Preparation and Acoustic Performance of Porous Aerogel Composites of Graphene Oxide and Cellulose
Abstract
:1. Introduction
2. Preparation and Characterisation of Materials
2.1. Materials Preparation of CEL-GO Composite Aerogels
2.2. Performance Characterisation of CEL-GO Composite Aerogels
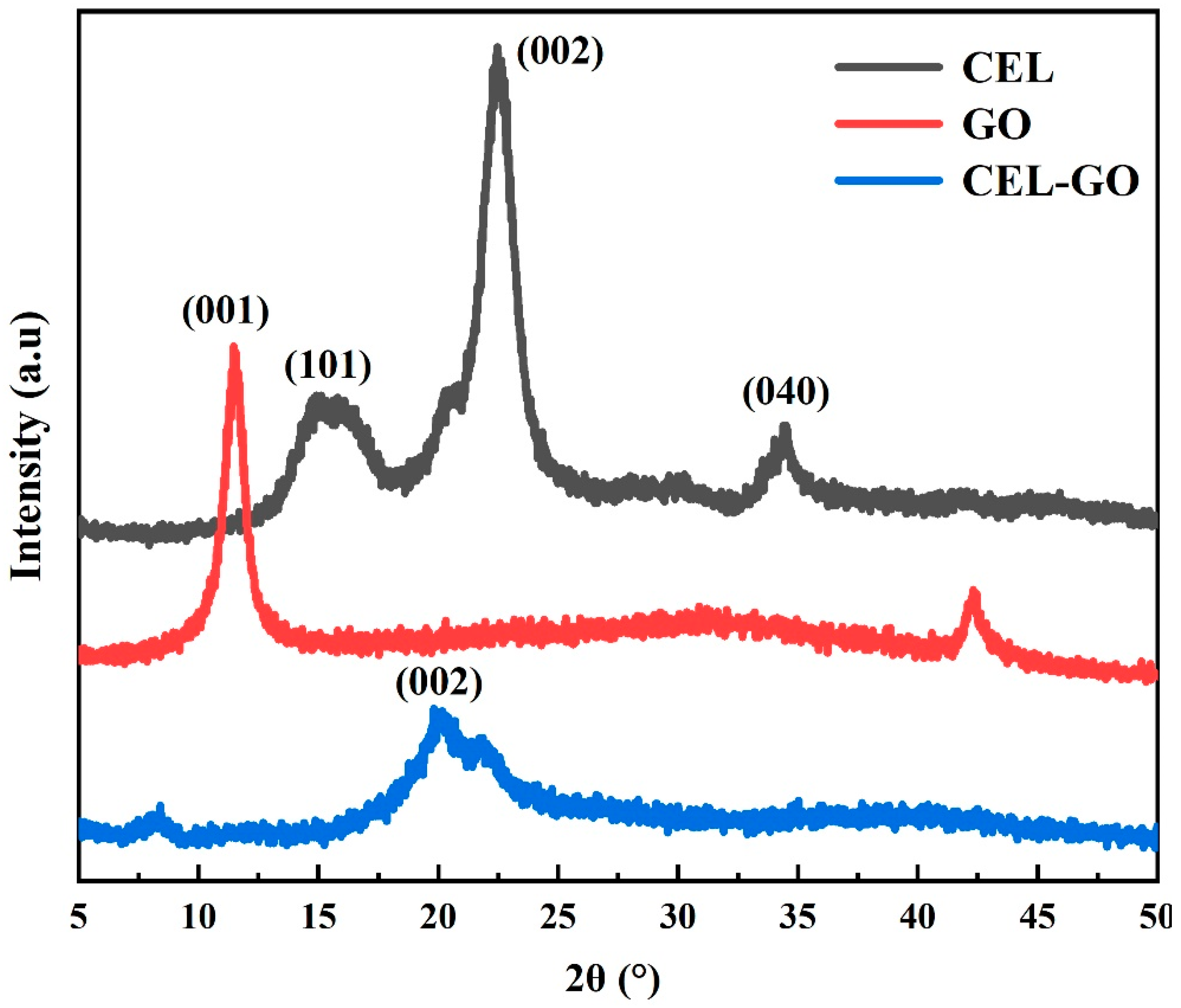
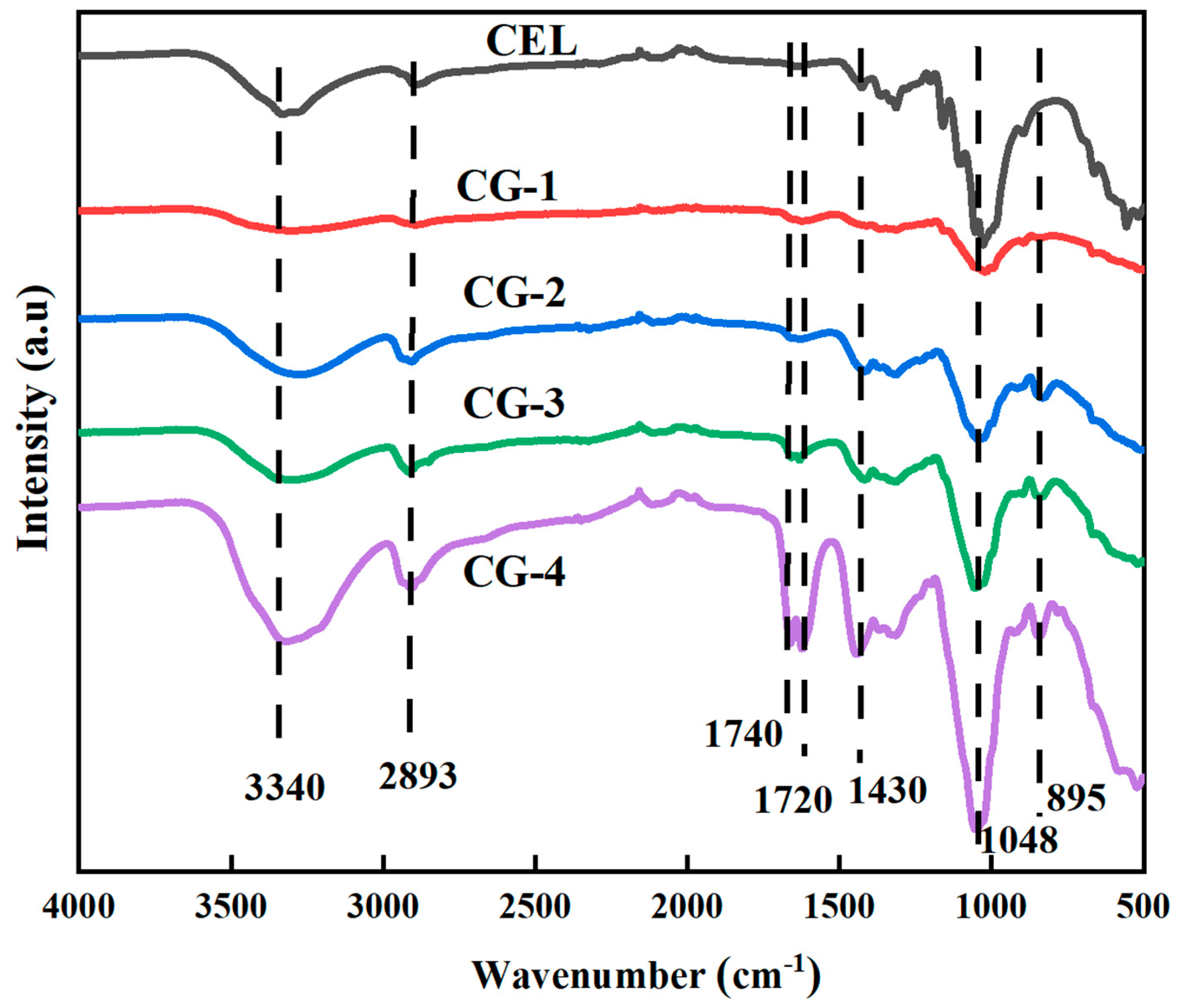
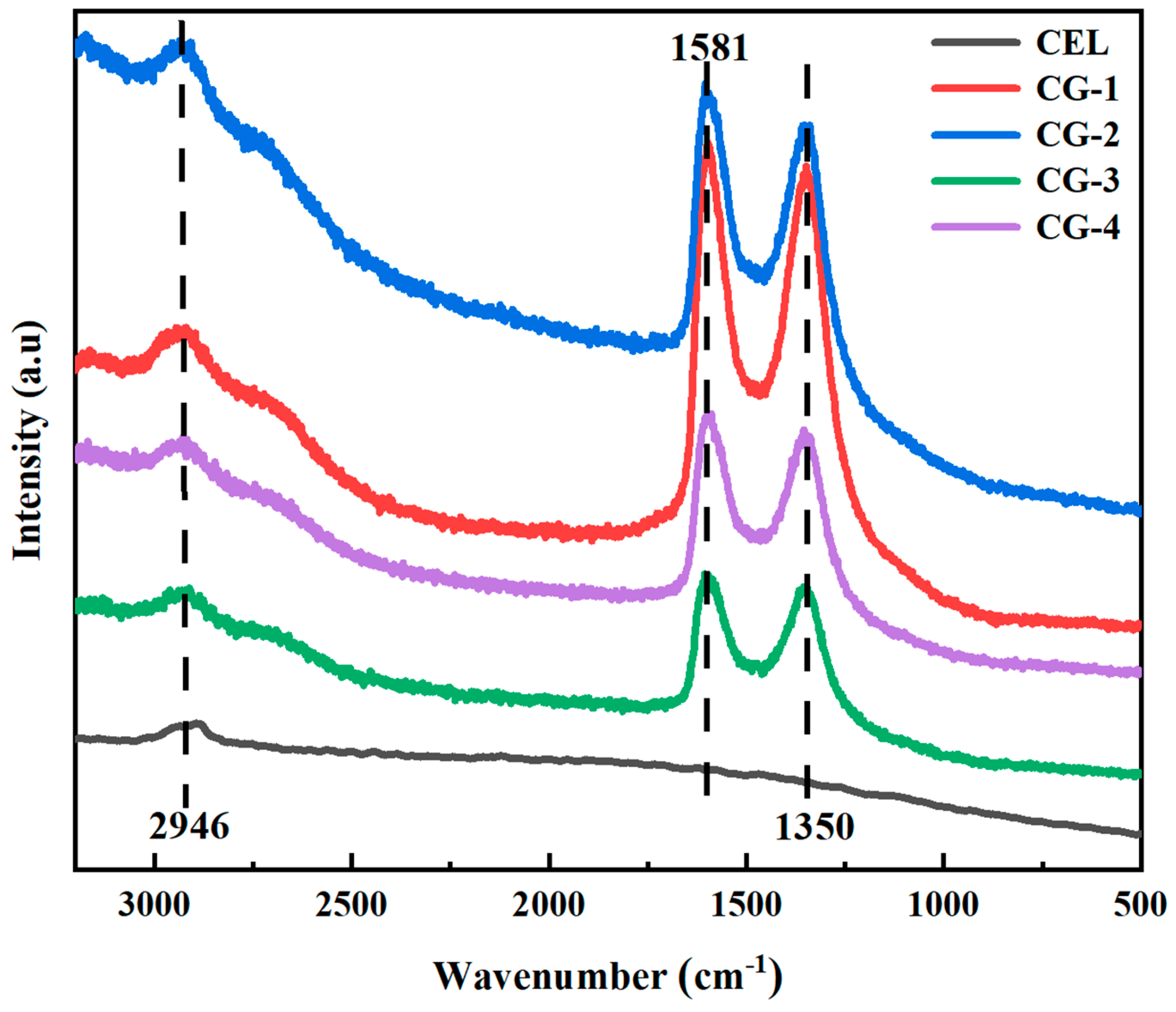
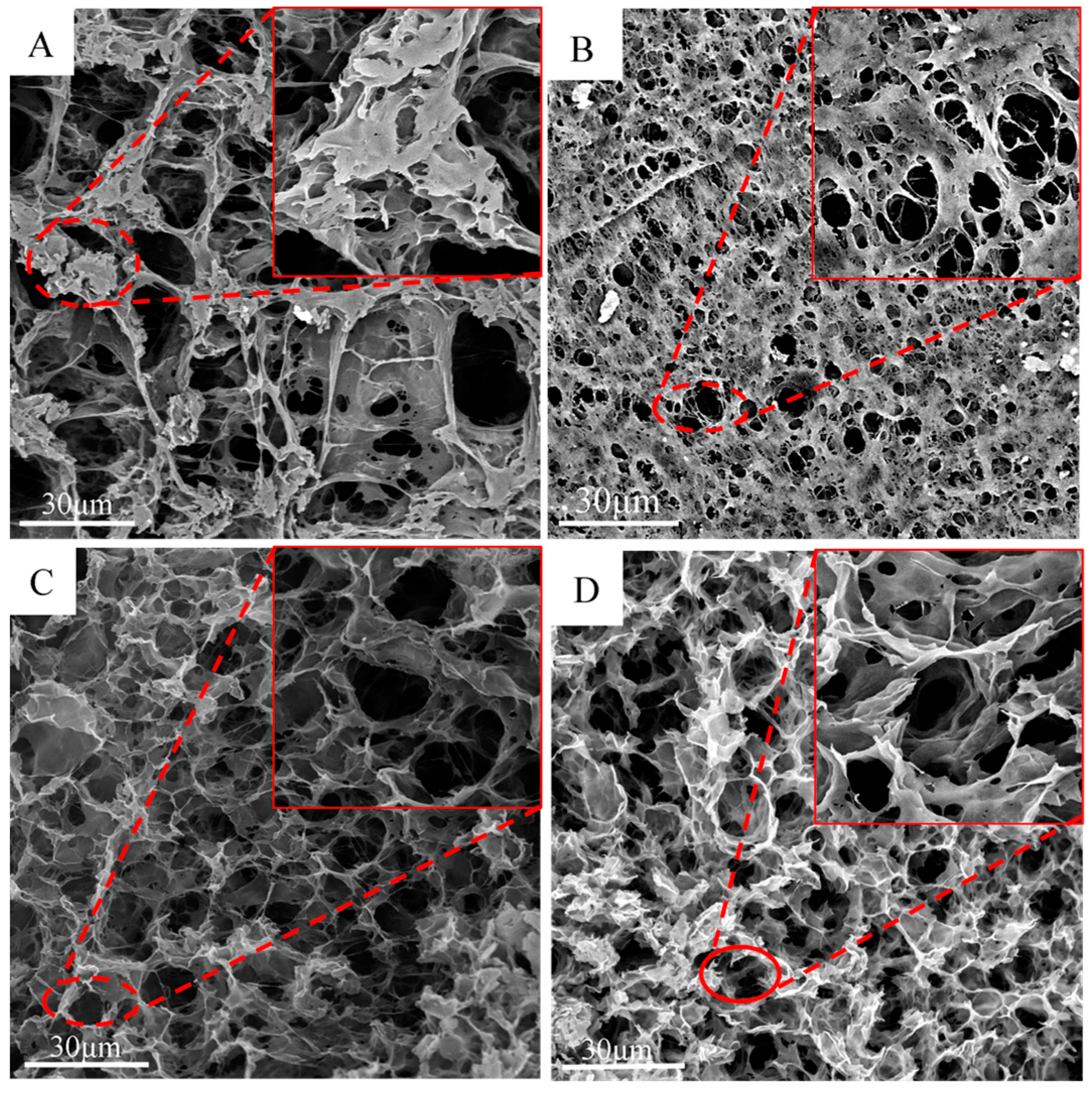
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Jiang, N. Research on Substation Noise and Design of the Control System. J. Mod. Power Syst. Clean Energy 2015, 31, 55–60. [Google Scholar]
- Sun, P. Preparation of Porous Sound-Absorbing Material Using Steel Slag and Its Sound Absorption Properties; University of Science and Technology Beijing: Beijing, China, 2016. [Google Scholar]
- Liu, K. Simulation Research on the Application of Porous Sound-absorbing Material Based on VA One. Transp. Sci. Technol. 2010, 2, 4. [Google Scholar]
- Cao, L. Porous materials for sound absorption. Compos. Commun. 2018, 10, 25–35. [Google Scholar] [CrossRef]
- Schiavoni, S. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Forouharmajd, F. Assessment of normal incidence absorption performance of sound absorbing materials. Int. J. Environ. Health Eng. 2016, 5, 10. [Google Scholar]
- Yang, T. Sound absorption properties of natural fibers: A review. Sustainability 2020, 12, 8477. [Google Scholar] [CrossRef]
- Asdrubali, F. A review of unconventional sustainable building insulation materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Liu, L. The development history and prospects of biomass-based insulation materials for buildings. Renew. Sustain. Energy Rev. 2017, 69, 912–932. [Google Scholar] [CrossRef]
- Hill, C. A comparison of the environmental impacts of different categories of insulation materials. Energy Build. 2018, 162, 12–20. [Google Scholar] [CrossRef]
- Papadopoulos, A.M. Environmental performance evaluation of thermal insulation materials and its impact on the building. Build. Environ. 2007, 42, 2178–2187. [Google Scholar] [CrossRef]
- Xia, Q. A strong, biodegradable and recyclable lignocellulosic bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Iñaki, G. Biomimetic Wood-Inspired Batteries: Fabrication, Electrochemical Performance, and Sustainability within a Circular Perspective. Adv. Sustain. Syst. 2021, 5, 2100236. [Google Scholar]
- CRIWI. The Physical and Mechanical Properties of the Main Tree Species in China; China Forestry Press: Beijing, China, 1982. [Google Scholar]
- Li, J. Wood Science, 3rd ed.; Science Press: Beijing, China, 2014. [Google Scholar]
- Zhao, G.J. Nanoscale, Nanowood, and Wood Inorganic Nanocomposites in Wood. J. Beijing For. Univ. 2002, 24, 204–207. [Google Scholar]
- Xue, Z.H. Theoretical Evaluation of Wood Voids. J. Inn. Mong. Agric. Univ. 2009, 30, 252–257. [Google Scholar]
- Lin, M.D. Estimating the sound absorption coeficients of perforated wooden panels by using artificial neural networks. Appl. Acoust. 2009, 70, 31–40. [Google Scholar] [CrossRef]
- Asdrubali, F. A review of structural, thermo-physical, acoustical, and environmental properties of wooden materials for building applications. Build. Environ. 2017, 114, 307–332. [Google Scholar] [CrossRef]
- Kang, C. Changes in anatomical features, air permeability and sound absorption capability of wood induced by delignification treatment. Kyushu Univ. 2008, 53, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H. High acoustic absorption properties of hackberry compared to nine diferent hardwood species: A novel finding for acoustical engineers. Appl. Acoust. 2020, 169, 107475. [Google Scholar] [CrossRef]
- Chen, C. Structure–property–function relationships of natural and engineered wood. Nat. Rev. Mater. 2020, 5, 642–666. [Google Scholar] [CrossRef]
- Li, J. In situ wood delignification toward sustainable applications. Acc. Mater. Res. 2021, 2, 606–620. [Google Scholar] [CrossRef]
- Guan, H. Highly compressible wood sponges with a spring-like lamellar structure as effective and reusable oil absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef]
- Song, J. Highly compressible, anisotropic aerogel with aligned cellulose nanofibers. ACS Nano 2018, 12, 140–147. [Google Scholar] [CrossRef]
- Li, T. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, 3724. [Google Scholar] [CrossRef]
- Zhu, M. Highly anisotropic, highly transparent wood composites. Adv. Mater. 2016, 28, 5181–5187. [Google Scholar] [CrossRef]
- Wang, J. Carbonate pre-treatment of wood for transformative structural applications through densification. Ind. Crops Prod. 2022, 183, 188. [Google Scholar] [CrossRef]
- Sun, J. Enhanced mechanical energy conversion with selectively decayed wood. Sci. Adv. 2021, 7, eabd9138. [Google Scholar] [CrossRef]
- Zhao, X. A scalable high-porosity wood for sound absorption and thermal insulation. Nat. Sustain. 2023, 6, 306–315. [Google Scholar] [CrossRef]
- Kolya, H. Ammonium persulfate treatment on carbohydrate polymers and lignin of wood improved sound absorption capacity. Int. J. Biol. Macromol. 2022, 205, 626–637. [Google Scholar] [CrossRef]
- Zhu, X.D. The current research status of sound absorption performance of wood plastic composite materials. J. For. Eng. 2017, 2, 10–15. [Google Scholar]
- Zhou, D.D.; Fang, L.; Yang, Q.Z. Preparation and performance of base magnesium sulfate porous sound absorbing materials. CIESC J. 2021, 72, 3041–3052. [Google Scholar]
- Lize, Q.; Chao, Z.J. Highly efficient acoustic absorber designed by backing cavity-like and filled-microperforated plate-like structure. Mater. Des. 2023, 225, 111484. [Google Scholar]
- Pang, K.; Liu, X.; Pang, J.; Samy, A.; Xie, J.; Liu, Y.; Peng, L.; Xu, Z. Highly Efficient Cellular Acoustic Absorber of Graphene Ultrathin Drums. Adv. Mater. 2022, 34, 2103740. [Google Scholar] [CrossRef]
- Zong, D.; Cao, L.; Yin, X.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Flexible ceramic nanofibrous sponges with hierarchically entangled graphene networks enable noise absorption. Nat. Commun. 2021, 12, 6599. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Wang, T.; Han, S.; Zhang, X.; Wang, J.; Sun, G. Polyurethane Foam with High-Efficiency Flame Retardant, Heat Insulation, and Sound Absorption Modified by Phosphorus-Containing Graphene Oxide. ACS Appl. Polym. Mater. 2024, 6, 1878–1890. [Google Scholar] [CrossRef]
- He, S.H. Development status and prospect of cellulose based foam and aerogel porous materials. In Collected Papers from the 19th Academic Annual Conference of the Chinese Paper Society; Chinese Paper Society: Beijing, China, 2020; pp. 451–463. [Google Scholar]
- ISO 845:2006; Cellular Plastics and Rubbers-Determination of Apparent Density. ISO: Geneva, Switzerland, 2006.
- Liu, T. Highly compressible anisotropic graphene aerogels fabricated by directional freezing for efficient absorption of organic liquids. Carbon 2016, 100, 456–464. [Google Scholar] [CrossRef]
- Vázquez, G. FTIR, 1H and 13C NMR Characterization of Acetosolv-Solubilized Pine and Eucalyptus Lignins. Holzforschung 1997, 51, 158–166. [Google Scholar] [CrossRef]
- Lin, Y. Preparation and characterisation of covalent polymer functionalized graphene oxide. J. Mater. Chem. 2011, 21, 3455–3461. [Google Scholar] [CrossRef]
- Jiang, S.T. Preparation and Absorption Propety of GO/Nanocellulose Composite Aerogel. New Chem. Mater. 2020, 48, 81–84+89. [Google Scholar]
- Silviana, S. Optimizing the environmentally friendly silica-cellulose aerogel composite for acoustic insulation material derived from newspaper and geothermal solid waste using a central composite design. J. Sol-Gel Sci. Technol. 2022, 103, 226–243. [Google Scholar] [CrossRef]
- Lin, D.H. Preparation and Properties of NFC Composite Aerogel Supercapacitors. Pap. Sci. Technol. 2017, 36, 28–35. [Google Scholar]
- Liu, X. Fabrication of hydrogel of hydroxypropyl cellulose (HPC) composited with graphene oxide and its application for methylene blue removal. J. Mater. Sci. 2015, 50, 6113–6123. [Google Scholar] [CrossRef]
- Klem, D. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Rapisarda, M. Multifunctional Thermal, Acoustic, and Piezoresistive Properties of In Situ-Modified Composite Aerogels with Graphene Oxide as the Main Phase. ACS Appl. Mater. Interfaces 2022, 14, 43646–43655. [Google Scholar] [CrossRef]
- Tao, Y. Recent progress in acoustic materials and noise control strategies—A review. Appl. Mater. Today 2021, 24, 101141. [Google Scholar] [CrossRef]
- Wang, J.Q. Research progress and prospects of performance of kinetic hydrate inhibitor and effect of functional group. J. Cent. South Univ. 2022, 53, 772–798. [Google Scholar]
- Yao, Q. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef]
- Rapisarda, M. Ultralight graphene oxide/polyvinyl alcohol aerogel for broadband and tuneable acoustic properties. Sci. Rep. 2021, 11, 10572. [Google Scholar] [CrossRef]
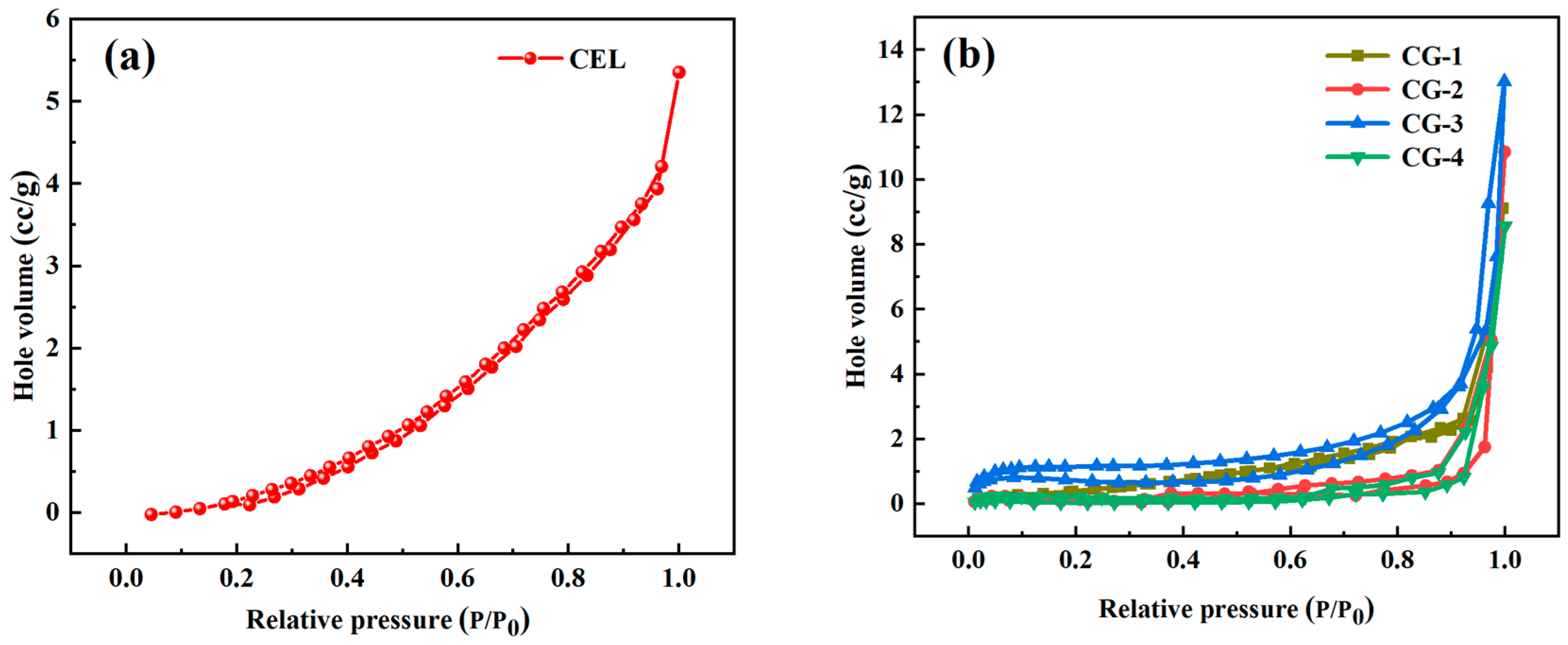

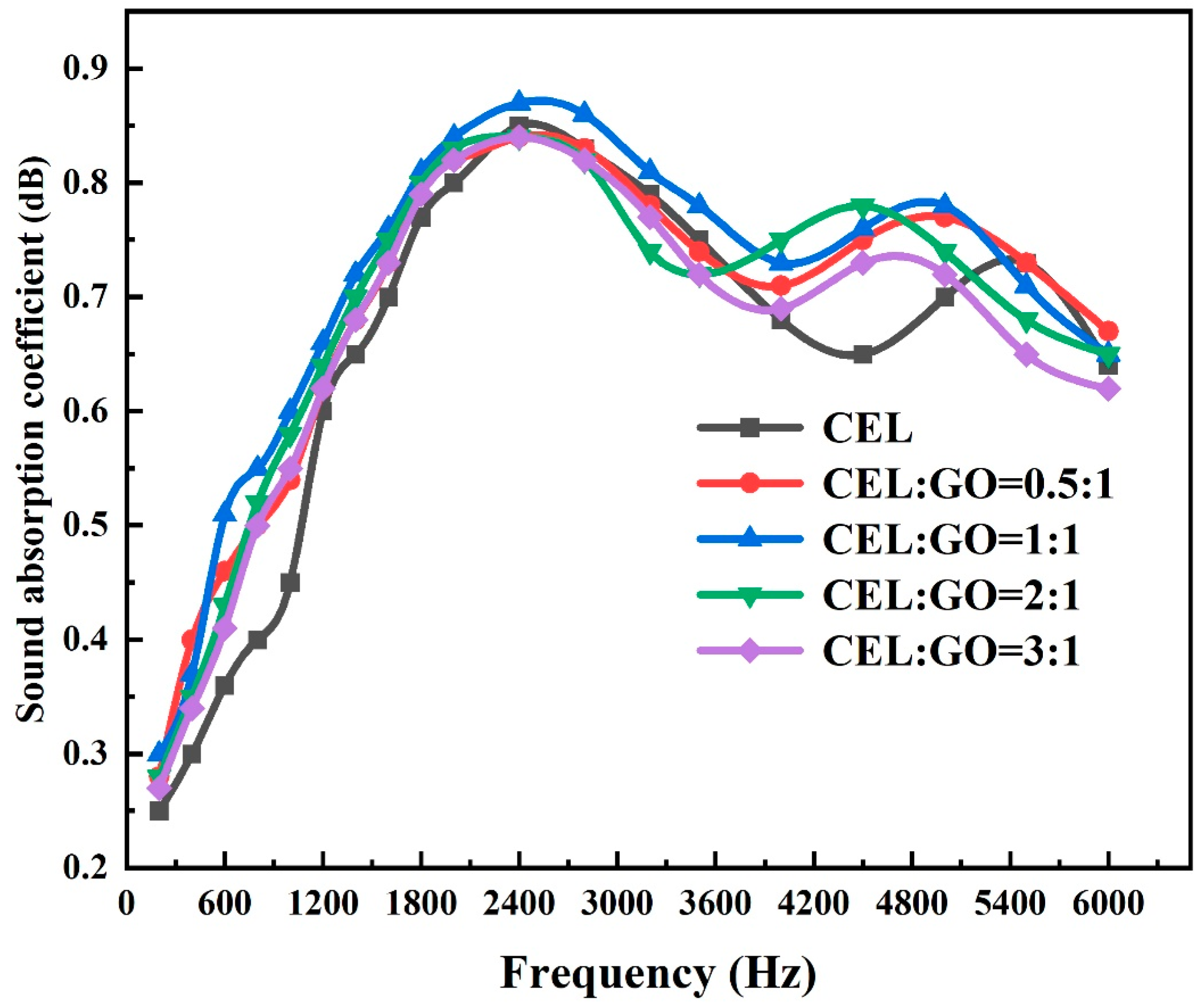

| Samples | Mass (g) | Densities (g/cm3) | Porosity (%) |
|---|---|---|---|
| CEL-CH | 2.22 | 0.078 | 94.4 |
| CEL-GO = 0.5:1 | 1.366 | 0.082 | 87.7 |
| CEL-GO = 1:1 | 1.21 | 0.085 | 89.4 |
| CEL-GO = 2:1 | 1.84 | 0.090 | 87.2 |
| CEL-GO = 3:1 | 1.05 | 0.095 | 85.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Lv, Y.; Xue, Z.; Pan, Y.; Liu, J.; Dai, M.; Qiu, F. Preparation and Acoustic Performance of Porous Aerogel Composites of Graphene Oxide and Cellulose. Coatings 2024, 14, 441. https://doi.org/10.3390/coatings14040441
Shao J, Lv Y, Xue Z, Pan Y, Liu J, Dai M, Qiu F. Preparation and Acoustic Performance of Porous Aerogel Composites of Graphene Oxide and Cellulose. Coatings. 2024; 14(4):441. https://doi.org/10.3390/coatings14040441
Chicago/Turabian StyleShao, Jinbao, Yuexiao Lv, Zhenhua Xue, Yanfei Pan, Jinwei Liu, Mayin Dai, and Fengqi Qiu. 2024. "Preparation and Acoustic Performance of Porous Aerogel Composites of Graphene Oxide and Cellulose" Coatings 14, no. 4: 441. https://doi.org/10.3390/coatings14040441





