High-Temperature Steam and Atmospheric Oxidation Characteristic of a Heat-Resistant SP2215 Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oxidation Experiments
2.3. Microstructure and Characterizations
3. Results
3.1. SP2215 Microstructure
3.2. Oxidation Weight Gain of the SP2215
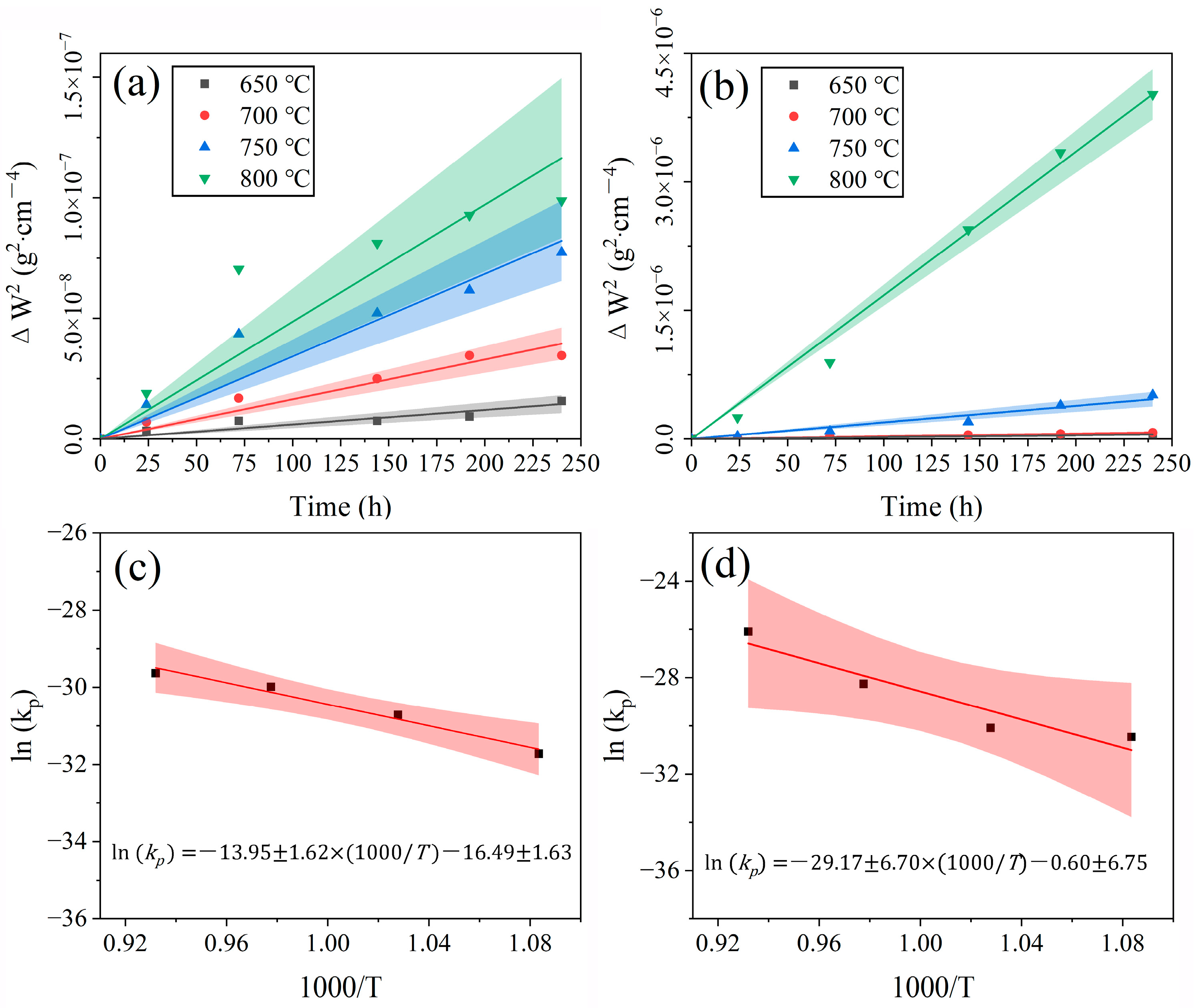
3.3. Oxidation Kinetics
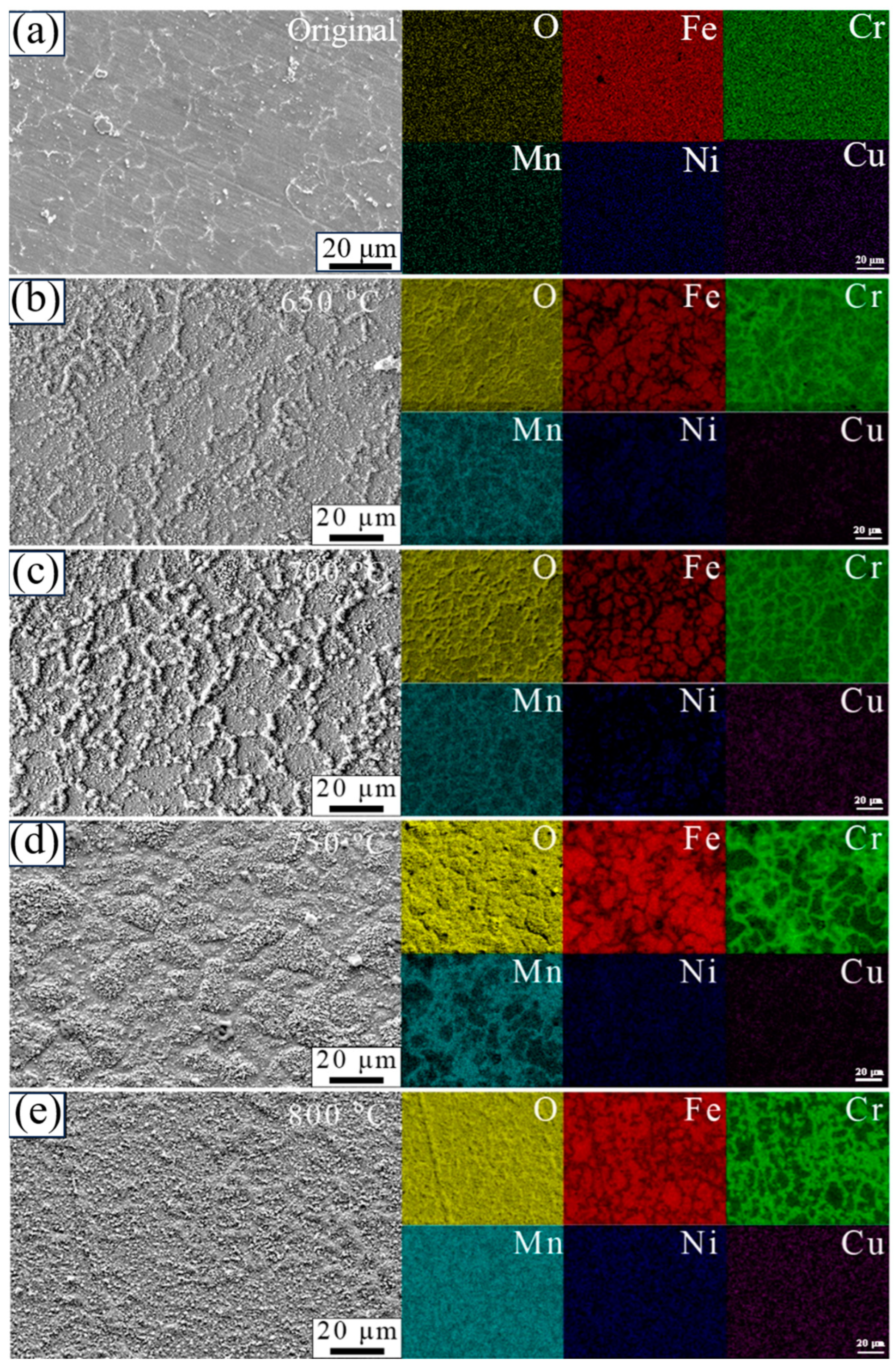
3.4. Surface Morphology of the Oxide Layers
3.5. GD-OES of the Oxide Layers
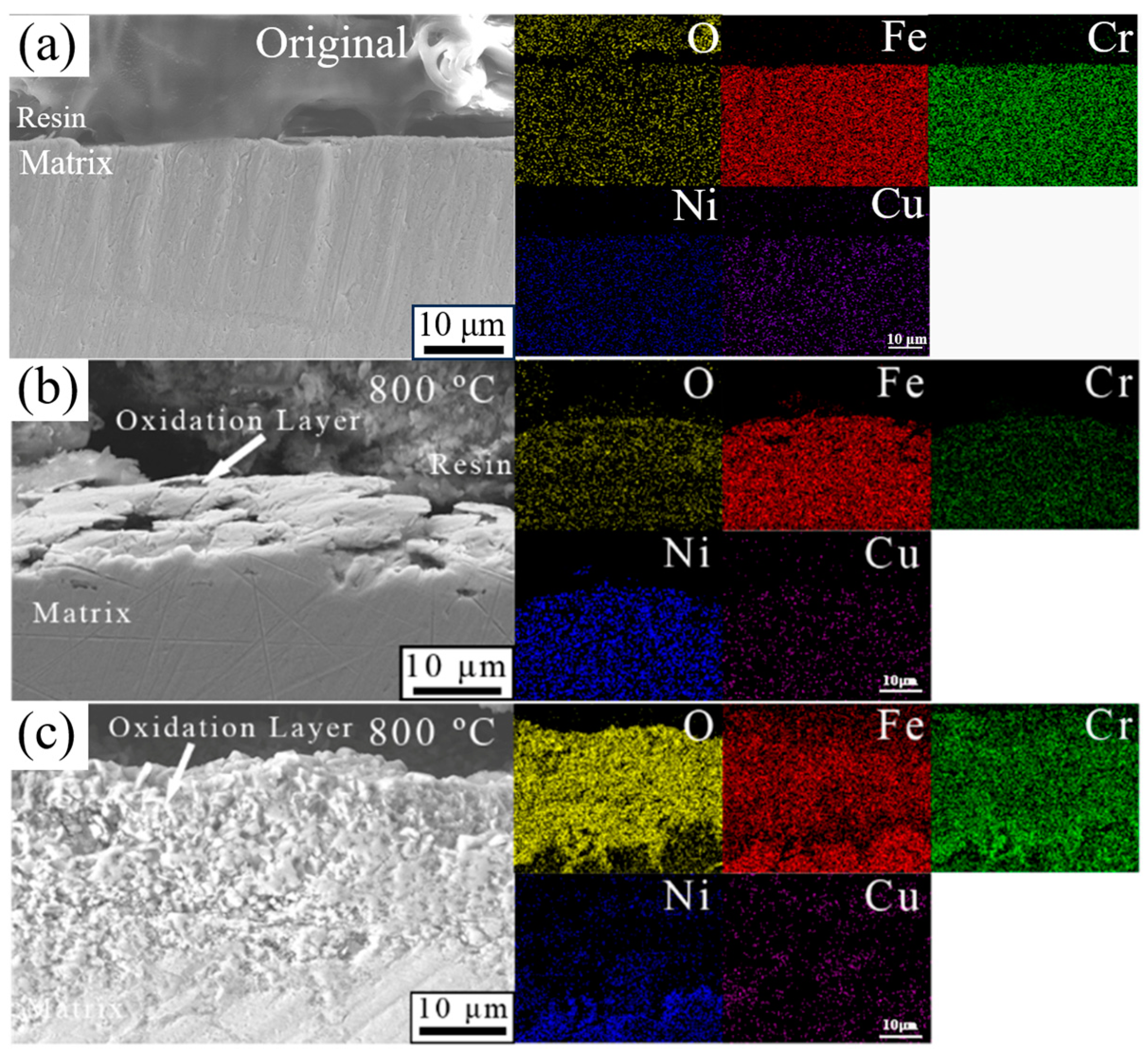
3.6. Cross-Sectional Microstructure
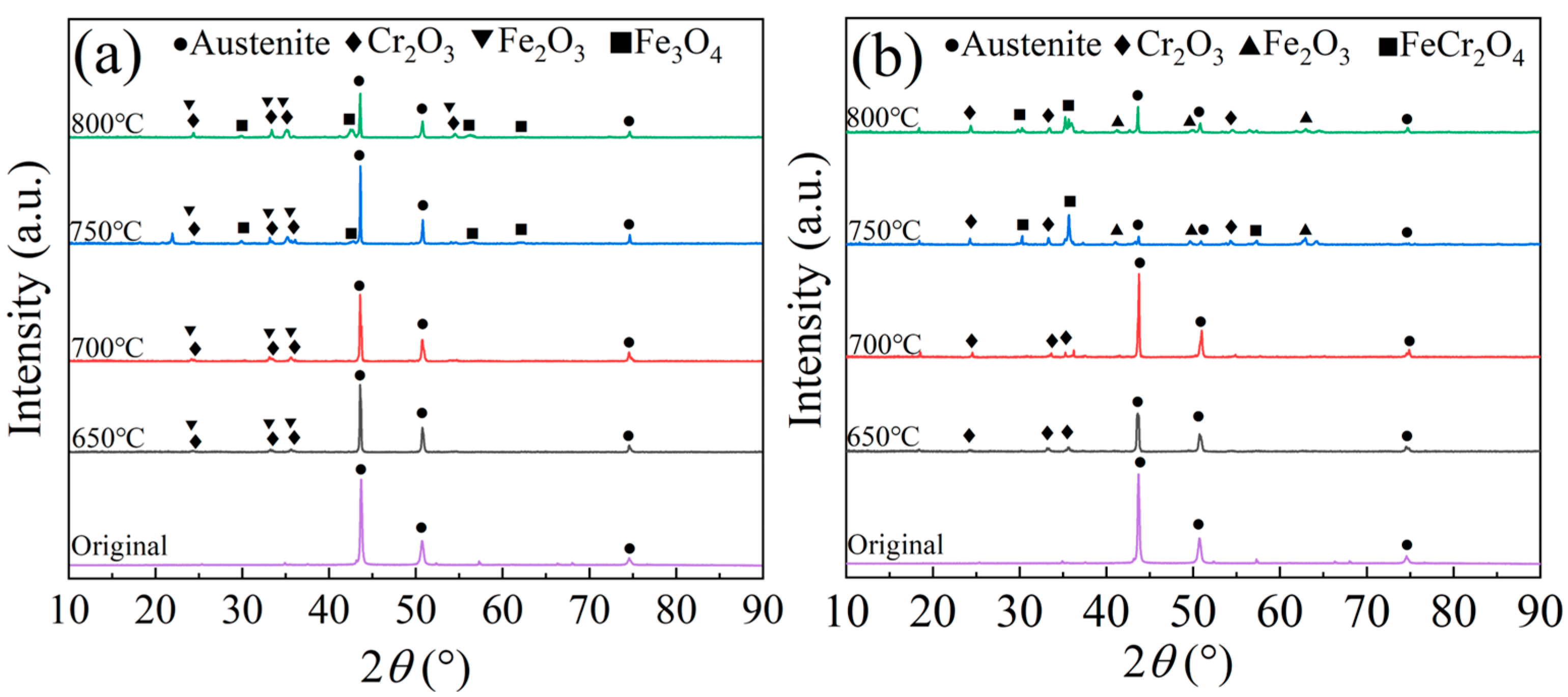
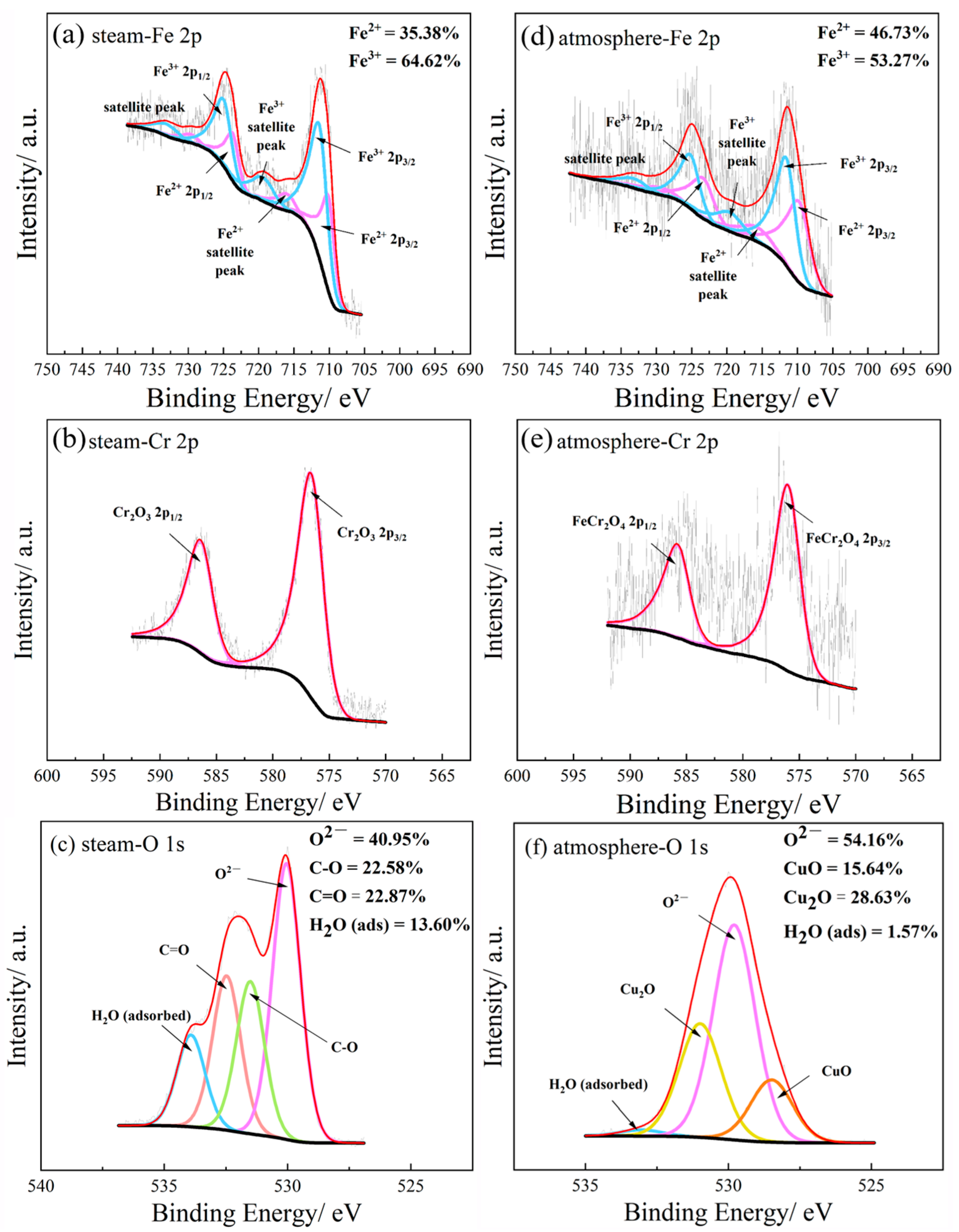
3.7. Composition of Oxide Layers
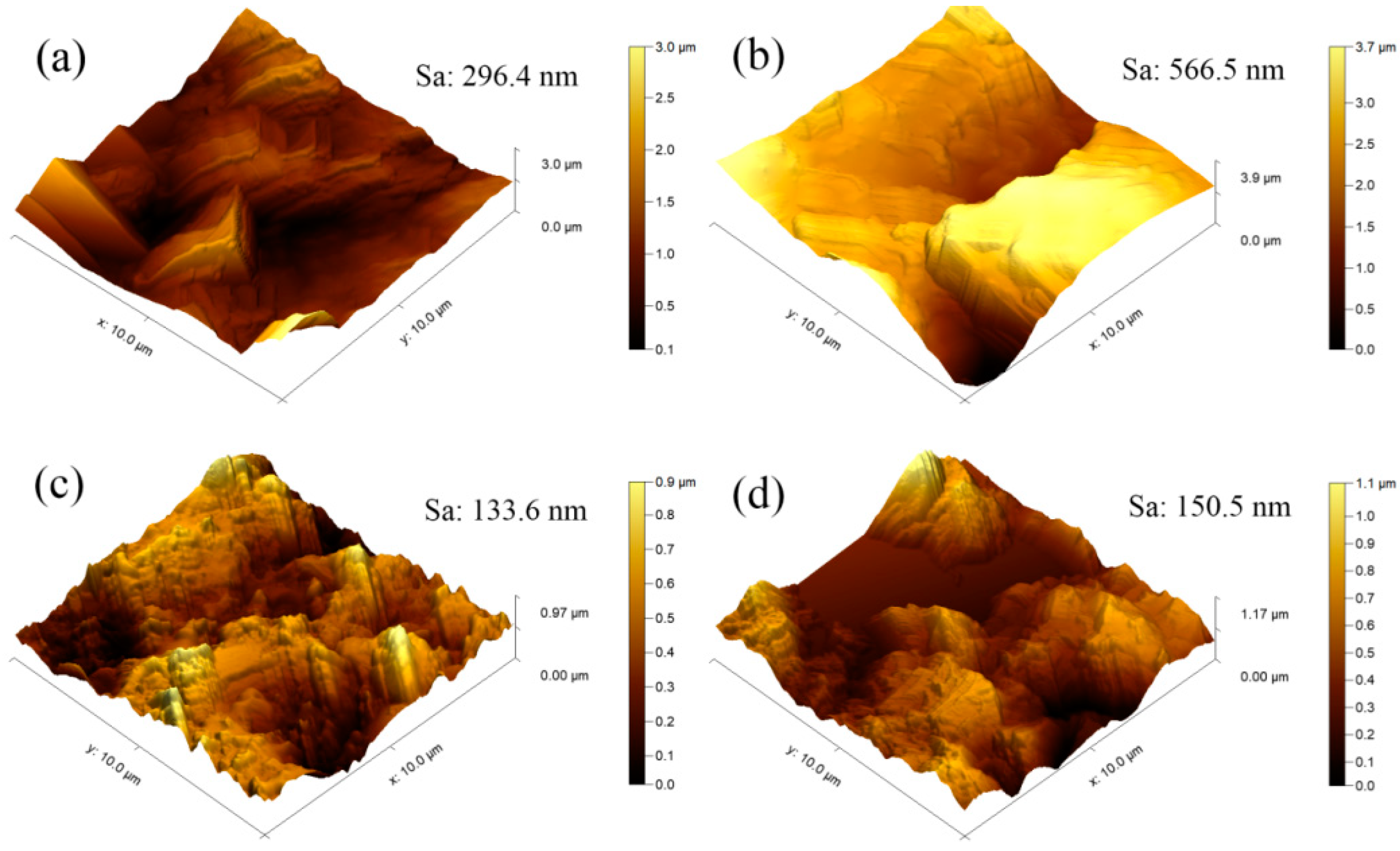
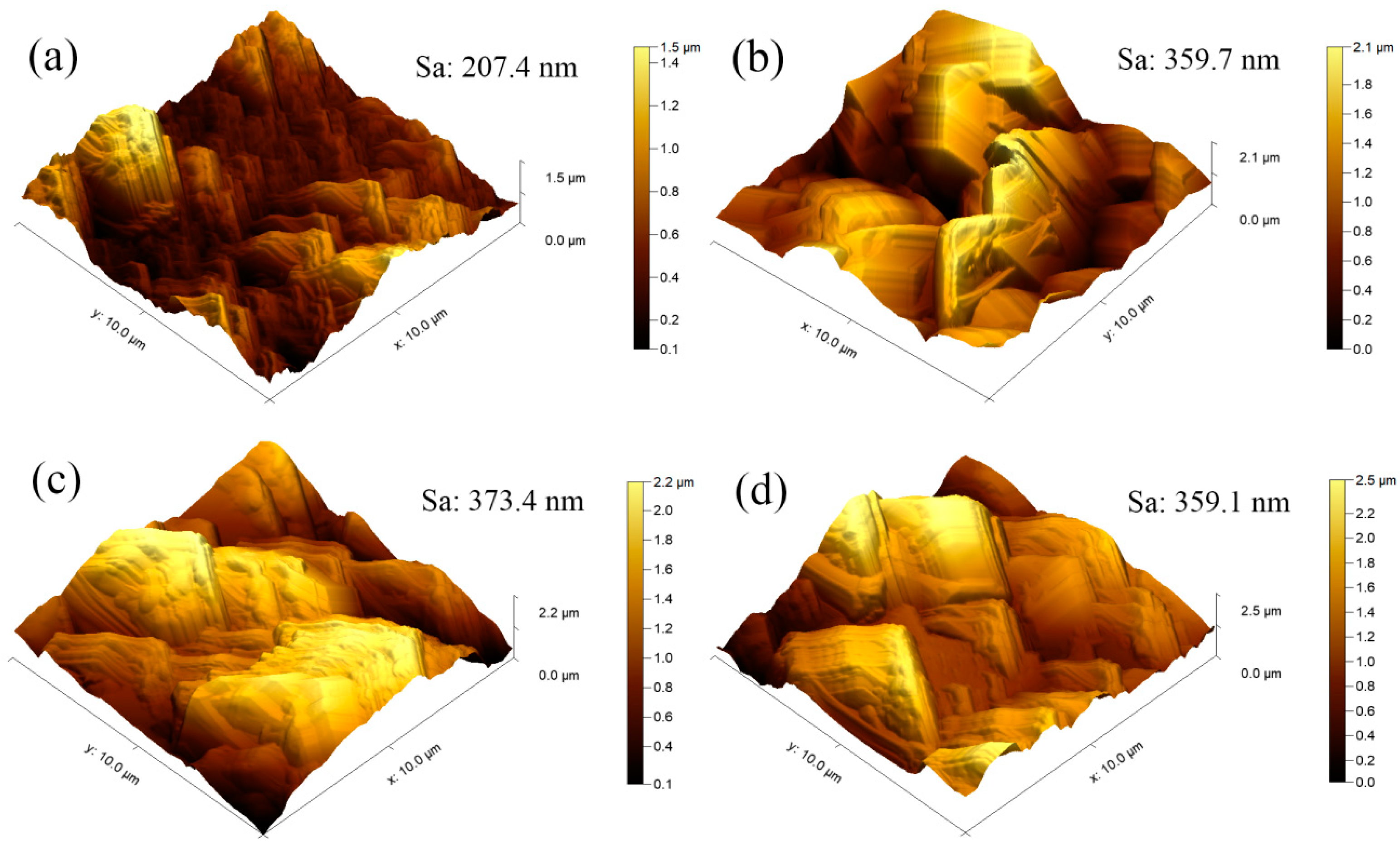
3.8. AFM Topography
4. Discussion
5. Conclusions
- The isothermal oxidation kinetics of SP2215 in the steam and atmospheric environments at 650–800 °C agrees with the parabolic law and belongs to the oxidation resistance category. The oxidation activation energy of SP2215 is estimated to be 242 ± 55.8·kJ·mol−1 and 116 ± 13.4 kJ·mol−1 in the atmospheric and steam environments, respectively.
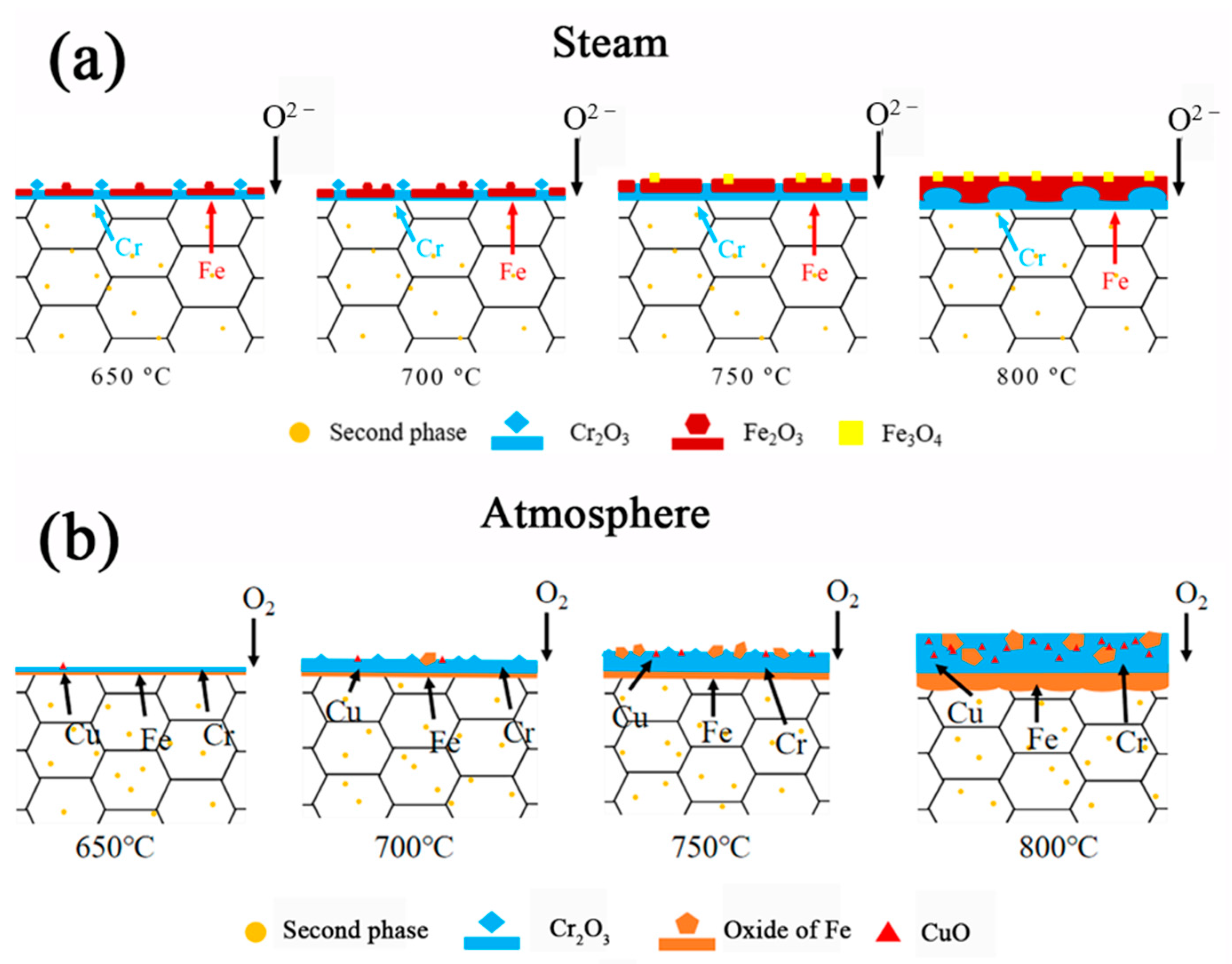
- In the steam environment, supercritical water molecules preferentially absorb at active grain boundaries, prompting Cr to diffuse rapidly through the grain boundary. As a result, Cr2O3 is rapidly deposited longitudinally at the grain boundary. Simultaneously, Fe2O3 is transversely diffused slowly within the grain. This process results in intergranular selective oxidation. With the increase in temperature, the phenomenon initially intensifies, then reverses with the depletion of Cr. Finally, Fe oxide predominates, forming a double oxide structure with an inner layer of Cr oxide and an outer layer of iron oxide in the grain boundary.
- In the atmospheric environment, oxygen is adsorbed uniformly on the surface. Given < and the quick diffusion path of Cr through the grain boundary, Cr oxide is preferentially generated and grows horizontally along the matrix–oxide interface to form a protective Cr2O3 layer. At the same time, only a small amount of iron participates in the oxidation process to form Fe2O3. With the increase in temperature, Cr is consumed gradually, and the quantity of iron oxide within the oxide layer increases correspondingly.
- We focused on the oxidation behavior of SP2215 in this study, but the detailed heat transfer characteristics under A-USC conditions are still unclear and methods for calculating metal temperatures with high accuracy are lacking [46]. Long-term testing verification (>1000 h) is needed for SP2215. In addition, different types of oxidation gas, such as SO2, are needed to explore its high-temperature oxidation behavior.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Du, C.; Zhang, K.; Zhang, Y.; Huang, Q. Steam oxidation behavior of T92 steel tube for high temperature reheater after long-term service in ultra-supercritical boilers. Corros. Sci. 2023, 221, 111352. [Google Scholar] [CrossRef]
- Lee, Y.; Han, S.; Jang, S.; Kim, W.; Choi, H.J.; Choi, S.K. Multidisciplinary materials and geometry optimization of superheater tubes for advanced ultra-supercritical power boilers. J. Mech. Sci. Technol. 2018, 32, 3359–3369. [Google Scholar] [CrossRef]
- Yuan, X.; Gong, X.; Li, D.; Zhang, N.; Zhu, Z. Effect of coating on the oxidation behavior of turbine rotor material in supercritical water. Mater. Corros. 2023, 74, 831–845. [Google Scholar] [CrossRef]
- Malede, Y.C.; Dahl, K.V.; Montgomery, M.; Grumsen, F.B.; Hald, J. Effect of service exposure on KCl corrosion attack of AISI 347H FG steel. J. Mater. Sci. 2019, 54, 13787–13809. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, L.; Zhou, R.; Hu, H.; Huang, Y. Effect of vanadium addition on the corrosion behavior of S30432 austenitic heat-resistant steel aged at 650 °C for different times. J. Mater. Sci. 2022, 57, 14096–14118. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, J.T.; Chen, M.H.; Wang, J.L.; Wang, F.H. Effect of pre-oxidation on hot corrosion resistance of HR3C stainless steel in sulfate salt with or without Fe2O3. Corros. Sci. 2021, 192, 109789. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mo, C.; Zhang, M.; Dong, M.; Pan, S.; Ran, S. High-temperature corrosion behavior of S30432 and TP310HCbN coatings in simulated 620 °C ultra-supercritical boiler coal ash/gas environment. Mater. Corros. 2022, 73, 1222–1235. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xiong, X.H.; Ping, S.B.; Gao, Y. Influence of chemical composition on intergranular corrosion susceptibility of novel Super304H austenitic heat-resistant steel. Corros. Eng. Sci. Technol. 2014, 49, 624–632. [Google Scholar] [CrossRef]
- Wang, X.; Wan, X.; Liu, Y.; Zhu, Y.; Zhang, F.; Lv, S.; Zhang, Y.; Du, Z. Failure mechanism of HR3C austenitic steel during creep at 650 °C. Fatigue Fract. Eng. Mater. Struct. 2023, 46, 2017–2022. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Z.; Zhang, J.; Wang, J.; Zhang, Z. Effect of on-site service for 16,000 and 38,000 h on microstructure and mechanical properties of austenitic steel HR3C reheater tubes. Eng. Fail. Anal. 2023, 149, 107247. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Xie, X.; Tang, Z. Fusion boundary evolution, precipitation behaviour, and interaction with dislocations in an Fe–22Cr–15Ni steel weldment during long-term creep. Prog. Nat. Sci. Mater. Int. 2019, 29, 41–49. [Google Scholar] [CrossRef]
- Du, J.K.; Zhang, Y.F.; Wang, S.L.; Zhou, S.L.; Liu, P.P.; Xie, X.S.; Geng, W.T.; Zhan, Q. Multiphase Strengthening of Nanosized Precipitates in a Cost-Effective Austenitic Heat-Resistant Steel. Steel Res. Int. 2020, 91, 2000122. [Google Scholar] [CrossRef]
- Xu, L.Y.; Yang, S.Q.; Zhao, L.; Han, Y.D.; Jing, H.Y.; Wang, K.M. Low cycle fatigue behavior and microstructure evolution of a novel Fe-22Cr-15Ni austenitic heat-resistant steel. J. Mater. Res. Technol. 2020, 9, 14388–14400. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Yang, L.; Du, W.; Wu, M.; Zhang, Q.; Song, Z. Comparative study on microstructure and mechanical properties of a novel nano-composite strengthening heat-resistant steel and two typical heat-resistant steels. Mater. Today Commun. 2023, 36, 106679. [Google Scholar] [CrossRef]
- Wei, L.; Wang, S.; Liu, G.; Hao, W.; Liang, K.; Yang, X.; Diao, W. Corrosion Behavior of High-Cr–Ni Materials in Biomass Incineration Atmospheres. ACS Omega 2022, 7, 21546–21553. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.-S.; Nuns, N.; Cristol, A.-L.; Cantrel, L.; Souvi, S.; Cristol, S.; Paul, J.-F. Multitechnique characterisation of 304L surface states oxidised at high temperature in steam and air atmospheres. Appl. Surf. Sci. 2016, 369, 510–519. [Google Scholar] [CrossRef]
- Pan, P.; Li, T.; Wang, Y.; Zhang, N.; Chen, H. Effect of temperature on hot corrosion of nickel-based alloys for 700 °C A-USC power plants. Corros. Sci. 2022, 203, 110350. [Google Scholar] [CrossRef]
- Shang, C.; Zhou, Z.; Wang, F.; Lu, Y. Evolution of oxide film of a new martensitic steel 9Cr3W3Co in supercritical water. Corros. Sci. 2022, 209, 110771. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, Q.; Li, S.L.; Zhang, Y.; Cai, Z.P.; Li, K.J.; Pan, J.L. Oxidation Behaviors of Different Grades of Ferritic Heat Resistant Steels in High-Temperature Steam and Flue Gas Environments. Acta Metall. Sin (Engl. Lett.) 2022, 35, 1103–1116. [Google Scholar] [CrossRef]
- Li, X.G.; Li, K.J.; Li, S.L.; Cai, Z.P.; Pan, J.L. Revealing the oxidation mechanism of ferritic heat resistant steel in high-temperature flue gas. Corros. Sci. 2022, 205, 110441. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Tavangar, R.; Akhtari, F.; Hejazi, S. High-temperature oxidation behavior of GTD-111 Ni-based superalloy with an ultrafine-grained surface at 900 °C. Corros. Sci. 2023, 212, 110935. [Google Scholar] [CrossRef]
- Su, M.; Zhao, J.; Tian, Z.; Gu, C. Short-term oxidation behavior of 304 stainless steel in N2-21 vol%O2 environment between 900 and 1200 °C. Corros. Sci. 2022, 208, 110612. [Google Scholar] [CrossRef]
- Zou, D.N.; Zhou, Y.Q.; Zhang, X.; Zhang, W.; Han, Y. High temperature oxidation behavior of a high Al-containing ferritic heat-resistant stainless steel. Mater. Charact. 2018, 136, 435–443. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Neroslavsky, O.; Li, M.; Godlewski, L.; Zhu, W. Effect of Spallation on Oxidation Kinetics of Heat-Resistant Cr–Ni Austenitic Steels on Air and Combustion Atmosphere. High Temp. Corros. Mater. 2022, 99, 79–99. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Q.; Zhou, Q.; Yao, X.; Wei, X.; Zhao, X.; Wang, Y.; Zhang, Z.; Bei, H. The effect of oxidation on microstructures of a Ni-based single crystal superalloy during heat-treatment and simulated service conditions. J. Mater. Sci. 2023, 58, 6343–6360. [Google Scholar] [CrossRef]
- Singh, D.; Cemin, F.; Jimenez, M.J.M.; Antunes, V.; Alvarez, F.; Orlov, D.; Figueroa, C.A.; Hosmani, S.S. High-temperature oxidation behaviour of nanostructure surface layered austenitic stainless steel. Appl. Surf. Sci. 2022, 581, 152437. [Google Scholar] [CrossRef]
- Nowak, W.J. Effect of Surface Roughness on Oxidation Resistance of Stainless Steel AISI 316Ti During Exposure at High Temperature. J. Mater. Eng. Perform. 2020, 29, 8060–8069. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Weiser, M.; Truong Nguyen, N.; Kiani-Rashid, A.-R.; Babakhani, A.; Virtanen, S. High temperature oxidation behaviour of AISI 321 stainless steel with an ultrafine-grained surface at 800 °C in Ar–20 vol.% O2. Corros. Sci. 2020, 163, 108282. [Google Scholar] [CrossRef]
- Zieliński, A.; Dudziak, T.P.; Golański, G.; Gazdowicz, J.; Kołodziej, A. Effects of Long-Term Ageing at High Temperatures on Oxide Scale Development and Evolution of Austenitic Steels Microstructure. Steel Res. Int. 2020, 91, 1900595. [Google Scholar] [CrossRef]
- Li, J.; Ma, H.; Wang, Y.; Xue, M.; Zhao, Q. Investigation on Oxidation Behavior of Super304H and HR3C Steel in High Temperature Steam from a 1000 MW Ultra-Supercritical Coal-Fired Boiler. Energies 2019, 12, 521. [Google Scholar] [CrossRef]
- Jun, J.; Lihui, Z. Strengthening mechanisms of precipitates in S30432 heat-resistant steel during short-term aging. Mater. Sci. Eng. A 2012, 539, 170–176. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Yu, H.-Y.; Dong, J.-X.; Liu, W.-Q.; Cheng, S.-C.; Liu, Z.-D.; Xie, X.-S. The precipitation strengthening behavior of Cu-rich phase in Nb contained advanced Fe–Cr–Ni type austenitic heat resistant steel for USC power plant application. Prog. Nat. Sci. Mater. Int. 2012, 22, 175–185. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L. Growth behavior and strengthening mechanism of Cu-rich particles in sanicro 25 austenitic heat-resistant steel after aging at 973 K. Mater. Sci. Eng. A 2020, 796, 139973. [Google Scholar] [CrossRef]
- GB/T 13303-91; Steels-Determination Method of Oxidation Resistance. The State Bureau of Quality and Technical Supervision: Beijing, China, 1991.
- Teng, J.W.; Gong, X.J.; Yang, B.B.; Yu, S.; Lai, R.L.; Liu, J.T.; Li, Y.P. High temperature oxidation behavior of a novel Ni-Cr-W-Al-Ti superalloy. Corros. Sci. 2022, 198, 110141. [Google Scholar] [CrossRef]
- Gamanov, Š.; Holzer, J.; Roupcová, P.; Svoboda, J. High temperature oxidation kinetics of Fe-10Al-4Cr-4Y2O3 ODS alloy at 1200–1400 °C. Corros. Sci. 2022, 206, 110498. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Hussain, S.; Jia, L.; Lin, D.; Yin, X.; Lin, Y.; Cheng, Z.; Wang, L. FeS2/carbon hybrids on carbon cloth: A highly efficient and stable counter electrode for dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1749–1756. [Google Scholar] [CrossRef]
- Langevoort, J.C.; Sutherland, I.; Hanekamp, L.J.; Gellings, P.J. On the oxide formation on stainless steels AISI 304 and incoloy 800H investigated with XPS. Appl. Surf. Sci. 1987, 28, 167–179. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, C.; Zhang, D.; Zhao, Y.; Khan, M.S.; Xu, D.; Xi, T.; Li, X.; Yang, K. Investigation on mechanical, corrosion resistance and antibacterial properties of Cu-bearing 2205 duplex stainless steel by solution treatment. RSC Adv. 2016, 6, 112738–112747. [Google Scholar] [CrossRef]
- Lou, Y.; Dai, C.; Chang, W.; Qian, H.; Huang, L.; Du, C.; Zhang, D. Microbiologically influenced corrosion of FeCoCrNiMo0.1 high-entropy alloys by marine Pseudomonas aeruginosa. Corros. Sci. 2020, 165, 108390. [Google Scholar] [CrossRef]
- Li, D.-S.; Chen, G.; Li, D.; Zheng, Q.; Gao, P.; Zhang, L.-L. Oxidation resistance of nickel-based superalloy Inconel 600 in air at different temperatures. Rare Met. 2018, 40, 3235–3240. [Google Scholar] [CrossRef]
- Sahu, B.P.; Ray, M.; Mitra, R. Structure and properties of Ni1-xTixN thin films processed by reactive magnetron co-sputtering. Mater. Charact. 2020, 169, 110604. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Hu, Z.-W.; Yu, S.-H. Comparison Study on the Stability of Copper Nanowires and Their Oxidation Kinetics in Gas and Liquid. ACS Nano 2016, 10, 3823–3834. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Sun, P.; Xu, D.; Ren, M.; Guo, Y.; Lin, G. Early oxidation mechanism of austenitic stainless steel TP347H in supercritical water. Corros. Sci. 2017, 128, 241–252. [Google Scholar] [CrossRef]
- Ericsson, T. A Study of the Cr-Depleted Surface Layers Formed on Four Cr-Ni Steels During Oxidation in Steam at 600 °C and 800 °C. Oxid. Met. 1970, 2, 401–417. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, T.; Wu, X.; Zhang, J.; Fan, H.; Zhang, Z. Coupled Heat Transfer Simulation of the Spiral Water Wall in a Double Reheat Ultra-supercritical Boiler. J. Therm. Sci. 2018, 27, 592–601. [Google Scholar] [CrossRef]
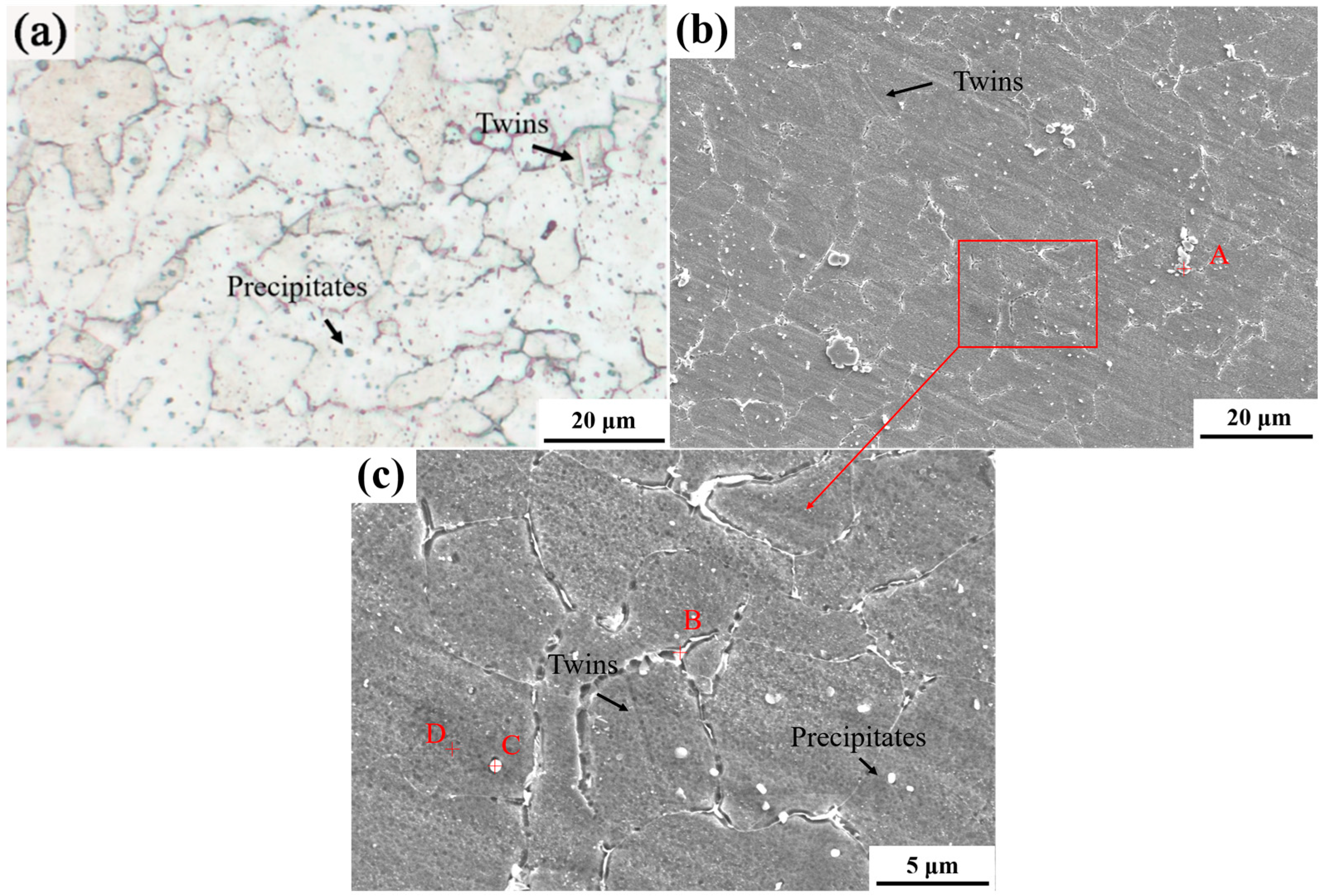
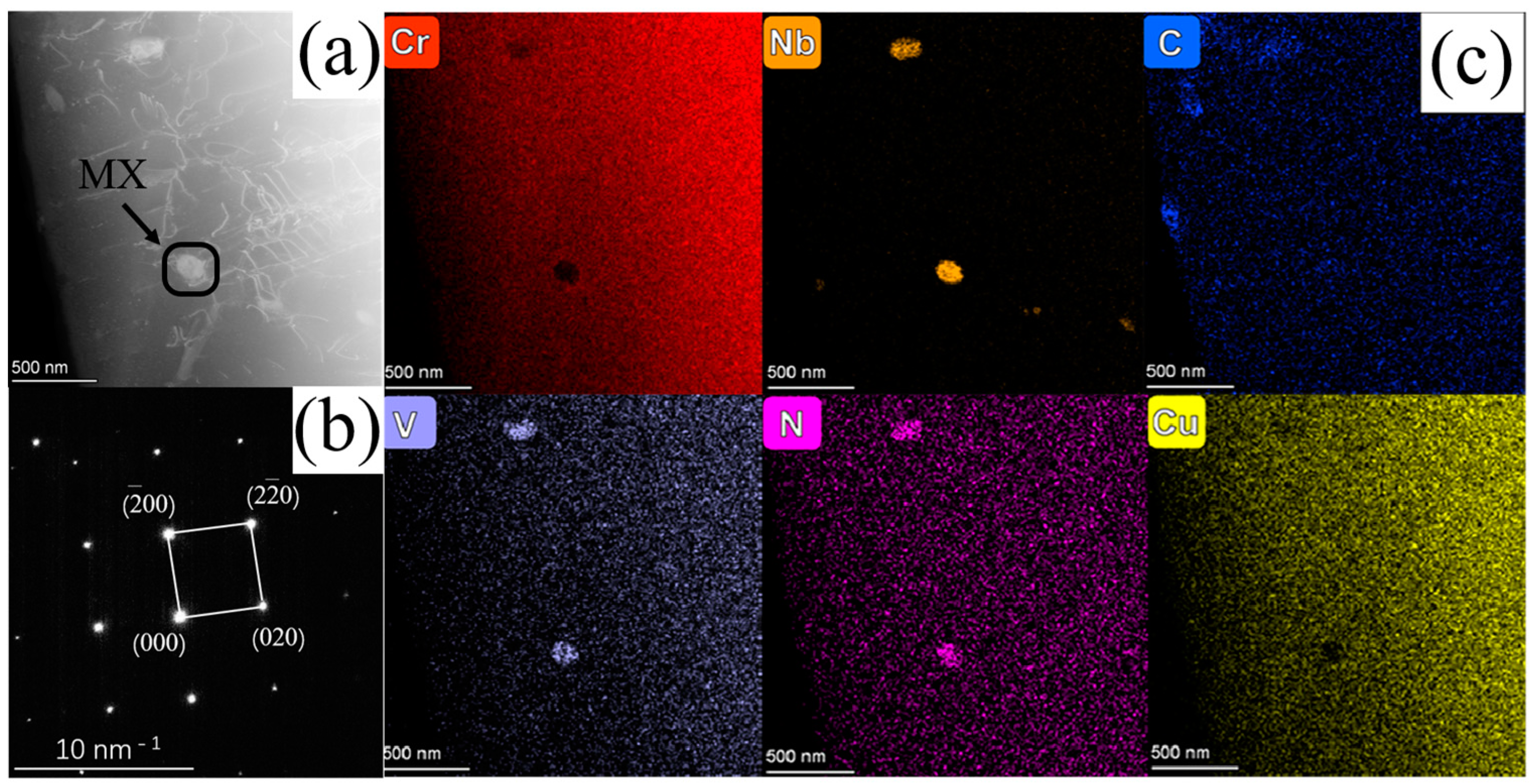




| C | Si | Mn | P | Cr | Mo | Ni | Co | Cu | Nb | V | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.07 | 0.41 | 0.60 | 0.02 | 22.80 | 0.34 | 15.00 | 0.01 | 3.49 | 0.52 | 0.30 | 0.32 | Bal. |
| Position | C | N | Cr | Ni | Cu | Nb | Fe | V |
|---|---|---|---|---|---|---|---|---|
| A | 7.26 | 5.69 | 24.17 | 0.54 | - | 44.55 | 5.46 | 2.14 |
| B | 2.78 | - | 25.77 | 14.27 | 3.11 | - | 54.07 | - |
| C | 12.1 | 7.24 | 20.59 | 2.32 | - | 28.12 | 12.85 | 1.31 |
| D | 3.93 | - | 22.79 | 14.76 | 3.44 | - | 55.08 | - |
| 24 h | 72 h | 144 h | 192 h | 240 h | |
|---|---|---|---|---|---|
| S-650 | 0.058 | 0.087 | 0.087 | 0.097 | 0.127 |
| S-700 | 0.084 | 0.130 | 0.158 | 0.186 | 0.186 |
| S-750 | 0.119 | 0.209 | 0.228 | 0.248 | 0.278 |
| S-800 | 0.138 | 0.265 | 0.285 | 0.305 | 0.314 |
| A-650 | 0.086 | 0.115 | 0.172 | 0.200 | 0.229 |
| A-700 | 0.120 | 0.179 | 0.210 | 0.240 | 0.270 |
| A-750 | 0.179 | 0.298 | 0.447 | 0.626 | 0.715 |
| A-800 | 0.501 | 0.943 | 1.562 | 1.827 | 2.004 |
| ln(k0) | Q (kJ/mol) | |
|---|---|---|
| Steam | 16.49 ± 1.63 | 116 ± 13.4 |
| Atmosphere | 0.60 ± 6.75 | 242 ± 55.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wu, M.; Huang, J.; Yang, L.; Zhao, P.; Chen, G.; Ding, B.; Du, W.; Lei, J.; Song, Z. High-Temperature Steam and Atmospheric Oxidation Characteristic of a Heat-Resistant SP2215 Steel. Coatings 2024, 14, 194. https://doi.org/10.3390/coatings14020194
Xu L, Wu M, Huang J, Yang L, Zhao P, Chen G, Ding B, Du W, Lei J, Song Z. High-Temperature Steam and Atmospheric Oxidation Characteristic of a Heat-Resistant SP2215 Steel. Coatings. 2024; 14(2):194. https://doi.org/10.3390/coatings14020194
Chicago/Turabian StyleXu, Liling, Minghua Wu, Jiazhen Huang, Lijing Yang, Pingping Zhao, Genbao Chen, Binhua Ding, Wenwen Du, Jinchang Lei, and Zhenlun Song. 2024. "High-Temperature Steam and Atmospheric Oxidation Characteristic of a Heat-Resistant SP2215 Steel" Coatings 14, no. 2: 194. https://doi.org/10.3390/coatings14020194






