Construction of Amphiphilic Indocyanine–Green–Based Langmuir Film and Drop–Casting Film with Photoelectric Conversion Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
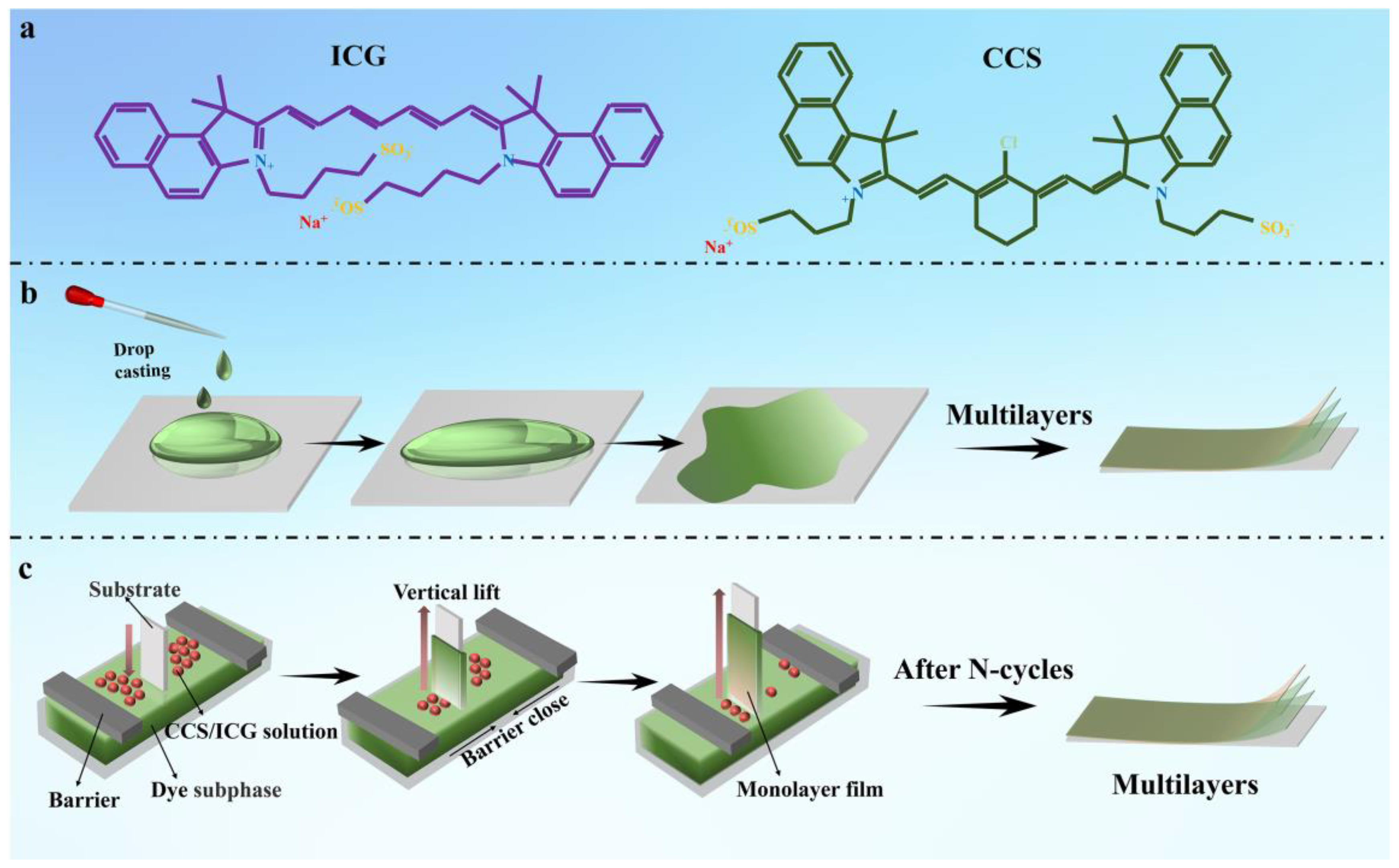
2.3. Preparation of ICS (CCS)/Dyes Langmuir Films
2.4. Preparation of ICS (CCS)/Dyes Drop–Casting Films
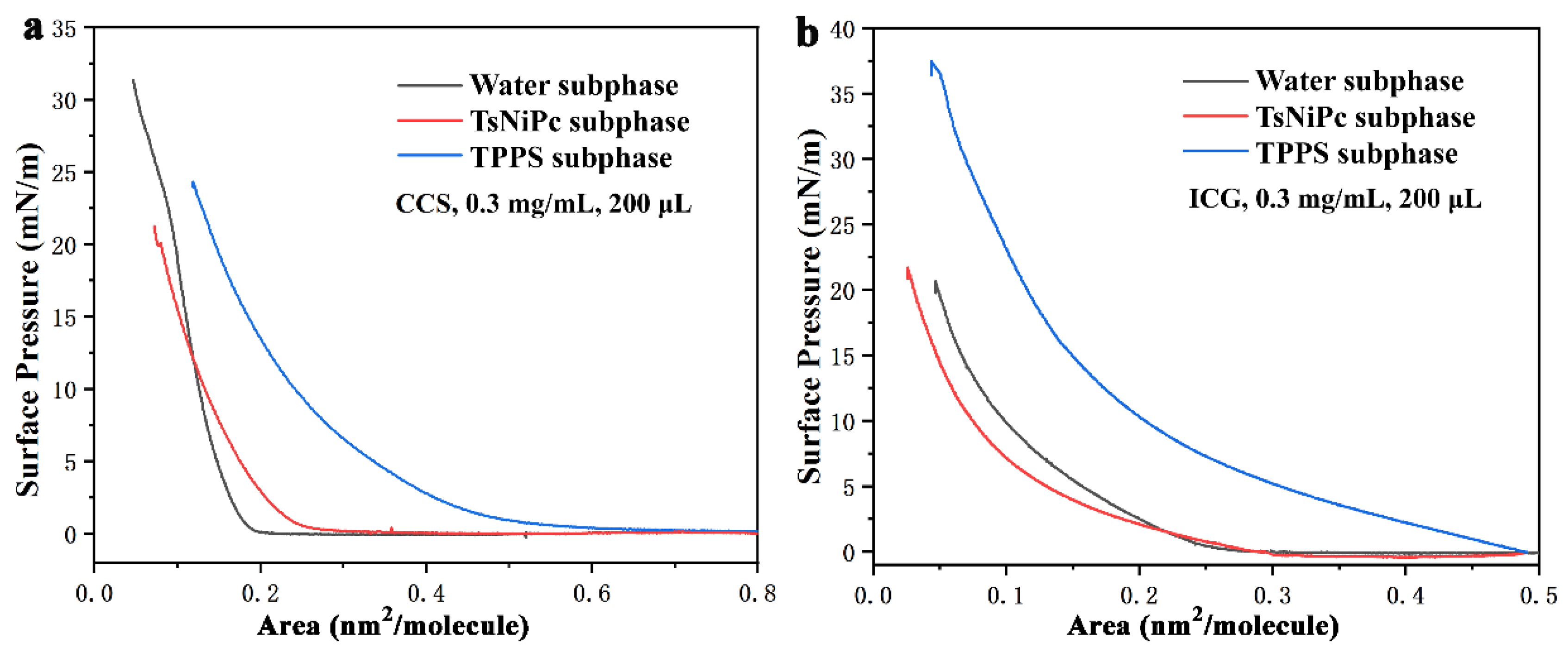
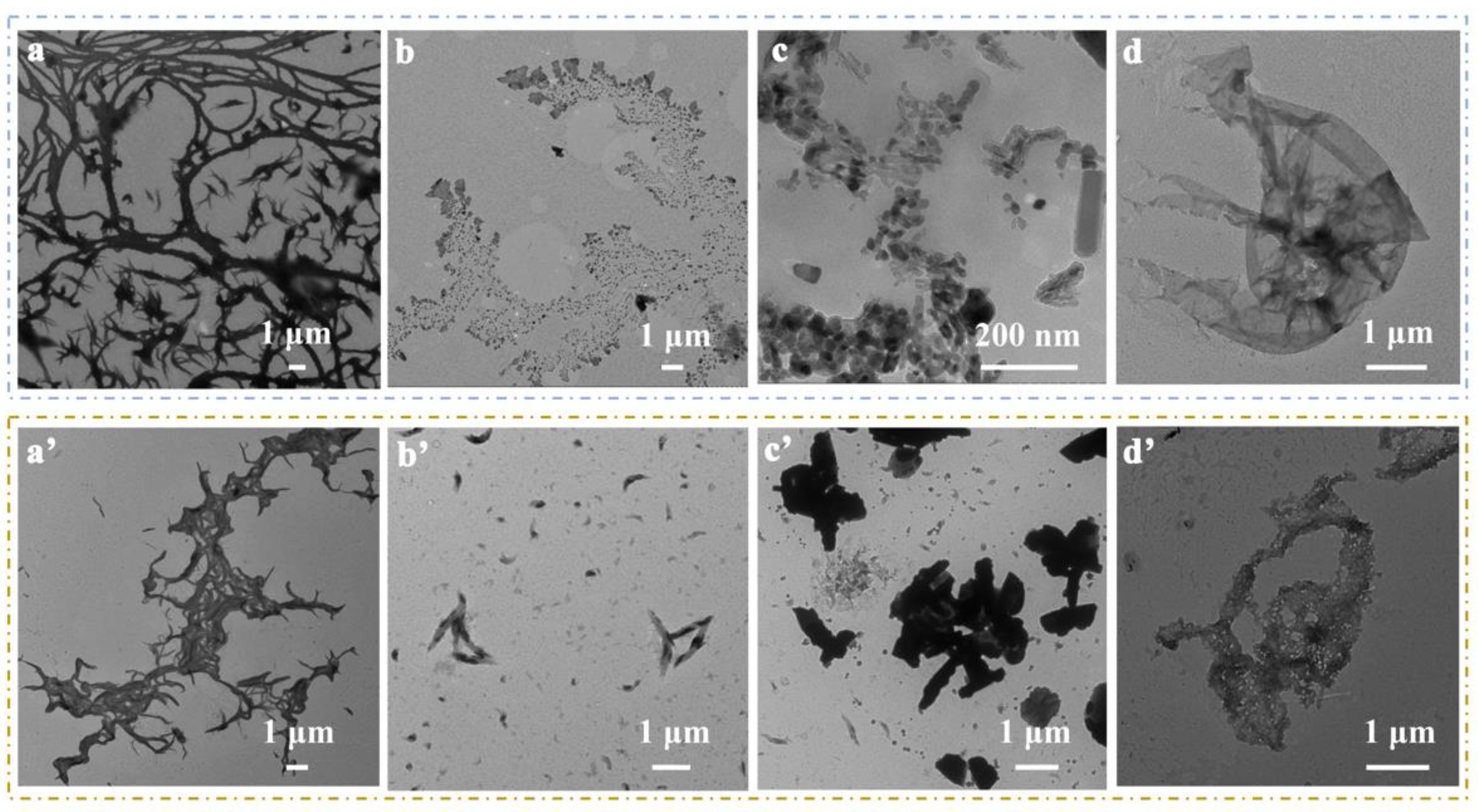
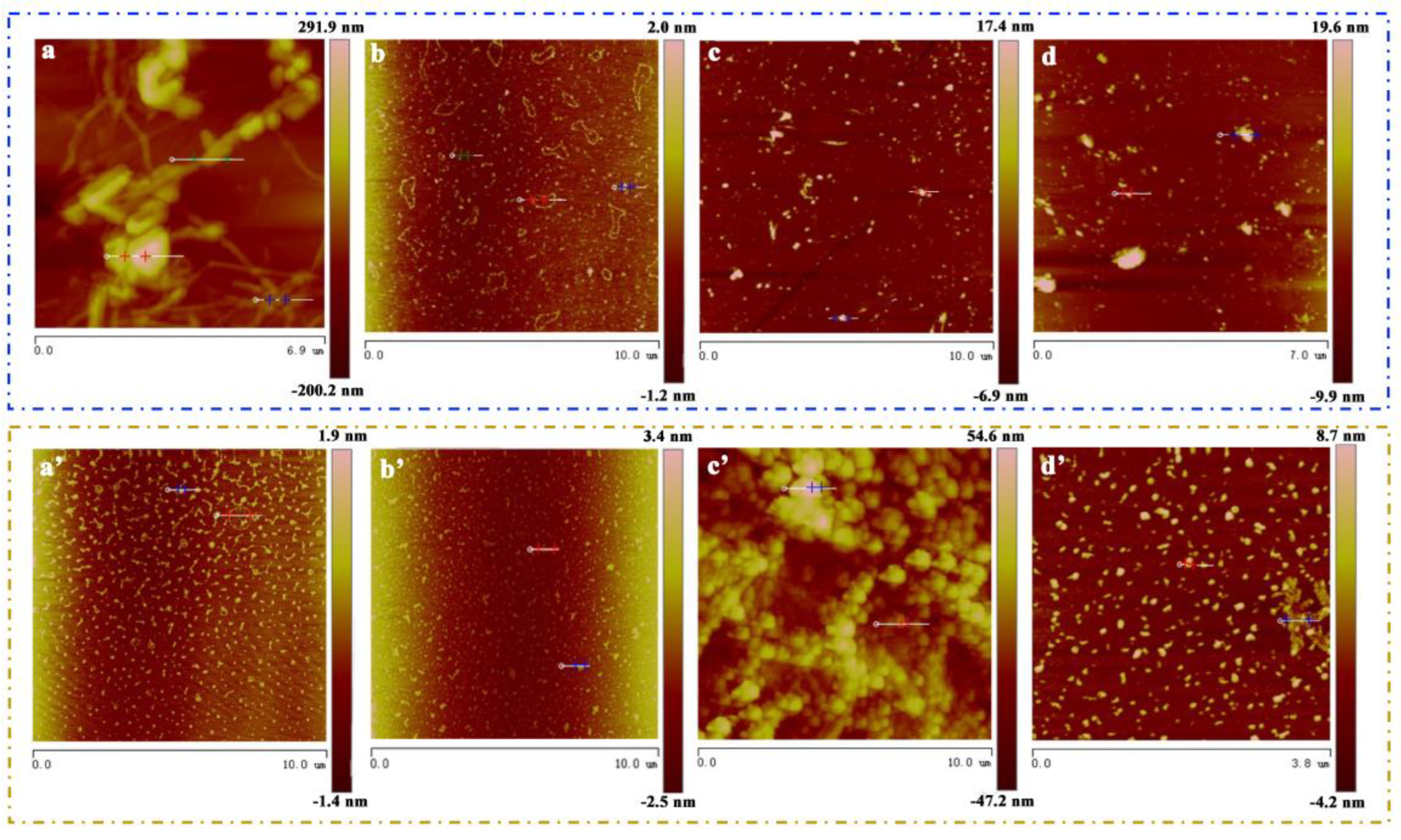
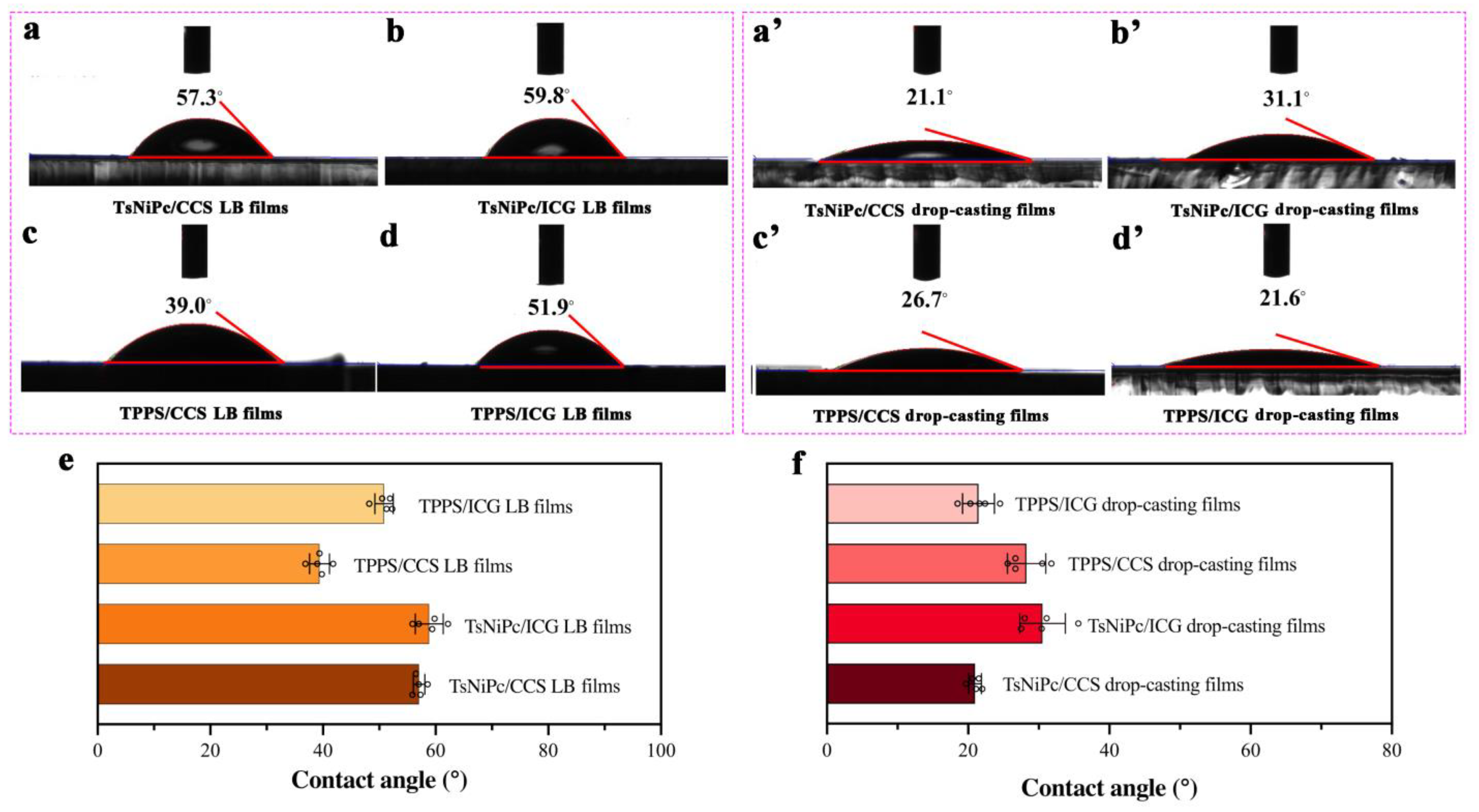
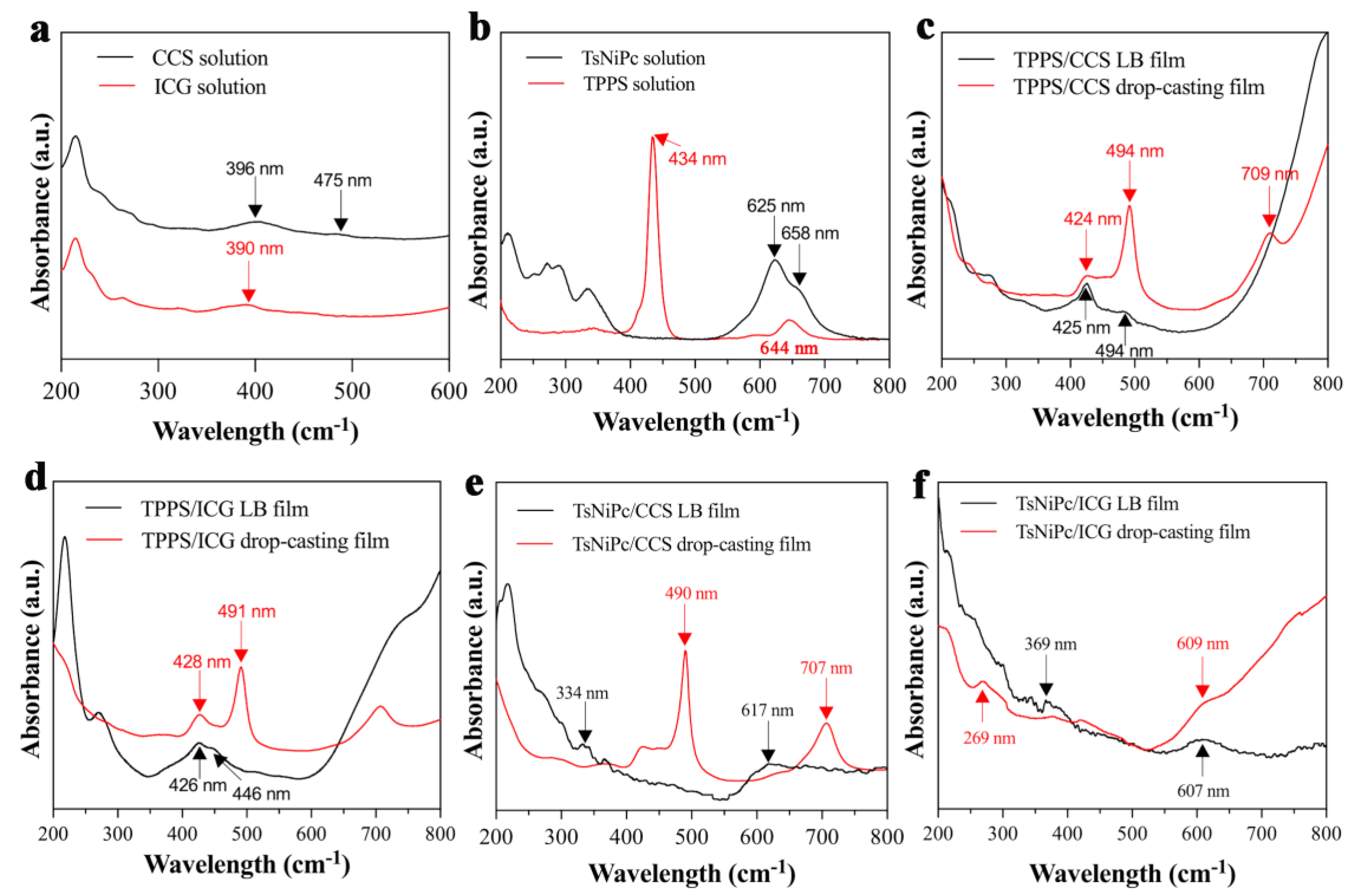
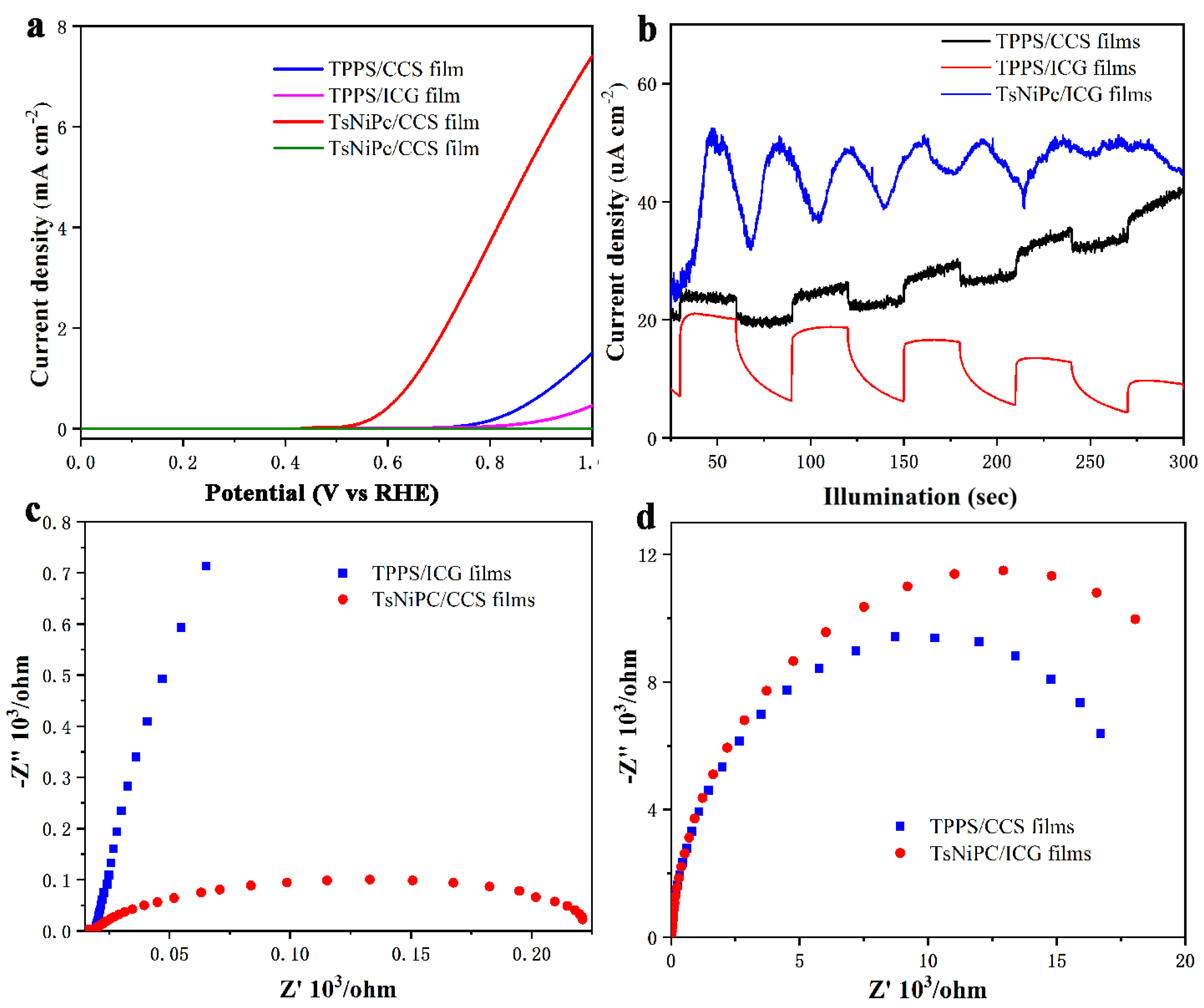
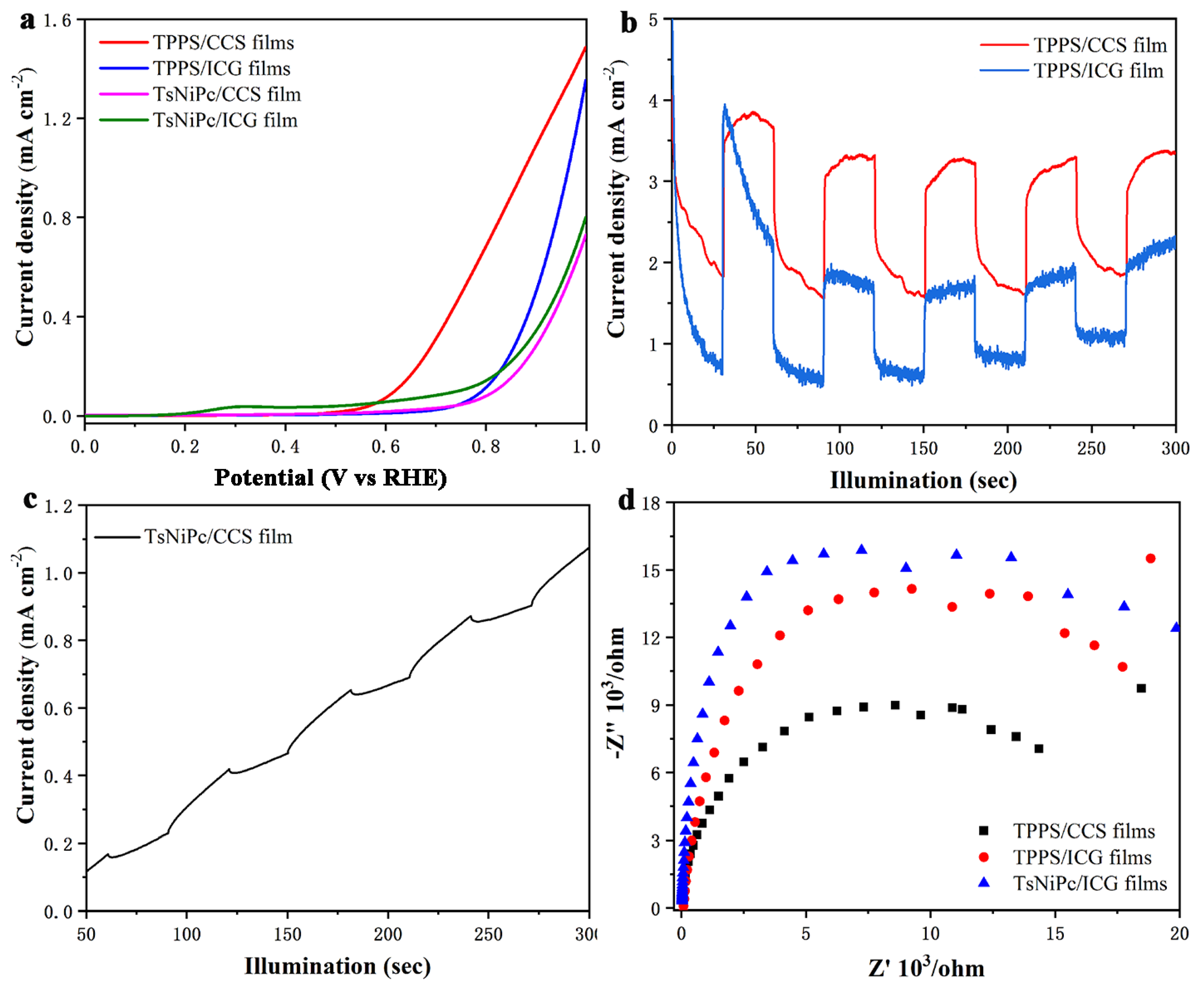
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, D.; Ji, L.; Ouyang, G.; Liu, M. Histidine proton Shuttle–Initiated switchable inversion of circularly polarized luminescence. ACS Appl. Mater. Interfaces 2020, 12, 18148–18156. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, L.; Dang, J.; Mi, L.; Han, J.; Mao, M.; Chen, B.; Liu, H. Reconfigurable and tunable photo–controlled hydrogrl using hydrogen bonding to drive molecule self–assembly and cross–linking. J. Mater. Sci. 2020, 55, 14740–14750. [Google Scholar] [CrossRef]
- Li, P.; Xu, G.; Wang, N.; Guan, B.; Zhu, S.; Chen, P.; Liu, M. 0D, 1D, and 2D Supramolecular Nanoassemblies of a Porphyrin: Controllable Assembly, and Dimensionality–Dependent Catalytic Performances. Adv. Funct. Mater. 2021, 31, 2100367. [Google Scholar] [CrossRef]
- Chang, R.; Zou, Q.; Zhao, L.; Liu, Y.; Xing, R.; Yan, X. Amino Acid–Encoded Supramolecular Photothermal Nanomedicine for Enhanced Cancer Therapy. Adv. Mater. 2022, 34, 2200139. [Google Scholar] [CrossRef]
- Xing, R.; Zou, Q.; Yuan, C.; Zhao, L.; Chang, R.; Yan, X. Self–Assembling Endogenous Biliverdin as a Versatile Near–Infrared Photothermal Nanoagent for Cancer Theranostics. Adv. Mater. 2019, 31, 1900822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Yang, W.; Li, S.; Xu, J.; Jiang, Y. Ordered and ultrathin reduced graphene oxide LB films as hole injection layers for organic light–emitting diode. Nanoscale Res. Lett. 2014, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Giner–Casares, J.; Brezesinski, G.; Möhwald, H. Langmuir monolayers as unique physical models. Curr. Opin. Colloid Interface Sci. 2014, 19, 176–182. [Google Scholar] [CrossRef]
- Nie, H.; Dou, X.; Tang, Z.; Jang, H.; Huang, J. High–Yield Spreading of Water–Miscible Solvents on Water for Langmuir–Blodgett Assembly. J. Am. Chem. Soc. 2015, 137, 10683–10688. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Valli, L.; Giancane, G. Spectroscopic Investigation of the Selective Interaction of Mercuric and Cupric Ions with a Porphyrin Active Layer. J. Phys. Chem. C 2014, 118, 12384–12390. [Google Scholar] [CrossRef]
- Ermakova, E.V.; Koroleva, E.O.; Shokurov, A.V.; Arslanov, V.V.; Bessmertnykh–Lemeune, A. Ultra–thin film sensors based on porphyrin–5–ylphosphonate diesters for selective and sensitive dual–channel optical detection of mercury(II) ions. Dye. Pigment. 2021, 186, 108967. [Google Scholar] [CrossRef]
- Graewe, L.; Hupfer, M.L.; Schulz, M.; Mahammed, A.; Gross, Z.; Presselt, M. Supramolecular Control of Photonic Response and Sensing of Nitricoxide using Iron(III) Corrole Monolayers and Their Stacks. ChemPlusChem 2023, 88, e202200260. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tachibana, H.; Sato, F.; Terrettaz, S. Photoinduced Self–Organization in Langmuir–Blodgett Films. J. Phys. Chem. B 1997, 101, 702–704. [Google Scholar] [CrossRef]
- Tachibana, H.; Yamanaka, Y.; Sakai, H.; Abe, M.; Matsumoto, M. J–Aggregate formation and morphological change on UV irradiation of the Langmuir–Blodgett films of spiropyran. Mol. Cryst. Liq. Cryst. 2000, 345, 149–154. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Bian, P.; Li, G.; Yang, J.; Li, Q.; Jiao, T. UV–response behavior and chiral structure determination of Langmuir–Blodgett films consisting of polypeptide and dye molecules. Colloids Surf. A 2022, 636, 128221. [Google Scholar] [CrossRef]
- Wang, R.; Yan, X.; Ge, B.; Zhou, J.; Wang, M.; Zhang, L.; Jiao, T. Facile Preparation of Self–Assembled Black Phosphorus–Dye Composite Films for Chemical Gas Sensors and Surface–Enhanced Raman Scattering Performances. ACS Sustain. Chem. Eng. 2020, 8, 4521–4536. [Google Scholar] [CrossRef]
- Gregory, B.W.; Vaknin, D.; Cotton, T.M.; Struve, W.S. Interfacial complexation of phospholipid Langmuir monolayers with water–soluble porphyrins and phthalocyanines: An X–ray reflectivity study. Thin Solid Film 1996, 284, 849–853. [Google Scholar] [CrossRef]
- Costa, S.M.B.; Andrade, S.M.; Togashi, D.M.; Paulo, P.M.R.; Laia, C.A.T.; Viseu, M.I.; da Silva, A.M.G. Optical spectroscopy and photochemistry of porphyrins and phthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 509–517. [Google Scholar] [CrossRef]
- Huc, V.; Armand, F.; Bourgoin, J.P.; Palacin, S. Covalent anchoring of phthalocyanines on silicon dioxide surfaces: Building up mono– and multilayers. Langmuir 2001, 17, 1928–1935. [Google Scholar] [CrossRef]
- Kroon, J.M.; Sudholter, E.J.R.; Schenning, A.; Nolte, R.J.M. Self–Organization of Amphiphilic Porphyrins at the Air–Water–Interface. Langmuir 1995, 11, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Wang, C.; Li, L.; Hao, Z.; Gu, J.; Wang, M.; Jiao, T. Chemical gas sensor, surface enhanced Raman scattering and photoelectrics of composite Langmuir–Blodgett films consisting of polypeptide and dye molecules. Colloids Surf. A 2023, 663, 131067. [Google Scholar] [CrossRef]
- Acikbas, Y.; Erdogan, M.; Capan, R.; Ozkaya, C.; Baygu, Y.; Kabay, N.; Gök, Y. Preparation of Zinc (II) phthalocyanine-based LB thin film: Experimental characterization, the determination of some optical properties and the investigation of the optical sensing ability. Optik 2021, 245, 167661. [Google Scholar] [CrossRef]
- Dutta, P.; Rai, R.; Pandey, S. Effect of ionic liquid on J–aggregation of meso–Tetrakis (4–sulfonatophenyl) porphyrin within aqueous mixtures of poly(ethyleneglycol). J. Phys. Chem. B 2011, 115, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Nakahara, H. Adsorption of sulforhodamine dyes in cationic Langmuir– blodgett films: Spectroscopic and structural studies. J. Phys. Chem. B 2002, 106, 92–100. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.; Mordon, S. Fluorescence Properties and Metabolic Features of Indocyanine Green (ICG) as Related to Angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Yan, L.; Wang, A.; Bai, S.; Yan, X. Trace solvent as a predominant factor to tune dipeptide self–assembly. ACS Nano 2016, 10, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, Y.; Wu, K.; Chen, Z.; Liu, S.; Cao, X.; Cheong, W.; Meng, T.; Luo, J.; Zheng, L.; et al. Regulating the coordination structure of single–atom Fe–NxCy catalytic sites for benzene oxidation. Nat. Commun. 2019, 10, 4290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Hu, Y.; Guo, J.; Wang, C.; Chen, S. Fiber-Spinning-Chemistry Method toward In Situ Generation of Highly Stable Halide Perovskite Nanocrystals. Adv. Sci. 2019, 6, 1901694. [Google Scholar] [CrossRef] [Green Version]
- Kahnt, A.; Kärnbratt, J.; Esdaile, L.; Hutin, M.; Sawada, K.; Anderson, H.; Albinsson, B. Temperature Dependence of Charge Separation and Recombination in Porphyrin Oligomer–Fullerene Donor–Acceptor Systems. J. Am. Chem. Soc. 2011, 133, 9863–9871. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, T.; Li, X.; Qian, H.; Bian, X.; Li, X.; Bai, H.; Ding, S.; Yan, Y. Femtomolar and locus-specific detection of N6-methyladenine in DNA by integrating double-hindered replication and nucleic acid-functionalized MB@Zr-MOF. J. Nanobiotechnol. 2021, 19, 408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, N.; Li, L.; Wang, C.; Han, D.; Jiao, T. Construction of Amphiphilic Indocyanine–Green–Based Langmuir Film and Drop–Casting Film with Photoelectric Conversion Properties. Coatings 2023, 13, 1423. https://doi.org/10.3390/coatings13081423
Chen J, Li N, Li L, Wang C, Han D, Jiao T. Construction of Amphiphilic Indocyanine–Green–Based Langmuir Film and Drop–Casting Film with Photoelectric Conversion Properties. Coatings. 2023; 13(8):1423. https://doi.org/10.3390/coatings13081423
Chicago/Turabian StyleChen, Jing, Na Li, Lin Li, Chongling Wang, Dongxue Han, and Tifeng Jiao. 2023. "Construction of Amphiphilic Indocyanine–Green–Based Langmuir Film and Drop–Casting Film with Photoelectric Conversion Properties" Coatings 13, no. 8: 1423. https://doi.org/10.3390/coatings13081423
APA StyleChen, J., Li, N., Li, L., Wang, C., Han, D., & Jiao, T. (2023). Construction of Amphiphilic Indocyanine–Green–Based Langmuir Film and Drop–Casting Film with Photoelectric Conversion Properties. Coatings, 13(8), 1423. https://doi.org/10.3390/coatings13081423






