Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
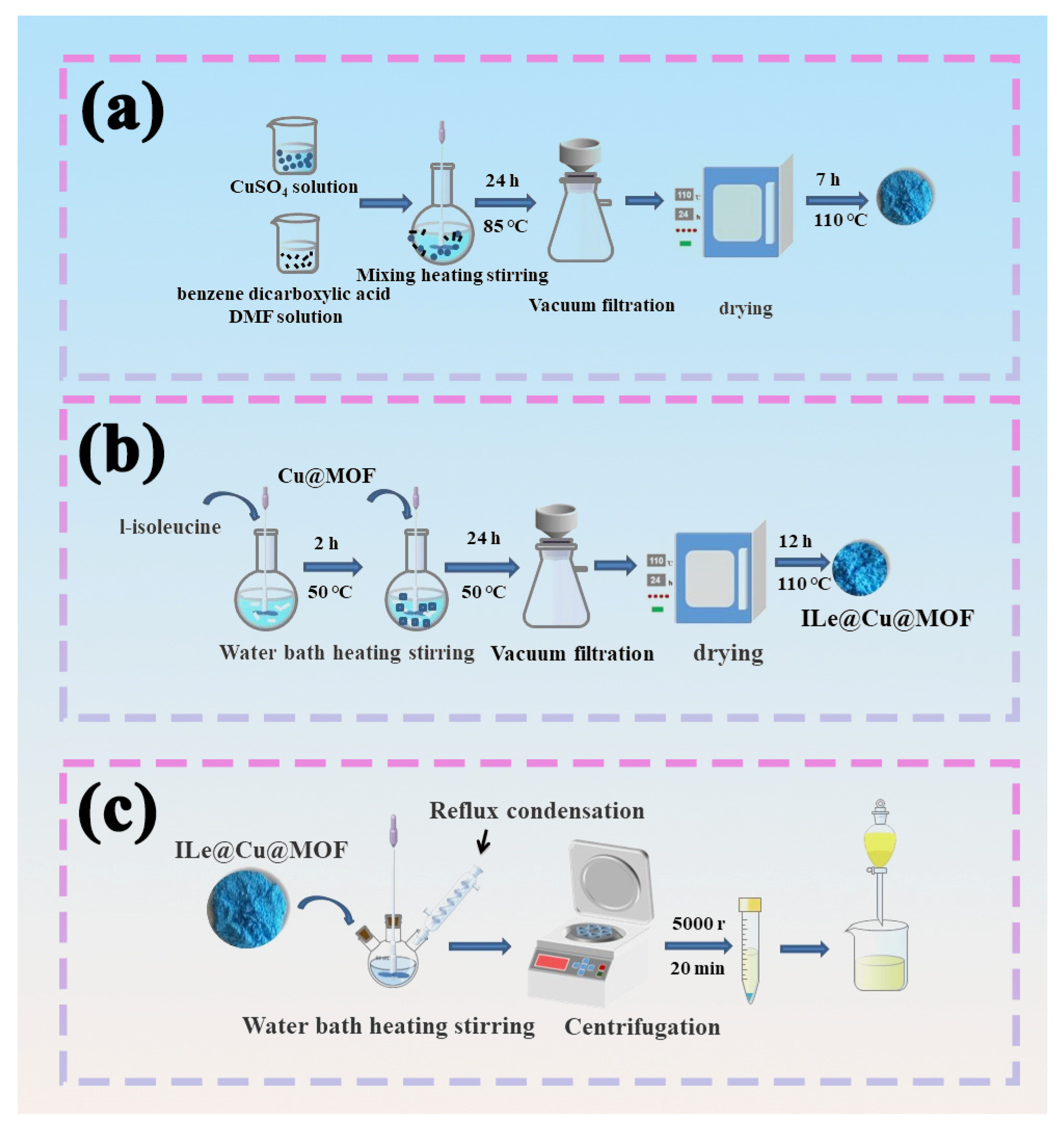
2.2. Synthesis of Catalysts
2.3. Preparation and Determination of Biodiesel
2.4. Catalyst Characterization
3. Results and Discussions
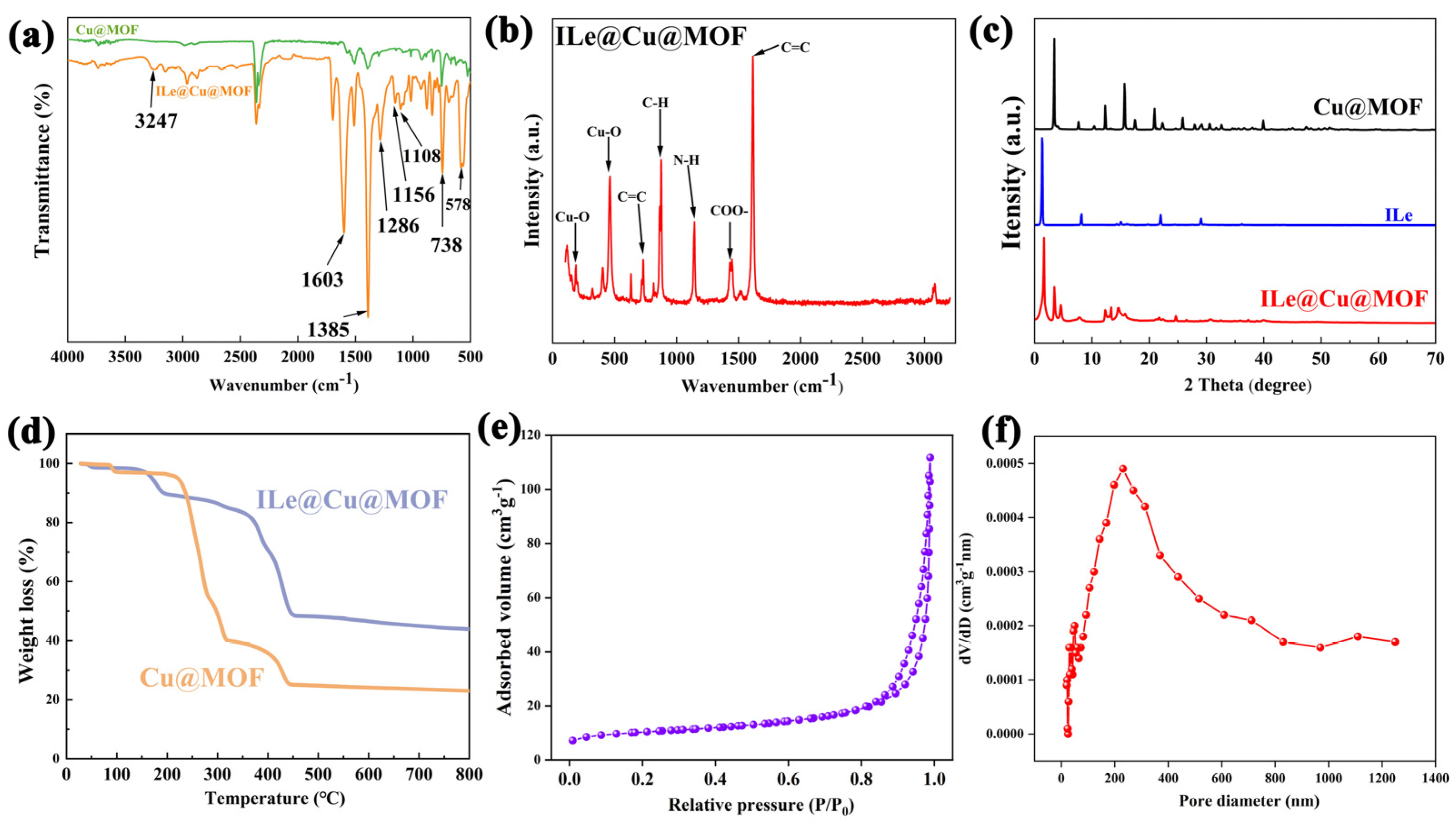
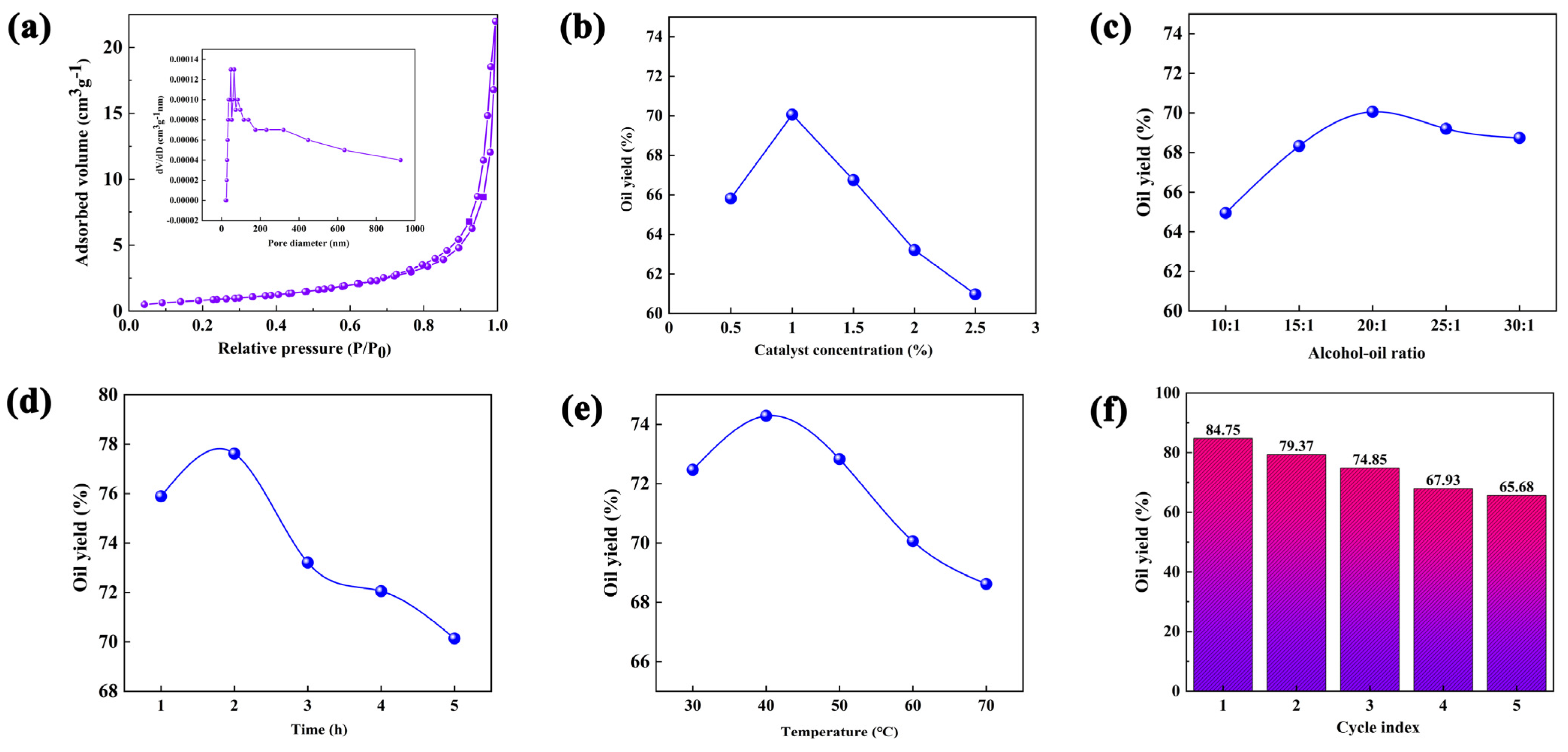
3.1. Characterization of Physicochemical Properties of Cu@MOF and ILe@Cu@MOF
3.2. Micromorphology of Cu@MOF and ILe@Cu@MOF
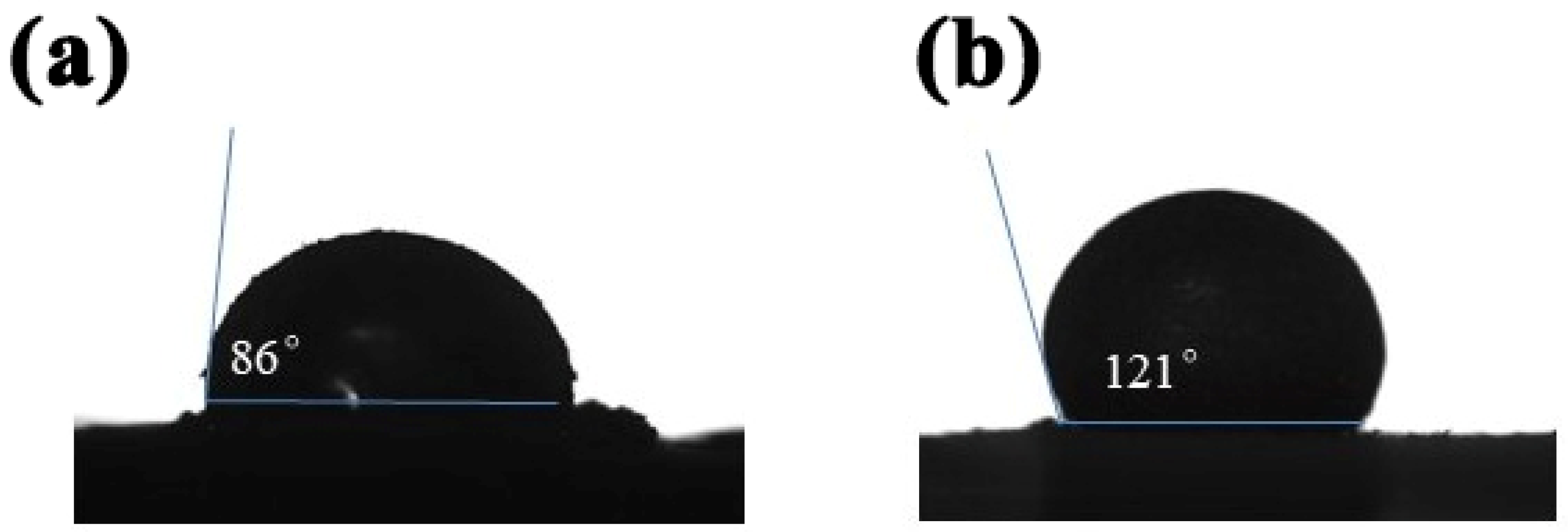
3.3. Analysis of Cu@MOF’s and ILe@Cu@MOF’s Hydrophobic Performance
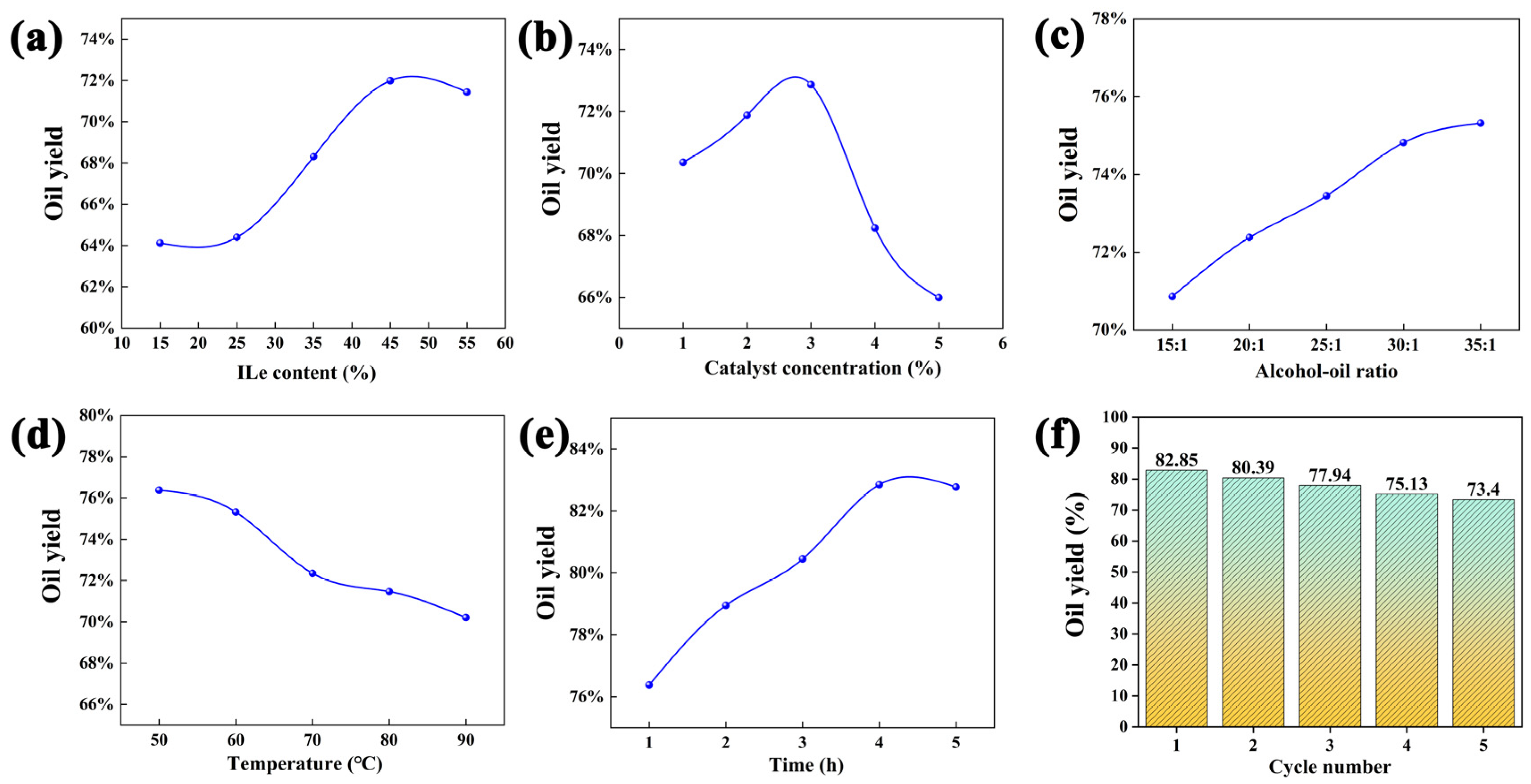
3.4. Effect of the Production Process Parameters on the Yield of Biodiesel
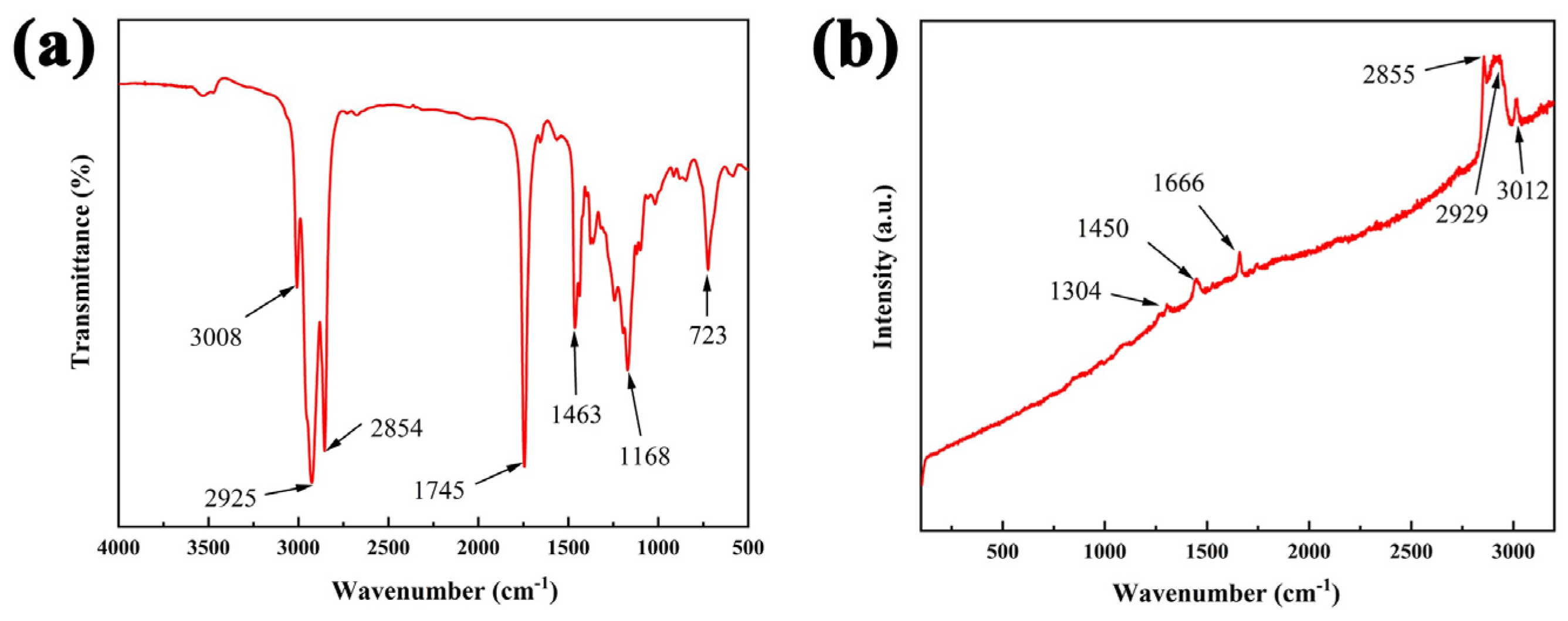
3.5. FTIR and Raman Characterization of Biodiesel
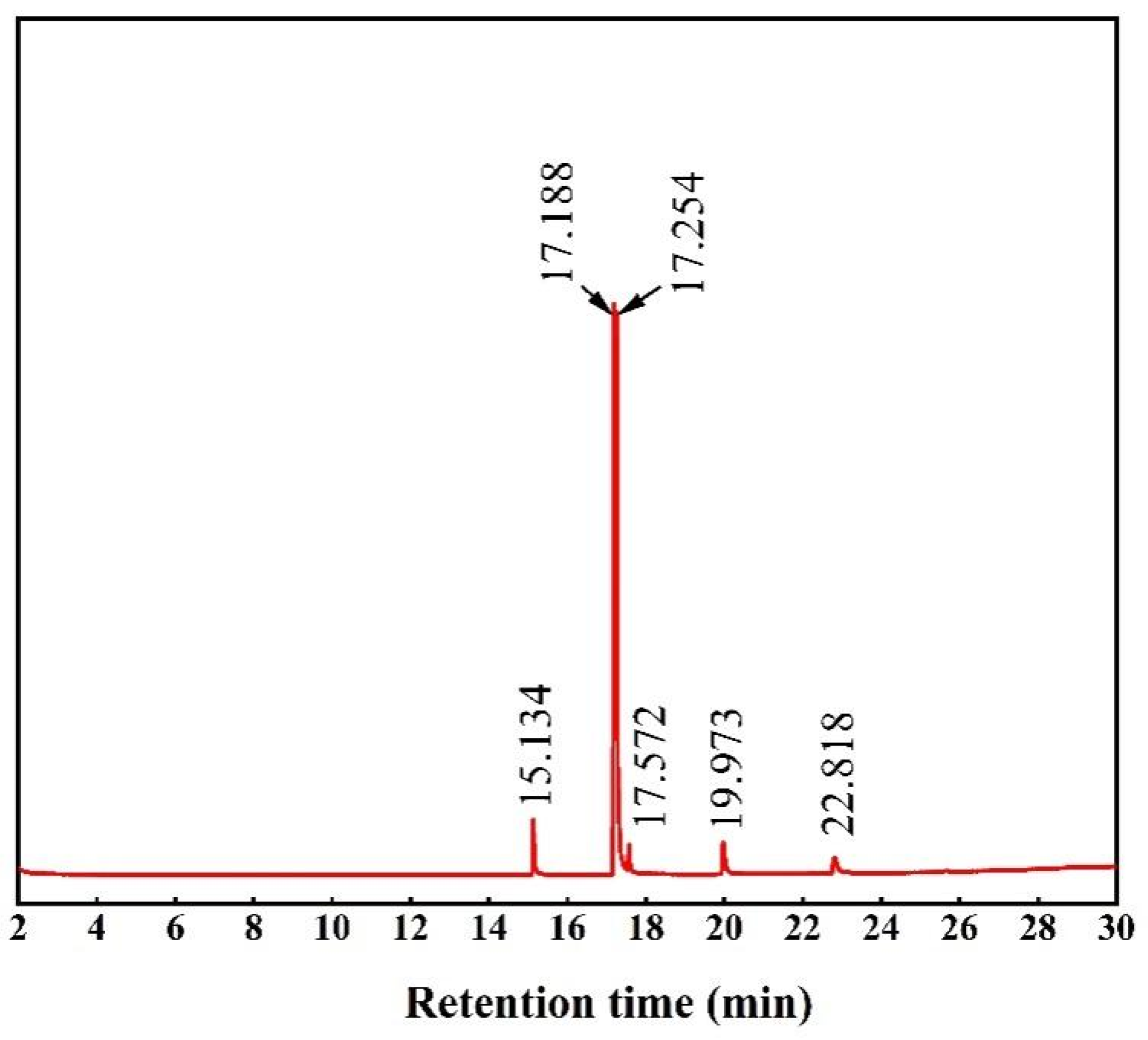
3.6. GC–MS Analysis of Biodiesel
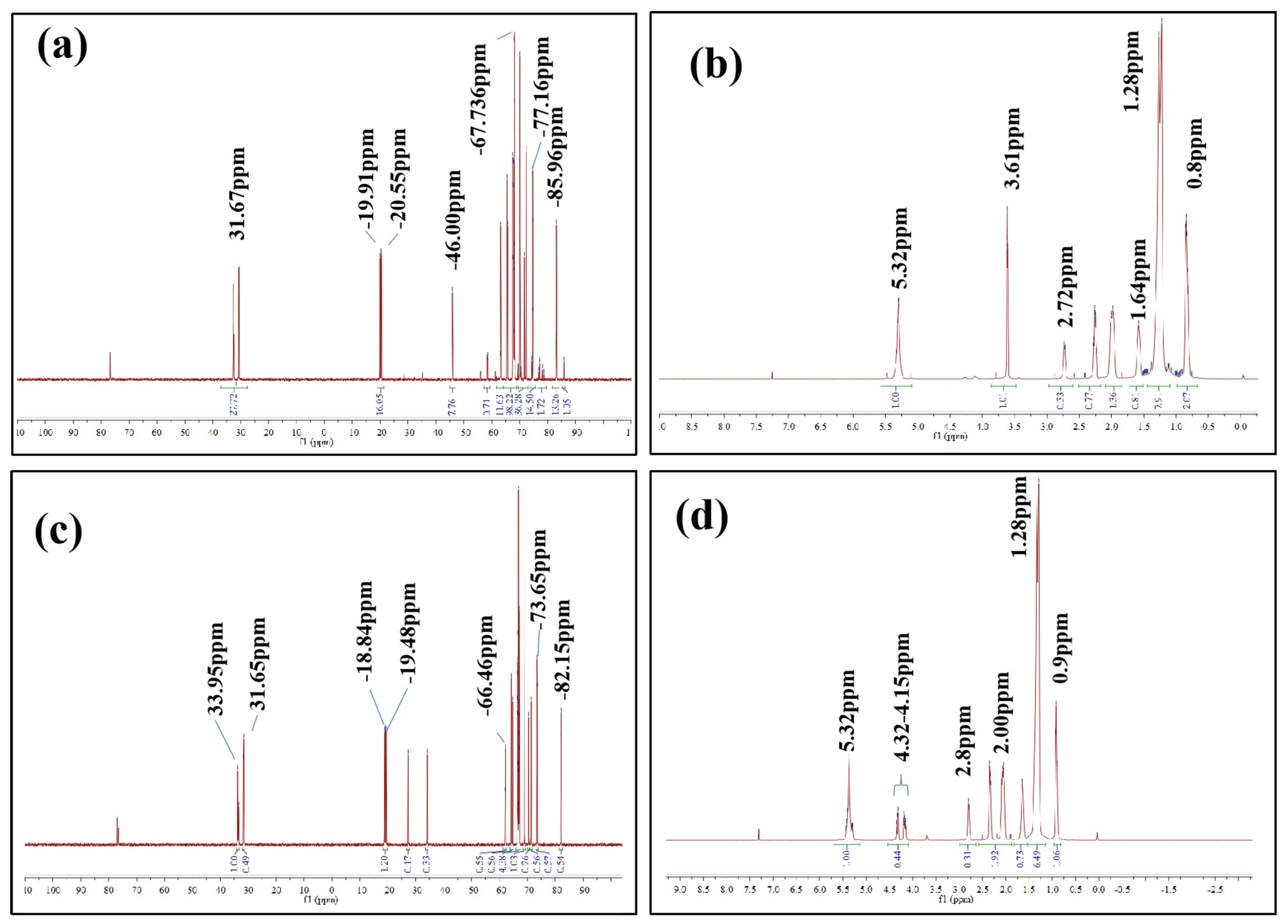
3.7. Comparison of NMR Analysis between Biodiesel and Xanthoceras Sorbifolia
3.8. Catalytic Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Sinha, S.K.; Subramanian, K.; Singh, H.M.; Tyagi, V.; Mishra, A. Progressive trends in biofuel policies in India: Targets and implementation strategy. Biofuels 2019, 10, 155–166. [Google Scholar] [CrossRef]
- Pereira, L.G.; Cavalett, O.; Bonomi, A.; Zhang, Y.; Warner, E.; Chum, H.L. Comparison of biofuel life-cycle GHG emissions assessment tools: The case studies of ethanol produced from sugarcane, corn, and wheat. Renew. Sustain. Energy Rev. 2019, 110, 1–12. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Hosseinpour, S. On the exergoeconomic and exergoenvironmental evaluation and optimization of biodiesel synthesis from waste cooking oil (WCO) using a low power, high frequency ultrasonic reactor. Energy Convers. Manag. 2018, 164, 385–398. [Google Scholar] [CrossRef]
- Sivakumar, P.; Anbarasu, K.; Renganathan, S. Bio-diesel production by alkali catalyzed transesterification of dairy waste scum. Fuel 2011, 90, 147–151. [Google Scholar] [CrossRef]
- Sohail, M.; Yun, Y.N.; Lee, E.; Kim, S.K.; Cho, K.; Kim, J.N. Synthesis of Highly Crystalline NH2-MIL-125 (Ti) with S-Shaped Water Isotherms for Adsorption Heat Transformation. Cryst. Growth Des. 2017, 17, 1208–1213. [Google Scholar] [CrossRef]
- Chen, L.; Yin, P.; Liu, X.; Yang, L.; Yu, Z.; Guo, X. Biodiesel production over copper vanadium phosphate. Energy 2011, 36, 175–180. [Google Scholar] [CrossRef]
- Danmaliki, G.I.; Saleh, T.A.; Shamsuddeen, A.A. Response surface methodology optimization of adsorptive desulfurization on nickel/activated carbon. Chem. Eng. J. 2017, 313, 993–1003. [Google Scholar] [CrossRef]
- Wang, S.; Ye, B.; An, C.; Wang, J.; Li, Q. Synergistic effects between Cu metal–organic framework (Cu-MOF) and carbon nanomaterials for the catalyzation of the thermal decomposition of ammonium perchlorate (AP). J. Mater. Sci. 2018, 54, 4928–4941. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Bayat, A.; Baghdadi, M.; Bidhendi, G.N. Tailored magnetic nano-alumina as an efficient catalyst for transesterification of waste cooking oil: Optimization of biodiesel production using response surface methodology. Energy Convers. Manag. 2018, 177, 395–405. [Google Scholar] [CrossRef]
- Khan, I.A.; Badshah, A.; Nadeem, M.A.; Haider, N.; Nadeem, M.A. A copper based metalorganic framework as single source for the synthesis of electrode materials for highperformance supercapacitors and glucose sensing applications. Int. J. Hydrogen Energy 2014, 39, 19609–19620. [Google Scholar] [CrossRef]
- Drenth, A.C.; Olsen, D.B.; Denef, K. Fuel property quantification of triglyceride blends with an emphasis on industrial oilseeds camelina, carinata, and pennycress. Fuel 2015, 153, 19–30. [Google Scholar] [CrossRef]
- Menon, S.S.; Chandran, S.V.; Koyappayil, A.; Berchmans, S. Copper-Based Metal-Organic Frameworks as Peroxidase Mimics Leading to Sensitive H2O2 and Glucose Detection. ChemistrySelect 2018, 3, 8319–8324. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, F.; Zeng, B. One-step synthesis of a copper-based metal–organic framework–graphene nanocomposite with enhanced electrocatalytic activity. RSC Adv. 2015, 5, 22060–22065. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Z.; Chen, Y.; Zhang, P.; Duan, S.; Liu, X. Preparation of Biodiesel Catalyzed by Solid Super Base of Calcium Oxide and Its Refining Process. Chin. J. Catal. 2006, 27, 391–396. [Google Scholar] [CrossRef]
- Devaraj, K.; Veerasamy, M.; Aathika, S.; Mani, Y.; Thanarasu, A.; Dhanasekaran, A. Study on effectiveness of activated calcium oxide in pilot plant biodiesel production. J. Clean. Prod. 2019, 225, 18–26. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Al-Jammal, N.; Al-Hamamre, Z.; Alnaief, M. Manufacturing of zeolite based catalyst from zeolite tuft for biodiesel production from waste sunflower oil. Renew. Energy 2016, 93, 449–459. [Google Scholar] [CrossRef]
- Rattanaphra, D.; Harvey, A.; Srinophakun, P. Simultaneous conversion of triglyceride/free fatty acid mixtures into biodiesel using sulfated zirconia. Top. Catal. 2010, 53, 773–782. [Google Scholar] [CrossRef]
- Jacobson, K.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production from waste cooking oil. Appl. Catal. B 2008, 85, 86–91. [Google Scholar] [CrossRef]
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Shi, Y.; Yuan, H.; Shi, J. Catalysts from renewable resources for biodiesel production. Energy Convers. Manag. 2018, 178, 277–289. [Google Scholar] [CrossRef]
- Perego, C.; Bosetti, A. Biomass to fuels: The role of zeolite and mesoporous materials. Microporous Mesoporous Mater. 2011, 144, 28–39. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Hu, H.; Xu, J.; Wang, Z.; Du, Y.; Cui, J.; Jia, S. Enhanced enzymatic performance of immobilized lipase on metal organic frameworks with superhydrophobic coating for biodiesel production. J. Colloid Interface Sci. 2021, 602, 426–436. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Gunawan, F.; Soetaredjo, F.E.; Santoso, S.P.; Chen, C.H.; Yuliana, M.; Kurniawan, A.; Lie, J.; Angkawijaya, A.E.; Ismadji, S. Efficient One-Step Conversion of a Low-Grade Vegetable Oil to Biodiesel over a Zinc Carboxylate Metal–Organic Framework. ACS Omega 2021, 6, 1834–1845. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Process optimisation and parametric effects on synthesis of lipase immobilised carbonaceous catalyst for conversion of rubber seed oil to biodiesel. Energy Convers. Manag. 2018, 176, 55–68. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. A novel biobased heterogeneous catalyst derived from Musa acuminata peduncle for biodiesel production–Process optimization using central composite design. Energy Convers. Manag. 2019, 189, 118–131. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Taufiq-Yap, Y.H.; Yaw, T.C.S.; Ismail, I. Synthesis of carbonaceous solid acid magnetic catalyst from empty fruit bunch for esterification of palm fatty acid distillate (PFAD). Energy Convers. Manag. 2019, 195, 480–491. [Google Scholar] [CrossRef]
- Kapadia, H.; Brahmbhatt, H.; Dabhi, Y.; Chourasia, S. Investigation of emulsion and effect on emission in CI engine by using diesel and bio-diesel fuel: A review. Egypt. J. Petrol. 2019, 28, 323–337. [Google Scholar] [CrossRef]
- Kim, Y.; Thomas, A.E.; Robichaud, D.J.; Iisa, K.; St John, P.C.; Etz, B.D.; Fioroni, G.M.; Dutta, A.; McCormick, R.L.; Mukarakate, C.; et al. A perspective on biomass-derived biofuels: From catalyst design principles to fuel properties. J. Hazard. Mater. 2020, 400, 123198. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, P.A.; Senra, M.; Soh, L. Cloud point and crystallization in fatty acid ethyl ester biodiesel mixtures with and without additives. Fuel 2018, 222, 243–249. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Yatish, K.V.; Lalithamba, H.S.; Suresh, R.; Latha, H.K.E. Ochrocarpus longifolius assisted green synthesis of CaTiO3 nanoparticle for biodiesel production and its kinetic study. Renew. Energy 2020, 147, 310–321. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Ngamcharussrivichai, C.; Azam, M. Synthesis of bifunctional nanocatalyst from waste palm kernel shell and its application for biodiesel production. RSC Adv. 2020, 10, 27183–27193. [Google Scholar] [CrossRef]
- Akinfalabi, S.-I.I.; Rashid, U.; Yunus, R.; Hin Taufiq-Yap, Y.; Taufiq-Yap, Y.H. Synthesis of biodiesel from palm fatty acid distillate using sulfonated palm seed cake catalyst. Renew. Energy 2017, 111, 611–619. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Dehghani, S.; Haghighi, M. Sono-enhanced dispersion of CaO over Zr-Doped MCM-41 bifunctional nanocatalyst with various Si/Zr ratios for conversion of waste cooking oil to biodiesel. Renew. Energy 2020, 153, 801–812. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- dos Santos, L.K.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Production of biodiesel from crude palm oil by a sequential hydrolysis/esterification process using subcritical water. Renew. Energy 2019, 130, 633–640. [Google Scholar] [CrossRef]
- Elkelawy, M.; Alm-Eldin Bastawissi, H.; Esmaeil, K.K.; Radwan, A.M.; Panchal, H.; Sadasivuni, K.K.; Ponnamma, D.; Walvekar, R. Experimental studies on thebiodiesel production parameters optimization of sunflower and soybean oil mixture and DI engine combustion, performance, and emission analysis fueled with diesel/biodiesel blends. Fuel 2019, 255, 115791. [Google Scholar] [CrossRef]
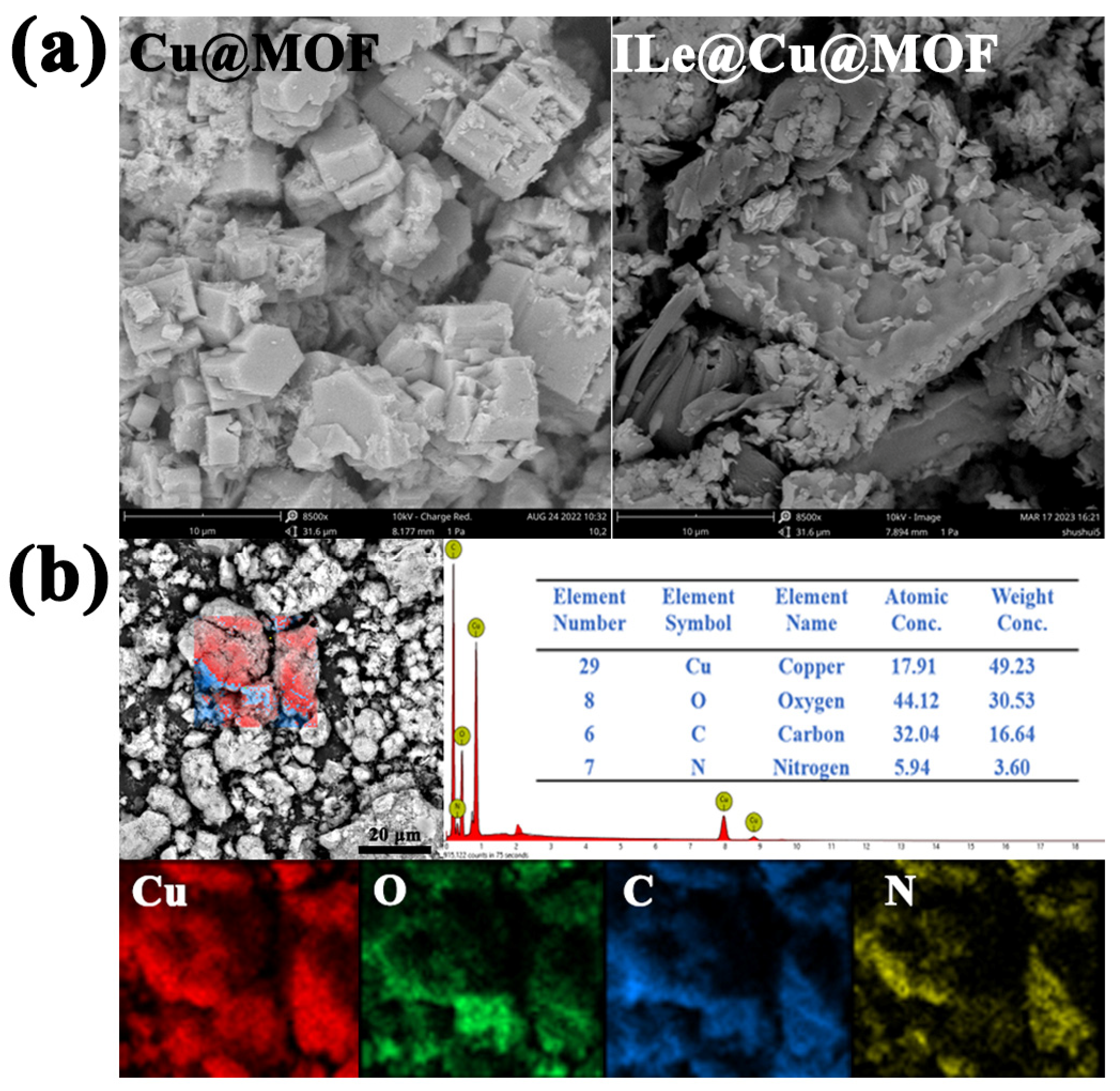

| Element Type | ILe@Cu@MOF Element Content (%) | Cu@MOF Element Content (%) |
|---|---|---|
| O | 30.53 | 52.9 |
| Cu | 49.23 | 36.51 |
| C | 16.64 | 10.19 |
| N | 3.6 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Wang, Y.; Ren, Z.; Shen, H.; Sheng, J.; Zhang, K.; Wang, J.; Wang, X. Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis. Coatings 2023, 13, 1437. https://doi.org/10.3390/coatings13081437
Hao Y, Wang Y, Ren Z, Shen H, Sheng J, Zhang K, Wang J, Wang X. Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis. Coatings. 2023; 13(8):1437. https://doi.org/10.3390/coatings13081437
Chicago/Turabian StyleHao, Yinan, Yan Wang, Zhiyuan Ren, Hongxia Shen, Jian Sheng, Kai Zhang, Jingwen Wang, and Ximing Wang. 2023. "Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis" Coatings 13, no. 8: 1437. https://doi.org/10.3390/coatings13081437
APA StyleHao, Y., Wang, Y., Ren, Z., Shen, H., Sheng, J., Zhang, K., Wang, J., & Wang, X. (2023). Preparation of ILe@Cu@MOF Catalyst and Its Application in Biodiesel Catalysis. Coatings, 13(8), 1437. https://doi.org/10.3390/coatings13081437





