The Effect of Electroplating Nickel on the Mechanical Properties of Brittle Mg-Based Bulk Metallic Glasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BMG Samples
2.2. Electroplating Treatment
2.3. Material Characterization Methods
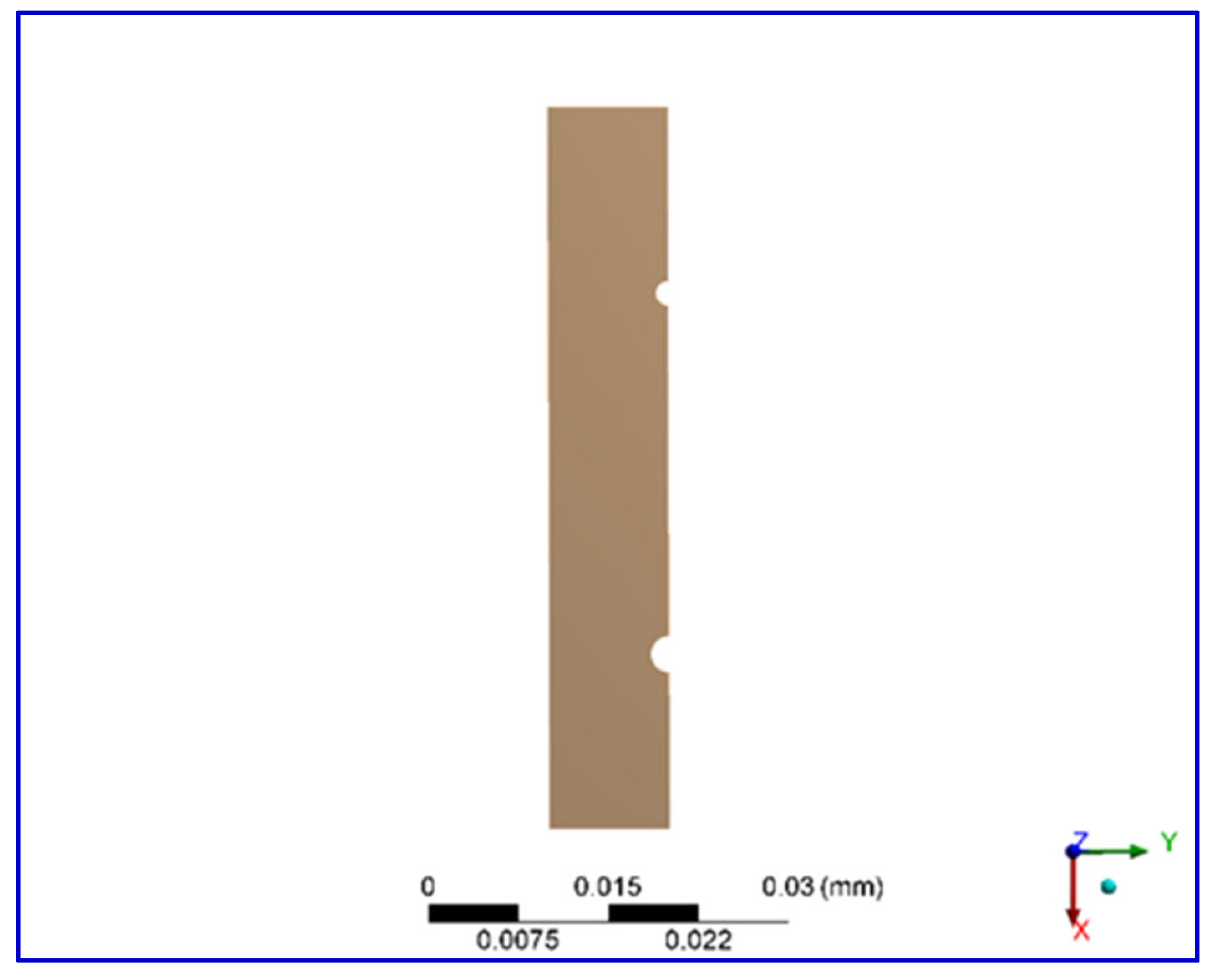
2.4. Finite Element Simulation Method
3. Results
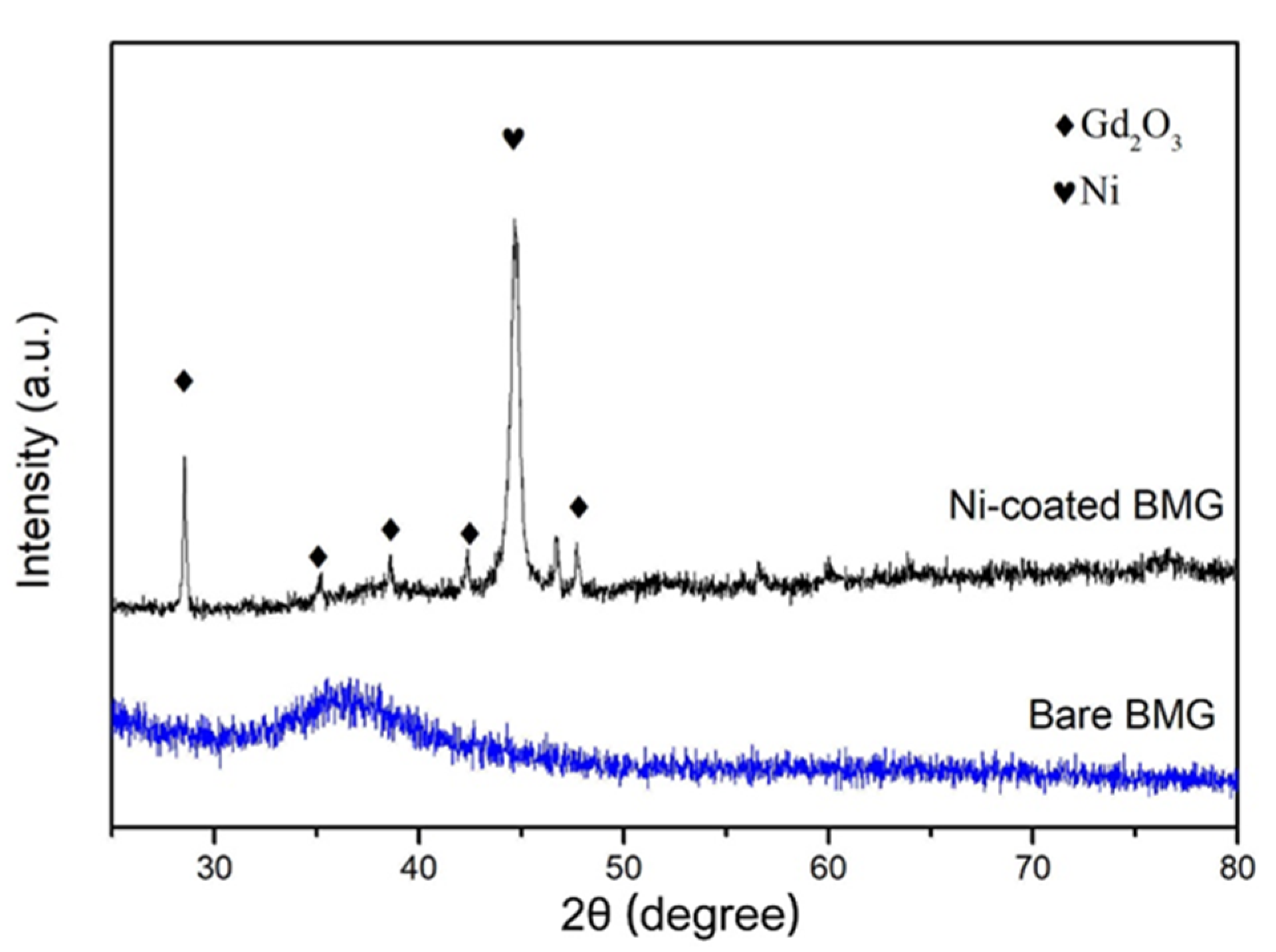
3.1. Microstructure of the Ni Coating
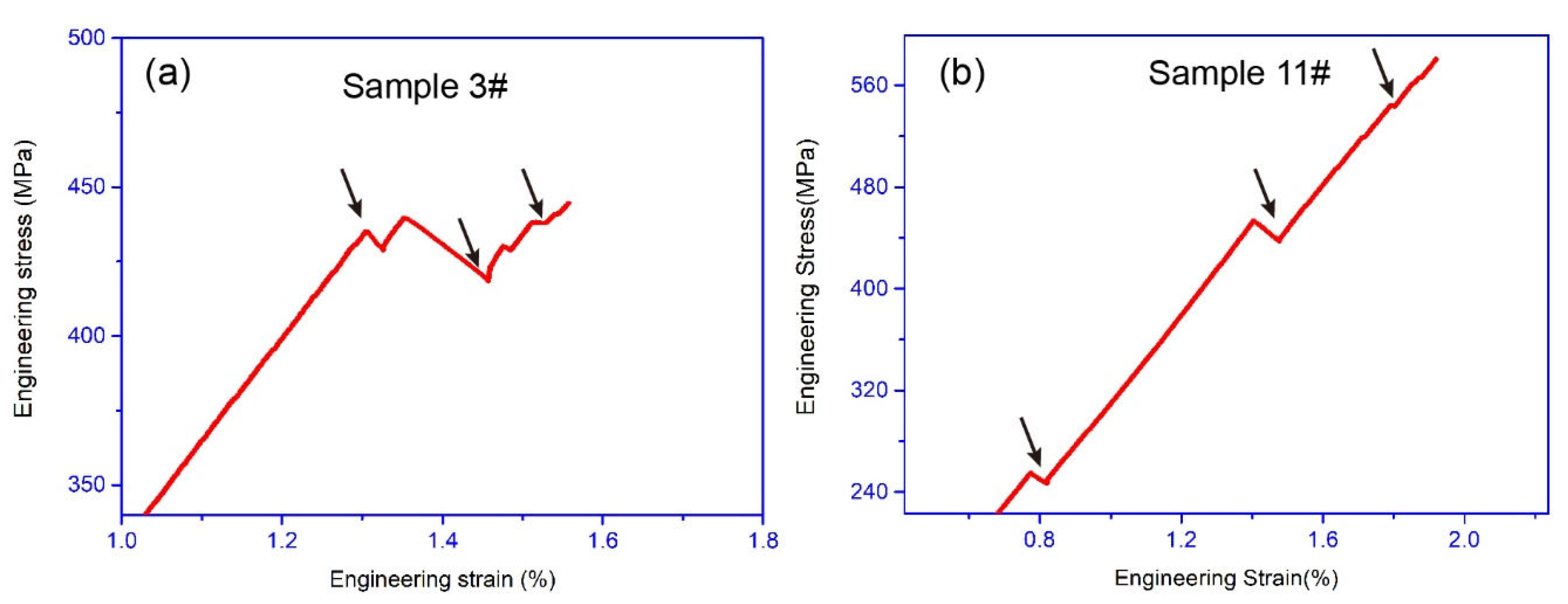
3.2. Mechanical Property
4. Discussion
5. Conclusions
- (1)
- The surface smoothness of the electroplated Ni coating was improved by adding the brightener nano alumina to enhance the adhesion between the coating and the Mg59.5Cu22.9Ag11Gd6.6 BMG. The main electroplating process parameters are nano Al2O3 1 g/L, an electroplating temperature of 50 °C, and an electroplating time of 3 h;
- (2)
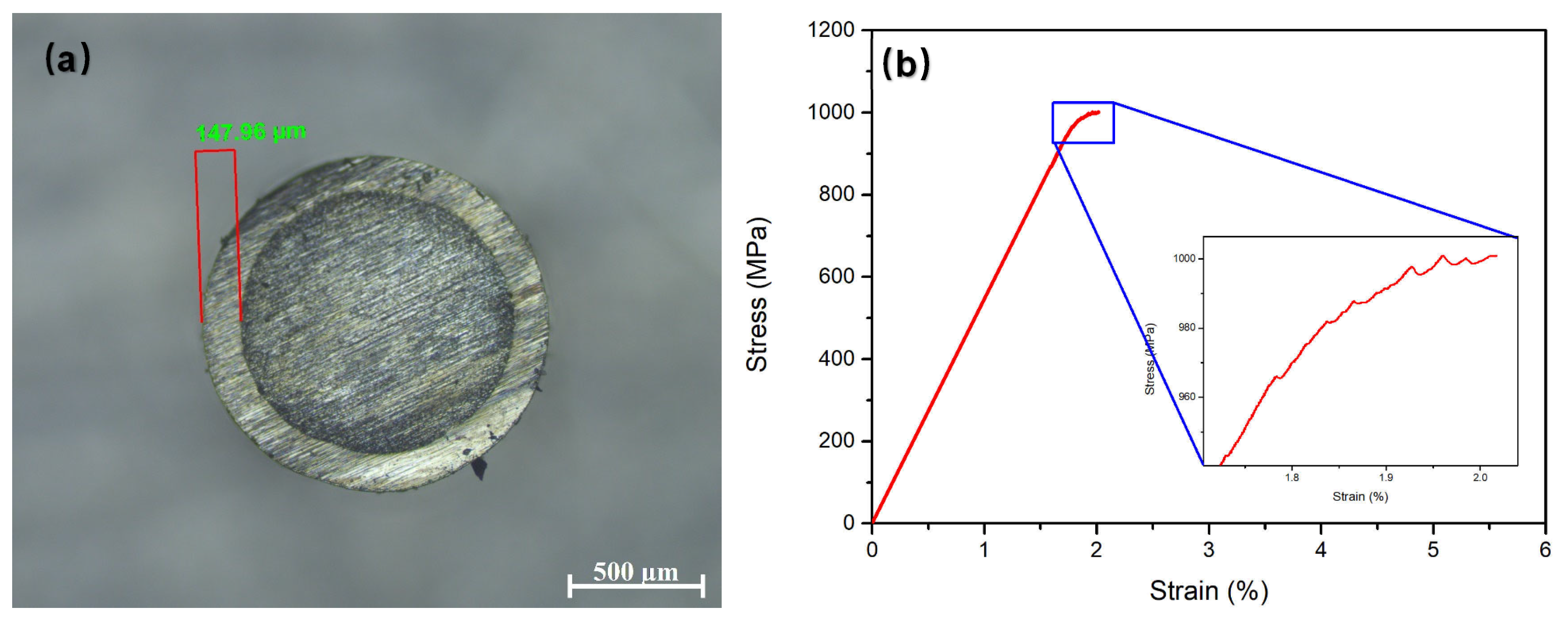
- The mechanical properties of the Ni-plated BMG were tested using the uniaxial compression method, and it was found that the coating thickness significantly affects the geometric constraint effect. The 50 μm Ni coating did not significantly improve the plasticity of the BMG, but it reduced the fluctuation value of fracture strength from 429 MPa to 276 MPa, with a reduction of up to 36%. The ~148 μm Ni coating can improve the plasticity of BMG up to 0.3%. This indicates that electroplating Ni can improve the strength stability and plastic deformation ability of brittle Mg-based BMGs;
- (3)
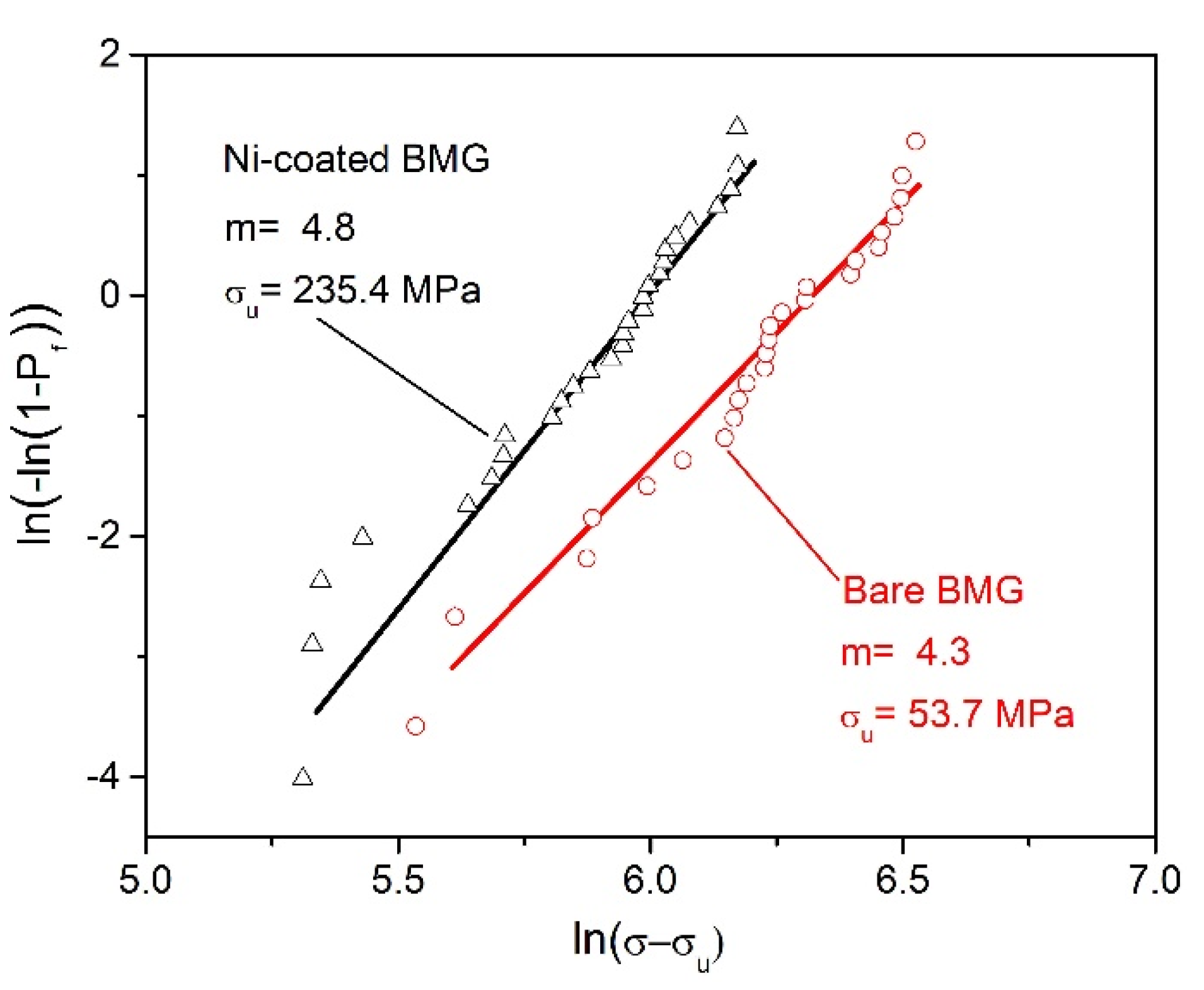
- The Weibull statistical analysis results showed that compared with the BMG sample without Ni plating, the safe stress value and Weibull modulus of the BMG sample after Ni plating increased from 54.7 MPa and 4.3 to 235.4 MPa and 4.8, respectively, indicating that the Ni plating treatment can significantly reduce the brittleness of Mg-based BMGs and improve their reliability;
- (4)
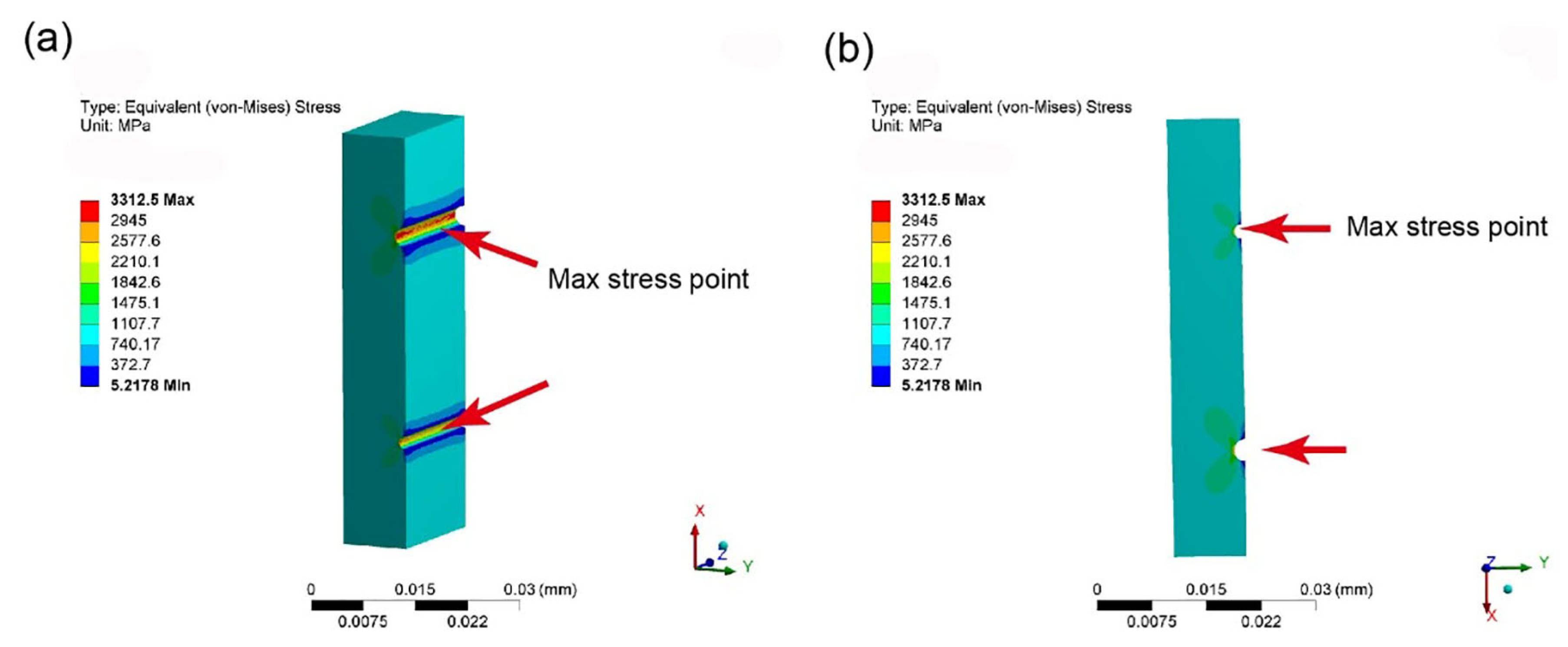
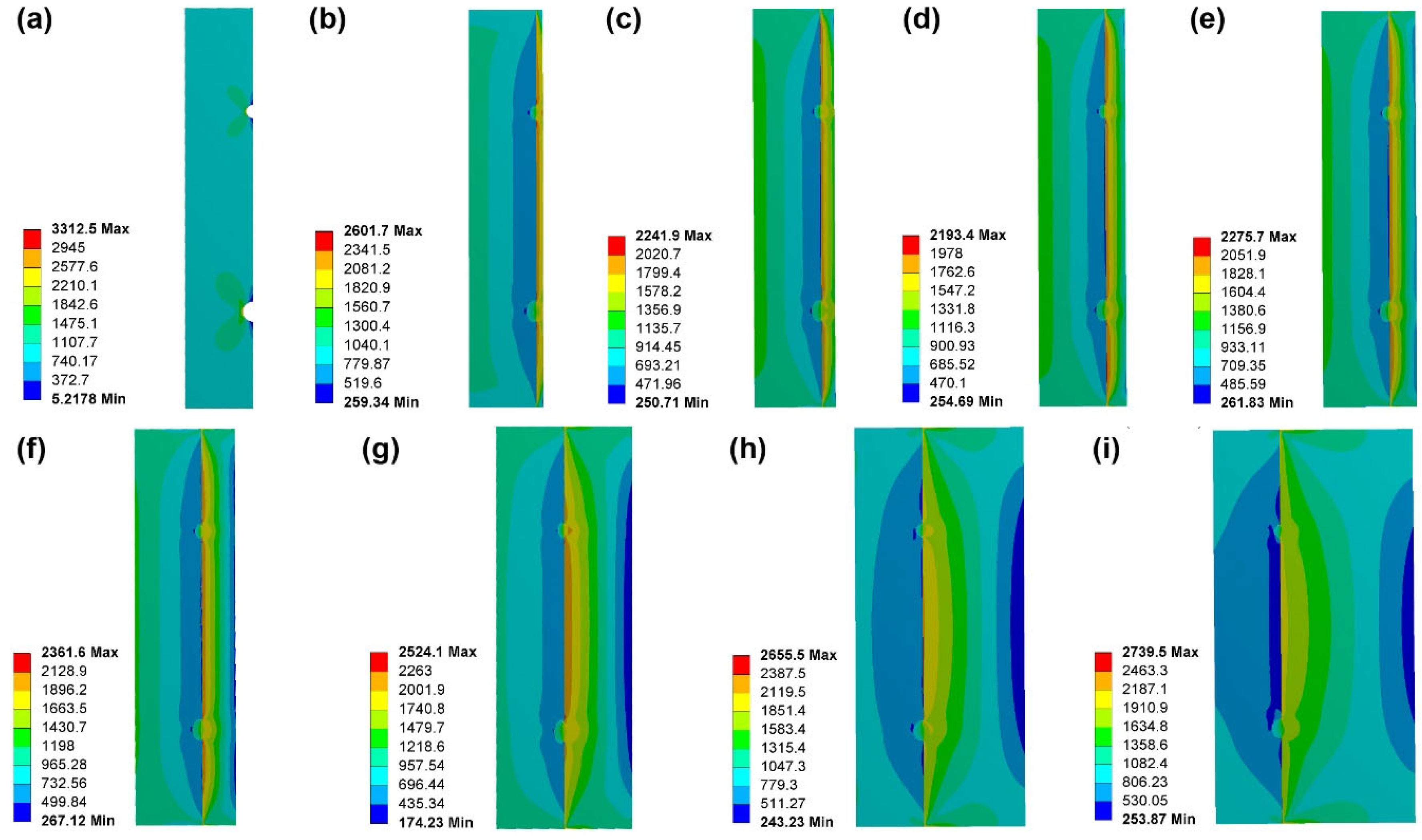
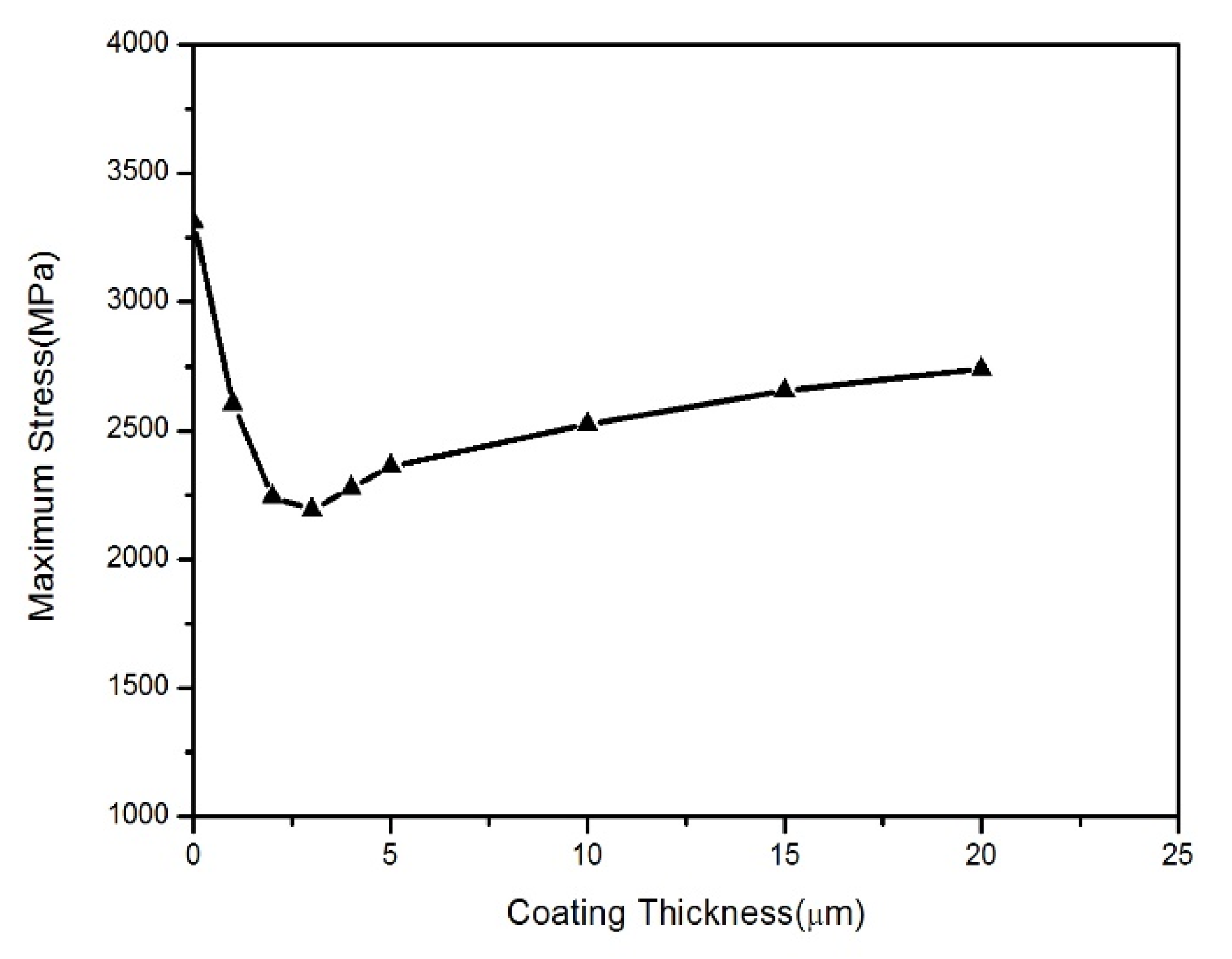
- The FEA results showed that as the thickness of the coating increases, the maximum stress concentration level first decreases rapidly and then slowly increases. When the coating thickness is about 30% of the thickness of BMG substrates, the maximum stress level is the lowest. This indicates that the coating thickness does not necessarily need to be particularly large for achieving an ideal geometric constraint, but rather an optimal value is required, i.e., 30% of the substrate thickness. This result provides a new understanding and perspective for the development of geometric constraint principles and technologies in BMGs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, W.L. Bulk Glass-Forming Metallic Alloys: Science and Technology 1998 MRS Medal Award Lecture, Presented at Symposium MM. In Proceedings of the Symposium MM on Bulk Metallic Glasses at the 1998 MRS Fall Meeting, Boston, MA, USA, 1–3 December 1999; pp. 311–339. [Google Scholar] [CrossRef]
- Xu, J.; Ramamurty, U.; Ma, E. The fracture toughness of bulk metallic glasses. JOM 2010, 62, 10–18. [Google Scholar] [CrossRef]
- Savaedi, Z.; Motallebi, R.; Mirzadeh, H.; Malekan, M. Superplasticity of bulk metallic glasses (BMGs): A review. J. Non-Crystal. Solids 2022, 583, 121503. [Google Scholar] [CrossRef]
- Ritchie, R.O. Toughening materials: Enhancing resistance to fracture. Philos. Trans. R. Soc. A 2021, 379, 20200437. [Google Scholar] [CrossRef]
- El Hafi, T.; Bajjou, O.; Jabraoui, H.; Louafi, J.; Mazroui, M.; Lachtioui, Y. Effects of cooling rate on the glass formation process and the microstructural evolution of Silver mono-component metallic glass. Chem. Phys. 2023, 569, 111873. [Google Scholar] [CrossRef]
- Lian, J.; Song, R.; Chen, Y.; Dai, L. Vortex Evolution Behavior in Self-Assembly of Flow Units in Metallic Glasses. Acta Mech. Solida Sin. 2023. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Liu, Y.; Li, C.; Kou, S. Rejuvenation and Malleability Enhancement of Zr-Based Metallic Glass by Sub-Tg Annealing and Cryogenic Thermal Cycle Treatment. J. Mater. Eng. Perform. 2023, 32, 8430–8440. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C. Atomistic investigation of the intrinsic toughening mechanism in metallic glass. Comp. Mater. Sci. 2016, 117, 188–194. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, D.; Wu, Y.; Geng, Y.; Chen, F.; Guo, P.; Qiao, Y.; Li, X.; Wang, Y. Nano-voids formation at the interaction sites of shear bands in a Zr-based metallic glass. Eur. Phys. J. Appl. Phys. 2022, 97, 91. [Google Scholar] [CrossRef]
- Gurbuz, E.; Sanyal, B. Tuning of lattice thermal conductivity of amorphous Fe0.85Zr0.15 by nanostructured voids, pressure and temperature. J. Non-Cryst. Solids 2023, 616, 122430. [Google Scholar] [CrossRef]
- Noell, P.J.; Sills, R.B.; Benzerga, A.A.; Boyce, B.L. Void nucleation during ductile rupture of metals: A review. Prog. Mater. Sci. 2023, 135, 101085. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, H.; Li, X.; Zhong, L.; Chen, H.; Zhang, Z.; Cao, P.; Ritchie, R.O.; Wang, J. Structural heterogeneity governing deformability of metallic glass. Matter 2023, 6, 1160–1172. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Zhang, L. Chemical and structural heterogeneity improve the plasticity of a Zr-based bulk metallic glass at low-temperature annealing. J. Non-Cryst. Solids 2023, 603, 122115. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Wang, Q.; Rao, J.; Xia, W.; Yan, Y.-Q.; Eckert, J.; Liu, C.; Ma, E.; Shan, Z.-W. Substantially enhanced homogeneous plastic flow in hierarchically nanodomained amorphous alloys. Nat. Commun. 2023, 14, 3670. [Google Scholar] [CrossRef]
- Wang, S.-G.; Shi, L.-L.; Xu, J. Mg-based bulk metallic glasses: Elastic properties and their correlations with toughness and glass transition temperature. J. Mater. Res. 2011, 26, 923–933. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhang, J.; Gong, P.; Yang, W.; Zhao, L.; Wang, X. Design Optimization and Mechanical Properties of SiC Particle Reinforced Ti-Based Metallic Glass Matrix Composite. Materials 2023, 16, 5323. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.L.; Liu, S.Q.; Zhao, L.C.; Cui, C.X.; Wang, X. Vacuum infiltration molding and mechanical property of short carbon fiber reinforced Ti-based metallic glass matrix composite. J. Mater. Proc. Technol. 2021, 295, 117151. [Google Scholar] [CrossRef]
- Yin, H.L.; Yang, W.; Zhao, L.C.; Hu, X.M.; Liu, S.Q.; Cui, C.X.; Wang, X. Fabrication and mechanical property of three-dimensional carbon fiber reinforced Mg-based bulk metallic glass matrix composite. Mater. Sci. Eng. A 2022, 839, 142853. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.-Y.; Wiest, A.; Duan, G.; Lind, M.-L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, H.; Liaw, P.K. Metallic glass matrix composites. Mater. Sci. Eng. R 2016, 100, 1–69. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Sun, Q.; Liu, K. Mechanical properties of Zr41.2Ti13.8Ni10Cu12.5Be22.5 bulk metallic glass with different geometric confinements. Results Phys. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Y.H.; Wang, G.; Bai, H.Y.; Wang, W.H. Enhance plasticity of bulk metallic glasses by geometric confinement. J. Mater. Res. 2007, 22, 2384–2388. [Google Scholar] [CrossRef]
- Li, J.; Cao, Q.; Zhou, Y. Microstructure of Cu60Zr20Ti20 bulk metallic glass rolled at different strain rates. Sci. China Ser. G 2008, 51, 394–399. [Google Scholar] [CrossRef]
- Nieh, T.G.; Schuh, C.; Wadsworth, J.; Li, Y. Strain rate-dependent deformation in bulk metallic glasses. Intermetallics 2002, 10, 1177–1182. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Mara, N.A.; Branagan, D.J.; Mukherjee, A.K. Strain rate effect on metallic glass ductility. Scr. Mater. 2004, 50, 1303–1307. [Google Scholar] [CrossRef]
- Nieh, T.G.; Yang, Y.; Lu, J.; Liu, C.T. Effect of surface modifications on shear banding and plasticity in metallic glasses: An overview. Prog. Nat. Sci. Mater. Int. 2012, 22, 355–363. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.H.; Greer, A.L. Making metallic glasses plastic by control of residual stress. Nat. Mater. 2006, 5, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Ge, Q.; Qu, S.; Jiang, J.Z. Stress-induced softening and hardening in a bulk metallic glass. Scr. Mater. 2008, 59, 1210–1213. [Google Scholar] [CrossRef]
- Wang, L.; Bei, H.; Gao, Y.F.; Lu, Z.P.; Nieh, T.G. Effect of residual stresses on the hardness of bulk metallic glasses. Acta Mater. 2011, 59, 2858–2864. [Google Scholar] [CrossRef]
- Mear, F.O.; Vaughan, G.; Yavari, A.R.; Greer, A.L. Residual-stress distribution in shot-peened metallic-glass plate. Philos. Mag. Lett. 2008, 88, 757–766. [Google Scholar] [CrossRef]
- Mear, F.O.; Doisneau, B.; Yavari, A.R.; Greer, A.L. Structural effects of shot-peening in bulk metallic glasses. J. Alloys Compd. 2009, 483, 256–259. [Google Scholar] [CrossRef]
- Fan, J.T.; Chen, A.Y.; Fu, M.W.; Lu, J. A novel structural gradient metallic glass composite with enhanced mechanical properties. Scr. Mater. 2009, 61, 608–611. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Jiang, H.; Liu, C.T.; Ruan, H.H.; Lu, J. Superior Tensile Ductility in Bulk Metallic Glass with Gradient Amorphous Structure. Sci. Rep. 2014, 4, 4757. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, A.; Wang, J.; Shen, J.; Lu, J. Improved plasticity and fracture toughness in metallic glasses via surface crystallization. Intermetallics 2011, 19, 1420–1427. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Yi, M.; Li, R.; Pang, S.; Wang, H.; Zhang, T. Optimization of mechanical properties of bulk metallic glasses by residual stress adjustment using laser surface melting. Scr. Mater. 2012, 66, 1057–1060. [Google Scholar] [CrossRef]
- Wu, G.; Li, R.; Liu, Z.; Chen, B.; Li, Y.; Cai, Y.; Zhang, T. Induced multiple heterogeneities and related plastic improvement by laser surface treatment in CuZr-based bulk metallic glass. Intermetallics 2012, 24, 50–55. [Google Scholar] [CrossRef]
- Raghavan, R.; Ayer, R.; Jin, H.W.; Marzinsky, C.N.; Ramamurty, U. Effect of shot peening on the fatigue life of a Zr-based bulk metallic glass. Scr. Mater. 2008, 59, 167–170. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Fan, C.; Choo, H.; Liaw, P.K. Nanocrystalline coating enhanced ductility in a Zr-based bulk metallic glass. J. Mater. Res. 2007, 22, 508–513. [Google Scholar] [CrossRef]
- Ren, L.W.; Wang, Z.; Meng, M.M.; Tian, H.; Yang, H.J.; Qiao, J.W. Plasticity enhancement in bulk metallic glasses by electroless plating with Ni-P amorphous films. J. Non-Cryst. Solids 2015, 430, 115–119. [Google Scholar] [CrossRef]
- Qiu, S.-B.; Yao, K.-F. Novel application of the electrodeposition on bulk metallic glasses. Appl. Surf. Sci. 2008, 255, 3454–3458. [Google Scholar] [CrossRef]
- Choi, Y.C.; Hong, S.I. Effect of crystallization and surface treatment on deformation and fracture of Zr-Ti-Cu-Ni-Be bulk metallic glass. Int. J. Mod. Phys. B 2009, 23, 1270–1275. [Google Scholar] [CrossRef]
- Choi, Y.C.; Hong, S.I. Enhancement of plasticity in Zr-base bulk metallic glass by soft metal plating. Scr. Mater. 2009, 61, 481–484. [Google Scholar] [CrossRef]
- Chen, W.; Chan, K.C.; Chen, S.H.; Guo, S.F.; Li, W.H.; Wang, G. Plasticity enhancement of a Zr-based bulk metallic glass by an electroplated Cu/Ni bilayered coating. Mater. Sci. Eng. A 2012, 552, 199–203. [Google Scholar] [CrossRef]
- Ren, L.W.; Yang, F.Q.; Jiao, Z.M.; Yang, H.J.; Wang, Z.H.; Qiao, J.W. Plasticity enhancement in Ni-P amorphous alloy/Ni/Zr-based metallic glass composites with a sandwich structure. Mater. Sci. Eng. A 2015, 643, 175–182. [Google Scholar] [CrossRef]
- Ren, L.W.; Meng, M.M.; Wang, Z.; Yang, F.Q.; Yang, H.J.; Zhang, T.; Qiao, J.W. Enhancement of plasticity in Zr-based bulk metallic glasses electroplated with copper coatings. Intermetallics 2015, 57, 121–126. [Google Scholar] [CrossRef]
- Chen, W.; Chan, K.C.; Guo, S.F.; Yu, P. Plasticity improvement of an Fe-based bulk metallic glass by geometric confinement. Mater. Lett. 2011, 65, 1172–1175. [Google Scholar] [CrossRef]
- Li, J.-F.; Wang, X.; Yang, G.-N.; Chen, N.; Liu, X.; Yao, K.-F. Enhanced plasticity of a Fe-based bulk amorphous alloy by thin Ni coating. Mater. Sci. Eng. A 2015, 645, 318–322. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Zhao, L.; Jiang, D.; Chen, P.; Wang, P.; Zhang, Z.; Liu, S.; Cui, C. Improved Plasticity of Ti-Based Bulk Metallic Glass at Room Temperature by Electroless Thin Nickel Coating. Metals 2017, 7, 562. [Google Scholar] [CrossRef]
- Cao, J.W.; Han, J.G.; Guo, Z.H.; Zhao, W.B.; Guo, Y.Q.; Xia, Z.H.; Qiao, J.W. Plasticity enhancement of high-entropy bulk metallic glasses by electroless plating with Ni-P amorphous films. Mater. Sci. Eng. A 2016, 673, 141–147. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. High corrosion resistance of electroless composite plating coatings on AZ91D magnesium alloys. Electrochim. Acta 2008, 53, 2135–2143. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, J.; Ma, E. High glass-forming ability correlated with fragility of Mg-Cu(Ag)-Gd alloys. J. Appl. Phys. 2007, 102, 113519. [Google Scholar] [CrossRef]
- Wang, S.-G.; Sun, M.-Y.; Song, Z.-Q.; Xu, J. Cast defects induced sample-size dependency on compressive strength and fracture toughness of Mg-Cu-Ag-Gd bulk metallic glass. Intermetallics 2012, 29, 123–132. [Google Scholar] [CrossRef]
- Wang, X. Surface Crystallization in Mg-Based Bulk Metallic Glass during Copper Mold Casting. Adv. Mater. Sci. Eng. 2014, 2014, 798479. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Hitosugi, T.; Chen, N.; Ketov, S.V.; Shluger, A.; Zadorozhnyy, V.Y.; Caron, A.; Gonzales, S.; Qin, C.L.; Inoue, A. Investigation of transparent magnetic material formed by selective oxidation of a metallic glass. Thin Solid Film. 2013, 531, 471–475. [Google Scholar] [CrossRef]
- Yamada, R.; Nomura, N.; Saida, J.; Kawasaki, A. Selective oxidation/crystallization and their patterning on metallic glass by laser irradiation. J. Alloys Compd. 2017, 727, 549–554. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Yao, K.-F. Chemical composition dependence of atomic oxygen erosion resistance in Cu-rich bulk metallic glasses. Chin. Sci. Bull. 2012, 57, 4801–4804. [Google Scholar] [CrossRef][Green Version]
- Lim, J.H.; Park, E.C.; Joo, J.; Jung, S.-B. Effect of Additives on Microstructure and Mechanical Properties of Nickel Plate/Mask Fabricated by Electroforming Process. J. Electrochem. Soc. 2009, 156, D108–D112. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Tripathy, B.C.; Singh, P.; Keshavarz, A.; Iglauer, S. Roles of organic and inorganic additives on the surface quality, morphology, and polarization behavior during nickel electrodeposition from various baths: A review. J. Appl. Electrochem. 2019, 49, 847–870. [Google Scholar] [CrossRef]
- Rajabalizadeh, Z.; Seifzadeh, D. Application of electroless Ni-P coating on magnesium alloy via CrO3/HF free titanate pretreatment. Appl. Surf. Sci. 2017, 422, 696–709. [Google Scholar] [CrossRef]
- Hu, R.; Su, Y.; Liu, Y.; Liu, H.; Chen, Y.; Cao, C.; Ni, H. Deposition Process and Properties of Electroless Ni-P-Al2O3 Composite Coatings on Magnesium Alloy. Nano Res. Lett. 2018, 13, 198. [Google Scholar] [CrossRef]
- Heakal, F.E.-T.; Maanoum, M.A. Role of Some Plating Parameters in the Properties of Ni-P/Al2O3 Nanocomposite Coatings on Mg alloy. Int. J. Electrochem. Sci. 2016, 11, 7198–7215. [Google Scholar] [CrossRef]
- Chen, J.; Yu, G.; Hu, B.; Liu, Z.; Ye, L.; Wang, Z. A zinc transition layer in electroless nickel plating. Surf. Coat. Tech. 2006, 201, 686–690. [Google Scholar] [CrossRef]
- Zheng, Q.; Cheng, S.; Strader, J.H.; Ma, E.; Xu, J. Critical size and strength of the best bulk metallic glass former in the Mg–Cu–Gd ternary system. Scr. Mater. 2007, 56, 161–164. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Hu, X.; Cheng, Y.; Liu, S.; Chen, P.; Cui, C. Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles. Materials 2017, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.F.; Li, Y.; Schuh, C.A. Strength, plasticity and brittleness of bulk metallic glasses under compression: Statistical and geometric effects. Philos. Mag. 2008, 88, 71–89. [Google Scholar] [CrossRef]
- Wang, J.-f.; Wu, X.; Pan, F.-s.; Tang, A.-t.; Ding, P.-d.; Liu, R.-p. Microstructure and mechanical properties of Mg-Cu-Y-Zn bulk metallic glass matrix composites prepared in low vacuum. Trans. Nonferr. Metal Soc. 2008, 18, S278–S282. [Google Scholar] [CrossRef]
- Yin, J.; Yuan, G.; Chen, L.; Zhou, Q. Effects of sample sizes on compressive mechanical behaviors of Mg-based bulk metallic glasses. Chin. J. Nonferrous Met. 2011, 21, 588–596. [Google Scholar]
- Park, E.S.; Kim, D.H. Formation of Mg-Cu-Ni-Ag-Zn-Y-Gd bulk glassy alloy by casting into cone-shaped copper mold in air atmosphere. J. Mater. Res. 2005, 20, 1465–1469. [Google Scholar] [CrossRef]
- Park, E.S.; Chang, H.J.; Kim, D.H. Mg-rich Mg-Ni-Gd ternary bulk metallic glasses with high compressive specific strength and ductility. J. Mater. Res. 2007, 22, 334–338. [Google Scholar] [CrossRef]
- Han, Z.; Tang, L.C.; Xu, J.; Li, Y. A three-parameter Weibull statistical analysis of the strength variation of bulk metallic glasses. Scr. Mater. 2009, 61, 923–926. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, X.; Wang, X.; Zhang, M.; Hu, H.; Gong, P.; Wang, X. Preparation and mechanical properties of tungsten-particle-reinforced Zr-based bulk-metallic-glass composites. Mater. Sci. Eng. A 2021, 815, 141312. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Yao, K. Effects of alloying elements on crystallization kinetics of Ti-Zr-Be bulk metallic glass. J. Mater. Sci. 2016, 51, 5321–5329. [Google Scholar] [CrossRef]
- Liu, S.; Tayyebi, M.; Assari, A.H.; Polkowska, A.; Lech, S.; Polkowski, W. Microstructure, Texture and Tensile Properties of Nickel/Titanium Laminated Composites Produced by Cross Accumulative Roll Bonding Process. Met. Mater. Int. 2023. [Google Scholar] [CrossRef]
- Ye, Q.; Li, X.; Tayyebi, M.; Assari, A.H.; Polkowska, A.; Lech, S.; Polkowski, W.; Tayebi, M. Effect of heat treatment parameters on microstructure evolution, tensile strength, wear resistance, and fracture behavior of Ni–Ti multilayered composites produced by cross-accumulative roll bonding. Archiv. Civ. Mech. Eng. 2022, 23, 27. [Google Scholar] [CrossRef]
- Huang, J.; Tayyebi, M.; Assari, A.H. Effect of SiC particle size and severe deformation on mechanical properties and thermal conductivity of Cu/Al/Ni/SiC composite fabricated by ARB process. J. Manuf. Process. 2021, 68, 57–68. [Google Scholar] [CrossRef]
- Luo, J.; Yarigarravesh, M.; Assari, A.H.; Amin, N.H.; Tayyebi, M.; Paidar, M. Investigating the solid-state diffusion at the interface of Ni/Ti laminated composite. J. Manuf. Process. 2022, 75, 670–681. [Google Scholar] [CrossRef]
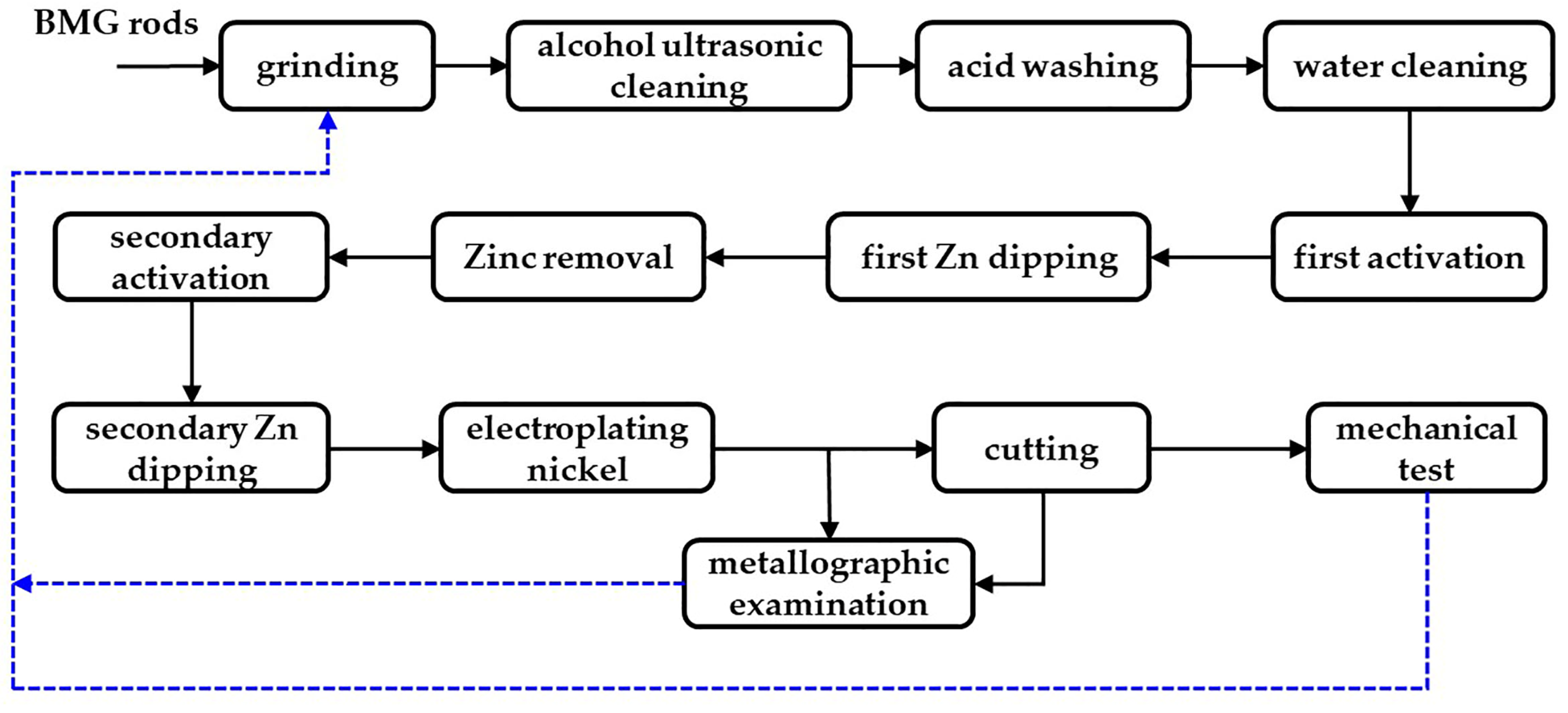



| Number | Process | Solution | Chemical Reagent | Content | Process Conditions |
|---|---|---|---|---|---|
| 1 | Acid washing | Acid washing solution | HNO3 | 30 mL/L | RT, 90 s, pH = 3 |
| 2 | First activation | Activation solution | Na2P2O7 | 120 g/L | 65 °C, 5 min, pH = 10 |
| Na2CO3 | 48 g/L | ||||
| NaF | 11 g/L | ||||
| 3 | First Zn dipping | Zinc dipping solution | ZnSO4·7H2O | 30 g/L | 65 °C, 10 min, pH = 11 |
| Na2P2O7 | 120 g/L | ||||
| NaF | 3 g/L | ||||
| Na2CO3 | 6 g/L | ||||
| 5 | Zinc removal | Zinc removal solution | HNO3 | 10 mL/L | RT, 90 s |
| 6 | Secondary activation | Activation solution | Same as the first activation | 65 °C, 5 min, pH = 10 | |
| 7 | Secondary zinc dipping | Zinc dipping solution | Same as the first Zn dipping | 65 °C, 5 min, pH = 11 | |
| 8 | Electroplating nickel | Plating solution | NiSO4·5H2O | 300 g/L | 50 °C, 1–3 h, pH = 5 |
| H3BO3 | 50 g/L | ||||
| C7H5NO3S | 0.3 g/L | ||||
| CH4N2S | 50 mg/L | ||||
| C12H25SO4Na | 0.2 g/L | ||||
| Nano Al2O3 | 100 mg/L | ||||
| Na2MoO4 | 1 g/L | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, J.; Jing, M.; Zhao, L.; Qi, Y.; Yang, W.; Wang, X. The Effect of Electroplating Nickel on the Mechanical Properties of Brittle Mg-Based Bulk Metallic Glasses. Coatings 2023, 13, 1598. https://doi.org/10.3390/coatings13091598
Zhang J, Li J, Jing M, Zhao L, Qi Y, Yang W, Wang X. The Effect of Electroplating Nickel on the Mechanical Properties of Brittle Mg-Based Bulk Metallic Glasses. Coatings. 2023; 13(9):1598. https://doi.org/10.3390/coatings13091598
Chicago/Turabian StyleZhang, Jingyao, Jing Li, Mei Jing, Lichen Zhao, Yumin Qi, Wei Yang, and Xin Wang. 2023. "The Effect of Electroplating Nickel on the Mechanical Properties of Brittle Mg-Based Bulk Metallic Glasses" Coatings 13, no. 9: 1598. https://doi.org/10.3390/coatings13091598
APA StyleZhang, J., Li, J., Jing, M., Zhao, L., Qi, Y., Yang, W., & Wang, X. (2023). The Effect of Electroplating Nickel on the Mechanical Properties of Brittle Mg-Based Bulk Metallic Glasses. Coatings, 13(9), 1598. https://doi.org/10.3390/coatings13091598








