonelli, C. UV light-induced grafting of fluorinated monomer onto cellulose sheets. Cellulose 2011, 18, 117–126. [Google Scholar] [CrossRef]
- Ferrero, F.; Periolatto, M.; Udrescu, C. Water and oil-repellent coatings of perfluoro-polyacrylate resins on cotton fibers: UV curing in comparison with thermal polymerization. Fibers Polym. 2012, 13, 191–198. [Google Scholar] [CrossRef]
- Deng, X.-R.; Yang, W.; Zhang, Q.-H.; Hui, H.-H.; Wei, Y.-W.; Wang, J.; Xu, Q.; Lei, X.-Y.; Chen, J.-J.; Zhu, J.-L. Fabrication of UV-curable silicone coating with high transmittance and laser-induced damage threshold for high-power laser system. J. Sol-Gel Sci. Technol. 2018, 88, 249–254. [Google Scholar] [CrossRef]
- Bruen, K.; Davidson, K.; Sydes, D.E.; Siemens, P.M. Benefits of UV-curable coatings. Eur. Coat. J. 2004, 4, 42. [Google Scholar]
- Liu, J.L.; Jiao, X.J.; Cheng, F.; Fan, Y.X.; Wu, Y.F.; Yang, X.F. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 10567. [Google Scholar] [CrossRef]







| Raw Material | Model | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| Fluorocarbon photocurable monomer | HEA-HDI-TEOH-6 | 25 | 20 | 15 | 10 | 5 |
| Trifunctional monomer | TMPTA | 5 | 10 | 15 | 20 | 25 |
| Decafunctional oligomers | UT53956 | 36.6 | 36.6 | 36.6 | 36.6 | 36.6 |
| Leveling agent | Tego 2100 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Initiator | Irgacure 184 | 2 | 2 | 2 | 2 | 2 |
| Deep initiator | Irgacure TPO | 1 | 1 | 1 | 1 | 1 |
| Hydrocarbon diluent | BA | 30 | 30 | 30 | 30 | 30 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Raw Material | Model | F3 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|
| Fluorocarbon photocurable monomer | HEA-HDI-TEOH-6 | 15 | 15 | 15 | 15 | 15 |
| Trifunctional monomer | TMPTA | 15 | 15 | 15 | 15 | 15 |
| Decafunctional oligomers | UT53956 | 36.6 | 36.6 | 36.6 | 36.6 | 36.6 |
| Leveling agent | Tego 2100 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Initiator | Irgacure 184 | 2 | 2 | 2 | 2 | 2 |
| Deep initiator | Irgacure TPO | 1 | 1 | 1 | 1 | 1 |
| hydrofluoric ether | HFE 72DA | 0 | 5 | 10 | 15 | 20 |
| Hydrocarbon diluent | BA | 30 | 25 | 20 | 15 | 10 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Test Method | F1 | F2 | F3 | F4 | F5 | |
|---|---|---|---|---|---|---|
| Hardness | GB/T 6739-2006 | HB | 1H | 2H | 2H | 3H |
| Adhesion | GB/T 1720/1989 | 0 class | 0 class | 0 class | 0 class | 0 class |
| Boiling water resistance | GB/T 23444-2009 | Slight whitening | No change | No change | No change | No change |
| 60° gloss | GB/T 9754-2007 | 86.7 | 88.1 | 92.5 | 93.2 | 93.8 |
| Sliding Angles | F3 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|
| Water | 33.5° | 33.1° | 32.8° | 32.2° | 31.6° |
| Cetane | 38.7° | 38.2° | 37.6° | 37.1° | 36.3° |
| Item | F3 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|
| Roughness ranking | 5 | 4 | 3 | 2 | 1 |
| 60° gloss | 92.5 | 92.1 | 90.9 | 90.7 | 90.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Yuan, W. UV-Curable Fluorocarbon Polyurethane Coatings for Marble Kitchen Countertops. Coatings 2023, 13, 1394. https://doi.org/10.3390/coatings13081394
Xi X, Yuan W. UV-Curable Fluorocarbon Polyurethane Coatings for Marble Kitchen Countertops. Coatings. 2023; 13(8):1394. https://doi.org/10.3390/coatings13081394
Chicago/Turabian StyleXi, Xiang, and Weizhong Yuan. 2023. "UV-Curable Fluorocarbon Polyurethane Coatings for Marble Kitchen Countertops" Coatings 13, no. 8: 1394. https://doi.org/10.3390/coatings13081394





