Spin Coating of Silica Nanocolloids on Mica: Self-Assembly of Two-Dimensional Colloid Crystal Structures and Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
- Program A: The first set of experiments had a rotational speed of 8000 rpm with a 50 rpm/s acceleration for a total of 520 s, resulting in a spin-up time of 160 s, with 8000 rpm maintained for 360 s.
- Program B: The second set of experiments had a rotational speed of 4000 rpm with different rotational accelerations (200 to 1000 rpm/s in 200 rpm/s steps) for 120 s, followed by 8000 rpm for 360 s.
- Program C: The third set of experiments had different rotational speeds (200 to 1000 rpm with 200 rpm steps, then 2000 to 8000 rpm with 2000 rpm steps) with a 1000 rpm/s acceleration for 120 s followed by 8000 rpm for 360 s.
2.3. Measurements
3. Results and Discussion
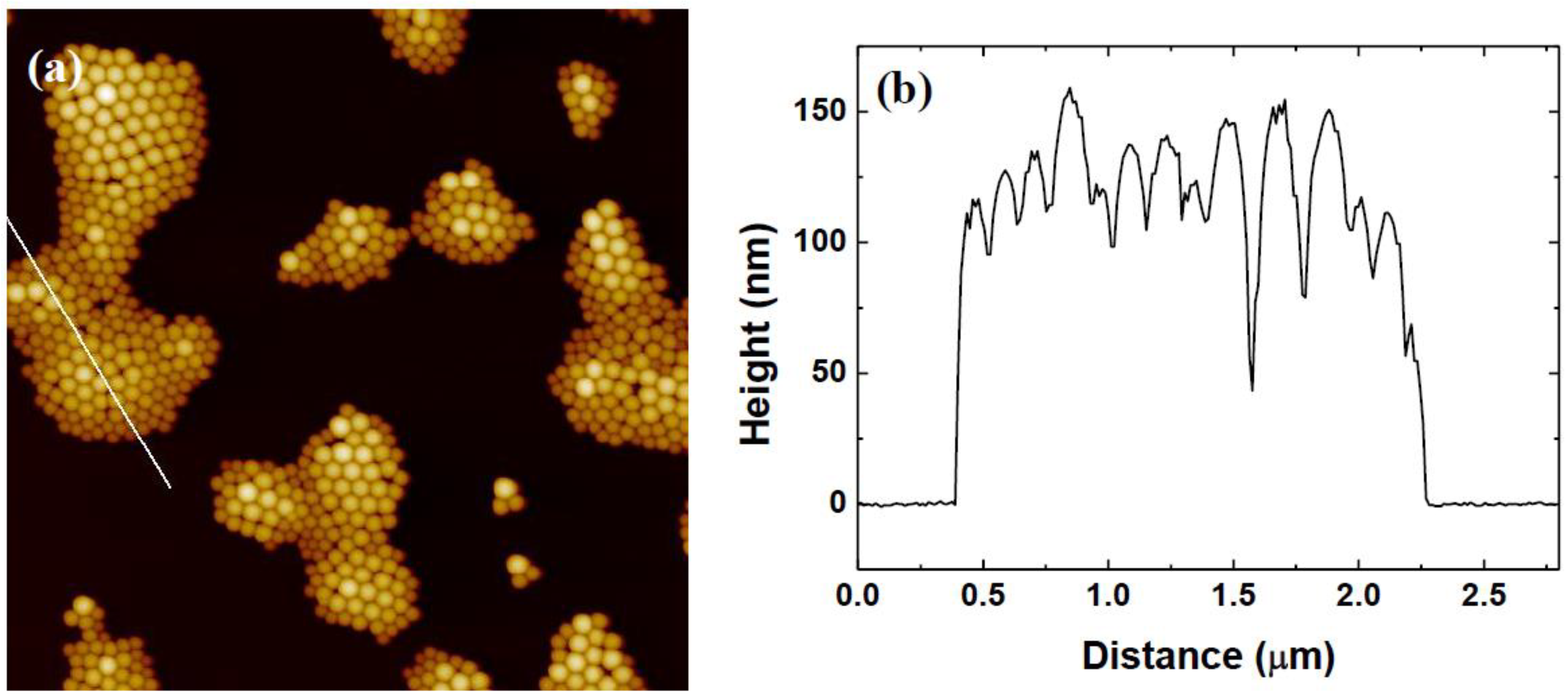
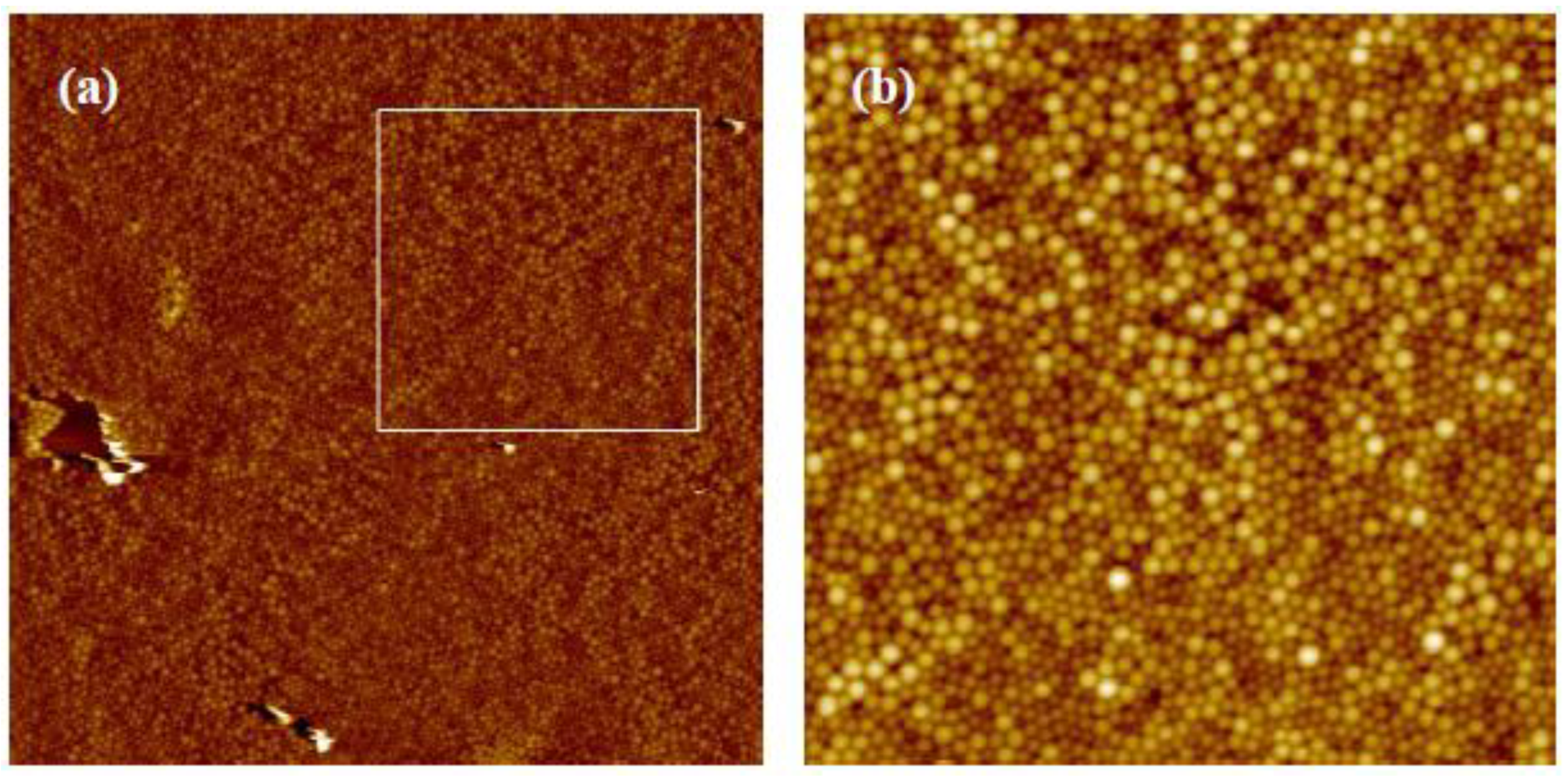
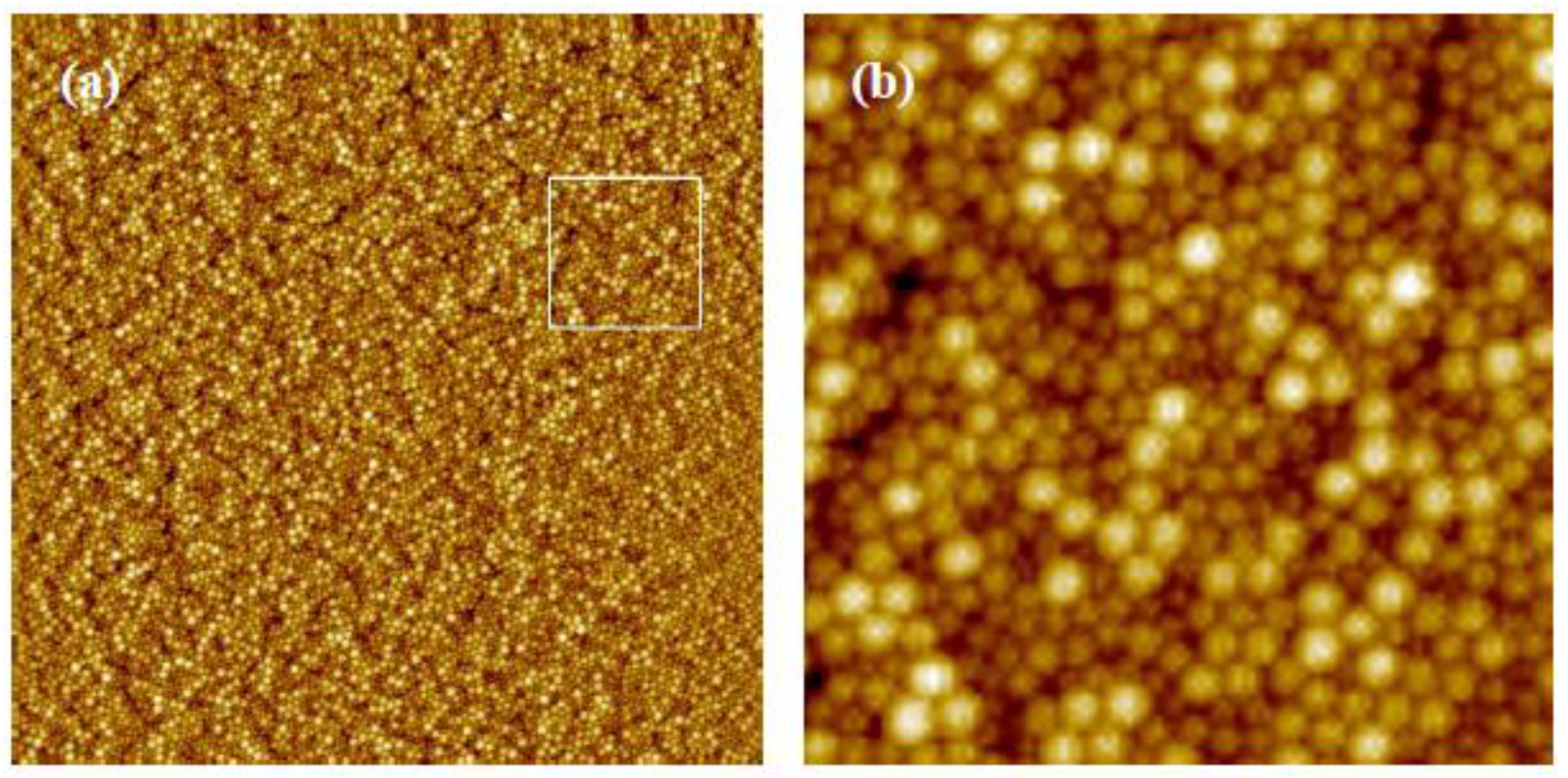
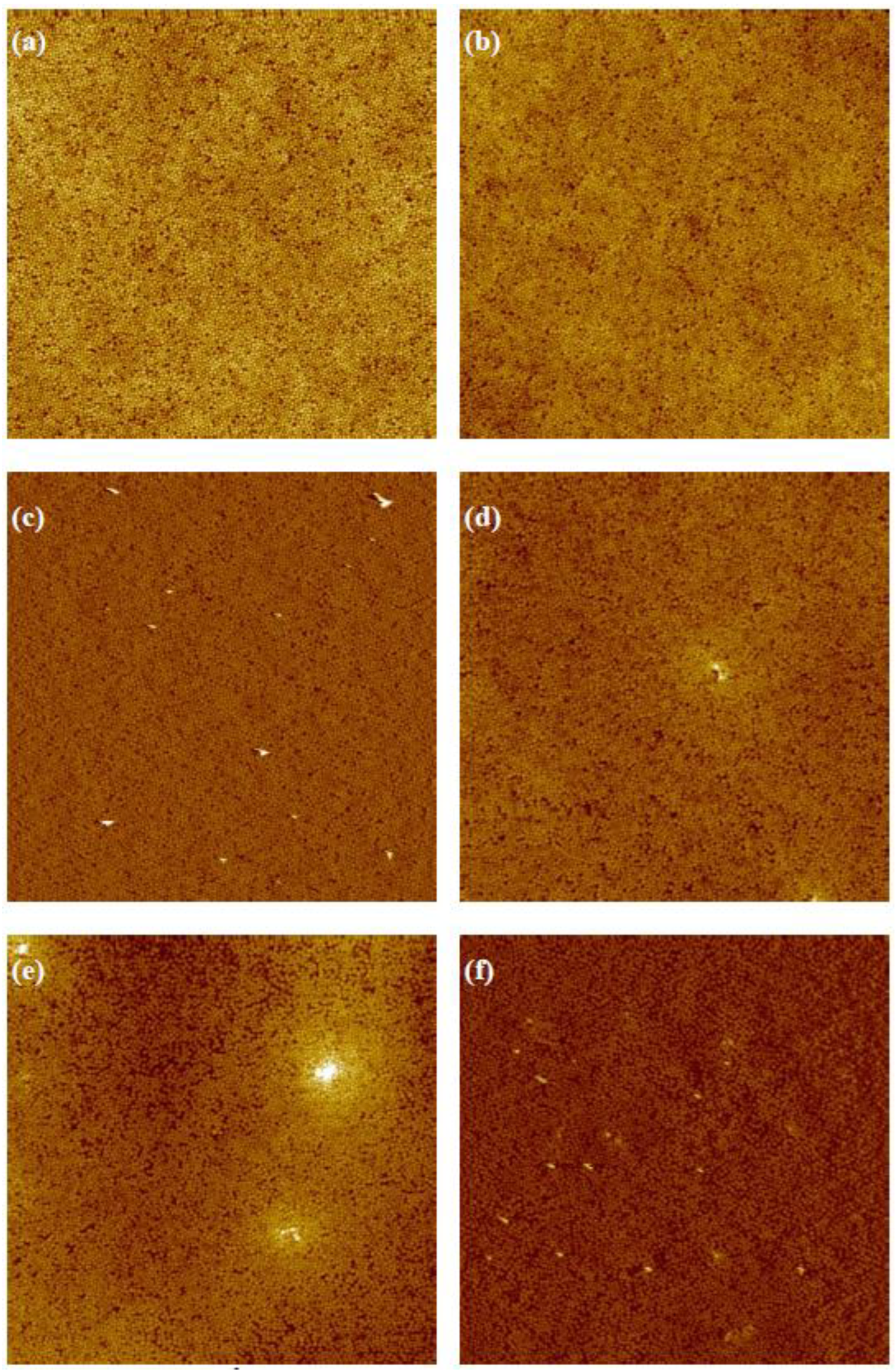
3.1. Variation of Colloidal Concentration (Program A)
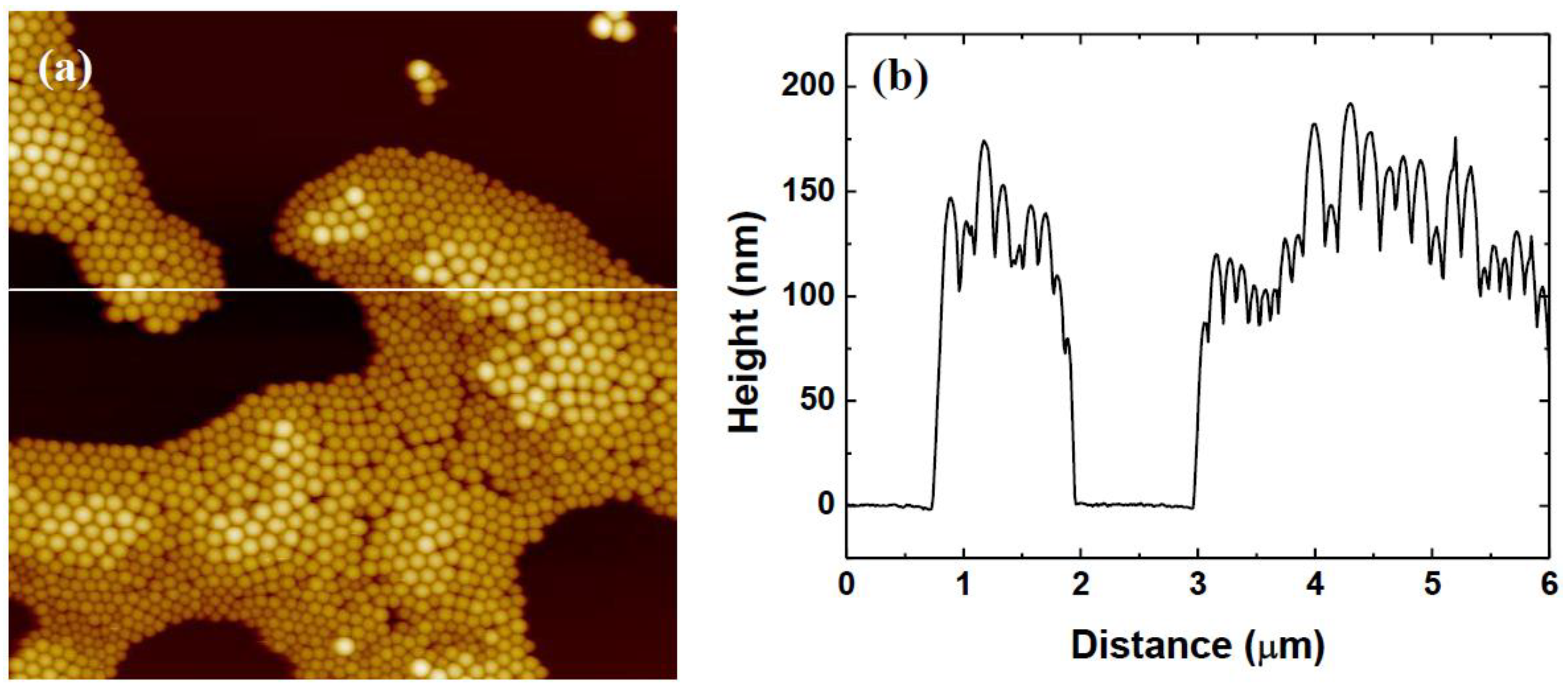
3.2. Variation of Rotational Acceleration (Program B)
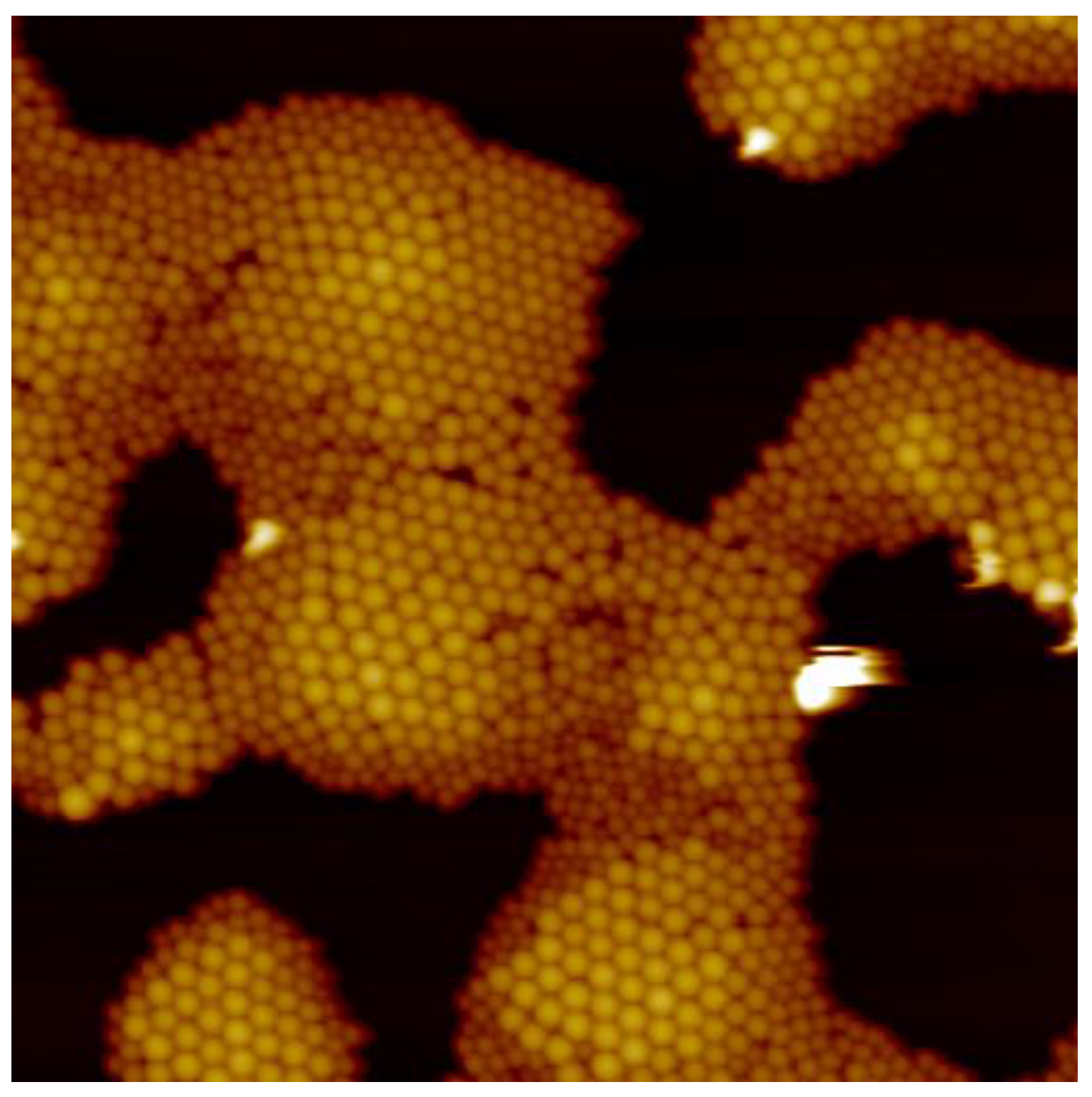
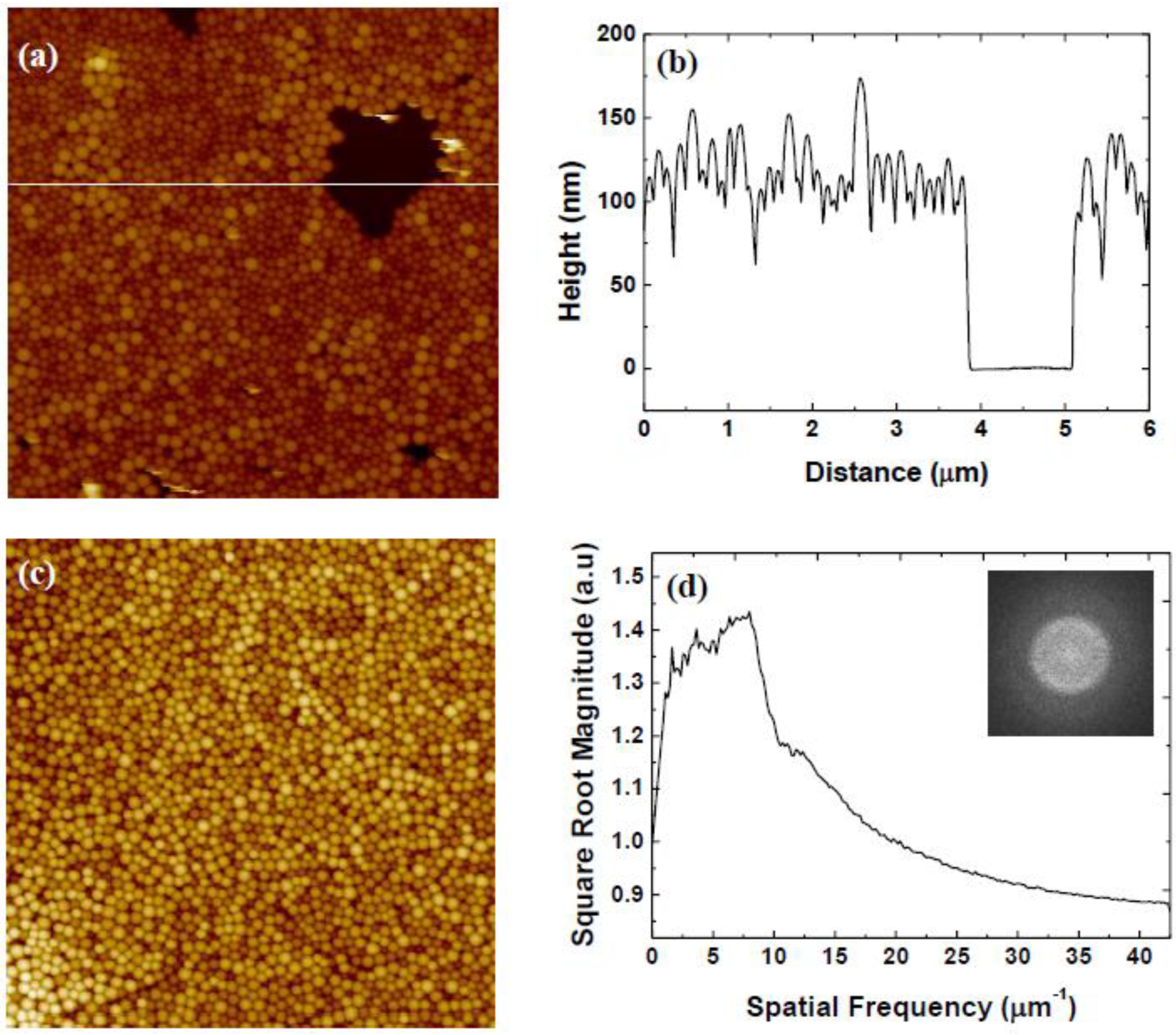
3.3. Variation of Rotational Speed (Program C)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, E.; Rathi, R.; Misharwal, J.; Sinhmar, B.; Kumari, S.; Dalal, J.; Kumar, A. Evolution in Lithography Techniques: Microlithography to Nanolithography. Nanomaterials 2022, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, E.M.; Jain, S.K.; Purohit, R.; Dhakad, S.K.; Rana, R.S. Nanolithography and its current advancements. Mater. Today Proc. 2020, 26, 2351–2356. [Google Scholar] [CrossRef]
- Yoo, S.T.; Park, K.C. Extreme Ultraviolet Lighting Using Carbon Nanotube-Based Cold Cathode Electron Beam. Nanomaterials 2022, 12, 4134. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal Self-Assembly Approaches to Smart Nanostructured Materials. Chem. Rev. 2022, 122, 4976–5067. [Google Scholar] [CrossRef]
- Dwivedi, M.; Singh, S.L.; Bharadwaj, A.S.; Kishore, V.; Singh, A.V. Self-Assembly of DNA-Grafted Colloids: A Review of Challenges. Micromachines 2022, 13, 1102. [Google Scholar] [CrossRef]
- Amadi, E.V.; Venkataraman, A.; Papadopoulos, C. Nanoscale self-assembly: Concepts, applications and challenges. Nanotechnology 2022, 33, 132001. [Google Scholar] [CrossRef]
- Koutsos, V.; Walker, J.; Glynos, E. Self-Assembly of Colloidal Nanoparticles on Surfaces: Towards Surface Nanopatterning. In Nanostructured Materials and Their Applications; Logothetidis, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 191–211. [Google Scholar]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Glynos, E.; Chremos, A.; Camp, P.J.; Koutsos, V. Surface Nanopatterning Using the Self-Assembly of Linear Polymers on Surfaces after Solvent Evaporation. Nanomanuf. Metrol. 2022, 5, 297–309. [Google Scholar] [CrossRef]
- Glynos, E.; Chremos, A.; Petekidis, G.; Camp, P.J.; Koutsos, V. Polymer-like to Soft Colloid-like Behavior of Regular Star Polymers Adsorbed on Surfaces. Macromolecules 2007, 40, 6947–6958. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, H.M.; Yang, G.G.; Han, K.H.; Yun, T.; Shin, J.Y.; Jeong, S.-J.; Kim, S.O. Smart Nanostructured Materials based on Self-Assembly of Block Copolymers. Adv. Funct. Mater. 2020, 30, 1902049. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Doerk, G.S. Thin film block copolymer self-assembly for nanophotonics. Nanotechnology 2022, 33, 292001. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, R.; Fanzio, P.; Sasso, L. Surface self-assembly of colloidal crystals for micro- and nano-patterning. Adv. Colloid Interface Sci. 2018, 251, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, J.C.; Van Duyne, R.P. Nanosphere lithography: A materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Technol. A 1995, 13, 1553–1558. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Ngqoloda, S.; Pescetelli, S.; Di Carlo, A.; Dinu, M.; Vladescu, A.; Parau, A.C.; Agresti, A.; Braic, M.; Arendse, C.J.; et al. Spin Coating Immobilisation of C-N-TiO2 Co-Doped Nano Catalyst on Glass and Application for Photocatalysis or as Electron Transporting Layer for Perovskite Solar Cells. Coatings 2020, 10, 1029. [Google Scholar] [CrossRef]
- Faraco, T.A.; Yoshioka, N.A.; Sábio, R.M.; Barud, H.d.S.; Maciel, I.O.; Quirino, W.G.; Fragneaud, B.; Aguiar, A.M.d.; Ribeiro, S.J.L.; Cremona, M.; et al. Monolayer of silica nanospheres assembled onto ITO-coated glass substrates by spin-coating. Nanotechnology 2021, 32, 205603. [Google Scholar] [CrossRef]
- Pliatsikas, N.; Kalfagiannis, N.; Arvanitidis, J.; Christofilos, D.; Koutsogeorgis, D.C.; Kagkoura, A.; Sefiane, K.; Koutsos, V.; Patsalas, P. Edge-engineered self-assembled hierarchical plasmonic SERS templates. Appl. Surf. Sci. Adv. 2021, 6, 100186. [Google Scholar] [CrossRef]
- Yadav, A.; Gerislioglu, B.; Ahmadivand, A.; Kaushik, A.; Cheng, G.J.; Ouyang, Z.; Wang, Q.; Yadav, V.S.; Mishra, Y.K.; Wu, Y.; et al. Controlled self-assembly of plasmon-based photonic nanocrystals for high performance photonic technologies. Nano Today 2021, 37, 101072. [Google Scholar] [CrossRef]
- Mayer, M.; Schnepf, M.J.; König, T.A.F.; Fery, A. Colloidal Self-Assembly Concepts for Plasmonic Metasurfaces. Adv. Opt. Mater. 2019, 7, 1800564. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Mouquinho, A.; Centeno, P.; Alexandre, M.; Haque, S.; Martins, R.; Fortunato, E.; Águas, H.; Mendes, M.J. Colloidal Lithography for Photovoltaics: An Attractive Route for Light Management. Nanomaterials 2021, 11, 1665. [Google Scholar] [CrossRef]
- Bolshakov, E.S.; Schemelev, I.S.; Ivanov, A.V.; Kozlov, A.A. Photonic Crystals and Their Analogues as Tools for Chemical Analysis. J. Anal. Chem. 2022, 77, 1215–1235. [Google Scholar] [CrossRef]
- Wan, Y.-Z.; Qian, W. From Self-Assembly of Colloidal Crystals toward Ordered Porous Layer Interferometry. Biosensors 2023, 13, 730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Han, L.; Tam, K.C. Superhydrophobic surfaces from sustainable colloidal systems. Curr. Opin. Colloid Interface Sci. 2022, 57, 101534. [Google Scholar] [CrossRef]
- Utsav; Khanna, S.; Paneliya, S.; Makani, N.H.; Mukhopadhyay, I.; Banerjee, R. Controlled restructuring of bidisperse silica nanospheres for size-selective nanowire growth. Mater. Chem. Phys. 2021, 273, 125063. [Google Scholar] [CrossRef]
- Xu, X.; Hou, S.; Wattanatorn, N.; Wang, F.; Yang, Q.; Zhao, C.; Yu, X.; Tseng, H.-R.; Jonas, S.J.; Weiss, P.S. Precision-Guided Nanospears for Targeted and High-Throughput Intracellular Gene Delivery. ACS Nano 2018, 12, 4503–4511. [Google Scholar] [CrossRef]
- Yu, J.; Lee, C.H.; Kan, C.-W.; Jin, S. Fabrication of Structural-Coloured Carbon Fabrics by Thermal Assisted Gravity Sedimentation Method. Nanomaterials 2020, 10, 1133. [Google Scholar] [CrossRef]
- Lu, Z.; Owens, H. Optimum processing parameters for coating polyester with silica nanoparticles using gravity sedimentation. J. Nanoparticle Res. 2019, 21, 212. [Google Scholar] [CrossRef]
- Hung, P.-S.; Liao, C.-H.; Chou, Y.-S.; Wang, G.-R.; Wang, C.-J.; Chung, W.-A.; Wu, P.-W. High throughput fabrication of large-area colloidal crystals via a two-stage electrophoretic deposition method. Electrochim. Acta 2019, 317, 52–60. [Google Scholar] [CrossRef]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. Structural transitions in a ring stain created at the contact line of evaporating nanosuspension sessile drops. Phys. Rev. E 2013, 87, 012301. [Google Scholar] [CrossRef] [PubMed]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. The effect of evaporation kinetics on nanoparticle structuring within contact line deposits of volatile drops. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 855–866. [Google Scholar] [CrossRef]
- Mutch, K.J.; Koutsos, V.; Camp, P.J. Deposition of Magnetic Colloidal Particles on Graphite and Mica Surfaces Driven by Solvent Evaporation. Langmuir 2006, 22, 5611–5616. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chujo, S.; Nishikawa, H.; Yamaguchi, Y.; Okubo, T. Spontaneous formation of large-area monolayers of well-ordered nanoparticles via a wet-coating process. J. Nanoparticle Res. 2004, 6, 479–487. [Google Scholar] [CrossRef]
- Fang, X.; Zheng, C.; Yin, Z.; Wang, Z.; Wang, J.; Liu, J.; Luo, D.; Liu, Y.J. Hierarchically Ordered Silicon Metastructures from Improved Self-Assembly-Based Nanosphere Lithography. ACS Appl. Mater. Interfaces 2020, 12, 12345–12352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sidnawi, B.; Fan, J.; Chen, R.; Scully, T.; Dietrich, S.; Gao, W.; Wu, Q.; Li, B. Wafer-Scale Full-Coverage Self-Limiting Assembly of Particles on Flexible Substrates. ACS Appl. Mater. Interfaces 2022, 14, 46095–46102. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Miyahara, M.T. Particulate pattern formation and its morphology control by convective self-assembly. Adv. Powder Technol. 2013, 24, 897–907. [Google Scholar] [CrossRef]
- Kim, M.H.; Im, S.H.; Park, O.O. Rapid Fabrication of Two- and Three-Dimensional Colloidal Crystal Films via Confined Convective Assembly. Adv. Funct. Mater. 2005, 15, 1329–1335. [Google Scholar] [CrossRef]
- Sun, J.; Tang, C.-j.; Zhan, P.; Han, Z.-l.; Cao, Z.-S.; Wang, Z.-L. Fabrication of Centimeter-Sized Single-Domain Two-Dimensional Colloidal Crystals in a Wedge-Shaped Cell under Capillary Forces. Langmuir 2010, 26, 7859–7864. [Google Scholar] [CrossRef]
- Bayat, F.; Chaghamirzaei, P.; Nikniazi, A.; Ahmadi-Kandjani, S.; Rashidi, M.-R.; Tajalli, H. Optimizing the concentration of colloidal suspensions in convective assembly of centimeter-sized uniform monolayer colloidal crystals. Appl. Surf. Sci. 2018, 434, 898–904. [Google Scholar] [CrossRef]
- Li, J.; Han, Y. Optical Intensity Gradient by Colloidal Photonic Crystals with a Graded Thickness Distribution. Langmuir 2006, 22, 1885–1890. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Connor, S.T.; Tang, M.X.; Cui, Y. Wafer-scale silicon nanopillars and nanocones by Langmuir–Blodgett assembly and etching. Appl. Phys. Lett. 2008, 93, 133109. [Google Scholar] [CrossRef]
- Gu, P.; Cai, X.; Chen, S.; Zhang, Z.; Chen, J.; Du, W.; Tang, C.; Yan, Z.; Chen, Z. Rapid fabrication of high-quality bare silica monolayer and multilayers at the water/air interface. Results Phys. 2020, 19, 103404. [Google Scholar] [CrossRef]
- Walker, J.; Schofield, A.B.; Koutsos, V. Nanostructures and Thin Films of Poly(Ethylene Glycol)-Based Surfactants and Polystyrene Nanocolloid Particles on Mica: An Atomic Force Microscopy Study. Coatings 2023, 13, 1187. [Google Scholar] [CrossRef]
- García Núñez, C.; Navaraj, W.T.; Liu, F.; Shakthivel, D.; Dahiya, R. Large-Area Self-Assembly of Silica Microspheres/Nanospheres by Temperature-Assisted Dip-Coating. ACS Appl. Mater. Interfaces 2018, 10, 3058–3068. [Google Scholar] [CrossRef]
- Osipov, A.A.; Gagaeva, A.E.; Speshilova, A.B.; Endiiarova, E.V.; Bespalova, P.G.; Osipov, A.A.; Belyanov, I.A.; Tyurikov, K.S.; Tyurikova, I.A.; Alexandrov, S.E. Development of controlled nanosphere lithography technology. Sci. Rep. 2023, 13, 3350. [Google Scholar] [CrossRef]
- Lan, N.T.T.; Hoang, C.M. Fabrication and Characteristics of Silica Nanoparticle Monolayer Assembled by Spin Coating. VNU J. Sci. Math. Phys. 2022, 38, 111–118. [Google Scholar] [CrossRef]
- Razaulla, T.; Bekeris, M.; Feng, H.; Beeman, M.; Nze, U.; Warren, R. Multiple Linear Regression Modeling of Nanosphere Self-Assembly via Spin Coating. Langmuir 2021, 37, 12419–12428. [Google Scholar] [CrossRef] [PubMed]
- Noppakuadrittidej, P.; Tonsomboon, K.; Ummartyotin, S. Importance of solvent singularity on the formation of highly uniform hexagonal close packed (HCP) colloidal monolayers during spin coating. Colloid Interface Sci. Commun. 2019, 30, 100177. [Google Scholar] [CrossRef]
- Khanna, S.; Utsav; Chaliyawala, H.; Paneliya, S.; Roy, D.; Mukhopadhyay, K.; Banerjee, R.; Mukhopadhyay, I. Systematic investigation of close-packed silica nanospheres monolayer under sintering conditions. J. Eur. Ceram. Soc. 2019, 39, 1411–1419. [Google Scholar] [CrossRef]
- Khanna, S.; Utsav; Marathey, P.; Chaliyawala, H.; Rajaram, N.; Roy, D.; Banerjee, R.; Mukhopadhyay, I. Fabrication of long-ranged close-packed monolayer of silica nanospheres by spin coating. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 520–527. [Google Scholar] [CrossRef]
- Chandramohan, A.; Sibirev, N.V.; Dubrovskii, V.G.; Petty, M.C.; Gallant, A.J.; Zeze, D.A. Model for large-area monolayer coverage of polystyrene nanospheres by spin coating. Sci. Rep. 2017, 7, 40888. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Alford, T.L.; Honsberg, C.B. Solvent-Controlled Spin-Coating Method for Large-Scale Area Deposition of Two-Dimensional Silica Nanosphere Assembled Layers. Langmuir 2014, 30, 5732–5738. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, P.; Di, D.; Wang, C.; Wang, H.; Wang, J.; Wu, X. Controllable fabrication of 2D colloidal-crystal films with polystyrene nanospheres of various diameters by spin-coating. Appl. Surf. Sci. 2013, 270, 6–15. [Google Scholar] [CrossRef]
- Ogi, T.; Modesto-Lopez, L.B.; Iskandar, F.; Okuyama, K. Fabrication of a large area monolayer of silica particles on a sapphire substrate by a spin coating method. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 71–78. [Google Scholar] [CrossRef]
- Colson, P.; Cloots, R.; Henrist, C. Experimental Design Applied to Spin Coating of 2D Colloidal Crystal Masks: A Relevant Method? Langmuir 2011, 27, 12800–12806. [Google Scholar] [CrossRef]
- Jurewicz, I.; King, A.A.K.; Worajittiphon, P.; Asanithi, P.; Brunner, E.W.; Sear, R.P.; Hosea, T.J.C.; Keddie, J.L.; Dalton, A.B. Colloid-Assisted Self-Assembly of Robust, Three-Dimensional Networks of Carbon Nanotubes over Large Areas. Macromol. Rapid Commun. 2010, 31, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Yang, Y.; Yan, H.; Guan, L.; Li, Y.; Qiu, X.; Wang, C. Quantifying Surface Charge Density by Using an Electric Force Microscope with a Referential Structure. J. Phys. Chem. C 2009, 113, 204–207. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Alcantar, N.A.; Maeda, N.; Mates, T.E.; Ruths, M. Preparing Contamination-free Mica Substrates for Surface Characterization, Force Measurements, and Imaging. Langmuir 2004, 20, 3616–3622. [Google Scholar] [CrossRef]
- Bergström, L.; Bostedt, E. Surface chemistry of silicon nitride powders: Electrokinetic behaviour and ESCA studies. Colloids Surf. 1990, 49, 183–197. [Google Scholar] [CrossRef]
- Johnson, C.A.; Lenhoff, A.M. Adsorption of Charged Latex Particles on Mica Studied by Atomic Force Microscopy. J. Colloid Interface Sci. 1996, 179, 587–599. [Google Scholar] [CrossRef]
- Antonietti, M.; Hartmann, J.; Neese, M.; Seifert, U. Highly Ordered Size-Dispersive Packings of Polydisperse Microgel Spheres. Langmuir 2000, 16, 7634–7639. [Google Scholar] [CrossRef]
- Yamaki, M.; Higo, J.; Nagayama, K. Size-Dependent Separation of Colloidal Particles In Two-Dimensional Convective Self-Assembly. Langmuir 1995, 11, 2975–2978. [Google Scholar] [CrossRef]
- Kralchevsky, P.A.; Paunov, V.N.; Ivanov, I.B.; Nagayama, K. Capillary meniscus interaction between colloidal particles attached to a liquid—Fluid interface. J. Colloid Interface Sci. 1992, 151, 79–94. [Google Scholar] [CrossRef]
- Denkov, N.; Velev, O.; Kralchevski, P.; Ivanov, I.; Yoshimura, H.; Nagayama, K. Mechanism of formation of two-dimensional crystals from latex particles on substrates. Langmuir 1992, 8, 3183–3190. [Google Scholar] [CrossRef]
- Rehg, T.J.; Higgins, G. Spin coating of colloidal suspensions. AIChE J. 1992, 38, 489–501. [Google Scholar] [CrossRef]
- Smith, B.; Vasut, J.; Hyde, T.; Matthews, L.; Reay, J.; Cook, M.; Schmoke, J. Dusty plasma correlation function experiment. Adv. Space Res. 2004, 34, 2379–2383. [Google Scholar] [CrossRef]
- Moon, J.; Park, J.-A.; Lee, S.-J.; Zyung, T. Insight into the Shear-Induced Ordering of Colloidal Particles by a Spin-Coating Method. Jpn. J. Appl. Phys. 2008, 47, 7968–7971. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a Viscous Liquid on a Rotating Disk. J. Appl. Phys. 2004, 29, 858–862. [Google Scholar] [CrossRef]
- Meyerhofer, D. Characteristics of resist films produced by spinning. J. Appl. Phys. 1978, 49, 3993–3997. [Google Scholar] [CrossRef]

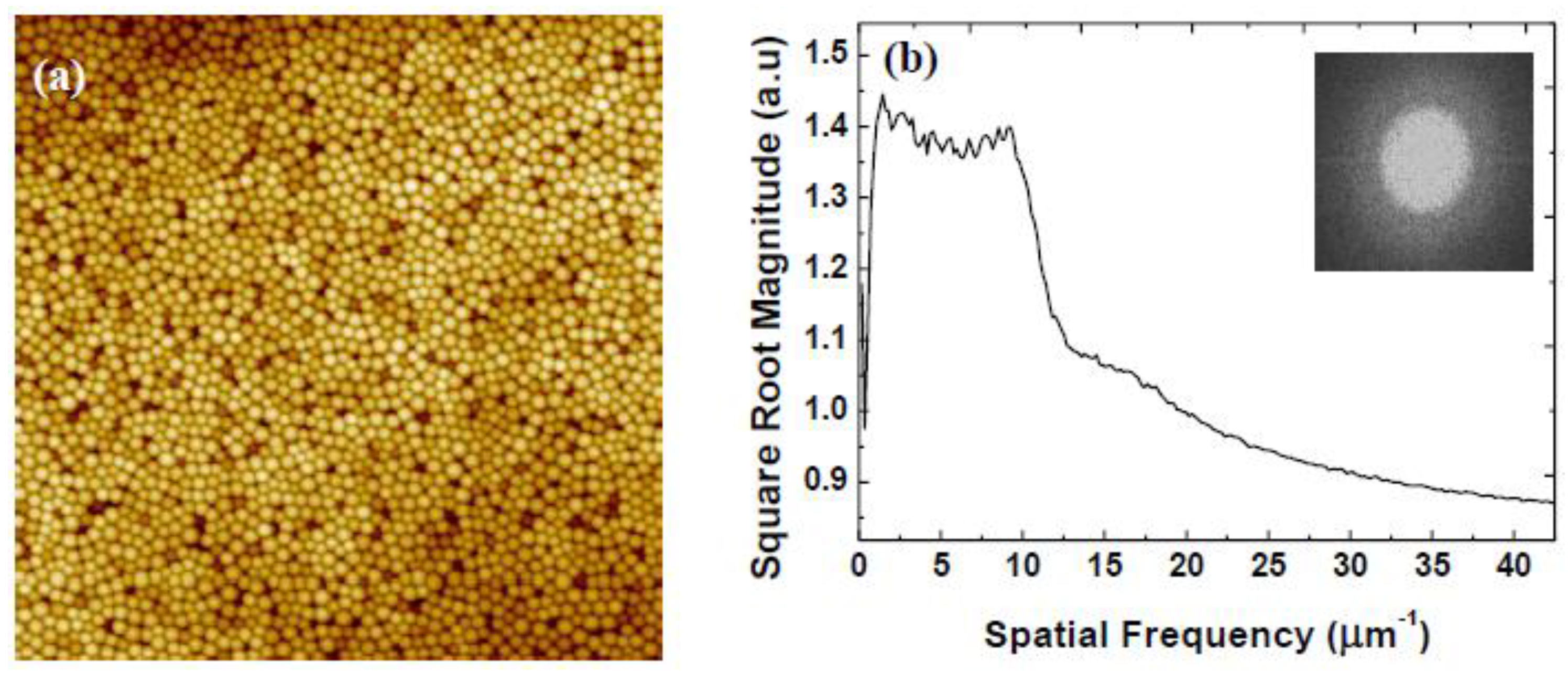
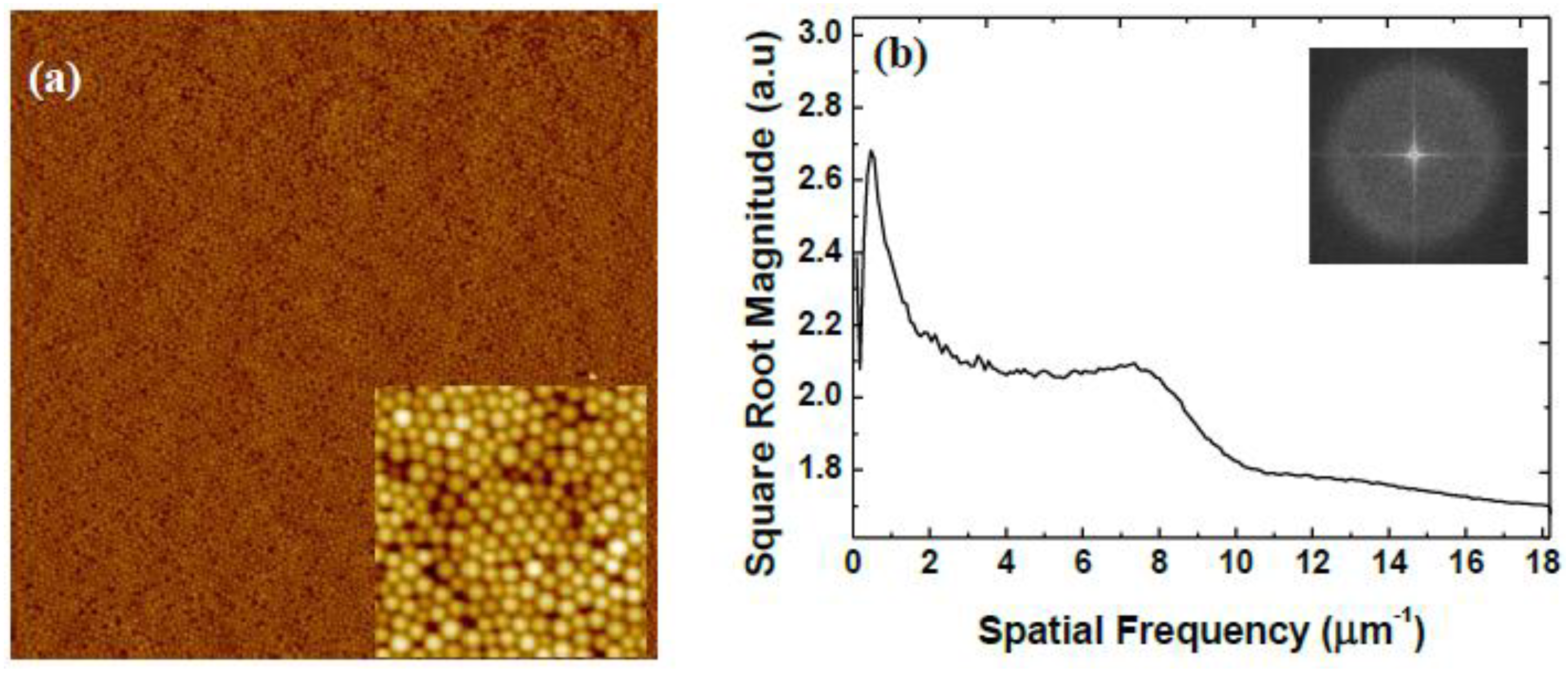

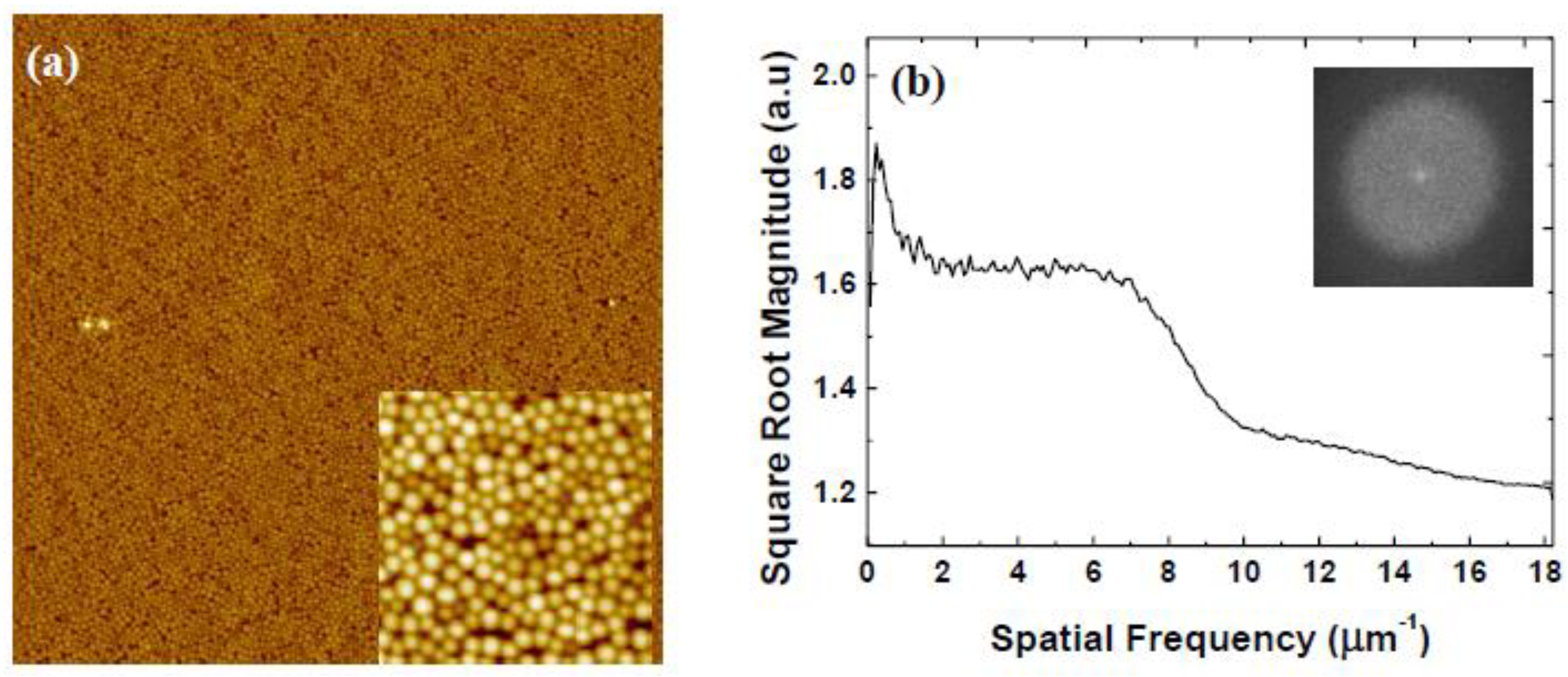
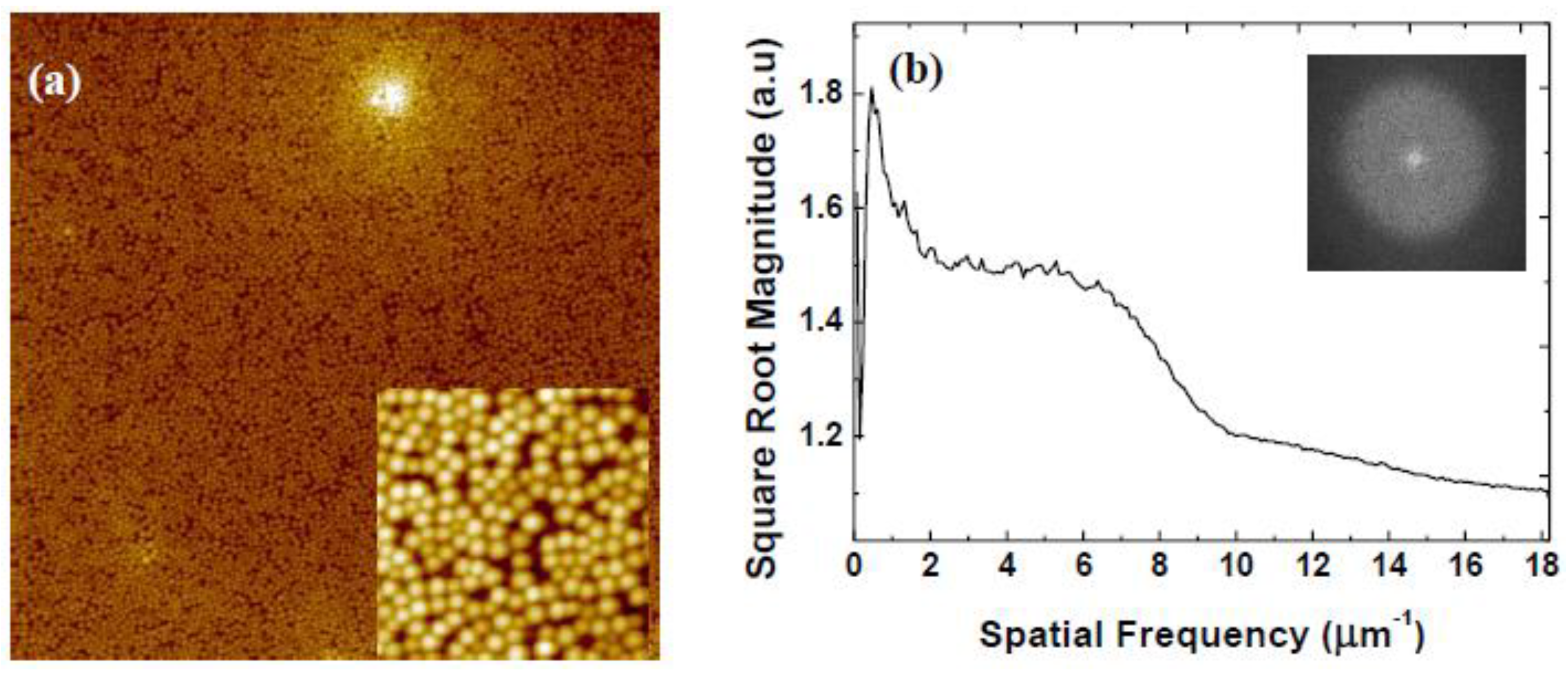


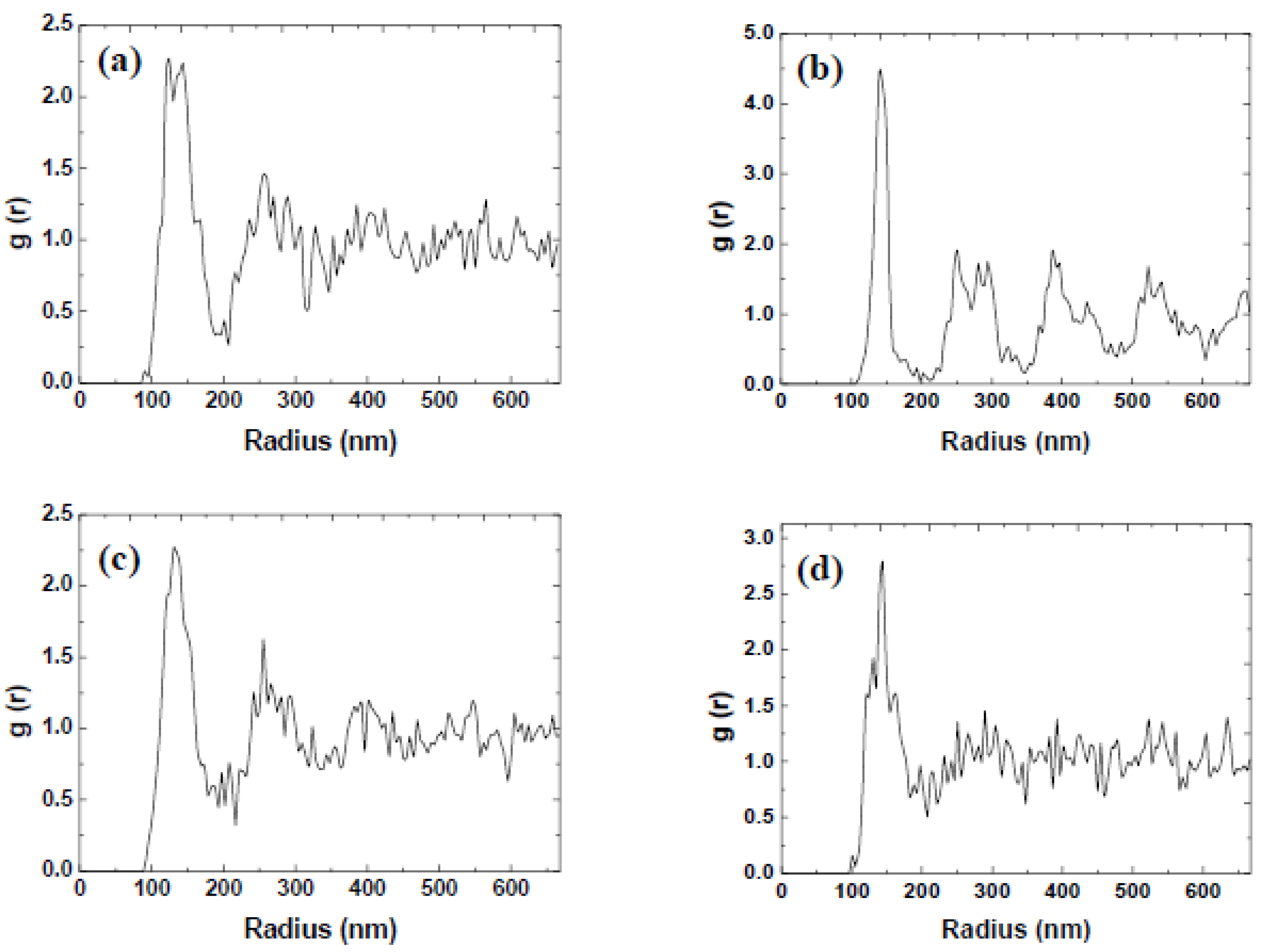
| Rotational Acceleration (rpm/s) | Characteristic Spacing (nm) |
|---|---|
| 200 | 133 |
| 400 | 138 |
| 600 | 140 |
| 800 | 168 |
| 1000 | 172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, J.; Koutsos, V. Spin Coating of Silica Nanocolloids on Mica: Self-Assembly of Two-Dimensional Colloid Crystal Structures and Thin Films. Coatings 2023, 13, 1488. https://doi.org/10.3390/coatings13091488
Walker J, Koutsos V. Spin Coating of Silica Nanocolloids on Mica: Self-Assembly of Two-Dimensional Colloid Crystal Structures and Thin Films. Coatings. 2023; 13(9):1488. https://doi.org/10.3390/coatings13091488
Chicago/Turabian StyleWalker, John, and Vasileios Koutsos. 2023. "Spin Coating of Silica Nanocolloids on Mica: Self-Assembly of Two-Dimensional Colloid Crystal Structures and Thin Films" Coatings 13, no. 9: 1488. https://doi.org/10.3390/coatings13091488






