Breaking through the Thermodynamics “Wilds” of Metal–Organic Chemical Vapor Deposition Precursors: Metal tris-Acetylacetonates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vapor Pressure Measurements (Transpiration Method)
2.3. Vapor Pressure Measurements (Knudsen Effusion Method)
3. Results and Discussion
3.1. Vapor Pressure
3.2. Sublimation/Vaporization Enthalpies and Entopies of Metal(III) β-Diketonates and Their Temperature Adjustment to T = 298.15 K. Evaluation of the Thermodynamic Characteristics
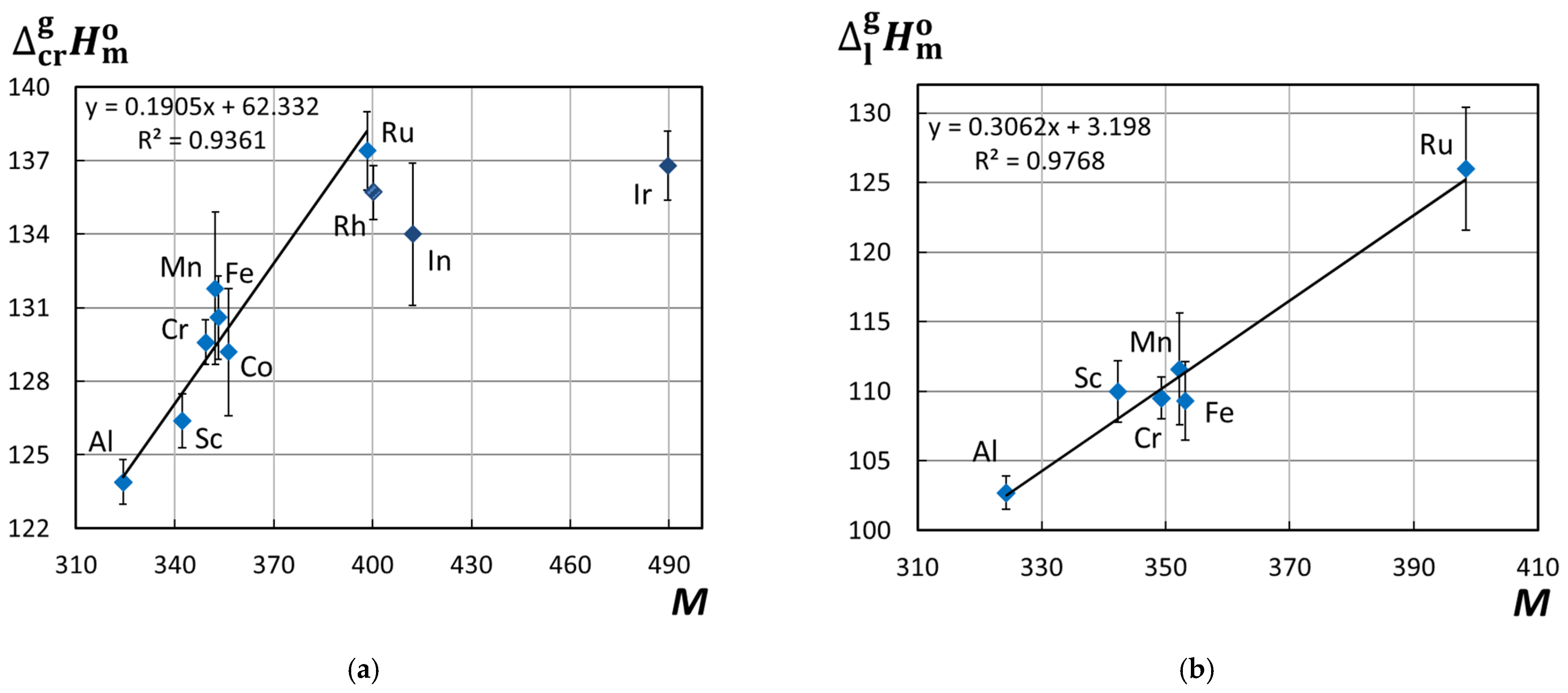
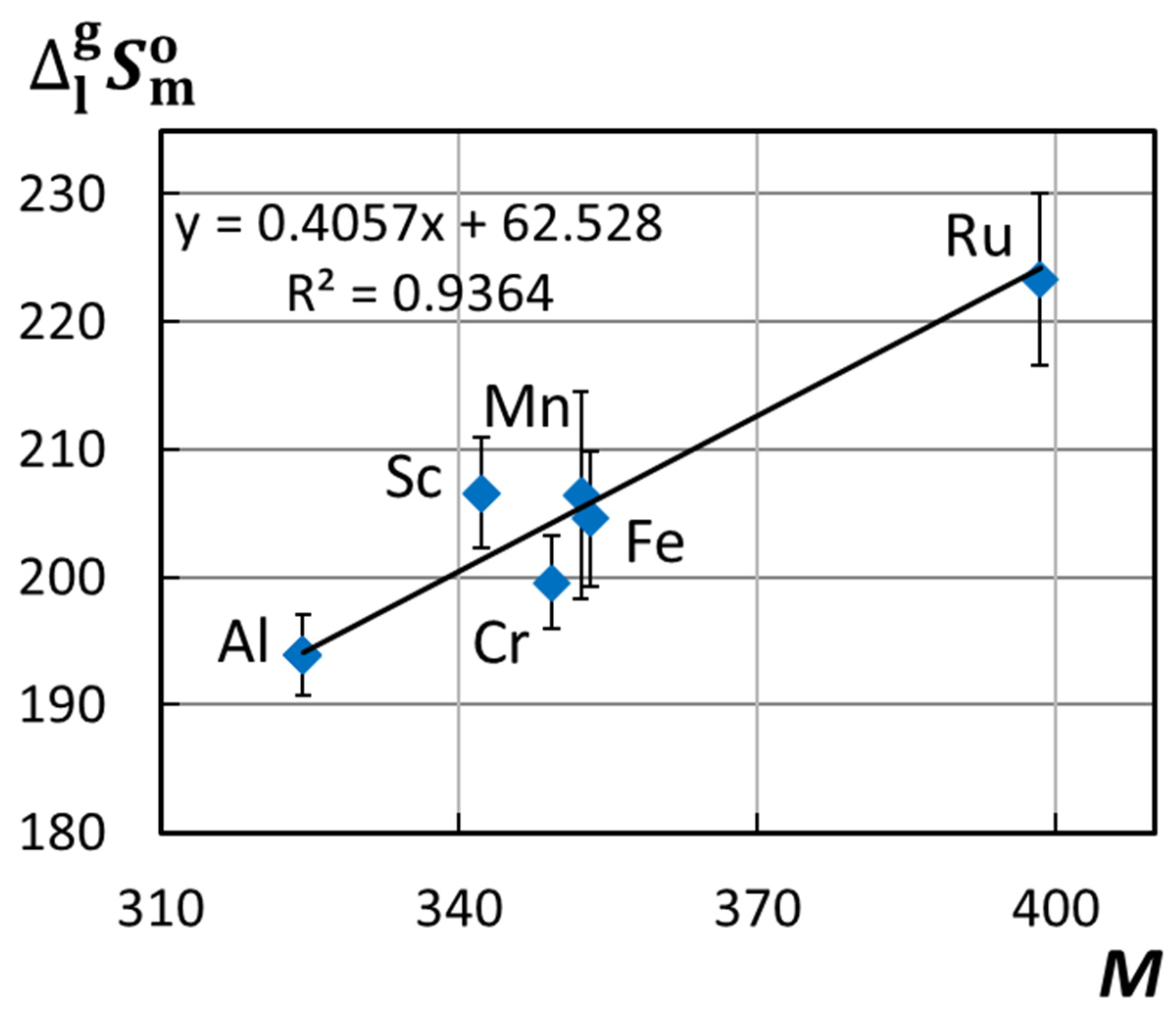
3.3. Structure–Property Relationships in Metal(III) Acetylacetonates. Influence of the Central Atom on the Sublimation/Vaporization Enthalpy and Entropy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karakovskaya, K.I.; Dorovskikh, S.I.; Vikulova, E.S.; Ilyin, I.Y.; Zherikova, K.V.; Basova, T.V.; Morozova, N.B. Volatile iridium and platinum MOCVD precursors: Chemistry, thermal properties, materials and prospects for their application in medicine. Coatings 2021, 11, 78. [Google Scholar] [CrossRef]
- Igumenov, I.K.; Lukashov, V.V. Modern solutions for functional coatings in CVD processes. Coatings 2022, 12, 1265. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Karakovskaya, K.I.; Korolkov, I.V.; Koretskaya, T.P.; Chepeleva, E.V.; Kuz’min, N.B.; Fedorenko, A.D.; Pischur, D.P.; Guselnikova, T.Y.; Maksimovskii, E.A.; et al. Application of biocompatible noble metal film materials to medical implants: TiNi surface modification. Coatings 2023, 13, 222. [Google Scholar] [CrossRef]
- Ievtushenko, A.; Dzhagan, V.; Khyzhun, O.; Baibara, O.; Bykov, O.; Zahornyi, M.; Yukhymchuk, V.; Valakh, M.; Zahn, D.R.T.; Naumenko, K.; et al. The effect of Ag doping on the structure, optical, and electronic properties of ZnO nanostructures deposited by atmospheric pressure MOCVD on Ag/Si substrates. Semicond. Sci. Technol. 2023, 38, 75008. [Google Scholar] [CrossRef]
- Vasilyeva, I.G.; Vikulova, E.S.; Pochtar, A.A.; Morozova, N.B. Mixed films based on MgO for secondary electron emission application: General trends and MOCVD prospects. Coatings 2021, 11, 176. [Google Scholar] [CrossRef]
- Selvakumar, J.; Raghunathan, V.S.; Nagaraja, K.S. Tris(2,4-pentanedionato)scandium(III) as a precursor for plasma-assisted liquid injection CVD to deposit nanocrystalline scandia thin films. Chem. Vap. Depos. 2009, 15, 262–268. [Google Scholar] [CrossRef]
- Berg, E.W.; Truemper, J.T. A study of the volatile characteristics of various metal β-diketone chelates. J. Phys. Chem. 1960, 64, 487–490. [Google Scholar] [CrossRef]
- Berg, E.W.; Truemper, J.T. Vapor pressure-temperature data for various metal β-diketone chelates. Anal. Chim. Acta 1965, 32, 245–252. [Google Scholar] [CrossRef]
- Frankhauser, W.A. Vapor Pressure Studies on Metal Chelates. Master’s Thesis, The Air Force Institute of Technology, Dayton, OH, USA, June 1965. [Google Scholar]
- Fontain, R.; Pommier, C.; Guiotchon, G. Vapor pressure and thermal stability of chromium and aluminum chelates. Bull. Soc. Chim. Fr. 1972, 8, 3011–3015. [Google Scholar]
- Naghibi-Bidokhti, H. Thermochemical Studies of Sublimation and Solvation of Some Metal β-Diketonate Complexes. Ph.D. Thesis, University of Surrey, Guildford, UK, February 1977. [Google Scholar]
- Volkov, S.V.; Mazurenko, E.A.; Bublik, Z.N. Influence of the nature of substituents in β-diketonates on their thermodynamic characteristics. In Structure, Properties and Applications of Metal β-Diketonates; Spitsyn, V., Ed.; Nauka: Moscow, Russia, 1978; pp. 119–122. (In Russian) [Google Scholar]
- Volkov, S.V.; Mazurenko, E.A.; Bublik, Z.N. Gas chromatographic determination of the thermodynamic characteristics of a number of metal β-diketonate complexes in the gas phase. Ukr. Khim. Zhurn. 1978, 44, 570–573. (In Russian) [Google Scholar]
- Sachinidis, J.; Hill, J.O. A re-evaluation of the enthalpy of sublimation of some metal acetylacetonate complexes. Thermochim. Acta 1980, 35, 59–66. [Google Scholar] [CrossRef]
- Teghil, R.; Ferro, D.; Bencivenni, L.; Pelino, M. A thermodynamic study of the sublimation processes of aluminium and copper acetylacetonates. Thermochim. Acta 1981, 44, 213–222. [Google Scholar] [CrossRef]
- Igumenov, I.; Chumachenko, Y.; Zemskov, S. Tenzimetric study of volatile metal β-diketonates. In Problems of Chemistry and Application of Metal β–Diketonates; Spitsyn, V., Ed.; Nauka: Moscow, Russia, 1982; pp. 100–120. (In Russian) [Google Scholar]
- Greenberg, J.H.; Lazarev, V.B.; Zavernjaev, A.J.; Shreider, V.A.; Chepik, S.D. Thermodynamic properties of aluminum tris-acetylacetonate. Z. Fiz. Khim. 1986, 60, 1386–1389. (In Russian) [Google Scholar]
- Alikhanyan, A.S.; Malkerova, I.P.; Greenberg, J.H.; Lazarev, V.B.; Bogdanov, V.A.; Gorgoraki, V.I.; Shreider, V.A. The thermodynamics of sublimation of acetylacetonates of Al, Cr, Y, Zr. Dokl. Akad. Nauk 1987, 292, 376–379. (In Russian) [Google Scholar]
- Mazurenko, E.A.; Gerasimchuk, A.I. Metal coordination compounds in the gas phase. Ukr. Khim. Zhurn. 1993, 59, 526–536. (In Russian) [Google Scholar]
- Fahlman, B.D.; Barron, A.R. Substituent effects on the volatility of metal β-diketonates. Adv. Mater. Opt. Electron. 2000, 10, 223–232. [Google Scholar] [CrossRef]
- Semyannikov, P.P.; Igumenov, I.K.; Trubin, S.V.; Chusova, T.P.; Semenova, Z.I. Thermodynamics of sublimation of aluminium triacetylacetonate. Thermochim. Acta 2006, 451, 80–83. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Siddiqui, R.A.; Atakan, B. Thermal stability, sublimation pressures and diffusion coefficients of some metal acetylacetonates. Surf. Coatings Technol. 2007, 201, 9055–9059. [Google Scholar] [CrossRef]
- Siddiqui, R.A. Experimental Investigations of Thermodynamic Properties of Organometallic Compounds. Ph.D. Thesis, The University of Duisburg-Essen, Duisburg/Essen, Germany, 10 June 2009. [Google Scholar]
- Gairola, A.; Kunte, G.V.; Umarji, A.M.; Shivashankar, S.A. Determination of the enthalpies of sublimation and evaporation from thermogravimetric data: Application to metalorganic complexes of Al and Cr. Thermochim. Acta 2009, 488, 17–20. [Google Scholar] [CrossRef]
- Makarenko, A.M.; Zaitsau, D.H.; Zherikova, K.V. Metal-organic chemical vapor deposition precursors: Diagnostic check for volatilization thermodynamics of scandium(III) β-diketonates. Coatings 2023, 13, 535. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Verevkin, S.P. Error or exemption to the rule? Development of a diagnostic check for thermochemistry of metal–organic compounds. RSC Adv. 2020, 10, 38158. [Google Scholar] [CrossRef] [PubMed]
- Zherikova, K.V.; Makarenko, A.M.; Morozova, N.B. Evaluating precursors for the sustainable gas-phase deposition: Phase transition thermodynamics of volatile iridium (III) β-diketonates. J. Therm. Anal. Calorim. 2022, 147, 14987–14998. [Google Scholar] [CrossRef]
- Fackler, J.P. Metal β-ketoenolate complexes. In Progress in Inorganic Chemistry; Cotton, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1966; Volume 7, pp. 361–425. [Google Scholar] [CrossRef]
- Semyannikov, P.P.; Igumenov, I.K.; Trubin, S.V.; Chusova, T.P.; Semenova, Z.I. Thermodynamics of chromium acetylacetonate sublimation. Thermochim. Acta 2005, 432, 91–98. [Google Scholar] [CrossRef]
- Melia, T.P.; Merrifield, R. Thermal properties of transition-metal compounds. Part I. Heat capacity, entropy, and standard heat of formation of tris(acetylacetonato)chromium(III). J. Chem. Soc. A 1968, 2819–2820. [Google Scholar] [CrossRef]
- Morgan, B.G.T.; Drew, H.D.K. Researches on residual affinity and coordination. Part V. Gallium acetylacetone and its analogues. J. Chem. Soc. 1921, 119, 1058–1066. [Google Scholar] [CrossRef]
- Rahman, A.; Ahmed, S.N.; Khair, M.A.; Zangrando, E.; Randaccio, L. The crystal structure of tris(acetylacetonato)aluminum(III). J. Bangladesh Acad. Sci. 1990, 14, 161–166. [Google Scholar]
- Bott, S.G.; Fahlman, B.D.; Pierson, M.L.; Barron, A.R. An accuracy assessment of the refinement of partial metal disorder in solid solutions of Al(acac)3 and Cr(acac)3. J. Chem. Soc. Dalton Trans. 2001, 14, 2148–2152. [Google Scholar] [CrossRef]
- Palenik, G.J.; Dymock, K.R. The structure of tris(2,4-pentanedionato)indium(III). J. Appl. Cryst. 1974, 7, 90–91. [Google Scholar] [CrossRef]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Determination of vapor pressures and vaporization enthalpies of the aliphatic branched C5 and C6 alcohols. J. Chem. Eng. Data 2001, 46, 1593–1600. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N. Transpiration method: Vapor pressures and enthalpies of vaporization of some low-boiling esters. Fluid Phase Equilib. 2008, 266, 64–75. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Sazonova, A.Y.; Emel´yanenko, V.N.; Zaitsau, D.H.; Varfolomeev, M.A.; Solomonov, B.N.; Zherikova, K.V. Thermochemistry of halogen-substituted methylbenzenes. J. Chem. Eng. Data 2015, 60, 89–103. [Google Scholar] [CrossRef]
- Zelenina, L.N.; Zherikova, K.V.; Chusova, T.P.; Trubin, S.V.; Bredikhin, R.A.; Gelfond, N.V.; Morozova, N.B. Comprehensive thermochemical study of sublimation, melting and vaporization of scandium(III) beta-diketonates. Thermochim. Acta 2020, 689, 178639. [Google Scholar] [CrossRef]
- Morozova, N.B.; Semyannikov, P.P.; Trubin, S.V.; Stabnikov, P.P.; Bessonov, A.A.; Zherikova, K.V.; Igumenov, I.K. Vapor pressure of some volatile iridium(I) compounds with carbonyl, acetylacetonate and cyclopentadienyl ligands. J. Therm. Anal. Calorim. 2009, 96, 261–266. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 261–269. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds. Sublimation, vaporization and fusion enthalpies from 1880 to 2015. Part 1. C1–C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Verevkin, S.P. Ferrocene: Temperature adjustments of sublimation and vaporization enthalpies. Fluid Phase Equilib. 2018, 472, 196–203. [Google Scholar] [CrossRef]
- Bespyatov, M.A. Low-temperature heat capacity and thermodynamic functions of tetrameric cobalt(II) acetylacetonate. J. Chem. Eng. Data 2020, 65, 5218–5225. [Google Scholar] [CrossRef]
- Zhilina, M.N.; Karyakin, N.V.; Maslova, V.A.; Shvetsova, K.G.; Busygina, G.I.; Nikolaev, P.N. Heat capacity and thermodynamic functions of iron(III) acetylacetonate. Russ. J. Phys. Chem. 1987, 61, 1633–1634. [Google Scholar]
- Melia, T.P.; Merrifield, R. Thermal properties of transition metal compounds: Heat capacity, entropy, enthalpy, free energy and heat of fusion of the tris(acetylacetonato)complexes of scandium(III), vanadium(III), manganese(III), iron(III) and cobalt(III) and the vapour pressure of tris(acetylacetonato)iron (III)–IV. J. Inorg. Nucl. Chem. 1970, 32, 2573–2579. [Google Scholar] [CrossRef]
- Zherikova, K.V.; Zelenina, L.N.; Pishchur, D.P.; Emel’yanenko, V.N.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Gelfond, N.V.; Morozova, N.B. Thermochemical study of rhodium(III) acetylacetonate. J. Chem. Thermodyn. 2016, 102, 442–450. [Google Scholar] [CrossRef]
- Wood, J.L.; Jones, M.M. Coordinate bond energies and inner orbital splitting in some tervalent transition metal acetylacetonates. Inorg. Chem. 1964, 3, 1553–1556. [Google Scholar] [CrossRef]
- Gotze, H.J.; Bloss, K.; Molketin, H. Vapor pressure determination of acetylacetonates. Z. Phyz. Chem. 1970, 73, 314–320. [Google Scholar] [CrossRef]
- Melia, T.P.; Merrifield, R. Vapour pressures of the tris(acetylacetonato)complexes of scandium(III),vanadium(III) and chromium(III). J.Inorg Nucl. Chem. 1970, 32, 1489–1493. [Google Scholar] [CrossRef]
- Ashcroft, S.J. The measurement of enthalpies of sublimation by thermogravimetry. Thermochim. Acta 1971, 2, 512–514. [Google Scholar] [CrossRef]
- Murray, J.P.; Hill, J.O. DSC determination of the fusion and sublimation enthalpy of tris(2,4-pentanedionato)Cr(III) and Fe(III). Thermochim. Acta 1984, 72, 341–347. [Google Scholar] [CrossRef]
- Lazarev, V.B.; Greenberg, J.H.; Ozerova, Z.P.; Sharpataya, G.A. DSC and vapour pressure investigation of some β-diketonates. J. Therm. Anal. 1988, 33, 797–799. [Google Scholar] [CrossRef]
- Malkerova, I.P.; Alikhanyan, A.S.; Sevastyanov, V.G.; Greenberg, J.H.; Gorgoraki, V.I. Features of thermal behaviour of 3d-transition metal acetylacetonates. Russ. J. Inorg. Chem. 1990, 35, 413–418. (In Russian) [Google Scholar]
- Semyannikov, P.P.; Igumenov, I.K.; Trubin, S.V.; Asanov, I.P. In situ mass spectrometry during thermal CVD of the tris-acetylacetonates of 3-d transition metals. J. Phys. IV 2001, 11, 995–1003. [Google Scholar] [CrossRef]
- Igumenov, I.K.; Gerasimenko, T.Y.; Isakova, V.G. Saturated vapor pressure of some β-diketonates of aluminium, gallium, indium. Izv. Sib. Otd. Akad. Nauk. SSSR Seriya Khimicheskikh Nauk. 1985, 1, 42–44. (In Russian) [Google Scholar]
- Selvakumar, J.; Raghunathan, V.S.; Nagaraja, K.S. Sublimation kinetics of scandium β-diketonates. J. Therm. Anal. Calorim. 2010, 100, 155–161. [Google Scholar] [CrossRef]
- Belova, N.V.; Girichev, G.V.; Giricheva, N.I.; Zaitseva, I.G.; Zyabko, I.O.; Krasnov, A.V.; Kuz’mina, N.P.; Shlykov, S.A. Mass-spectrometric study of scandium β-diketonates vaporization. Izv. Vyss. Uchebnykh Zaved. Khimiya I Khimicheskaya Tekhnologiya 2012, 55, 50–54. (In Russian) [Google Scholar]
- Murata, S.; Sakiyama, M.; Seki, S. Sublimation calorimetric studies using a calvet microcalorimeter. Thermochim. Acta 1985, 88, 121–126. [Google Scholar] [CrossRef]
- Fedotova, N.E.; Morozova, N.B.; Igumenov, I.K.; Gerasimov, P.A.; Gerasimova, A.I. Thermodynamic study of iron(III) tris-β-diketonates. Koord. Khim. 1993, 19, 622–629. (In Russian) [Google Scholar]
- Ribeiro Da Silva, M.A.V.; Monte, M.J.S.; Huinink, J. Vapour pressures and standard molar enthalpies of sublimation of two crystalline iron(III) β-diketonates. The mean molar (Fe-O) bond-dissociation enthalpies. J. Chem. Thermodyn. 1996, 28, 413–419. [Google Scholar] [CrossRef]
- Gillan, E.G.; Bott, S.G.; Barron, A.R. Volatility studies on gallium chalcogenide cubanes: Thermal analysis and determination of sublimation enthalpies. Chem. Mater. 1997, 9, 796–806. [Google Scholar] [CrossRef]
- Stabnikov, P.A.; Sysoev, S.V.; Vanina, N.S.; Trubin, S.V.; Semyannikov, P.P.; Igumenov, I.K. Vapor pressures of beta-diketonates of trivalent iron. Electron. J Investig. Russ. 2001, 238–245. (In Russian). Available online: https://cyberleninka.ru/article/n/davleniya-parov-beta-diketonatov-trehvalentnogo-zheleza (accessed on 1 May 2023).
- Beech, G.; Lintonbon, R.M. Thermal and kinetic studies of some metal complexes of 2,4-pentanedione. Thermochim. Acta 1971, 3, 97–105. [Google Scholar] [CrossRef]
- Murray, J.P.; Hill, J.O. DSC determination of the sublimation enthalpy of tris(2,4-pentanedionato)cobalt(III) and bis(2,4-pentanedionato) nickel(II) and copper(II). Thermochim. Acta 1987, 109, 383–390. [Google Scholar] [CrossRef]
- Bykov, A.F.; Morozova, N.B.; Igumenov, I.K.; Sysoev, S.V. Investigation of thermal properties of ruthenium(III) β-diketonate precursors for preparation of RuO2 films by CVD. J. Therm. Anal. 1996, 46, 1551–1565. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.V.; Giera, E.; Monte, M.J.S. Vapour pressures and standard molar enthalpy of sublimation of crystalline tris(pentane-2,4-dionato)ruthenium(III). J. Alloys Compd. 1993, 197, 105–107. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Siddiqui, R.A.; Atakan, B. Thermal stability, sublimation pressures, and diffusion coefficients of anthracene, pyrene, and some metal β-diketonates. J. Chem. Eng. Data 2009, 54, 2795–2802. [Google Scholar] [CrossRef]
- Morozova, N.B.; Zherikova, K.V.; Semyannikov, P.P.; Trubin, S.V.; Igumenov, I.K. Study of temperature dependencies of saturated vapor pressure of ruthenium(III) beta-diketonate derivatives. J. Therm. Anal. Calorim. 2009, 98, 395–399. [Google Scholar] [CrossRef]
- Morozova, N.B.; Zharkova, G.I.; Semyannikov, P.P.; Sysoev, S.V.; Igumenov, I.K.; Fedotova, N.E.; Gelfond, N.V. Vapor pressure of precursors for CVD on the base of platinum group metals. J. Phys. IV 2001, 11, 609–616. [Google Scholar] [CrossRef]
- Murray, J.P.; Hill, J.O. DSC determination of the fusion and sublimation enthalpy of bis(2,4-pentanedionato)beryllium(II) and tris(2,4-pentanedionato)aluminium(III). Thermochim. Acta 1983, 63, 211–218. [Google Scholar] [CrossRef]
- Sabolović, J.; Mrak, Ž.; Koštrun, S.; Janeković, A. Is the enthalpy of fusion of tris(acetylacetonato)metal(III) complexes affected by their potential energy in the crystal state? Inorg. Chem. 2004, 43, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Zherikova, K.V.; Zelenina, L.N.; Morozova, N.B.; Chusova, T.P. Thermal properties of volatile ruthenium(III) complexes. J. Therm. Anal. Calorim. 2011, 108, 1325–1329. [Google Scholar] [CrossRef]
- Gobble, C.; Chickos, J.; Verevkin, S.P. Vapor pressures and vaporization enthalpies of a series of dialkyl phthalates by correlation gas chromatography. J. Chem. Eng. Data 2014, 59, 1353–1365. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel´yanenko, V.N.; Zaitsau, D.H. Thermochemistry of substituted benzamides and substituted benzoic acids: Like tree, like fruit? Chem. Phys. Chem. 2018, 19, 619–630. [Google Scholar] [CrossRef]
- Verevkin, S.P. Improved Benson`s increments for the estimation of the standard enthalpies of formation and enthalpies of vaporization of alkyl ethers, acetals, ketals, and orthoesters. J. Chem. Eng. Data 2002, 47, 1071–1097. [Google Scholar] [CrossRef]
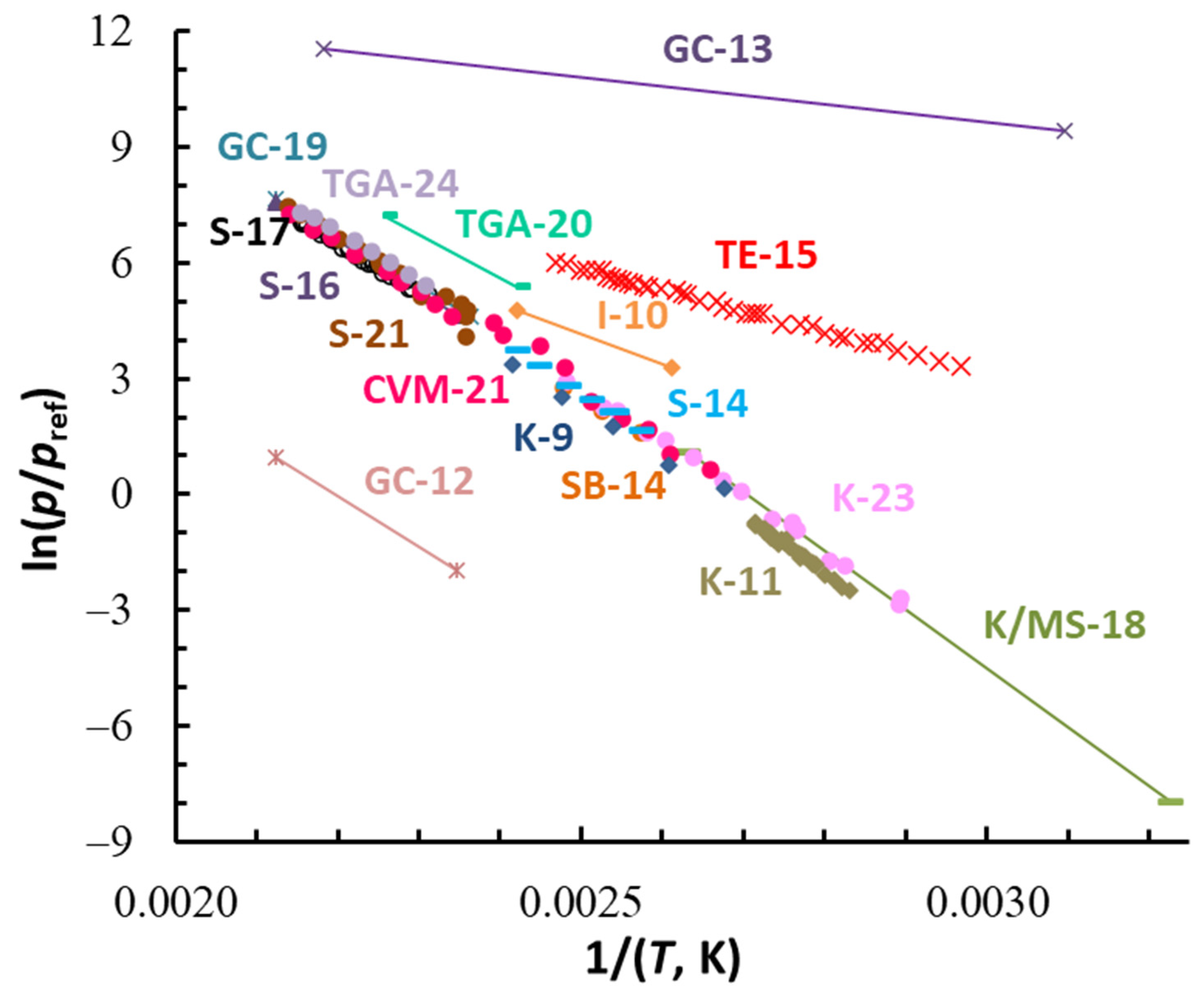
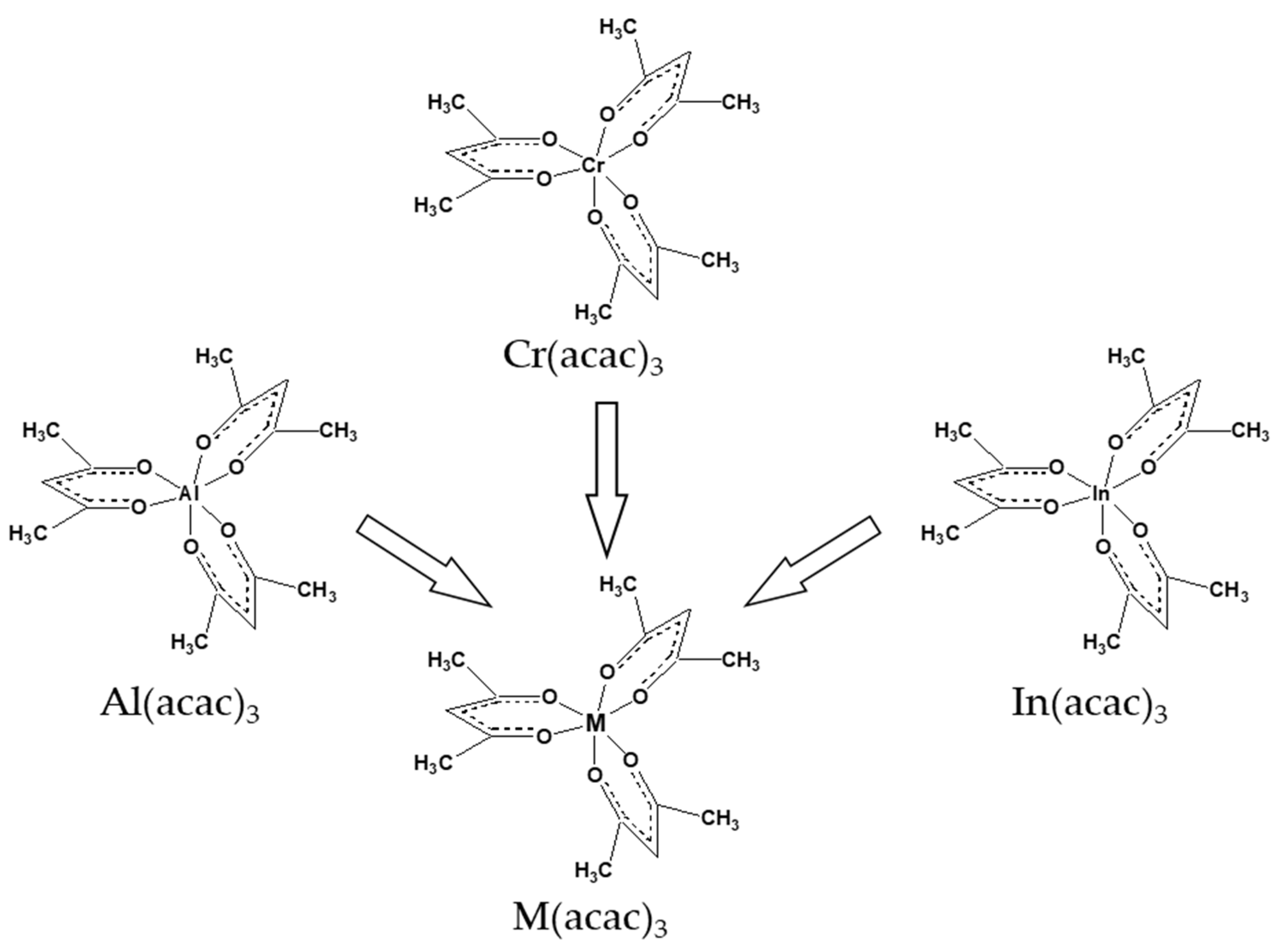
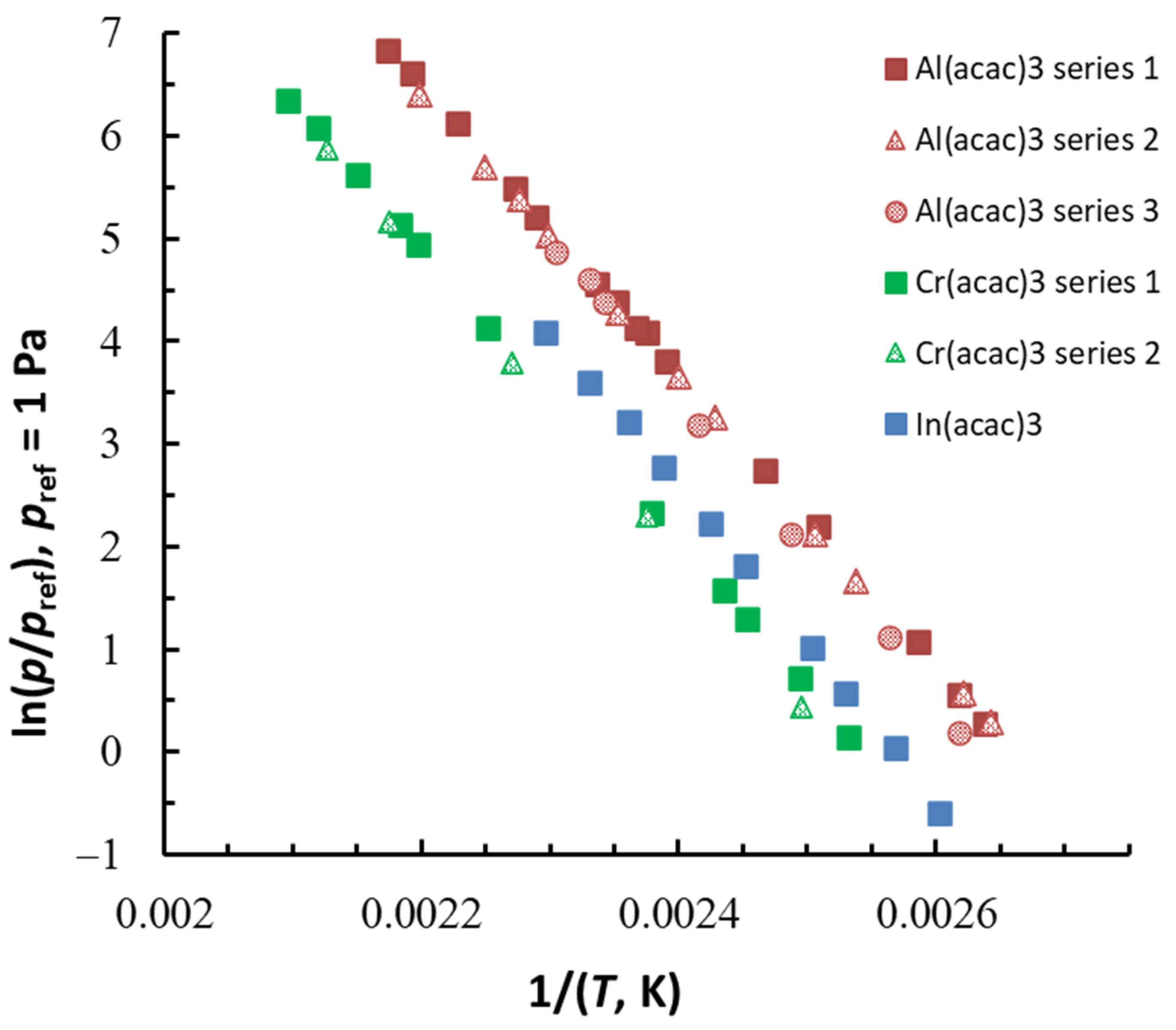
| Compound | ΔT (Tav), K | n b | (Tav), kJ·Mol−1 | (Tav), J·Mol−1 K−1 | (298.15 K), kJ·Mol−1 c | (298.15 K), J·K−1·Mol−1 c |
|---|---|---|---|---|---|---|
| Al(acac)3 series 1 | 379–460 (419.0) | 15 | 117.6 ± 0.8 | 217.0 ± 1.6 | 125.5 ± 1.4 | 239.3 ± 2.6 |
| Al(acac)3 series 2 | 378–455 (416.6) | 11 | 115.3 ± 1.1 | 211.1 ± 1.9 | 123.0 ± 1.6 | 232.9 ± 2.9 |
| Al(acac)3 series 3 | 382–434 (408.0) | 7 | 124.5 ± 4.2 | 232.0 ± 7.7 | 131.6 ± 5.2 | 252.5 ± 9.8 |
| Cr(acac)3 series 1 | 395–477 (436.0) | 11 | 118.6 ± 1.0 | 206.0 ± 1.7 | 127.6 ± 1.5 | 230.8 ± 2.6 |
| Cr(acac)3 series 2 | 401–470 (435.5) | 5 | 122.1 ± 2.0 | 213.0 ± 3.5 | 131.1 ± 2.5 | 237.7 ± 4.3 |
| In(acac)3 | 384–436 (409.6) | 10 | 126.7 ± 2.3 | 229.7 ± 4.2 | 134.0 ± 2.9 | 250.4 ± 5.2 |
| Complex (State) CAS | Method b | T-Range (T), c K | (T), d kJ·mol−1 | (T), d J·K−1·mol−1 | (298.15 K), e kJ·mol−1 | (298.15 K), e J·K−1·mol−1 | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Al(acac)3 (cr) 13963-57-0 | I | 422–493 (458) | 19.14 | - | (30 ± 20) | - | [7,8] |
| K | 374–414 (394) | 104.74 | - | 111.0 ± 7 | 203.2 ± 13 | [9] | |
| I | 383–413 (398) | 66.0 | - | (74 ± 20) | (122 ± 40) | [10] | |
| K | 353–368 (360.5) | 120.0 ± 3.0 | - | 128.4 ± 5.2 | 247.9 ± 9.8 | [11] | |
| GC | 403–473 (438) | 24.2 | 52.7 | (33 ± 20) | (78 ± 40) | [12] | |
| GC | 323–458 (391) | 19.2 | 42.2 | (25 ± 20) | (59 ± 40) | [13] | |
| SB | 388–413 (401) | 105 ± 2 | - | 111.2 ± 7 | 206 ± 13 | [14] | |
| S | 388–404 (396) | 111 ± 4 | - | 117.6 ± 3.3 | 223.1 ± 6.2 | [14] | |
| TE | 337–405 (371) | 47.0 ± 0.5 | - | (52 ± 20) | (80 ± 40) | [15] | |
| S | 426–471 (449) | 108.05 ± 0.36 | 196.54 ± 0.86 | 117.9 ± 1.9 | 223.1 ± 4.0 | [16] | |
| S | 432–464 (448) | 102.0 ± 3.3 | 182.7 ± 4.9 | 111.9 ± 3.6 | 209.6 ± 6.8 | [17] | |
| K/MS | 310–380 (345) | 126.8 ± 1.0 | - | 129.6 ± 1.4 | 256.6 ± 2.7 | [18] | |
| GC | 423–471 (447) | 105.2 ± 3.6 | 191.1 ± 2.7 | 115 ± 10 | 218 ± 20 | [19] | |
| TGA | 413–443 (428) | 93 | 220 | (102 ± 10) | (198 ± 20) | [20] | |
| S | 424–468 (446) | 108.2 ± 4.6 | 197.7 ± 8.4 | 117.7 ± 5.1 | 223.5 ± 9.8 | [21] | |
| CVM | 376–467 (422) | 106.0 ± 2.4 | 191.9 ± 4.3 | 113.8 ± 2.8 | 214.2 ± 5.4 | [21] | |
| K | 345–410 (378) | 117.31 ± 1.67 | - | 121.2 ± 2.3 | 232.8 ± 4.4 | [22,23] | |
| TGA | 433–463 (448) | 107.1 | - | 114.0 ± 5.3 | 216.7 ± 9.8 | [24] | |
| T | 379–460 (419) | 117.6 ± 0.4 | 217.0 ± 0.8 | 125.5 ± 0.7 | 239.3 ± 1.3 | This work, series 1 | |
| T | 378–455 (417) | 115.3 ± 0.5 | 211.1 ± 1.0 | 123.0 ± 0.8 | 232.9 ± 1.4 | This work, series 2 | |
| K/MS | 382–434 (408) | 124.5 ± 2.1 | 232.0 ± 3.9 | 131.6 ± 2.6 | 252.5 ± 4.9 | This work, Series 3 | |
| 123.9 ± 0.9 f | 236.4 ± 1.6 f | This work | |||||
| Al(acac)3 (l) 13963-57-0 | S | 471–536 (504) | 77.54 ± 0.33 | 131.59 ± 0.71 | 104.3 ± 1.1 | 199.8 ± 4.8 | [16] |
| S | 468–515 (492) | 78.7 ± 1.1 | 133.6 ± 1.1 | 104.0 ± 1.2 | 198.7 ± 4.7 | [17] | |
| GC | 471–538 (505) | 78.5 ± 3.6 | 133.8 ± 2.7 | 105 ± 10 | 202 ± 20 | [19] | |
| TGA | 471–513 (492) | 80.2 | – | 104.2 ± 4.4 | 200.1 ± 8.7 | [24] | |
| 104.2 ± 1.6 f | 199.4 ± 6.2 f | This work | |||||
| IC | 100.7 ± 1.9 | 192.0 ± 3.7 | This work | ||||
| Cr(acac)3 (cr) 21679-31-2 | I | 389–397 (393) | 27.76 ± 2.9 | – | (31 ± 20) | (14 ± 40) | [47] |
| K | 374–414 (394) | 91.46 | – | (97.5 ± 7) | (154.0 ± 13) | [9] | |
| T | 365–421 (385) | 112.1 | 291.2 | 118 ± 8 | 206 ± 16 | [48] | |
| SB/SPM | 390–403 (397) | 110.8 ± 0.8 | 185.2 ± 1.6 | 117 ± 10 | 205 ± 20 | [49] | |
| TGA | 335–356 (346) | 85.9 | – | (89 ± 10) | - | [50] | |
| I | 363–393 (378) | 39.7 | – | (45 ± 20) | (60 ± 40) | [10] | |
| K | 374–398 (386) | 123 ± 3 | – | 129.5 ± 4.4 | 234.2 ± 7.9 | [11] | |
| GC | 443–493 (468) | 28.8 | 62.3 | (40 ± 20) | (76 ± 40) | [12] | |
| GC | 323–463 (393) | 28.8 | 62.3 | (35 ± 20) | (64 ± 40) | [13] | |
| S | 462–486 (474) | 127.40 ± 0.80 | 224.00 ± 1.67 | 138.9 ± 7.5 | 254 ± 13 | [16] | |
| DSC | 551 | 141.5 ± 5.6 | – | (158 ± 20) | - | [51] | |
| K | 325–375 (350) | 126.8 ± 2.1 | – | 130.1 ± 2.4 | 265.0 ± 5.0 | [18] | |
| S | 357–486 (422) | 113.0 ± 2.4 | 194.8 ± 5.1 | 120.6 ± 2.9 | 217.2 ± 5.3 | [52] | |
| K/MS | 350–375 (363) | 126.8 ± 2.1 | – | 131.0 ± 3.4 | 242.2 ± 6.2 | [53] | |
| GC | 428–483 (456) | 126.4 ± 3.6 | 233.1 ± 2.7 | 137 ± 10 | 261 ± 20 | [19] | |
| TGA | 413–443 (428) | 91 | 216 | (99 ± 10) | (239 ± 20) | [20] | |
| K/MS | 350–410 (380) | 118.7 ± 2.5 | 206.9 ± 5.9 | 124.0 ± 1.7 | 223.1 ± 3.2 | [54] | |
| K/MS | 320–388 (354) | 127.4 ± 0.6 | 231.5 ± 1.1 | 131.3 ± 1.1 | 242.6 ± 2.2 | [29] | |
| CVM | 384–476 (430) | 127.4 ± 0.5 | 230.5 ± 0.9 | 135.8 ± 1.3 | 254.1 ± 2.9 | [29] | |
| K | 356–411 (384) | 128.20 ± 1.60 | – | 130.3 ± 3.3 | 239.4 ± 6.0 | [22,23] | |
| TGA | 443–465 (454) | 120.8 | – | 124.2 ± 8.9 | 223 ± 16 | [24] | |
| T | 395–477 (436) | 118.6 ± 0.5 | 206.0 ± 0.9 | 127.6 ± 0.8 | 230.8 ± 1.3 | This work, series 1 | |
| T | 401–470 (434) | 122.1 ± 1.0 | 213.0 ± 1.8 | 131.1 ± 1.3 | 237.7 ± 2.2 | This work, series 2 | |
| 129.6 ± 0.9 d | 236.2 ± 1.7 d | This work | |||||
| Cr(acac)3 (l) 21679-31-2 | GC | 483–553 (518) | 84.2 ± 3.6 | 136.8 ± 2.7 | 113 ± 10 | 209 ± 20 | [19] |
| S | 490–536 (513) | 82.2 ± 2.0 | 131.8 ± 4.0 | 110.3 ± 1.5 | 204.4 ± 5.2 | [52] | |
| TGA | 489–552 (521) | 89.9 | – | 114.0 ± 4.1 | 212.0 ± 8.3 | [24] | |
| S | 491–563 (527) | 81.84 ± 0.27 | 131.21 ± 0.53 | 111.5 ± 1.4 | 205.3 ± 5.4 | [16] | |
| 111.1 ± 2.0 f | 206.1 ± 6.7 f | This work | |||||
| IC | 107.1 ± 2.4 | 197.1 ± 4.2 | This work | ||||
| In(acac)3 (cr) 14405-45-9 | GC | 423–478 (451) | 95.7 ± 3.6 | 162.6 ± 2.7 | (105 ± 10) | (190 ± 20) | [19] |
| T | 384–436 (410) | 126.7 ± 1.2 | 229.7 ± 2.1 | 134.0 ± 2.9 | 250.4 ± 5.2 | This work | |
| In(acac)3 (l) 14405-45-9 | S | 435–490 (463) | 86.6 ± 0.2 | 145.2 ± 0.4 | (107.9 ± 1.1) | (202.2 ± 4.2) | [55] |
| GC | 478–533 (506) | 85.1 ± 3.6 | 141.1 ± 2.7 | (112 ± 10) | (210 ± 20) | [19] | |
| Sc(acac)3 (cr) 14284-94-7 | SB/SPM | 380–398 (389) | 99.6 ± 0.8 | 167.4 ± 1.7 | 105.5 ± 10 | 184.6 ± 20 | [49] |
| TGA | 413–443 (428) | 95 | 224 | (103.4 ± 10) | (247.3 ± 20) | [20] | |
| K/MS | 330–390 (360) | 123.8 ± 2.1 | 228.0 ± 5.8 | 127.6 ± 2.4 | 240.2 ± 4.4 | [54] | |
| TGA | 420–450 (424) | 118 ± 4 | – | 126.3 ± 6.7 | – | [56] | |
| K/MS | 345–391 (386) | 119.2 ± 2.1 | – | 124.9 ± 2.2 | – | [57] | |
| K/MS | 330–390 (360) | 124.3 ± 4.4 | 230.7 ± 8.1 | 128.3 ± 4.5 | 242.9 ± 8.4 | [38] | |
| S | 422–460 (441) | 103.2 ± 2.7 | 179.9 ± 4.7 | 112.5 ± 5.1 | 205.5 ± 8.9 | [38] | |
| T | 385–458 (422) | 117.6 ± 0.7 | 212.7 ± 1.2 | 125.6 ± 0.9 | 233.4 ± 2.1 | [25] | |
| T | 394–456 (425) | 120.7 ± 0.8 | 217.9 ± 1.5 | 128.9 ± 1.1 | 240.7 ± 2.0 | [25] | |
| TGA | 403.2 | 119.2 ± 0.8 | 126.1 ± 1.1 | – | [25] | ||
| 126.4 ± 1.1 f | 236.7 ± 2.7 f | [25], this work | |||||
| Sc(acac)3 (l) 14284-94-7 | TGA | 460–520 (490) | 85 ± 4 | – | 109.8 ± 5.3 | – | [56] |
| S | 463–490 (477) | 87.9 ± 1.7 | 146.4 ± 1.2 | 111.7 ± 2.8 | 208.5 ± 4.3 | [38] | |
| 111.3 ± 5.0 f | 208.5 ± 8.6 | [25], this work | |||||
| IC | 109.7 ± 2.5 | 206.0 ± 5.0 | [25], this work | ||||
| Fe(acac)3 (cr) 14024-18-1 | SB/SPM | 378–405 (392) | 99.0 ± 0.8 | 162.8 ± 1.6 | 105 ± 10 | 181 ± 20 | [45] |
| T | 363–423 (393) | 114.2 | 296.6 | 120 ± 8 | 215 ± 16 | [48] | |
| TGA | 335–356 (346) | 114.9 | – | 118 ± 10 | – | [50] | |
| K | 406–441 (424) | 117 ± 16 | 205 ± 30 | 125 ± 16 | 228 ± 30 | [26] | |
| SB | 373–402 (388) | 121 ± 5 | – | 128.2 ± 7 | 237.4 ± 13 | [14] | |
| I | 381–402 (392) | 112 ± 6 | – | 118 ± 10 | 246 ± 21 | [14] | |
| T | 400–458 (429) | 100 | – | 109 ± 20 | – | [26] | |
| C | 298 | 138 ± 5 | – | 138 ± 5 | – | [58] | |
| K/MS | 309–360 (335) | 126.4 ± 1.6 | – | 130.8 ± 2.0 | 245.4 ± 3.7 | [53] | |
| LT | 338–355 (347) | 114.2 ± 1.5 | – | 117 ± 10 | 227 ± 20 | [59] | |
| GC | 453–488 (471) | 132.9 ± 3.6 | 232.6 ± 2.7 | 144 ± 10 | 262 ± 20 | [19] | |
| TE | 369–388 (378.5) | 124.6 ± 1.3 | – | 129.8 ± 1.9 | 244.4 ± 3.6 | [60] | |
| K | 369–388 (379) | 124.7 ± 1.2 | – | 129.7 ± 1.8 | 240.1 ± 3.3 | [60] | |
| TGA | 430–450 (440) | 118 | – | 127 ± 10 | – | [61] | |
| TGA | 413–443 (428) | 112 | 259 | 120 ± 10 | 283 ± 20 | [20] | |
| K/MS | 340–405 (373) | 130.5 ± 2.5 | 241.2 ± 5.9 | 135.5 ± 2.7 | 256.1 ± 5.2 | [54] | |
| T | 400–458 (429) | 124.1 ± 1.2 | 223.7 ± 2.7 | 132.2 ± 2.0 | 246.4 ± 4.0 | [62] | |
| 130.6 ± 1.7 f | 244.4 ± 3.3 f | [26], this work | |||||
| Fe (acac)3 (l) 14024-18-1 | TGA | 452–535 (494) | 82 ± 1 | – | 108 ± 5 | – | [63] |
| GC | 488–548 (518) | 93.3 ± 3.6 | 154.1 ± 2.7 | 122 ± 10 | 226 ± 20 | [19] | |
| 110.8 ± 8.9 f | 226 ± 20 | [26], this work | |||||
| IC | 109.1 ± 2.9 | 203.0 ± 5.5 | [26], this work | ||||
| Ir(acac)3 (cr) 15635-87-7 | 136.8 ± 1.4 | 234.3 ± 2.8 | [27], this work | ||||
| Mn(acac)3 (cr) 14284-89-0 | I | 383-391 (387) | 77.8 ± 0.8 | – | (83.5 ± 20) | (174.1 ± 40) | [47] |
| T | 355-445 (385) | 113.0 | 293.3 | 118.6 ± 8 | 309 ± 16 | [48] | |
| TGA | 335-356 | 117.3 | – | 120.4 ± 10 | – | [50] | |
| K/MS | 320-380 (350) | 124.7 ± 1.9 | – | 128.0 ± 2.2 | 238.1 ± 4.0 | [53] | |
| K/MS | 340-400 (370) | 132.2 ± 2.5 | 246.4 ± 9.6 | 137.0 ± 2.4 | 259.4 ± 4.4 | [54] | |
| 131.3 ± 3.1 f | 249.6 ± 5.8 f | This work | |||||
| Mn(acac)3 (l) 14284-89-0 | IC | 19.7 ± 2.6 | 43.2 ± 5.7 | 111.6 ± 4.0 | 206.4 ± 8.1 | This work | |
| Co(acac)3 (cr) 21679-46-9 | I | 378-393 (386) | 74.9 ± 4.6 | – | (81.8 ± 20) | (159.2 ± 40) | [47] |
| T | 350-430 (390) | 107.1 | 274.5 | 112.8 ± 8 | 291.2 ± 16 | [48] | |
| TGA | 335-361 (348) | 86.3 | – | (89.6 ± 10) | – | [45] | |
| DSC | 453 | 142.6 ± 6.9 | – | (151.9 ± 20) | – | [64] | |
| K/MS | 318-382 (350) | 134.6 ± 2.1 | – | 138.0 ± 2.4 | 255.6 ± 4.3 | [53] | |
| TGA | 433-463 (448) | 138 | 311 | 147.3 ± 10 | (336.3 ± 20) | [20] | |
| K/MS | 350-415 (383) | 120.1 ± 3.8 | 207.1 ± 9.6 | 125.5 ± 1.6 | 222.7 ± 4.3 | [54] | |
| 129.2 ± 2.6 f | 241.0 ± 6.0 f | This work | |||||
| Ru(acac)3 (cr) 14284-93-6 | T | 423-493 (458) | 127.0 ± 0.5 | 212.5 ± 1.0 | 137.1 ± 0.9 | 240.9 ± 2.6 | [65] |
| K | 398-413 (406) | 139.7 ± 2.5 | – | 150.0 ± 6.7 | 270 ± 12 | [66] | |
| K | 394-441 (418) | 148.81 ± 1.68 | – | 158.1 ± 6.7 | 277 ± 12 | [67] | |
| K/MS | 377-434 (406) | 129.1 ± 1.0 | 210.0 ± 2.5 | 136.2 ± 1.8 | 229.4 ± 3.2 | [68] | |
| 137.4 ± 1.6 f | 238.3 ± 3.9 f | This work | |||||
| Ru(acac)3 (l) 14284-93-6 | IC | 11.4 ± 4.1 | 15.0 ± 5.4 | 126.0 ± 4.4 | 223.3 ± 6.7 | This work | |
| Rh(acac)3 (cr) 14284-92-5 | T | 412-483 (448) | 128.28 ± 0.50 | 211.63 ± 1.13 | 138.2 ± 0.9 | 238.9 ± 2.5 | [69] |
| K/MS | 348-414 (381) | 121.65 ± 0.51 | 199.45 ± 1.32 | 127.0 ± 1.2 | 215.7 ± 2.3 | [69] | |
| S | 473-498 (486) | 100.8 ± 1.9 | 156.1 ± 2.2 | (113 ± 20) | (189 ± 40) | [69] | |
| T | 398-463 (431) | 127.1 ± 0.6 | 207.9 ± 1.0 | 135.9 ± 1.0 | 232.4 ± 2.4 | [46] | |
| S | 458-521 (490) | 127.0 ± 1.0 | 208.0 ± 1.6 | 139.8 ± 1.2 | 241.1 ± 3.1 | [46] | |
| 135.7 ± 1.1 f | 230.5 ± 2.5 f | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarenko, A.M.; Trubin, S.V.; Zherikova, K.V. Breaking through the Thermodynamics “Wilds” of Metal–Organic Chemical Vapor Deposition Precursors: Metal tris-Acetylacetonates. Coatings 2023, 13, 1458. https://doi.org/10.3390/coatings13081458
Makarenko AM, Trubin SV, Zherikova KV. Breaking through the Thermodynamics “Wilds” of Metal–Organic Chemical Vapor Deposition Precursors: Metal tris-Acetylacetonates. Coatings. 2023; 13(8):1458. https://doi.org/10.3390/coatings13081458
Chicago/Turabian StyleMakarenko, Alexander M., Sergey V. Trubin, and Kseniya V. Zherikova. 2023. "Breaking through the Thermodynamics “Wilds” of Metal–Organic Chemical Vapor Deposition Precursors: Metal tris-Acetylacetonates" Coatings 13, no. 8: 1458. https://doi.org/10.3390/coatings13081458





