Preparation of Antistatic Polyester Fiber via Layer-by-Layer Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Treatment of Polyester Fiber Substrate
2.3. Preparation of the Self-Assembly Solution
2.4. Formation of Antistatic Coatings on Polyester Fibers via Layer-by-Layer Self-Assembly
2.5. Characterization and Measurements
2.6. Surface Resistance Test
2.7. Water Absorption Performance Test
3. Results and Discussion
3.1. Surface Morphology and Structure Analysis
3.2. Analysis of Surface Resistance
3.3. Analysis of Surface Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Wang, Q.Z.; Wang, B.; Liu, Y.; Xu, Y.; Liu, Y.; Zhu, P. Blending alginate fibers with polyester fibers for flame-retardant filling materials: Thermal decomposition behaviors and fire performance. Polym. Degrad. Stab. 2021, 193, 109470. [Google Scholar] [CrossRef]
- Fan, Y.; Shen, J.; Xu, H. Synthesis and dilute aqueous solution properties of cationic antistatic surfactant functionalized with hydroxyl and ether groups. Tenside Surfactants Deterg. 2023, 60, 64–73. [Google Scholar] [CrossRef]
- Lv, J.; Zhou, Q.; Zhi, T.; Gao, D.; Wang, C. Environmentally friendly surface modification of polyethylene terephthalate (PET) fabric by low-temperature oxygen plasma and carboxymethyl chitosan. J. Clean. Prod. 2016, 118, 187–196. [Google Scholar] [CrossRef]
- Khoddami, A.; Avinc, O.; Ghahremanzadeh, F. Improvement in poly (lactic acid) fabric performance via hydrophilic coating. Prog. Org. Coat. 2011, 72, 299–304. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, N.; Wei, Q.; Chu, L. Surface Functionalisation of Polyester Nonwoven Fabrics by Sputter Coating of Titanium Dioxide. Polym. Polym. Compos. 2009, 17, 347–351. [Google Scholar] [CrossRef]
- Li, S.; Huang, S.; Xu, F.; Xiao, H.; Zhang, F.; Zhang, G. Preparing polyester/carbon multifunctional fabrics by phosphoric acid carbonization. Cellulose 2019, 26, 8907–8917. [Google Scholar] [CrossRef]
- Kan, C.W. Evaluating antistatic performance of plasma-treated polyester. Fibers Polym. 2007, 8, 629–634. [Google Scholar] [CrossRef]
- An, W.; Ma, J.; Xu, Q.; Fan, Q. Flame retardant, antistatic cotton fabrics crafted by layer-by-layer assembly. Cellulose 2020, 27, 8457–8469. [Google Scholar] [CrossRef]
- Su, M.; Chen, X.; Zhang, L.; Min, J. Synthesis of Active Graphene with Para-Ester on Cotton Fabrics for Antistatic Properties. Nanomaterials 2020, 10, 1147. [Google Scholar] [CrossRef]
- Sano, Y.; Lee, C.; Kimura, Y.; Saegusa, T. A novel spin-finishing method for antistatic modification of polyester fiber. Surface reaction onto amine-containing copoly(amide-ethers) blended with polyester fiber. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1997, 246, 109–123. [Google Scholar] [CrossRef]
- Sano, Y.; Konda, M.; Lee, C.; Kimura, Y.; Saegusa, T. A facile antistatic modification of polyester fiber based on ion-exchange reaction of sulfonate-modified polyester and various cationic surfactants. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1997, 251, 181–191. [Google Scholar] [CrossRef]
- Lindgren, J.; Persson, P.; Öhman, L.O. Interactions of calcium (II), copper (II) and aluminium (III) ions with two chemically modified wood fibres. Nord. Pulp Pap. Res. J. 2001, 16, 225–234. [Google Scholar] [CrossRef]
- Xing, X.; Yang, H.; Tao, M.; Zhang, W. An overwhelmingly selective colorimetric sensor for Ag+ using a simple modified polyacrylonitrile fiber. J. Hazard. Mater. 2015, 297, 207–216. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper (II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, F.; Zhou, R.; Liu, P.; Xiong, Q.; Zhang, W.; Ye, X.; Gao, H. Fabrication of recyclable Fe3+ chelated aminated polypropylene fiber for efficient clean-up of phosphate wastewater. Front. Chem. Sci. Eng. 2023, 17, 840–852. [Google Scholar] [CrossRef]
- Xu, T.; Qian, D.; Hu, Y.; Zhu, Y.; Zhong, Y.; Zhang, L.; Xu, H.; Mao, Z. Assembled hybrid films based on sepiolite, phytic acid, polyaspartic acid and Fe3+ for flame-retardant cotton fabric. J. Polym. Eng. 2022, 42, 744–754. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Rahman, M.Z.; Song, L.; Hu, Y. An environmentally friendly approach to fabricating flame retardant, antibacterial and antifungal cotton fabrics via self-assembly of guanazole-metal complex. J. Clean. Prod. 2020, 273, 122832. [Google Scholar] [CrossRef]
- Aslam, S.; Hussain, T.; Ashraf, M.; Tabassum, M.; Rehman, A.; Iqbal, K.; Javid, A. Multifunctional Finishing of Cotton Fabric. Autex Res. J. 2019, 19, 191–200. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, Q.; Song, L.; Zhao, H. Fabrication of layer-by-layer self-assembled coating modified cotton fabric with flame retardancy and hydrophobicity based on sepiolite. Polym.-Plast. Technol. Mater. 2021, 60, 1368–1376. [Google Scholar] [CrossRef]
- Chen, X.; Ding, F.; Zhang, S.; Liu, Y.; Hou, X.; Ren, X. Flame-retardant, antibacterial and hydrophobic multifunctional coatings on cotton fabrics via layer-by-layer self-assembly. Cellulose 2023, 30, 6679–6694. [Google Scholar] [CrossRef]
- Wang, X.; Romero, M.Q.; Zhang, X.Q.; Wang, R.; Wang, D.Y. Intumescent multilayer hybrid coating for flame retardant cotton fabrics based on layer-by-layer assembly and sol–gel process. RSC Adv. 2015, 5, 10647–10655. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process. I. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles on Charged Surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Jeonghwan, K.; Arcadio, S.; Jungwoo, C.; Dowoo, N.; Arman, B.; Bruggen, B.V. Embedding TiO2 nanoparticles versus surface coating by layer-by-layer deposition on nanoporous polymeric films. Microporous Mesoporous Mater. 2013, 173, 121–128. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Xu, Z.; Wang, Y.; Dong, S. Surface plasmon resonance and electrochemistry characterization of layer-by-layer self-assembled DNA and Zr4+ thin films, and their interaction with cytochrome C. Talanta 2007, 74, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Iamphaojeen, Y.; Siriphannon, P. Immobilization of zinc oxide nanoparticles on cotton fabrics using poly 4-styrenesulfonic acid polyelectrolyte. Int. J. Mater. Res. 2012, 103, 643–647. [Google Scholar] [CrossRef]
- Yang, J.M.; Tsai, R.Z.; Hsu, C.C. Protein adsorption on polyanion/polycation layer-by-layer assembled polyelectrolyte films. Colloids Surf. B Biointerfaces 2016, 142, 98. [Google Scholar] [CrossRef] [PubMed]
- Janesch, J.; Czabany, I.; Hansmann, C.; Mautne, A.; Rosenau, T.; Altmutter, W.G. Transparent layer-by-layer coatings based on biopolymers and CeO2 to protect wood from UV light. Prog. Org. Coat. 2020, 138, 105409. [Google Scholar] [CrossRef]
- Teli, M.; Prasad, N.; Vyas, U. Electrokinetic properties of modified polyester fibers. J. Appl. Polym. Sci. 1993, 50, 449–457. [Google Scholar] [CrossRef]
- AATCC 76-2018(E2019); Test Method for Electrical Surface Resistivity of Fabrics. American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2019.
- GB/T 12703.4-2010; Textile-Evaluation for Electrostatic Properties—Part 4: Resistivity. Standards Press of China: Beijing, China, 2010.
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Hu, X.; Zhu, F.; Su, J.; Han, J. A novel strategy for fabrication of antistatic and antibacterial fabric via layer-by-layer self-assembly. Surf. Coat. Technol. 2023, 453, 129143. [Google Scholar] [CrossRef]
- Eom, S. Using Chitosan as an Antistatic Finish for Polyester Fabric. AATCC Rev. 2001, 1, 57. [Google Scholar]
- GB/T 12490-2014; Textiles-Tests for Colour Fastness-Colour Fastness to Domestic and Commercial Laundering. Standards Press of China: Beijing, China, 2014.
- Abdel-Halima, E.; Abdel-Mohdya, F.; Al-Deyabb, S.; El-Newehy, E. Chitosan and monochlorotriazinyl–cyclodextrin finishes improve antistatic properties of cotton/polyester blend and polyester fabrics. Carbohydr. Polym. 2010, 82, 202–208. [Google Scholar] [CrossRef]
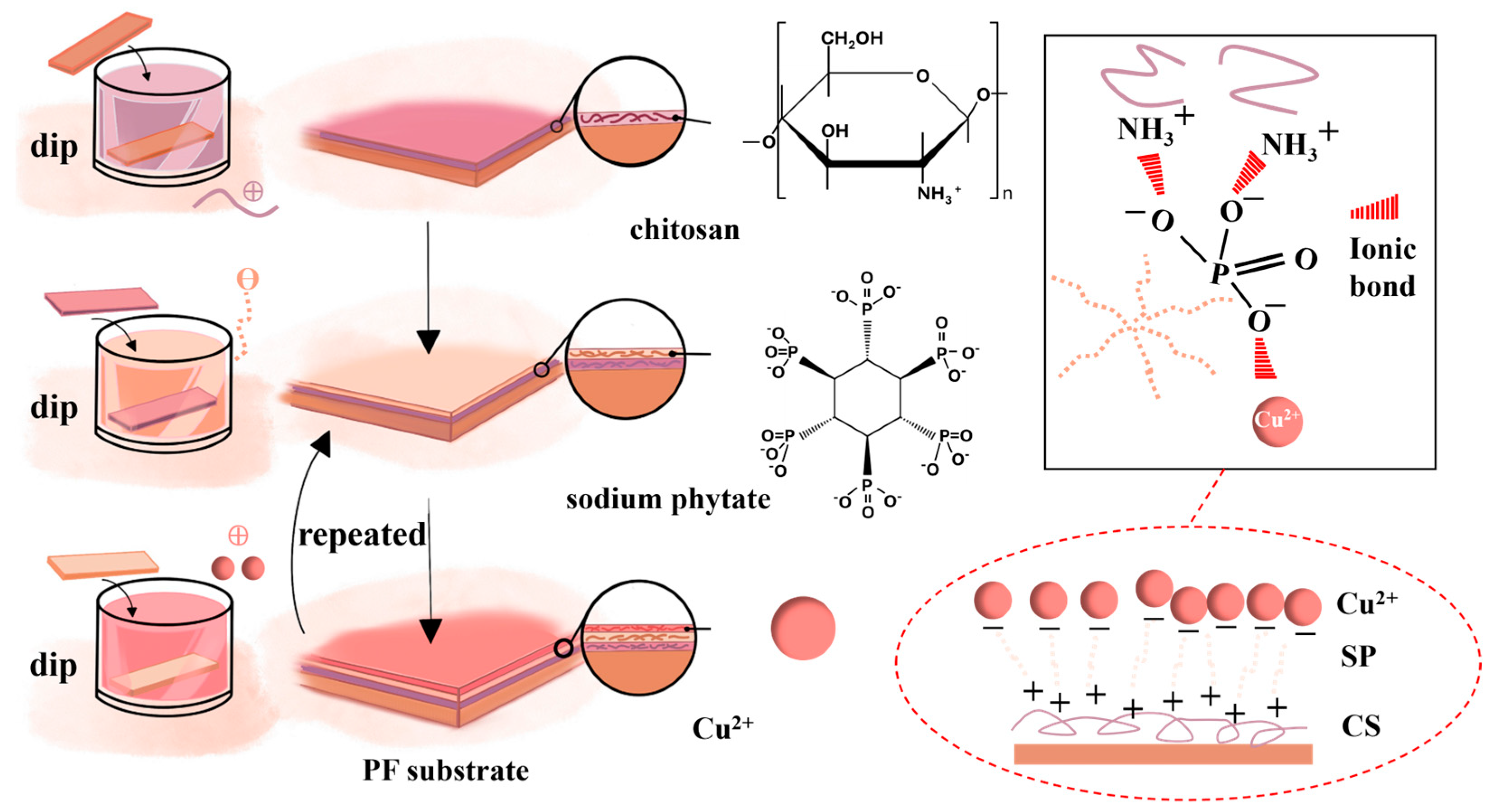
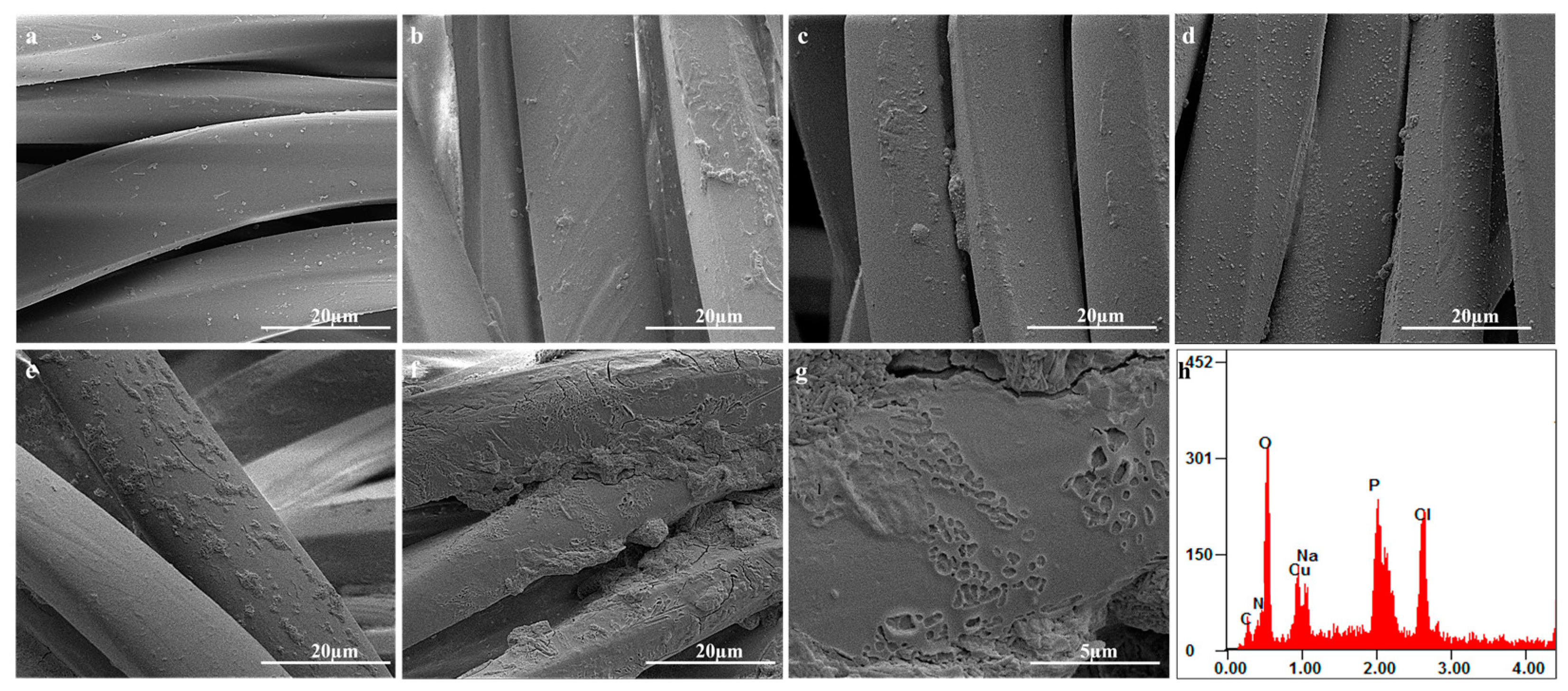

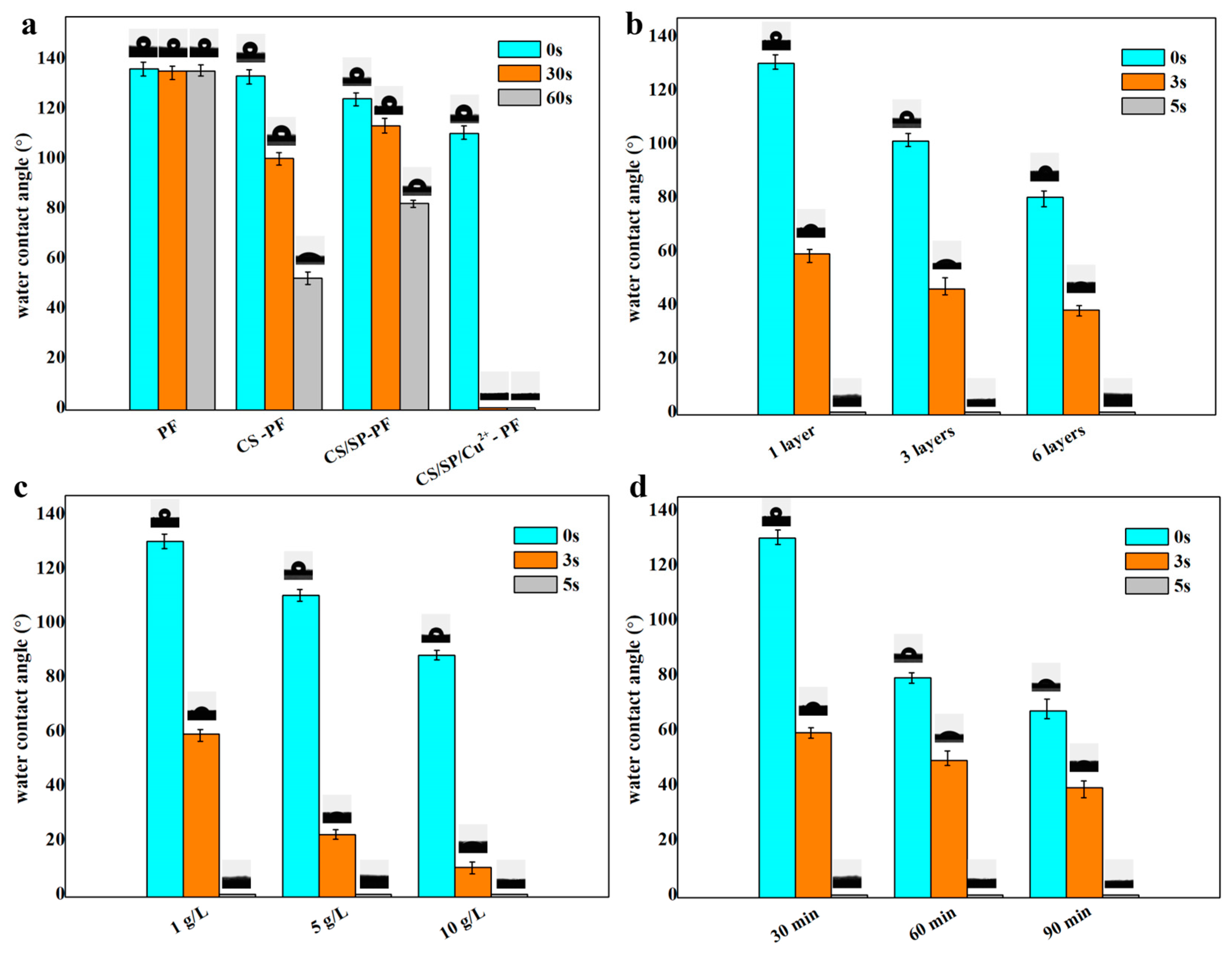
| LBL Self-Assembly Structure | Concentration (g/L) | Impregnation Time(min) | Layers | Surface Resistance Value (Ω) | ||
|---|---|---|---|---|---|---|
| CS | SP | CuCl2 | ||||
| original | / | / | / | / | / | outrange |
| CS | 1 | / | / | 90 | 1 | 1012 |
| CS | 5 | / | / | 90 | 1 | 1011 |
| CS | 10 | / | / | 90 | 1 | 1010 |
| CS/SP | 1 | 1 | / | 90 | 6 | 1011 |
| CS/SP | 5 | 5 | / | 90 | 6 | 1010 |
| CS/SP | 10 | 10 | / | 90 | 6 | 1010 |
| CS/SP/Cu2+ | 1 | 1 | 1 | 90 | 6 | 109 |
| CS/SP/Cu2+ | 5 | 5 | 5 | 90 | 6 | 108 |
| CS/SP/Cu2+ | 10 | 10 | 10 | 90 | 6 | 106 |
| CS/SP/Cu2+ | 10 | 10 | 10 | 30 | 6 | 108 |
| CS/SP/Cu2+ | 10 | 10 | 10 | 60 | 6 | 107 |
| CS/SP/Cu2+ | 10 | 10 | 10 | 90 | 1 | 108 |
| CS/SP/Cu2+ | 10 | 10 | 10 | 90 | 3 | 107 |
| CS/SP/Cu2+ after washing 10 times | 10 | 10 | 10 | 90 | 6 | 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, J.; Liu, Y.; Weng, M.; Fu, Y. Preparation of Antistatic Polyester Fiber via Layer-by-Layer Self-Assembly. Coatings 2024, 14, 1236. https://doi.org/10.3390/coatings14101236
Wang W, Zhang J, Liu Y, Weng M, Fu Y. Preparation of Antistatic Polyester Fiber via Layer-by-Layer Self-Assembly. Coatings. 2024; 14(10):1236. https://doi.org/10.3390/coatings14101236
Chicago/Turabian StyleWang, Wei, Jialong Zhang, Yifan Liu, Mengyun Weng, and Yanchun Fu. 2024. "Preparation of Antistatic Polyester Fiber via Layer-by-Layer Self-Assembly" Coatings 14, no. 10: 1236. https://doi.org/10.3390/coatings14101236






