Novel Antibacterial Resin Coating for Dental Provisional Crowns to Suppress Biofilms and Inhibit Secondary Caries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Resin-Based Coating
- TEMPSMART (GC America) (denoted as “Commercial Control”);
- UV resin + 0% DMADDM (denoted as “Experimental Control”);
- UV resin + 2.5% DMADDM (denoted as “UV + 2.5% DMADDM”);
- UV resin + 5% DMADDM (denoted as “UV + 5% DMADDM”);
- UV resin + 7.5% DMADDM (denoted as “UV + 7.5% DMADDM”);
- UV resin + 10% DMADDM (denoted as “UV + 10% DMADDM”).
2.2. Samples Preparation and Analysis of Surface Roughness
2.3. Contact Angle Assays
2.4. Resin-Based Coating Thickness
2.5. Samples Preparation for S. mutans Biofilm Testing
2.6. Inoculation of S. mutans and Biofilm Formation
2.7. S. mutans Biofilm Colony-Forming Units (CFU)
2.8. Metabolic Activity of S. mutans Biofilms (MTT)
2.9. Lactic Acid Production by S. mutans Biofilms
2.10. Live/dead Staining of Biofilm
2.11. Scanning Electron Microscopy (SEM) of S. mutans Biofilms
2.12. Cytotoxicity of Human Gingival Fibroblasts
2.13. Statistical Analyses
3. Results
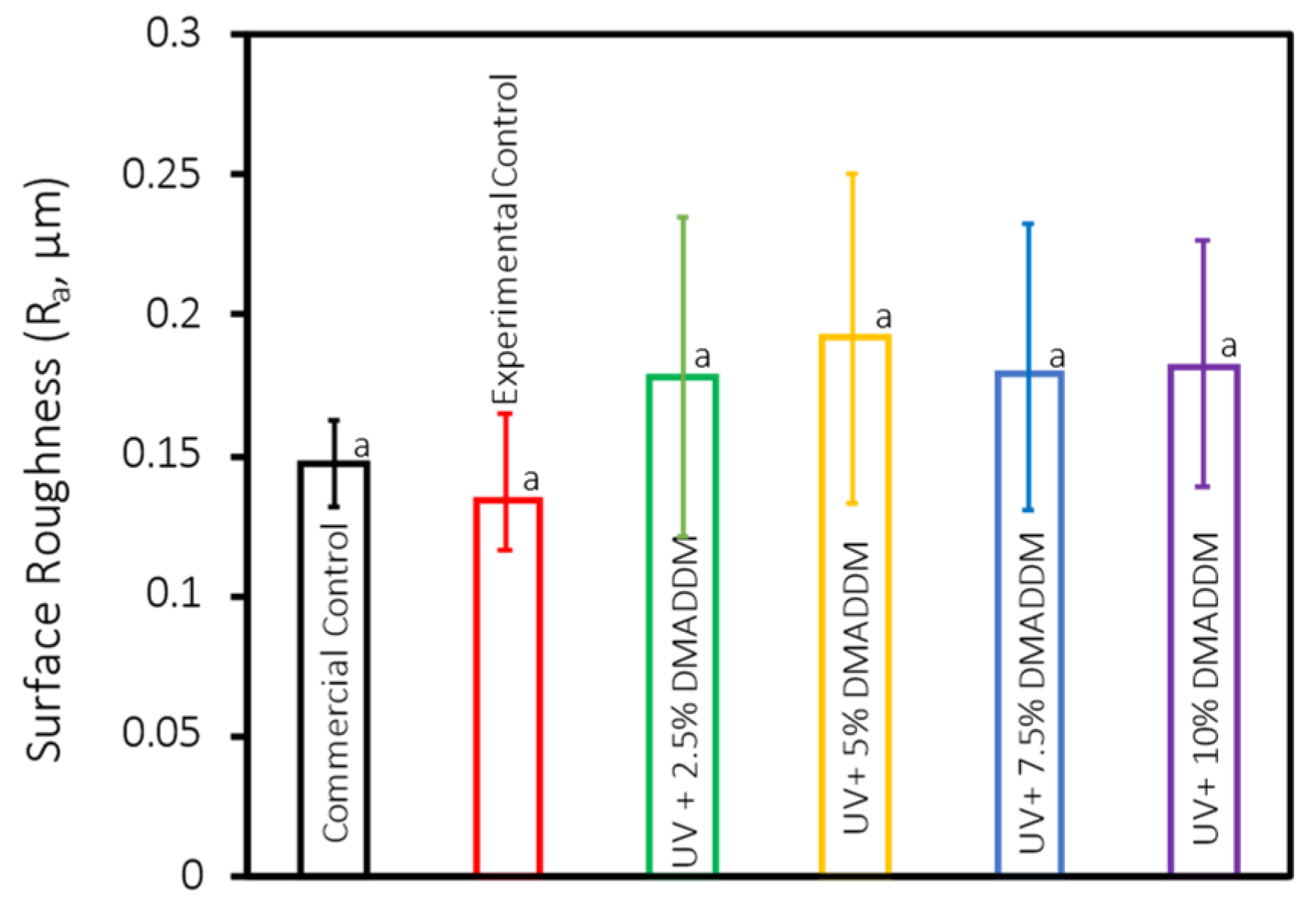
3.1. Surface Roughness
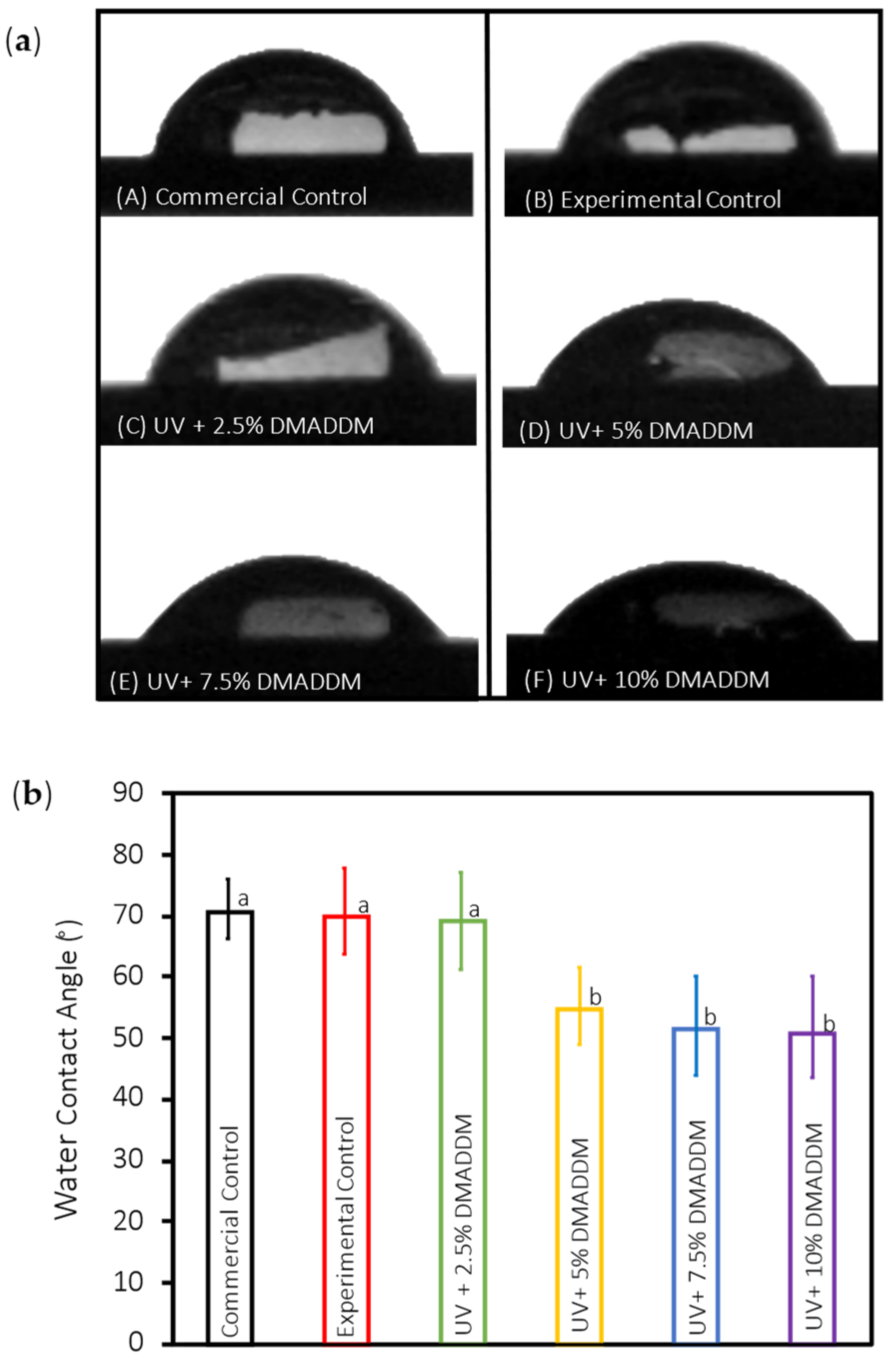
3.2. Contact Angle Assays
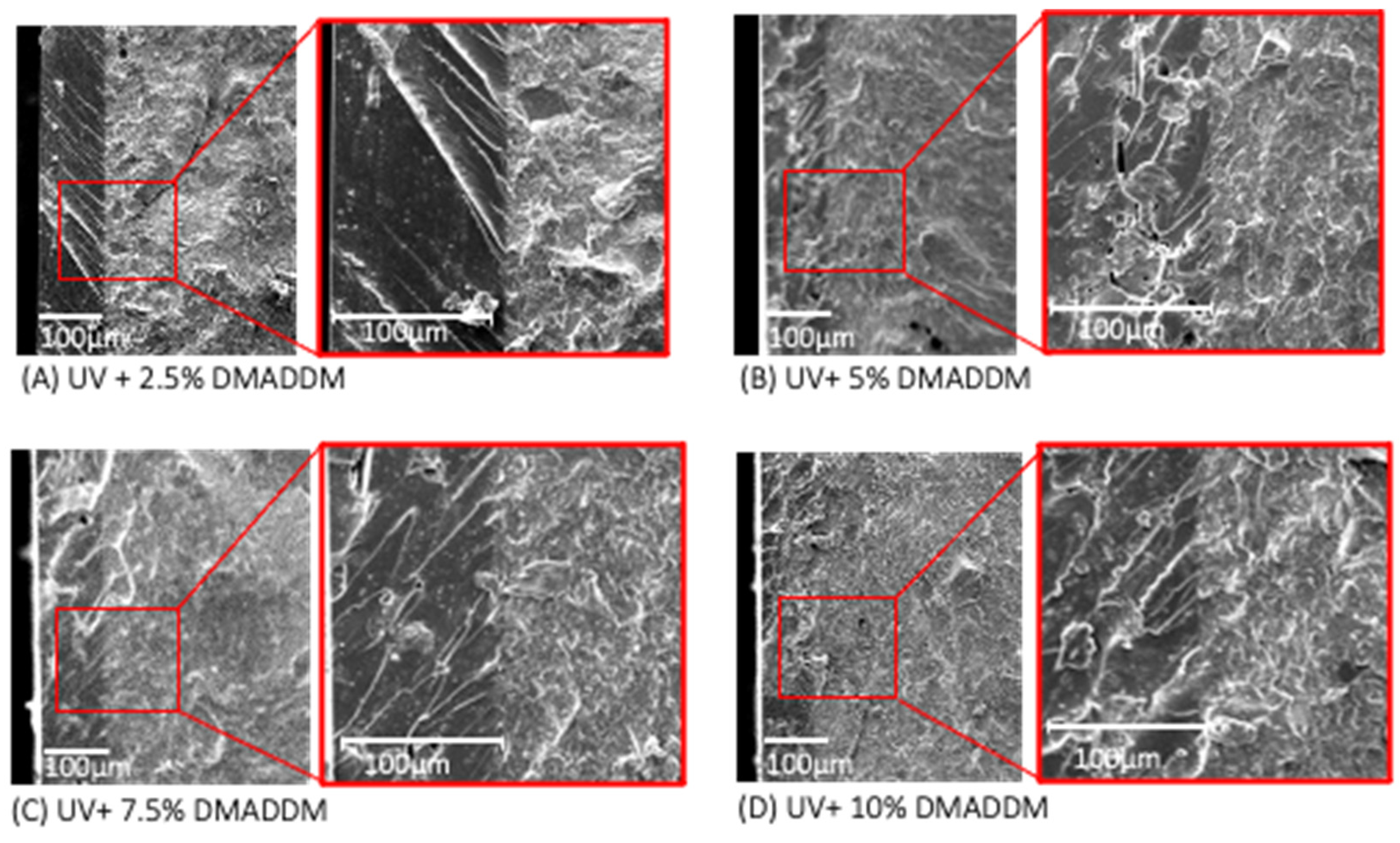
3.3. SEM Analysis of Resin-Based Coating Thickness
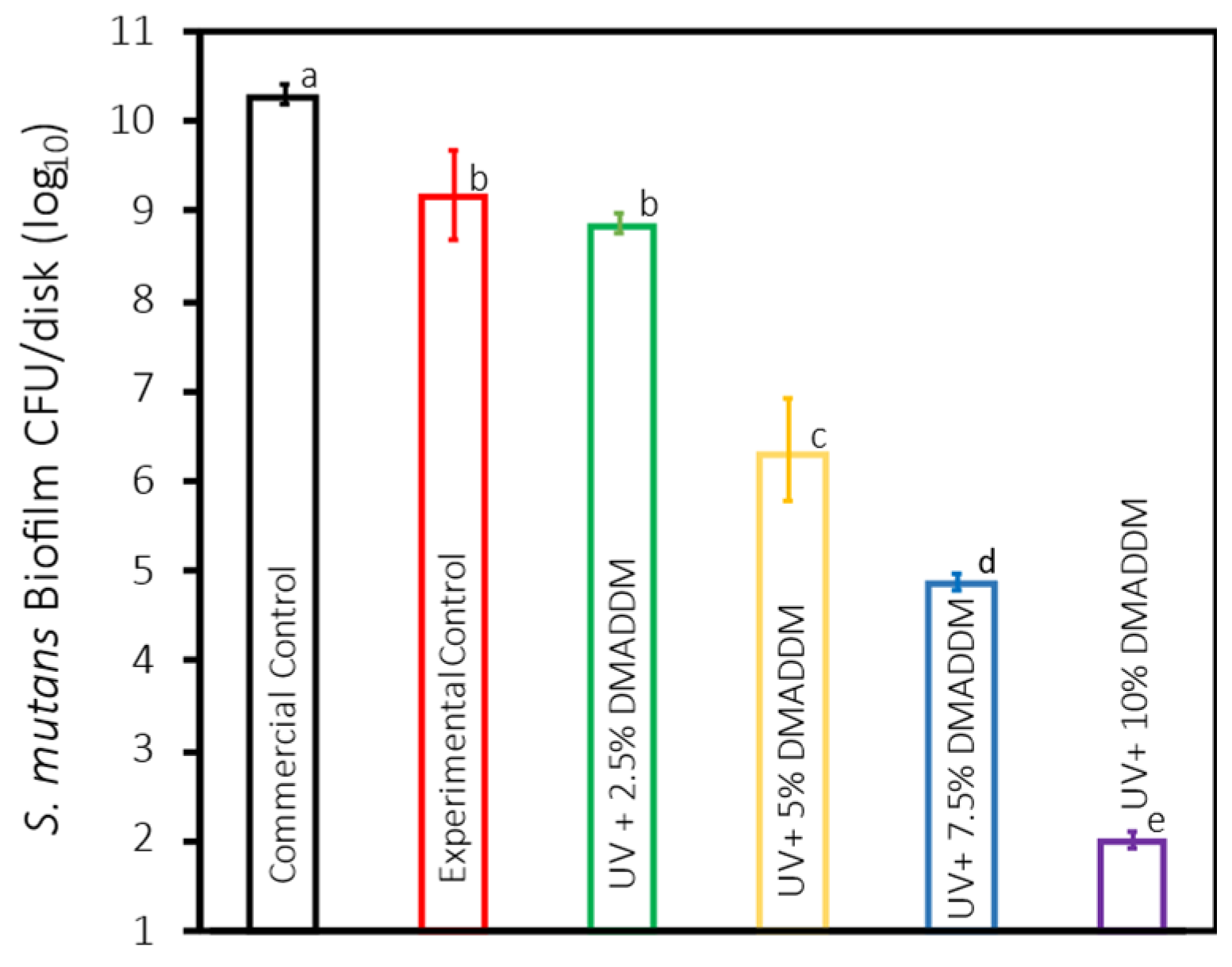
3.4. S. mutans Biofilm Colony-Forming Units (CFU)
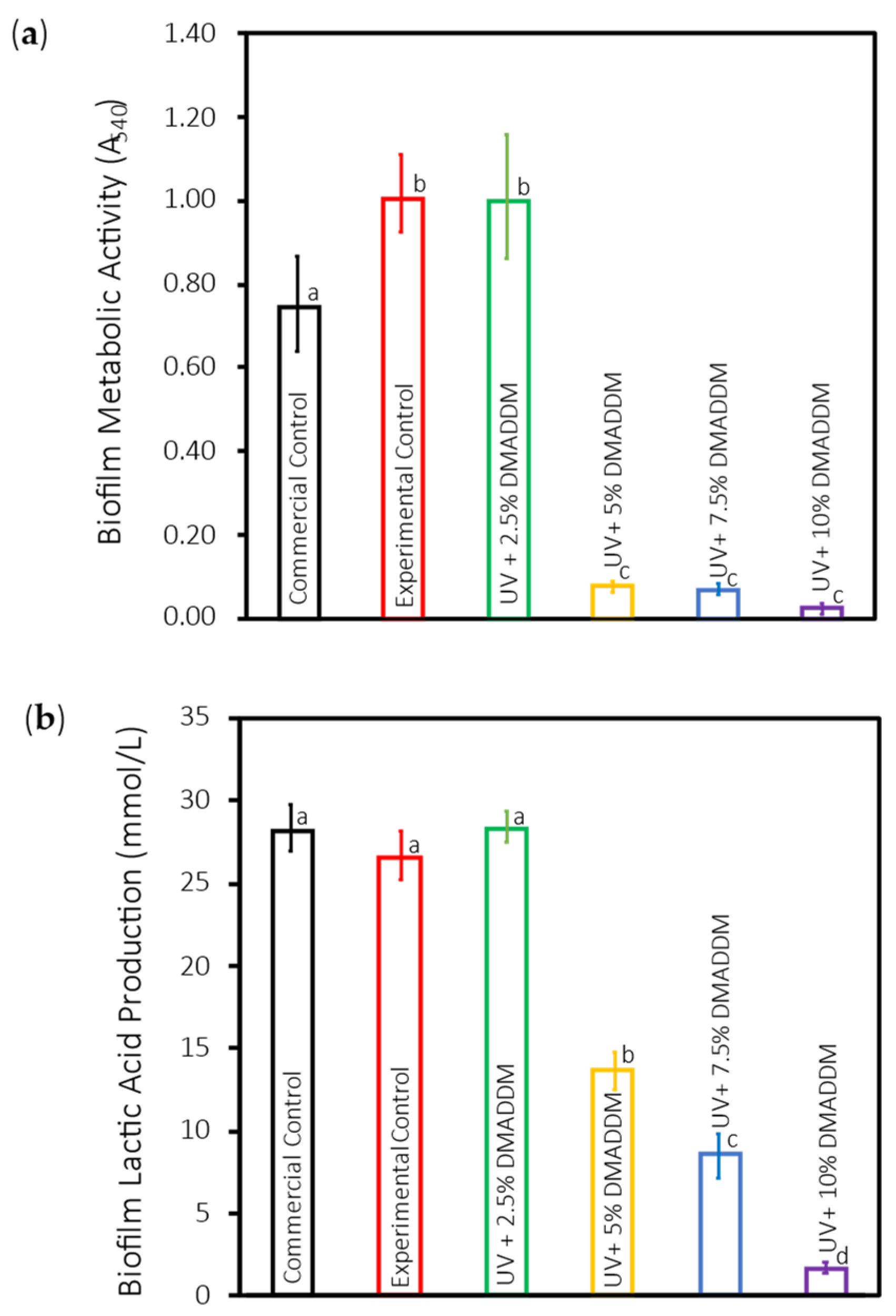
3.5. Metabolic Activity of S. mutans Biofilms (MTT)
3.6. Lactic Acid Production by S. mutans Biofilms
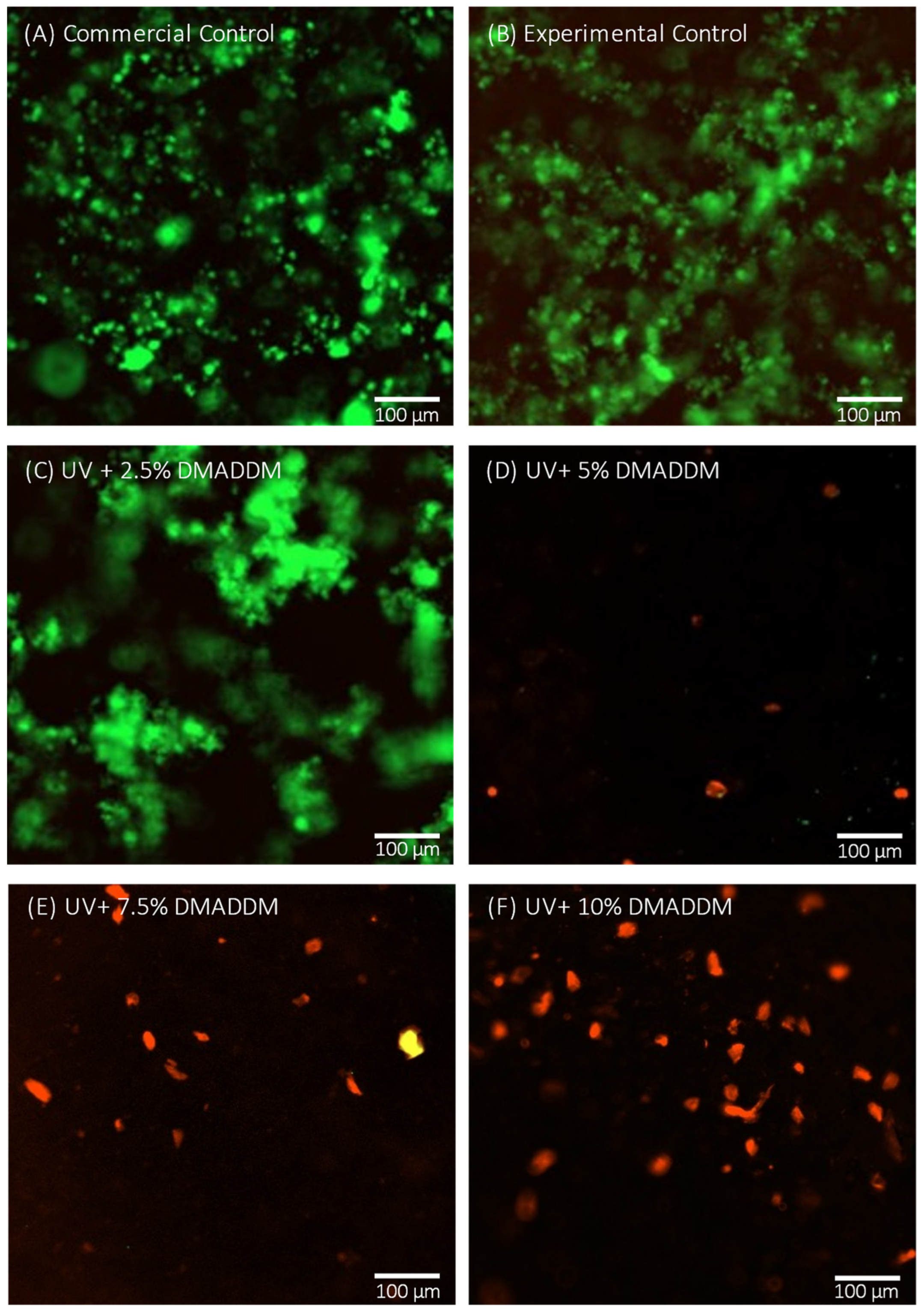
3.7. Live/Dead Staining Assay
3.8. SEM Observation of S. mutans Biofilms
3.9. Cytotoxicity of Human Gingival Fibroblasts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hensel, F.; Koenig, A.; Doerfler, H.-M.; Fuchs, F.; Rosentritt, M.; Hahnel, S. CAD/CAM Resin-Based Composites for Use in Long-Term Temporary Fixed Dental Prostheses. Polymers 2021, 13, 3469. [Google Scholar] [CrossRef] [PubMed]
- Edelhoff, D.; Beuer, F.; Schweiger, J.; Brix, O.; Stimmelmayr, M.; Guth, J.-F. CAD/CAM-generated high-density polymer restorations for the pretreatment of complex cases: A case report. Quintessence Int. 2012, 43, 457–467. [Google Scholar]
- Singh, Y.; Brar, A.; A Mattoo, K.; Singh, M.; Khurana, P.R.S.; Singh, M. Clinical reliability of different facial measurements in determining vertical dimension of occlusion in dentulous and edentulous subjects. Int. J. Prosthodont. Restor. Dent. 2014, 4, 68–77. [Google Scholar] [CrossRef]
- Aldahian, N.; Khan, R.; Mustafa, M.; Vohra, F.; Alrahlah, A. Influence of Conventional, CAD-CAM, and 3D printing fabrication techniques on the marginal integrity and surface roughness and wear of interim crowns. Appl. Sci. 2021, 11, 8964. [Google Scholar] [CrossRef]
- Sham, A.S.K.; Chu, F.C.S.; Chai, J.; Chow, T.W. Color stability of provisional prosthodontic materials. J. Prosthet. Dent. 2004, 91, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in vitro evaluation of CAD/CAM vs conventional provisional crowns. J. Appl. Oral Sci. 2016, 24, 258–263. [Google Scholar] [CrossRef]
- Mörmann, W.H. The evolution of the CEREC system. J. Am. Dent. Assoc. 2006, 137, 7s–13s. [Google Scholar] [CrossRef]
- Madhav, V.N.V.; Digholkar, S.; Palaskar, J. Evaluation of the flexural strength and microhardness of provisional crown and bridge materials fabricated by different methods. J. Indian Prosthodont. Soc. 2016, 16, 328–334. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jang, W.; Im, Y.-G.; Lim, H.-P. Antibacterial Effect of Zirconia Nanoparticles on Polyethyl Methacrylate Resin for Provisional Crowns. Int. J. Nanomed. 2022, 17, 6551–6560. [Google Scholar] [CrossRef]
- Burns, D.R.; A Beck, D.; Nelson, S.K. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: Report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2003, 90, 474–497. [Google Scholar] [CrossRef]
- McLean, J.W. The failed restoration: Causes of failure and how to prevent them. Int. Dent. J. 1990, 40, 354–358. [Google Scholar] [PubMed]
- Buergers, R.; Rosentritt, M.; Handel, G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J. Prosthet. Dent. 2007, 98, 461–469. [Google Scholar] [CrossRef]
- Bollenl, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Jubhari, E.H.; Machmud, E.; Hasminar, H.; Arafi, A.; Kristianti, C.A.; Arpa, S.; Amiruddin, M. The effect of fabrication techniques of temporary crowns on the gingiva health. J. Dentomaxillofacial Sci. 2019, 4, 133. [Google Scholar] [CrossRef]
- Karaman, T.; Eser, B.; Altintas, E.; Atala, M.H. Evaluation of the effects of finish line type and width on the fracture strength of provisional crowns. Odontology 2021, 109, 76–81. [Google Scholar] [CrossRef]
- Anadioti, E.; Aquilino, S.A.; Gratton, D.G.; Holloway, J.A.; Denry, I.; Thomas, G.W.; Qian, F. 3D and 2D Marginal fit of pressed and CAD/CAM lithium disilicate crowns made from digital and conventional impressions. J. Prosthodont. 2014, 23, 610–617. [Google Scholar] [CrossRef]
- e Silva, J.S.A.; Erdelt, K.; Edelhoff, D.; Araújo, É.; Stimmelmayr, M.; Vieira, L.C.C.; Güth, J.-F. Marginal and internal fit of four-unit zirconia fixed dental prostheses based on digital and conventional impression techniques. Clin. Oral Investig. 2014, 18, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.C.; da Silva-Neto, J.P.; Dantas, T.S.; Naves, L.Z.; Neves, F.D.D.; da Mota, A.S. Bacterial Adhesion and Surface Roughness for Different Clinical Techniques for Acrylic Polymethyl Methacrylate. Int. J. Dent. 2016, 2016, 8685796. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; Van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Köroğlu, A.; Sahin, O.; Dede, D.; Yilmaz, B. Effect of different surface treatment methods on the surface roughness and color stability of interim prosthodontic materials. J. Prosthet. Dent. 2016, 115, 447–455. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [Google Scholar] [CrossRef]
- Morgan, T.; Wilson, M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.; Douglas, C.W.I. Relationship between the ability of oral streptococci to interact with platelet glycoprotein Ibalpha and with the salivary low-molecular-weight mucin, MG2. FEMS Immunol. Med. Microbiol. 2006, 48, 390–399. [Google Scholar] [CrossRef]
- Quirynen, M.; De Soete, M.; Dierickx, K.; Van Steenberghe, D. The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. J. Clin. Periodontol. 2001, 28, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Wieland, M.; Ximénez-Fyvie, L.A.; Schwartz, Z.; Boyan, B.D. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin. Oral Implant. Res. 2012, 23, 301–307. [Google Scholar] [CrossRef]
- Weerkamp, A.; Uyen, H.; Busscher, H. Effect of zeta potential and surface energy on bacterial adhesion to uncoated and saliva-coated human enamel and dentin. J. Dent. Res. 1988, 67, 1483–1487. [Google Scholar] [CrossRef]
- Carlén, A.; Nikdel, K.; Wennerberg, A.; Holmberg, K.; Olsson, J. Surface characteristics and in vitro biofilm formation on glass ionomer and composite resin. Biomaterials 2001, 22, 481–487. [Google Scholar] [CrossRef]
- Busscher, H.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Hamada, S.; Slade, H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, M.; Shen, Y.; Zhang, L.; Pan, Y.; Sun, Y.; Zhang, K. Regulatory Effect of Irresistin-16 on Competitive Dual-Species Biofilms Composed of Streptococcus mutans and Streptococcus sanguinis. Pathogens 2022, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, H.; Zhu, C.G.; Zhou, X.; Wang, H.; Han, Q.; Ren, B.; Cheng, L. Anti-bacterial and anti-microbial aging effects of resin-based sealant modified by quaternary ammonium monomers. J. Dent. 2021, 112, 103767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef]
- Wu, T.; Li, B.; Zhou, X.; Hu, Y.; Zhang, H.; Huang, Y.; Xu, H.H.; Guo, Q.; Li, M.; Feng, M.; et al. Evaluation of Novel Anticaries Adhesive in a Secondary Caries Animal Model. Caries Res. 2018, 52, 14–21. [Google Scholar] [CrossRef]
- Namba, N.; Yoshida, Y.; Nagaoka, N.; Takashima, S.; Matsuura-Yoshimoto, K.; Maeda, H.; Van Meerbeek, B.; Suzuki, K.; Takashiba, S. Antibacterial effect of bactericide immobilized in resin matrix. Dent. Mater. 2009, 25, 424–430. [Google Scholar] [CrossRef]
- Santoro, O.; Izzo, L. Antimicrobial Polymer Surfaces Containing Quaternary Ammonium Centers (QACs): Synthesis and Mechanism of Action. Int. J. Mol. Sci. 2024, 25, 7587. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacteria and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef]
- Alhussein, A.; Alsahafi, R.; Balhaddad, A.A.; Mokeem, L.; Schneider, A.; Jabra-Rizk, M.-A.; Masri, R.; Hack, G.D.; Oates, T.W.; Sun, J.; et al. Novel Bioactive Nanocomposites Containing Calcium Fluoride and Calcium Phosphate with Antibacterial and Low-Shrinkage-Stress Capabilities to Inhibit Dental Caries. Bioengineering 2023, 10, 991. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, B.; Zhou, X.; Xu, H.H.K.; Chen, Y.; Han, Q.; Li, B.; Weir, M.D.; Li, M.; Feng, M.; et al. Effect of Antimicrobial Denture Base Resin on Multi-Species Biofilm Formation. Int. J. Mol. Sci. 2016, 17, 1033. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- AlSahafi, R.; Wang, X.; Mitwalli, H.; Alhussein, A.; Balhaddad, A.A.; Melo, M.A.S.; Oates, T.W.; Sun, J.; Xu, H.; Weir, M.D. Novel antibacterial low-shrinkage-stress resin-based cement. Dent. Mater. 2022, 38, 1689–1702. [Google Scholar] [CrossRef]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef]
- Almutairi, N.; Alhussein, A.; Alenizy, M.; Ba-Armah, I.; Sun, J.; Weir, M.D.; Xu, H.H.K. Novel Resin-Based Antibacterial Root Surface Coating Material to Combat Dental Caries. J. Funct. Biomater. 2024, 15, 168. [Google Scholar] [CrossRef]
- Chen, H.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Amirfazli, A. Surface tension measurement with a smartphone using a pendant drop. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 213–217. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Opdam, N.J.; Bronkhorst, E.M.; Loomans, B.A.; Huysmans, M.C. 12-year survival of composite vs. amalgam restorations. J. Dent. Res. 2010, 89, 1063–1067. [Google Scholar] [CrossRef]
- Deligeorgi, V.; A Mjör, I.; Wilson, N.H. An overview of reasons for the placement and replacement of restorations. Prim. Dent. Care 2001, 8, 5–11. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; Teughels, W.; De Munck, J.; Van Meerbeek, B.; Van Landuyt, K.L. Is secondary caries with composites a material-based problem? Dent. Mater. 2015, 31, e247–e277. [Google Scholar] [CrossRef]
- Metz, I.; Rothmaier, K.; Pitchika, V.; Crispin, A.; Hickel, R.; Garcia-Godoy, F.; Bücher, K.; Kühnisch, J. Risk factors for secondary caries in direct composite restorations in primary teeth. Int. J. Paediatr. Dent. 2015, 25, 451–461. [Google Scholar] [CrossRef]
- Mjör, I.A.; Toffenetti, F. Secondary caries: A literature review with case reports. Quintessence Int. 2000, 31, 165–179. [Google Scholar]
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Barutçugil, Ç.; Tayfun, D.; Tuncer, N.; Dündar, A. Bacterial adhesion and surface properties of computer-aided design-computer-aided manufacturing restorative materials. J. Oral Sci. 2024, 66, 157–162. [Google Scholar] [CrossRef]
- Zissis, A.J.; Polyzois, G.L.; A Yannikakis, S.; Harrison, A. Roughness of denture materials: A comparative study. Int. J. Prosthodont. 2001, 13, 136–140. [Google Scholar]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Arends, J.; Darius, P.L.; Van Steenberghe, D. The influence of surface free-energy on planimetric plaque growth in man. J. Dent. Res. 1989, 68, 796–799. [Google Scholar] [CrossRef]
- Everaert, E.P.J.M.; Mahieu, H.F.; Chung, R.P.W.; Verkerke, G.J.; van der Mei, H.C.; Busscher, H.J. A new method for in vivo evaluation of biofilms on surface-modified silicone rubber voice prostheses. Eur. Arch. Oto-Rhino-Laryngol. 1997, 254, 261–263. [Google Scholar] [CrossRef]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef]
- Gyo, M.; Nikaido, T.; Okada, K.; Yamauchi, J.; Tagami, J.; Matin, K. Surface response of fluorine polymer-incorporated resin composites to cariogenic biofilm adherence. Appl. Environ. Microbiol. 2008, 74, 1428–1435. [Google Scholar] [CrossRef]
- Chandra, J.; Patel, J.D.; Li, J.; Zhou, G.; Mukherjee, P.K.; McCormick, T.S.; Anderson, J.M.; Ghannoum, M.A. Modification of surface properties of biomaterials influences the ability of Candida albicans to form biofilms. Appl. Environ. Microbiol. 2005, 71, 8795–8801. [Google Scholar] [CrossRef]
- Tang, H.; Wang, A.; Liang, X.; Cao, T.; Salley, S.O.; McAllister, J.P., 3rd; Ng, K.Y. Effect of surface proteins on Staphylococcus epidermidis adhesion and colonization on silicone. Colloids Surf B Biointerfaces 2006, 51, 16–24. [Google Scholar] [CrossRef]
- Baveja, J.; Willcox, M.; Hume, E.; Kumar, N.; Odell, R.; Poole-Warren, L. Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials 2004, 25, 5003–5012. [Google Scholar] [CrossRef]
- Grivet, M.; Morrier, J.J.; Benay, G.; Barsotti, O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. J. Mater. Sci. Mater. Med. 2000, 11, 637–642. [Google Scholar] [CrossRef]
- Thomas, R.Z.; Van Der Mei, H.C.; Van Der Veen, M.H.; De Soet, J.J.; Huysmans, M.C. Bacterial composition and red fluorescence of plaque in relation to primary and secondary caries next to composite: An in situ study. Oral Microbiol. Immunol. 2008, 23, 7–13. [Google Scholar] [CrossRef]
- Garcia, I.M.; Assad-Loss, T.F.; Schneider, L.F.J.; Collares, F.M.; Cavalcante, L.M.A.; Tostes, M.A. Cytotoxicity evaluation, antibacterial effect, and degree of conversion of QAM-containing adhesives. Braz. Oral Res. 2024, 38, e001. [Google Scholar] [CrossRef]
- Shim, J.; Kim, H.; Park, S.; Yun, H.; Ryu, J. Comparison of Various Implant Provisional Resin Materials for Cytotoxicity and Attachment to Human Gingival Fibroblasts. Int. J. Oral Maxillofac. Implant. 2019, 34, 390–396. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba-Armah, I.; Alenizy, M.; Almutairi, N.; Alqarni, H.; Alhussein, A.; Masri, R.; Hack, G.D.; Oates, T.W.; Sun, J.; Weir, M.D.; et al. Novel Antibacterial Resin Coating for Dental Provisional Crowns to Suppress Biofilms and Inhibit Secondary Caries. Coatings 2024, 14, 1370. https://doi.org/10.3390/coatings14111370
Ba-Armah I, Alenizy M, Almutairi N, Alqarni H, Alhussein A, Masri R, Hack GD, Oates TW, Sun J, Weir MD, et al. Novel Antibacterial Resin Coating for Dental Provisional Crowns to Suppress Biofilms and Inhibit Secondary Caries. Coatings. 2024; 14(11):1370. https://doi.org/10.3390/coatings14111370
Chicago/Turabian StyleBa-Armah, Ibrahim, Mohammad Alenizy, Nader Almutairi, Heba Alqarni, Abdullah Alhussein, Radi Masri, Gary D. Hack, Thomas W. Oates, Jirun Sun, Michael D. Weir, and et al. 2024. "Novel Antibacterial Resin Coating for Dental Provisional Crowns to Suppress Biofilms and Inhibit Secondary Caries" Coatings 14, no. 11: 1370. https://doi.org/10.3390/coatings14111370
APA StyleBa-Armah, I., Alenizy, M., Almutairi, N., Alqarni, H., Alhussein, A., Masri, R., Hack, G. D., Oates, T. W., Sun, J., Weir, M. D., & Xu, H. H. K. (2024). Novel Antibacterial Resin Coating for Dental Provisional Crowns to Suppress Biofilms and Inhibit Secondary Caries. Coatings, 14(11), 1370. https://doi.org/10.3390/coatings14111370





