Insight into Anti-Corrosion Behavior and Mechanism of 8-Hydroxyquinoline Inhibitor on AZ91D Alloy in Different Concentrations of Sodium Chloride Solution
Abstract
:1. Introduction
2. Experimental Theoretical Details
2.1. Materials and Sample Preparation
2.2. Hydrogen Evolution Test
2.3. Electrochemical Test
2.4. Surface Morphology and Components Analysis
2.5. DFT Computations
3. Results and Discussion
3.1. Hydrogen Evolution Measurement
3.2. Electrochemical Evaluation of Corrosion Inhibition
3.2.1. OCP Measurement
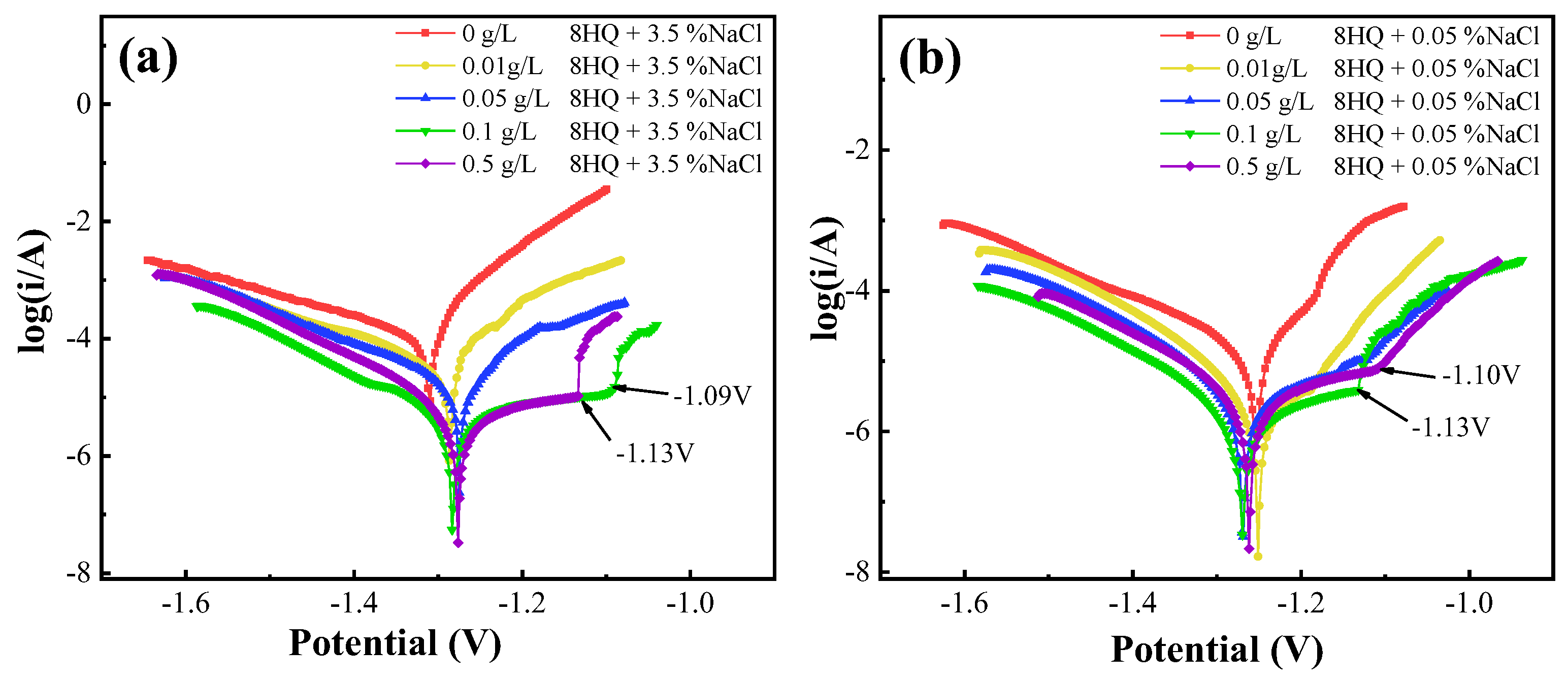
3.2.2. PDP Measurement
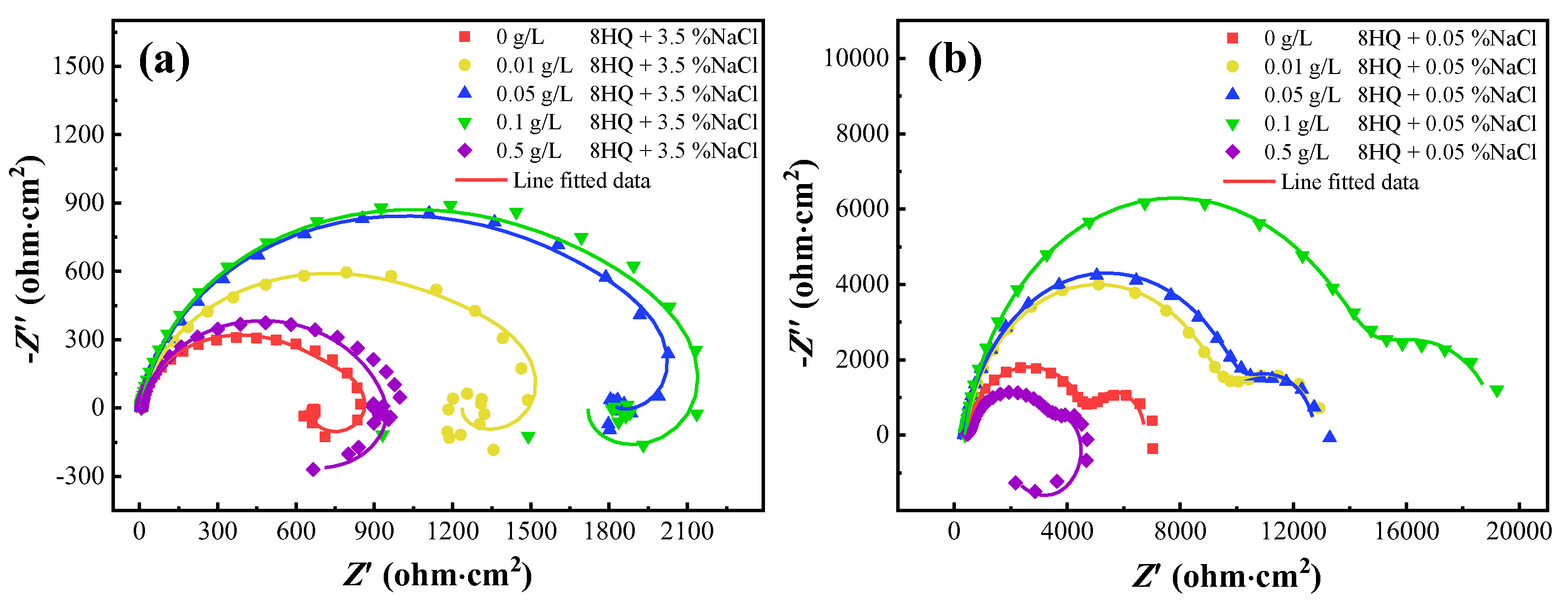
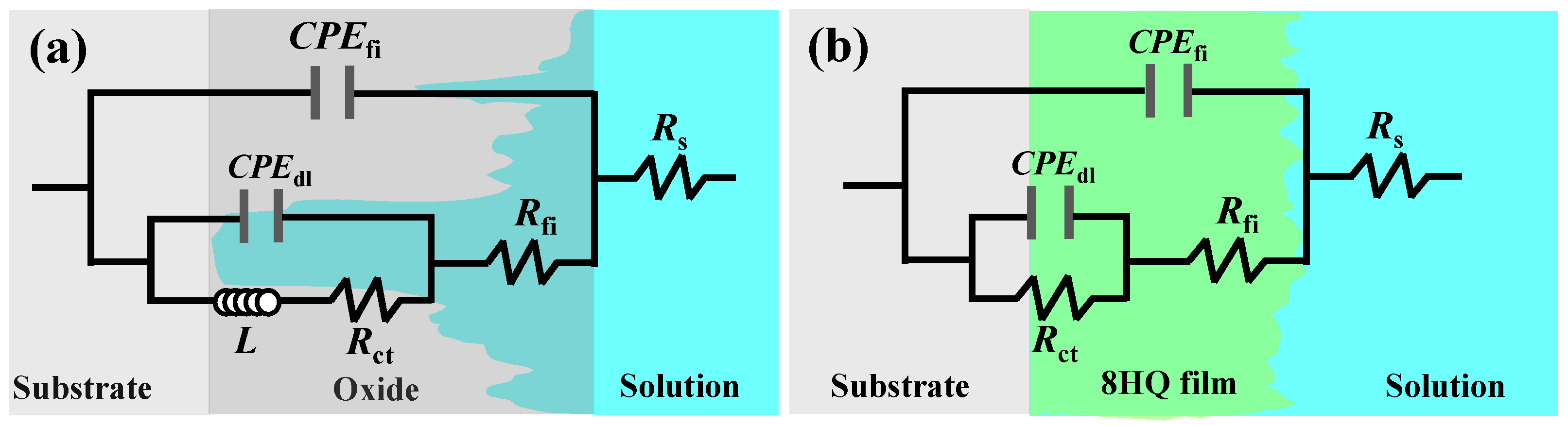
3.2.3. EIS Measurement
3.3. Surface Morphology and Components Analysis
3.3.1. SEM Analysis
3.3.2. Three-Dimensional Morphology
3.4. Adsorption Isotherms Studies
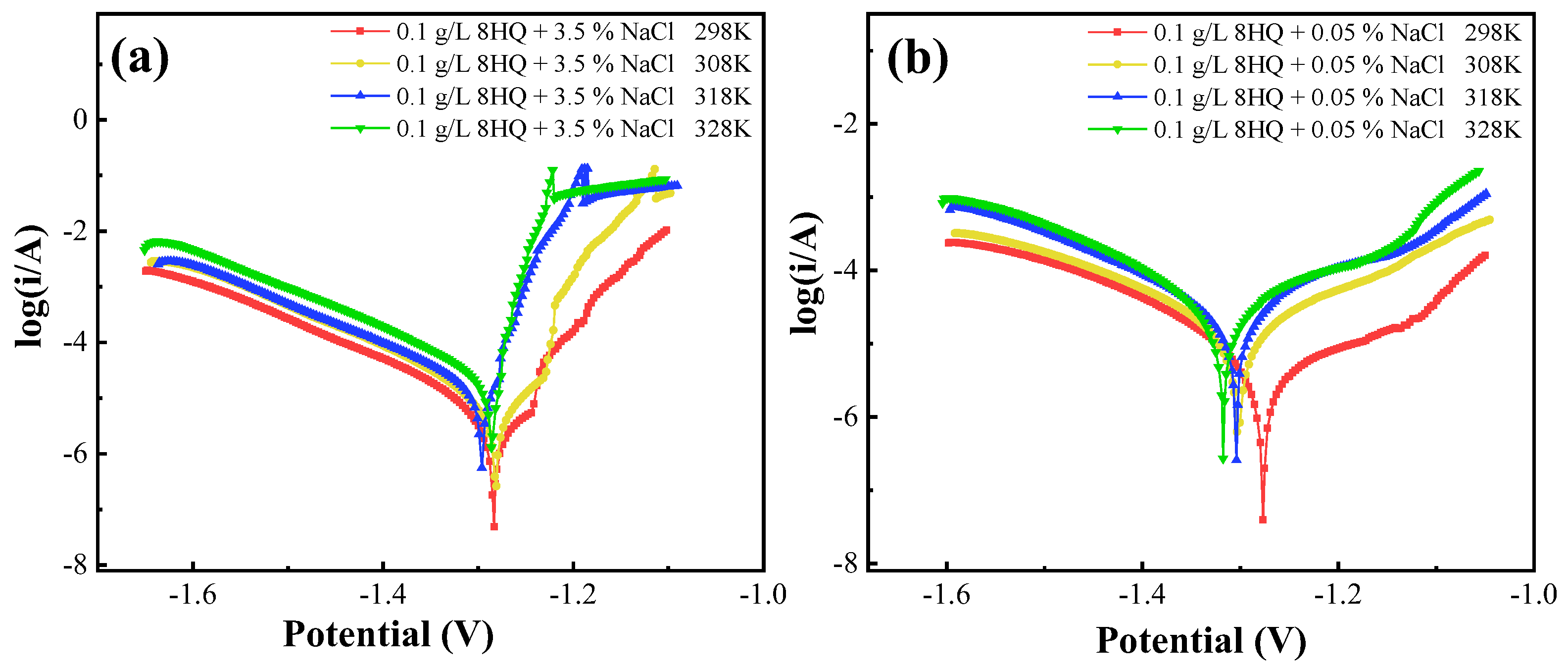
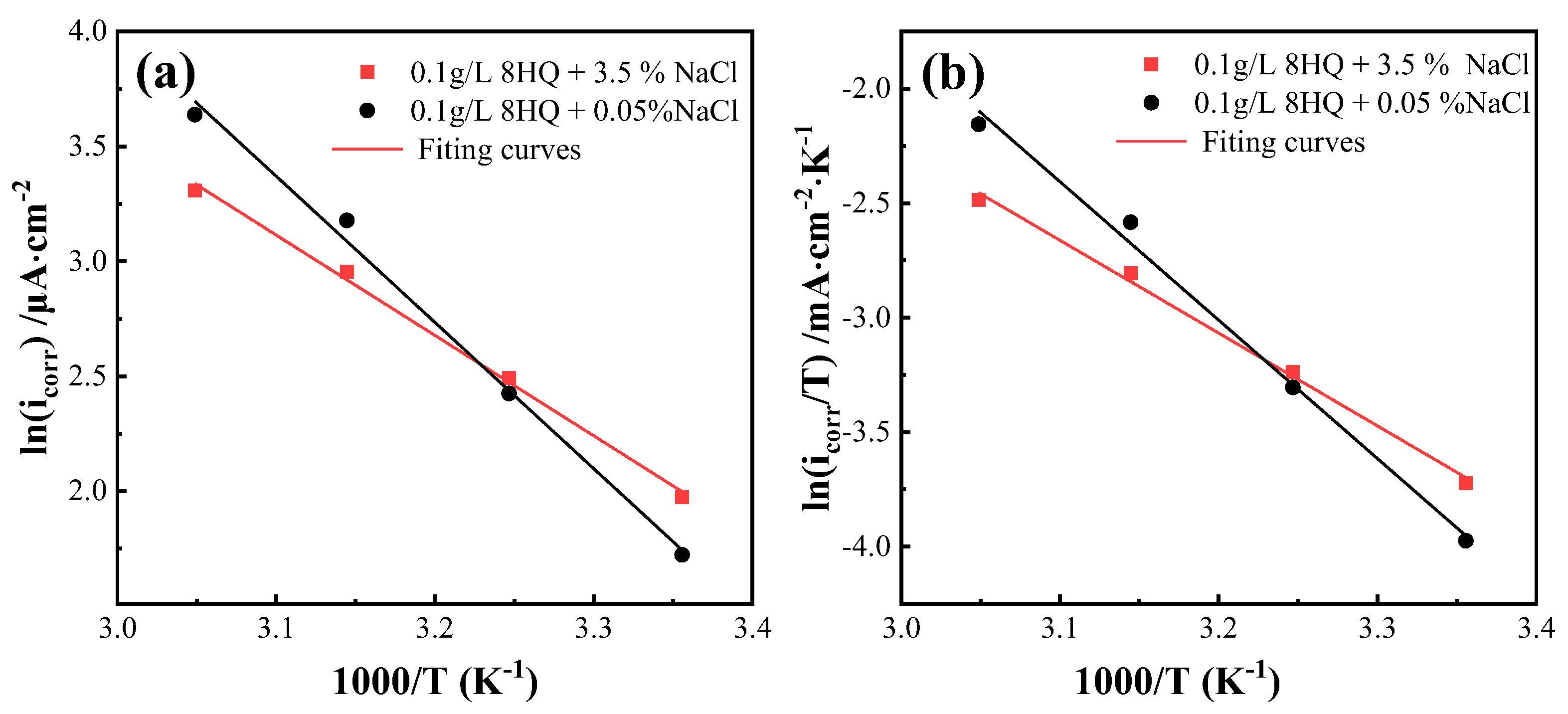
3.5. Temperature Effects and Activation Parameters
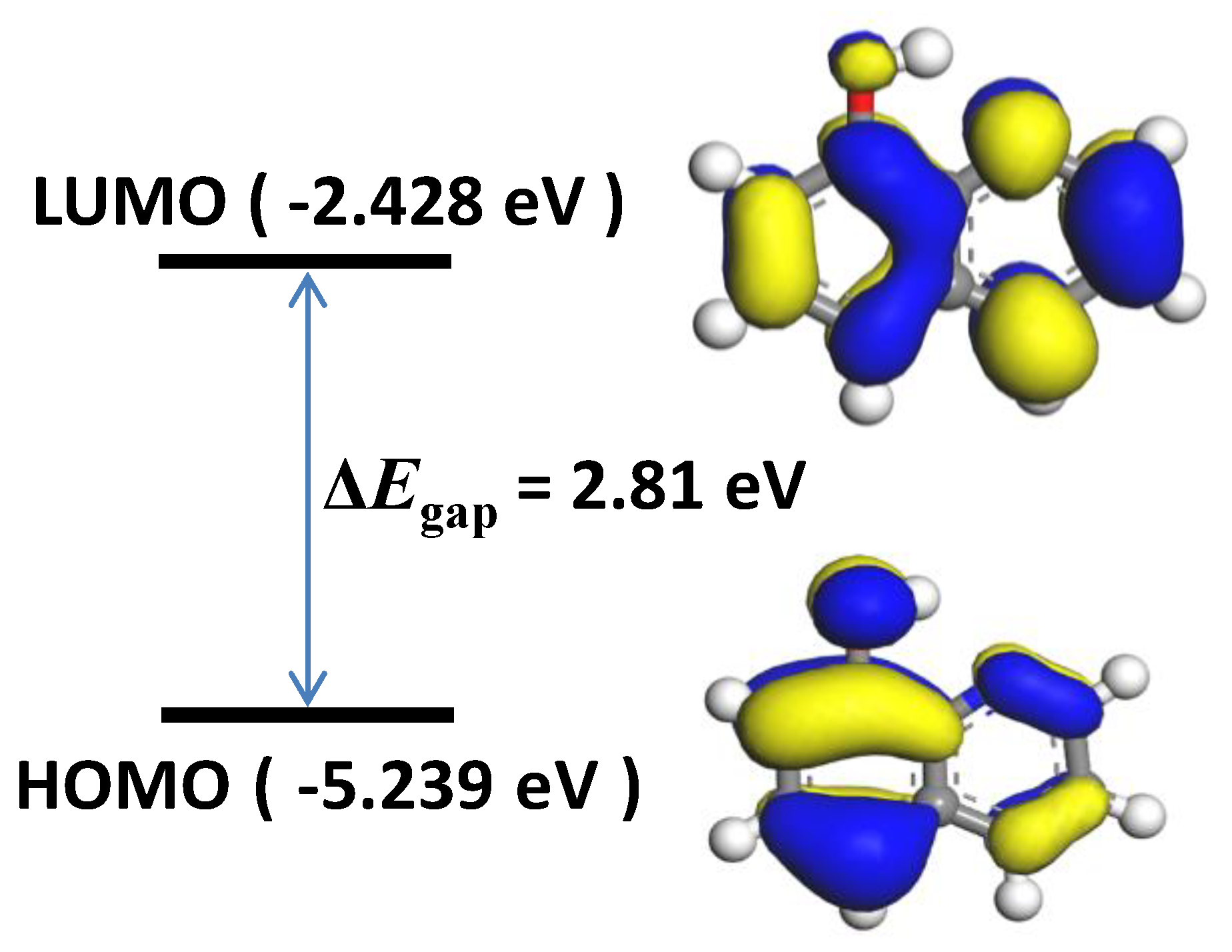
3.6. Quantum Chemical Calculations
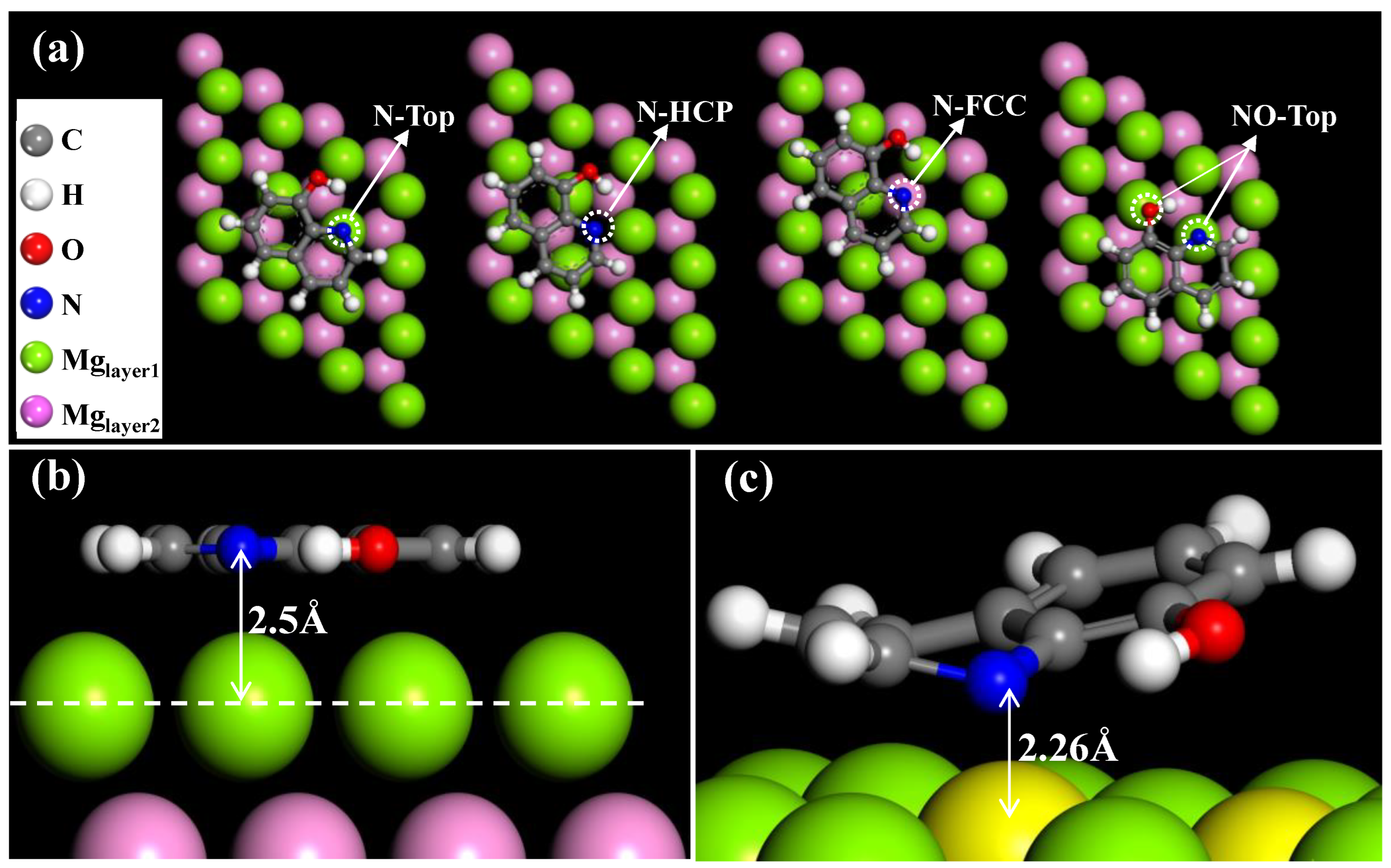
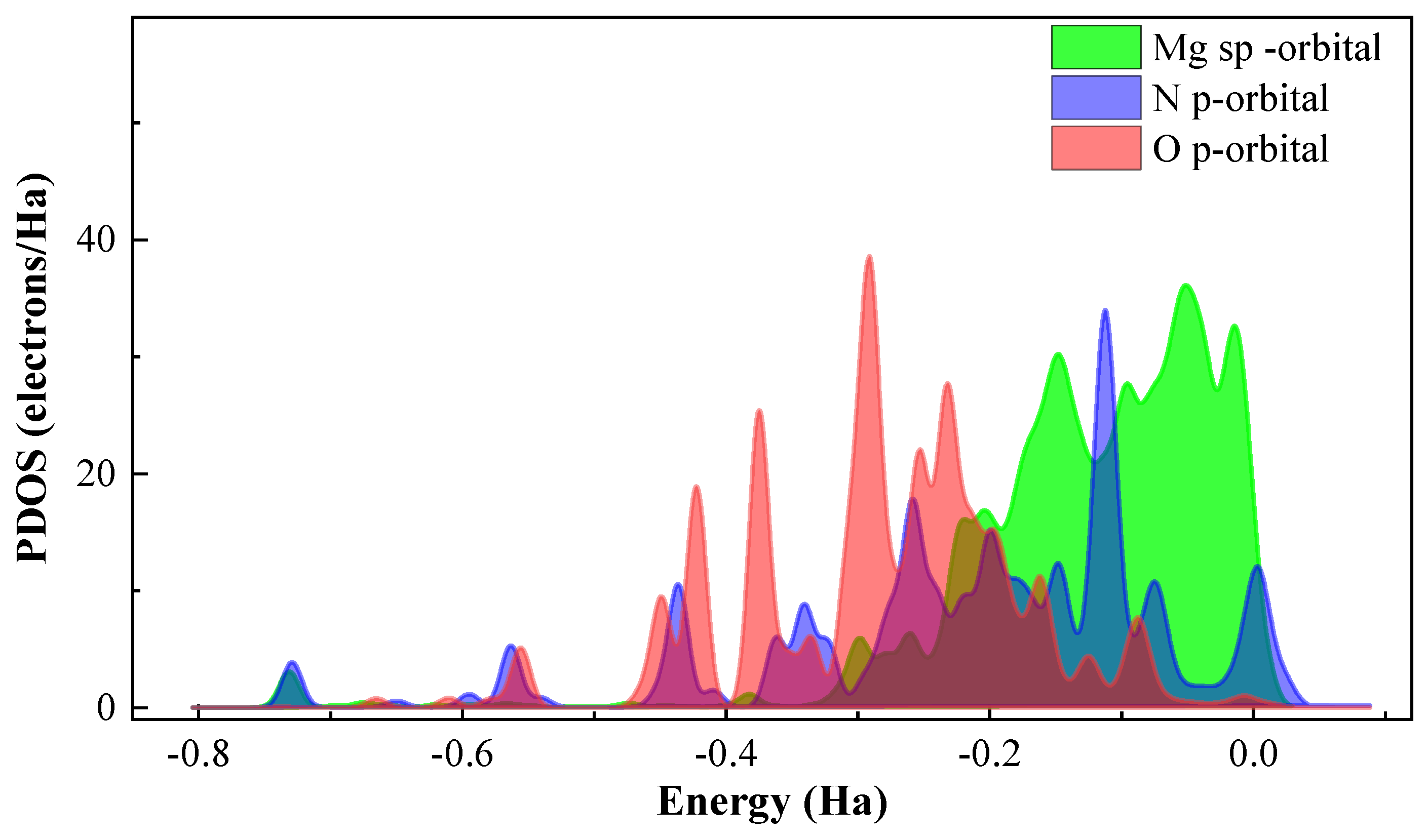
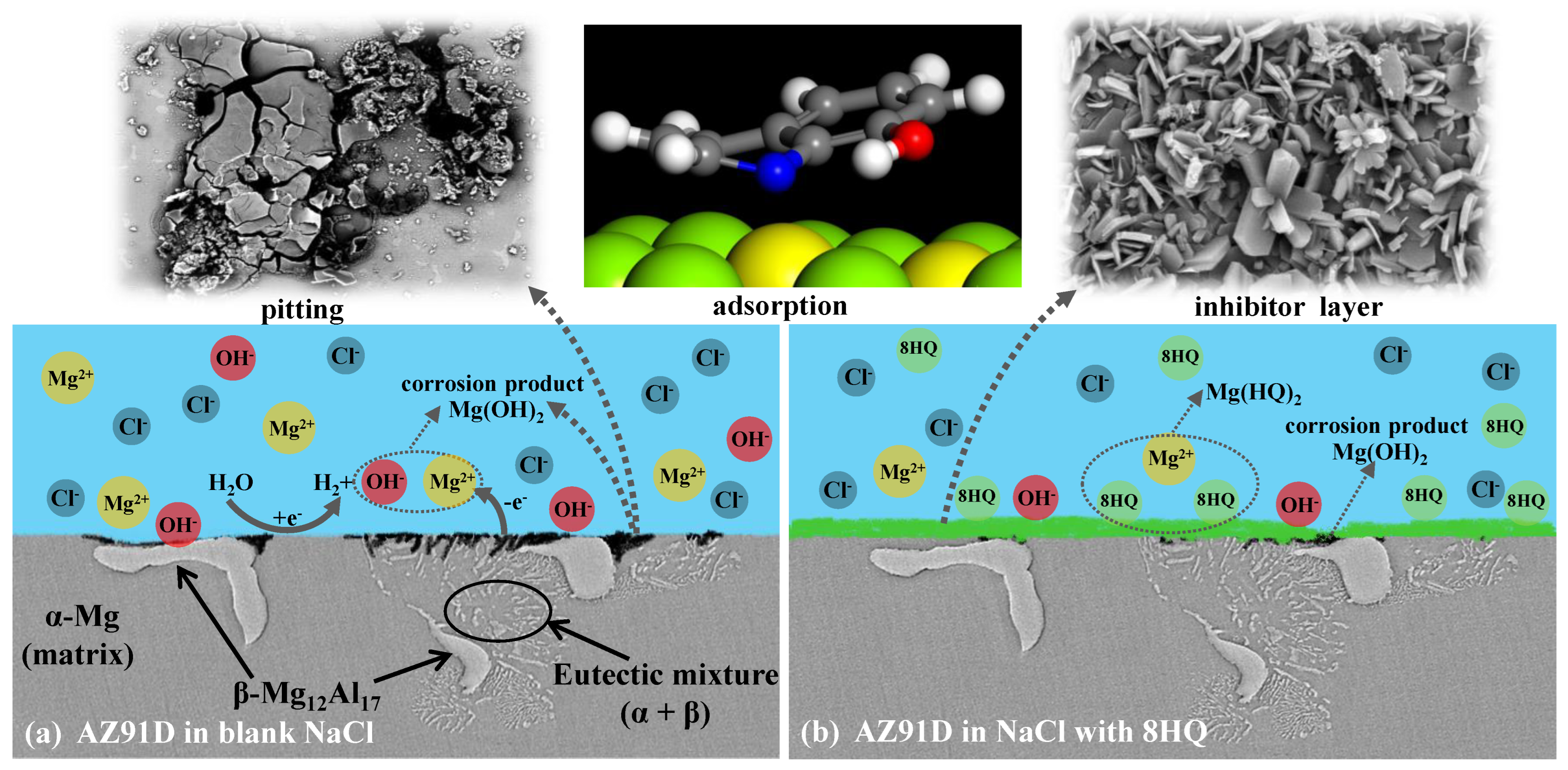
3.7. Possible Corrosion and Inhibition Mechanism
4. Conclusions
- 8-hydroxyquinoline is a kind of mixed corrosion inhibitor, which can form a protective layer on an AZ91D alloy surface to delay corrosion. The inhibition efficiency of 8HQ is greatly related to the concentration of inhibitor. According to the thermodynamic results, the adsorption process includes both physical and chemical adsorptions and the activation energy in 0.05 wt.% NaCl is greater than that in 3.5 wt.% NaCl.
- There is a competitive adsorption relationship between 8HQ and [Cl−], and the adsorption morphology is obviously affected by the concentration of [Cl−]. The lower concentration of [Cl−] can promote the adsorption of 8HQ on an AZ91D alloy, making its adsorption more orderly and faster, so as to form a denser protective layer with excellent anti-corrosion properties.
- The DFT results showed that the most stable adsorption configuration of 8HQ is NO-Top (N and O are located on the top site of Mg (001)). The N and O atoms are the active sites, and there is strong coupling between them and Mg atoms, which is consistent with the experimental results.
- The results and conclusions provide theoretical and experimental support for the development of a new generation of green corrosion inhibitors. However, whether 8HQ has good durability and can maintain the corrosion inhibition effect in more complex environments needs further research. In addition, from the point of view of quantum computing, it is necessary to establish a more complete model using molecular dynamics methods to study the coexistence of multiple water molecules and sodium chloride molecules.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Song, H.; Xu, Z.; Benabou, L.; Yin, Z.; Guan, H.; Yan, H.; Chao, L.; Hu, Z.; Wang, X. Sodium dodecyl sulfate (SDS) as an effective corrosion inhibitor for Mg-8Li-3Al alloy in aqueous NaCl: A combined experimental and theoretical investigation. J. Magnes. Alloys 2023, 11, 287–300. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.; Ferreira, M.G.S.; Carmezim, M.J. Composition and corrosion resistance of cerium conversion films on the AZ31 magnesium alloy and its relation to the salt anion. Appl. Surf. Sci. 2008, 254, 1806–1814. [Google Scholar] [CrossRef]
- Adsul, S.H.; Soma Raju, K.R.C.; Sarada, B.V.; Sonawane, S.H.; Subasri, R. Evaluation of self-healing properties of inhibitor loaded nanoclay-based anticorrosive coatings on magnesium alloy AZ91D. J. Magnes. Alloys 2018, 6, 299–308. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-Garcia, R.; Connolly, B.J.; Matthews, A.; Yerokhin, A. Smart Functionalization of Ceramic-Coated AZ31 Magnesium Alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef]
- Ji, X.; Wang, W.; Li, W.; Zhao, X.; Shi, H. pH-responsible self-healing performance of coating with dual-action core-shell electrospun fibers. J. Taiwan Inst. Chem. Eng. 2019, 104, 227–239. [Google Scholar] [CrossRef]
- Song, Y.; Tang, Y.; Fang, L.; Wu, F.; Zeng, X.; Hu, J.; Zhang, S.F.; Jiang, B.; Luo, H. Enhancement of corrosion resistance of AZ31 Mg alloys by one-step in situ synthesis of ZnAl-LDH films intercalated with organic anions (ASP, La). J. Magnes. Alloys 2020, 9, 658–667. [Google Scholar] [CrossRef]
- Wang, X.; Jing, C.; Chen, Y.; Wang, X.; Zhao, G.; Zhang, X.; Wu, L.; Liu, X.; Dong, B.; Zhang, Y. Active corrosion protection of super-hydrophobic corrosion inhibitor intercalated Mg–Al layered double hydroxide coating on AZ31 magnesium alloy. J. Magnes. Alloys 2020, 8, 291–300. [Google Scholar] [CrossRef]
- Gerengi, H.; Mielniczek, M.; Gece, G.; Solomon, M.M. Experimental and Quantum Chemical Evaluation of 8-Hydroxyquinoline as a Corrosion Inhibitor for Copper in 0.1 M HCl. Ind. Eng. Chem. Res. 2016, 55, 9614–9624. [Google Scholar] [CrossRef]
- Cicileo, G.P.; Rosales, B.M.; Varela, F.E.; Vilche, J.R. Inhibitory action of 8-HYDROXYQUINOLINE on the copper corrosion process. Corros. Sci. 1998, 40, 1915–1926. [Google Scholar] [CrossRef]
- Soliman, H.N. Influence of 8-hydroxyquinoline addition on the corrosion behavior of commercial Al and Al-HO411 alloys in NaOH aqueous media. Corros. Sci. 2011, 53, 2994–3006. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taheri, P.; Mol, J.M.C.; Montemor, M.F. Comparison of the synergistic effects of inhibitor mixtures tailored for enhanced corrosion protection of bare and coated AA2024-T3. Surf. Coat. Technol. 2016, 303, 342–351. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q.; Dai, Y.; Luo, F.; Zhang, H.X. High efficiency corrosion inhibitor 8-hydroxyquinoline and its synergistic effect with sodium dodecylbenzenesulphonate on AZ91D magnesium alloy. Corros. Sci. 2010, 52, 1603–1609. [Google Scholar] [CrossRef]
- Obot, I.B.; Ankah, N.K.; Sorour, A.A.; Gasem, Z.M.; Haruna, K. 8-Hydroxyquinoline as an alternative green and sustainable acidizing oilfield corrosion inhibitor. Sustain. Mater. Technol. 2017, 14, 1–10. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Si, Y.; Mu, G.; Liu, G. The synergistic inhibition between 8-hydroxyquinoline and chloride ion for the corrosion of cold rolled steel in 0.5M sulfuric acid. Mater. Chem. Phys. 2006, 95, 29–38. [Google Scholar] [CrossRef]
- Zong, Q.; Wang, L.; Sun, W.; Liu, G. Active deposition of bis (8-hydroxyquinoline) magnesium coating for enhanced corrosion resistance of AZ91D alloy. Corros. Sci. 2014, 89, 127–136. [Google Scholar] [CrossRef]
- Duan, Y.H. Adsorption of fluorine and chlorine on Mg (0001) surface: A density functional theory investigation. Trans. Nonferrous Met. Soc. China 2014, 24, 1844–1852. [Google Scholar] [CrossRef]
- Chen, X.; Zou, W.; Lin, Q.; Li, R.; Xia, G.; Yu, X. The effect of oxygen coverages on hydrogenation of Mg (0001) surface. Appl. Surf. Sci. 2019, 487, 510–518. [Google Scholar] [CrossRef]
- Hrimla, M.; Bahsis, L.; Boutouil, A.; Laamari, M.R.; Julve, M.; Stiriba, S.E. Corrosion inhibition performance of a structurally well-defined 1,2,3-triazole derivative on mild steel-hydrochloric acid interface. J. Mol. Struct. 2021, 1231, 129895. [Google Scholar] [CrossRef]
- Su, H.; Wang, L.; Wu, Y.; Zhang, Y.; Zhang, J. Insight into inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: Experimental and theoretical investigation. Corros. Sci. 2020, 165, 108410. [Google Scholar] [CrossRef]
- El Faydy, M.; Lakhrissi, B.; Jama, C.; Zarrouk, A.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Electrochemical, surface and computational studies on the inhibition performance of some newly synthesized 8-hydroxyquinoline derivatives containing benzimidazole moiety against the corrosion of carbon steel in phosphoric acid environment. J. Mater. Res. Technol. 2020, 9, 727–748. [Google Scholar] [CrossRef]
- Gao, X.; Wu, Y.; Huang, Q.; Jiang, Y.; Ma, D.; Ren, T. The inhibition behavior of novel ionic liquids for magnesium alloy in NaCl solution: Experimental and theoretical investigation. J. Mol. Liq. 2021, 324, 114732. [Google Scholar] [CrossRef]
- Kavitha, T.; Aruna Priyankaa, B.K.; Yuvarani, N.; Sudha, N.; Inmozhi, C.; Mathammal, R. Investigation on crystal growth characterisation and molecular docking insights of 8-hydroxyquinoline. Mater. Today Proc. 2021, 36, 480–487. [Google Scholar] [CrossRef]
- Chiter, F.; Lacaze-Dufaure, C.; Tang, H.; Pébère, N. DFT studies of the bonding mechanism of 8-hydroxyquinoline and derivatives on the (111) aluminum surface. Phys. Chem. Chem. Phys. 2015, 17, 22243–22258. [Google Scholar] [CrossRef]
- Chiter, F.; Bonnet, M.L.; Lacaze-Dufaure, C.; Tang, H.; Pébère, N. Corrosion protection of Al(111) by 8-hydroxyquinoline: A comprehensive DFT study. Phys. Chem. Chem. Phys. 2018, 20, 21474–21486. [Google Scholar] [CrossRef]
- You, J.; Liu, Z. Atomistic simulation of corrosion protection of Al2Cu aluminum alloy by 8-hydroxyquinoline. Appl. Surf. Sci. 2021, 540, 148315. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Gao, X. Corrosion inhibition of magnesium alloy in NaCl solution by ionic liquid: Synthesis, electrochemical and theoretical studies. J. Alloys Compd. 2019, 791, 681–689. [Google Scholar] [CrossRef]
- Osipenko, M.A.; Kharytonau, D.S.; Kasach, A.A. Inhibitive effect of sodium molybdate on corrosion of AZ31 magnesium alloy in chloride solutions. Electrochim. Acta 2022, 414, 140175. [Google Scholar] [CrossRef]
- Duan, Y.H.; Zhou, S.G.; Sun, Y.; Peng, M.J. The electronic structure and phase diagram of chlorine adsorption on Mg (0001) surface. Comput. Mater. Sci. 2014, 84, 108–114. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Jiang, Y.; Qian, Y.; Guo, X.; Wang, L.; Zhang, J. Orange peel extracts as biodegradable corrosion inhibitor for magnesium alloy in NaCl solution: Experimental and theoretical studies. J. Taiwan Inst. Chem. Eng. 2020, 115, 35–46. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Zimowska, M.; Ryl, J.; Zieliński, A.; Osipenko, M.A.; Adamiec, J.; Wrzesińska, A.; Claesson, P.M.; Kurilo, I.I. Aqueous molybdate provides effective corrosion inhibition of WE43 magnesium alloy in sodium chloride solutions. Corros. Sci. 2021, 190, 109664. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 magnesium alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C. Anodic desorption of inhibitor III. Mechanism of inhibitor desorption. J. Chin. Soc. Corros. Prot. 1996, 16, 6. [Google Scholar]
- Shang, W.; He, C.; Wen, Y.; Wang, Y.; Zhang, Z. Performance evaluation of triethanolamine as corrosion inhibitor for magnesium alloy in 3.5 wt% NaCl solution. Rsc Adv. 2016, 6, 113967–113980. [Google Scholar] [CrossRef]
- Baril, G.; Pébère, N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, H.R.; Liu, J.H. Corrosion behavior of aluminum alloy 2024-T3 by 8-hydroxy-quinoline and its derivative in 3.5% chloride solution. Trans. Nonferrous Met. Soc. China 2007, 17, 318–325. [Google Scholar] [CrossRef]
- Verma, C.; Sorour, A.A.; Ebenso, E.E.; Quraishi, M.A. Inhibition performance of three naphthyridine derivatives for mild steel corrosion in 1M HCl: Computation and experimental analyses. Results Phys. 2018, 10, 504–511. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; Santos, N.E.; Maldonado, A.C.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H- 1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Gao, S.; Guo, X.; Zhang, J. Experimental and theoretical studies on corrosion inhibition behavior of three imidazolium-based ionic liquids for magnesium alloys in sodium chloride solution. J. Mol. Liq. 2021, 345, 116998. [Google Scholar] [CrossRef]
- Talebian, M.; Raeissi, K.; Atapour, M.; Fernández-Pérez, B.M.; Salarvand, Z.; Meghdadi, S.; Amirnasr, M.; Souto, R.M. Inhibitive effect of sodium (E)-4-(4-nitrobenzylideneamino)benzoate on the corrosion of some metals in sodium chloride solution. Appl. Surf. Sci. 2018, 447, 852–865. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About some corrosion mechanisms of AZ91D magnesium alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion inhibition of Schiff bases for Mg-Zn-Y-Nd alloy in normal saline: Experimental and theoretical investigations. Corros. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Formation of flower-like structures for optimizing the corrosion resistance of Mg alloy. Mater. Lett. 2018, 221, 196–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Li, N.; Jiang, Y.; Qian, Y.; Wang, L.; Zhang, J. Synergistic inhibition effect of L-Phenylalanine and zinc salts on chloride-induced corrosion of magnesium alloy: Experimental and theoretical investigation. J. Taiwan Inst. Chem. Eng. 2021, 121, 48–60. [Google Scholar] [CrossRef]
- Dhar, H.P.; Conway, B.E.; Joshi, K.M. On the form of adsorption isotherms for substitutional adsorption of molecules of different sizes. Electrochim. Acta 1973, 18, 789–798. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Dafali, A.; Bentiss, F. Inhibitive Properties and adsorption of purpald as a corrosion inhibitor for copper in nitric acid medium. Ind. Eng. Chem. Res. 2013, 52, 2560–2568. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Guo, L.; Dahmani, K.; Nouneh, K.; Briche, S.; Lakhrissi, B.; Dkhireche, N.; Ebn Touhami, M. Effect of alkyl group position on adsorption behavior and corrosion inhibition of new naphthol based on 8-hydroxyquinoline: Electrochemical, surface, quantum calculations and dynamic simulations. J. Mol. Liq. 2021, 335, 116552. [Google Scholar] [CrossRef]
- Shen, S.; Zuo, Y.; Zhao, X. The effects of 8-hydroxyquinoline on corrosion performance of a Mg-rich coating on AZ91D magnesium alloy. Corros. Sci. 2013, 76, 275–283. [Google Scholar] [CrossRef]
- Gopiraman, M.; Selvakumaran, N.; Kesavan, D.; Karvembu, R. Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl)benzamide on mild steel in acidic medium. Prog. Org. Coat. 2012, 73, 104–111. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Noor, E.; Al-Moubaraki, A. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Soltani, N.; Salavati-Niasari, M. The inhibitive effect of some bis-N,S-bidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1073–1082. [Google Scholar] [CrossRef]
- Duan, Y.H.; Sun, Y.; Zhou, S.G. Different coverages of fluorine adsorption on Mg (0001) surface. Comput. Mater. Sci. 2013, 72, 81–85. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Zhang, D.; Li, K.; Gao, L.; Lin, T. Electrochemical and DFT studies of quinoline derivatives on corrosion inhibition of AA5052 aluminium alloy in NaCl solution. Appl. Surf. Sci. 2015, 357, 2176–2183. [Google Scholar] [CrossRef]
- Thirumalaikumarasamy, D.; Shanmugam, K.; Balasubramanian, V. Influence of chloride ion concentration on immersion corrosion behaviour of plasma sprayed alumina coatings on AZ31B magnesium alloy. J. Magnes. Alloys 2014, 2, 325–334. [Google Scholar] [CrossRef]





| Element | Al | Zn | Mn | Si | Fe | Mg |
|---|---|---|---|---|---|---|
| Composition % | 8.62 | 0.90 | 0.20 | 0.005 | <0.001 | Bal. |
| NaCl (wt. %) | C8HQ (g/L) | pH (0 h) | pH (72 h) | ΔpH | C8HQ (0 h) | C8HQ (72 h) | ΔC8HQ |
|---|---|---|---|---|---|---|---|
| 3.5 | 0 | 6.24 | 8.88 | 2.64 | - | - | - |
| 0.01 | 5.82 | 8.68 | 2.86 | 0.009 | 0.003 | 0.006 | |
| 0.05 | 5.85 | 7.71 | 1.86 | 0.052 | 0.023 | 0.029 | |
| 0.1 | 5.99 | 7.57 | 1.58 | 0.098 | 0.061 | 0.037 | |
| 0.5 | 6.35 | 7.09 | 0.74 | 0.497 | 0.364 | 0.133 | |
| 0.05 | 0 | 6.09 | 9.31 | 3.22 | - | - | - |
| 0.01 | 6.56 | 9.17 | 2.61 | 0.011 | 0.003 | 0.008 | |
| 0.05 | 7.26 | 9.03 | 1.77 | 0.049 | 0.014 | 0.035 | |
| 0.1 | 7.52 | 8.29 | 0.77 | 0.102 | 0.024 | 0.078 | |
| 0.5 | 8.71 | 8.75 | 0.05 | 0.498 | 0.124 | 0.374 |
| NaCl | C8HQ | Ecorr | Icorr | βc | ηp | θ |
|---|---|---|---|---|---|---|
| (wt. %) | (g/L) | (V) | (μA·cm−2) | (mV·dec−1) | (%) | |
| 3.5 | 0 | −1.31 | 115.08 | −261 | - | - |
| 0.01 | −1.29 | 43.01 | −274 | 62.63 | 0.6263 | |
| 0.05 | −1.28 | 20.10 | −203 | 82.53 | 0.8253 | |
| 0.1 | −1.28 | 3.88 | −143 | 96.63 | 0.9663 | |
| 0.5 | −1.28 | 7.79 | −150 | 93.23 | 0.9323 | |
| 0.05 | 0 | −1.25 | 17.90 | −231 | - | - |
| 0.01 | −1.25 | 5.76 | −155 | 67.82 | 0.6782 | |
| 0.05 | −1.27 | 3.47 | −139 | 80.61 | 0.8061 | |
| 0.1 | −1.27 | 1.85 | −142 | 89.66 | 0.8966 | |
| 0.5 | −1.26 | 2.62 | −141 | 85.35 | 0.8535 |
| NaCl | C8HQ | Rs | Rfi | Rct | L | CPEfi | CPEdl | ||
|---|---|---|---|---|---|---|---|---|---|
| (wt.%) | (g/L) | (Ω·cm2) | (KΩ·cm2) | (Ω·cm2) | (H·cm−2) | Cfi (μF·cm−2) | n | Cdl (μF·cm−2) | n |
| 3.5 | 0 | 6.54 | 0.59 | 66.8 | 35.1 | 5.52 | 0.928 | 4.03 | 0.313 |
| 0.01 | 6.68 | 1.23 | 2.83 | 37.2 | 5.49 | 0.908 | 5.48 | 0.363 | |
| 0.05 | 7.06 | 1.68 | 28.2 | 63.7 | 5.64 | 0.912 | 3.72 | 0.354 | |
| 0.1 | 6.99 | 1.71 | 157 | 101 | 5.49 | 0919 | 1.97 | 0.274 | |
| 0.5 | 7.71 | 0.83 | 42.3 | 27.8 | 5.27 | 0.934 | 11.91 | 0.391 | |
| 0.05 | 0 | 389 | 4.55 | 1780 | - | 3.72 | 0.855 | 2530 | 0.980 |
| 0.01 | 390 | 9.36 | 3190 | - | 3.62 | 0.900 | 1180 | 0.944 | |
| 0.05 | 352 | 10.10 | 2290 | - | 3.64 | 0.897 | 849 | 0.900 | |
| 0.1 | 391 | 14.71 | 4140 | - | 3.82 | 0.899 | 502 | 0.924 | |
| 0.5 | 472 | 1.25 | 0.17 × 10−4 | 24,300 | 2.07 | 0.812 | 0.174 | 0.114 | |
| NaCl (wt.%) | T (K) | Ecorr (V) | Icorr (μA·cm−2) | βc (mV·dec−1) |
|---|---|---|---|---|
| 3.5 | 298 | −1.28 | 7.2 | −0.142 |
| 308 | −1.28 | 12.1 | −0.141 | |
| 318 | −1.29 | 19.2 | −0.142 | |
| 328 | −1.28 | 27.3 | −0.140 | |
| 0.05 | 298 | −1.276 | 5.6 | −0.131 |
| 308 | −1.303 | 16.4 | −0.149 | |
| 318 | −1.305 | 24.1 | −0.143 | |
| 328 | −1.318 | 38.3 | −0.135 |
| Compounds | Ea | ΔHa | ΔSa |
|---|---|---|---|
| KJ·mol−1 | KJ·mol−1 | KJ·mol−1·K−1 | |
| 0.1 g/L 8HQ + 3.5 wt.% NaCl | 26.25 | 33.67 | −117.61 |
| 0.1 g/L 8HQ + 0.05 wt.% NaCl | 44.56 | 50.22 | −64.06 |
| Configurations | Adsorption Energy (Ea/KJ·mol−1) | Work Function (Φ/Ha) | Distance between N and Mg (d/Å) |
|---|---|---|---|
| N-Top | −91.27 | 0.125 | 3.09 |
| N-FCC | −88.58 | 0.129 | 3.50 |
| N-HCP | −77.62 | 0.128 | 3.62 |
| NO-Top | −114.41 | 0.128 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, P.; Li, J.; Wang, S.; Li, W.; Li, C. Insight into Anti-Corrosion Behavior and Mechanism of 8-Hydroxyquinoline Inhibitor on AZ91D Alloy in Different Concentrations of Sodium Chloride Solution. Coatings 2023, 13, 1595. https://doi.org/10.3390/coatings13091595
Wang Y, Wang P, Li J, Wang S, Li W, Li C. Insight into Anti-Corrosion Behavior and Mechanism of 8-Hydroxyquinoline Inhibitor on AZ91D Alloy in Different Concentrations of Sodium Chloride Solution. Coatings. 2023; 13(9):1595. https://doi.org/10.3390/coatings13091595
Chicago/Turabian StyleWang, Yimeng, Ping Wang, Jianping Li, Shaoqing Wang, Weiming Li, and Chun Li. 2023. "Insight into Anti-Corrosion Behavior and Mechanism of 8-Hydroxyquinoline Inhibitor on AZ91D Alloy in Different Concentrations of Sodium Chloride Solution" Coatings 13, no. 9: 1595. https://doi.org/10.3390/coatings13091595


.jpg)


