A Phase-Field Regularized Cohesion Model for Hydrogen-Assisted Cracking
Abstract
:1. Introduction
2. Materials and Methods
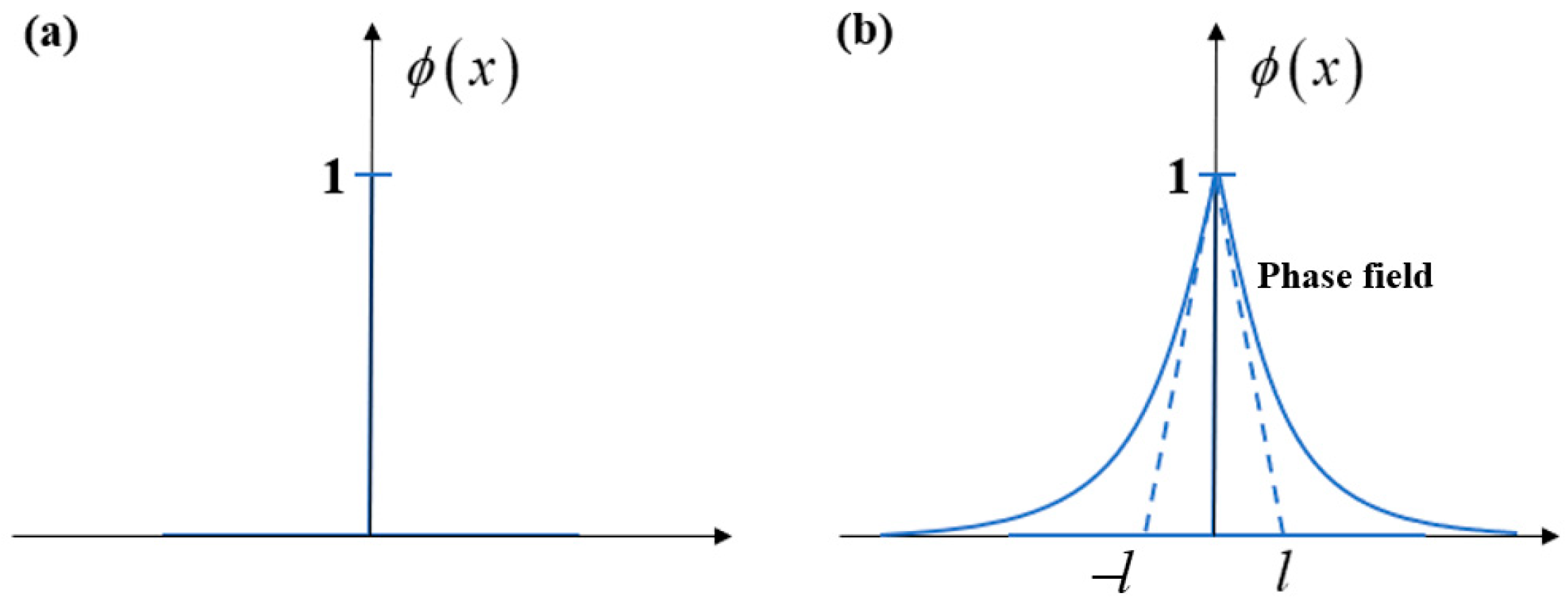
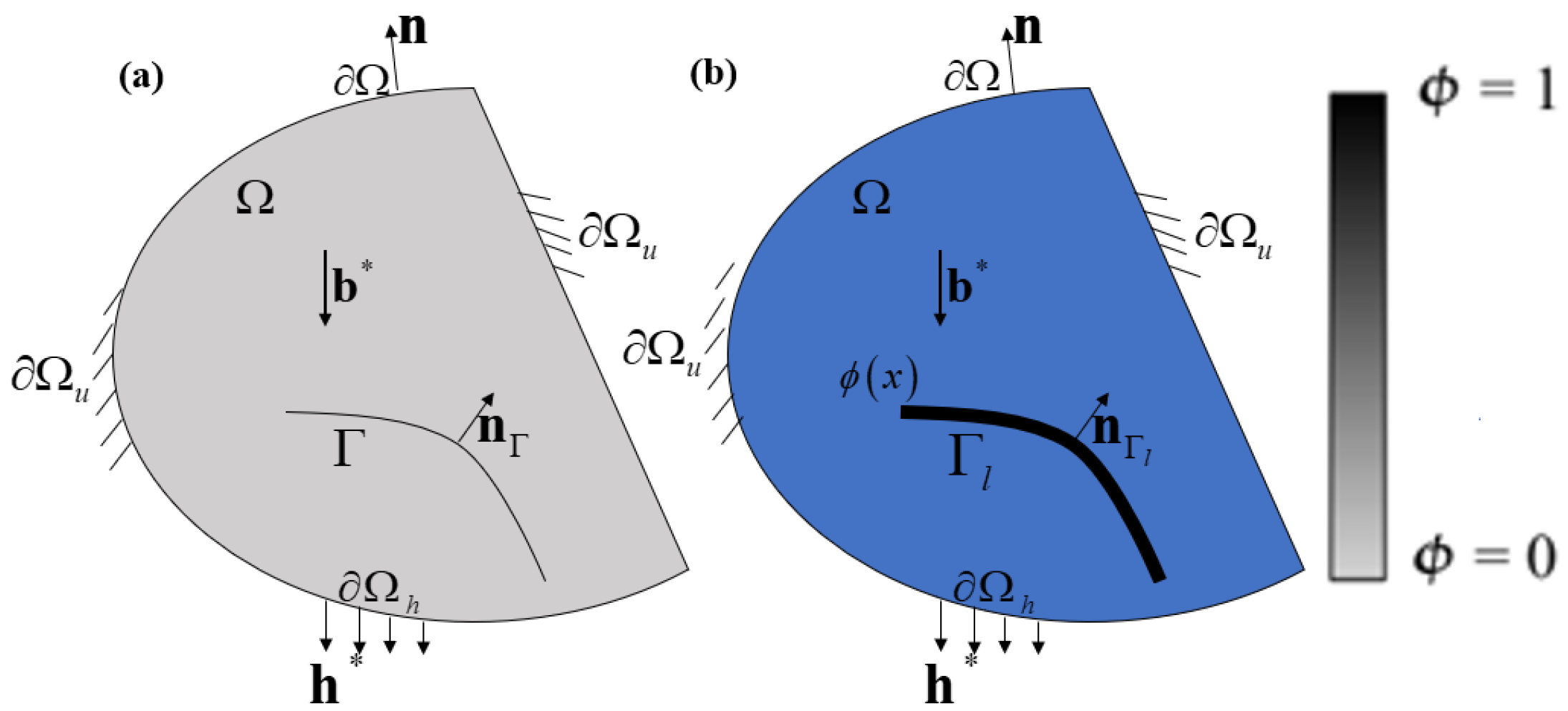
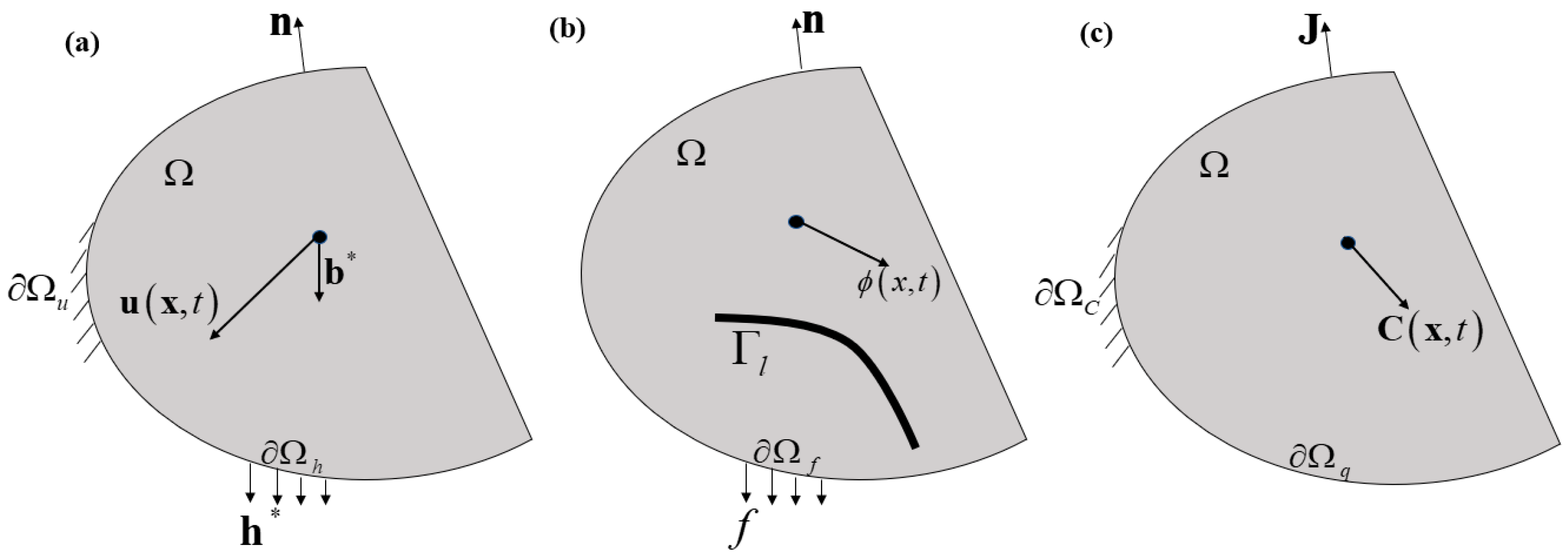
2.1. Phase-Field Model of Fractures
2.2. Hydrogen-Dependent Surface Energy Degradation
2.3. Stress-Assisted Hydrogen Diffusion Coupled with Phase-Field Fractures
2.4. Energy Imbalance and Constitutive Theory
2.5. Degradation of Hydrogen-Dependent Material Properties
3. Results and Discussion
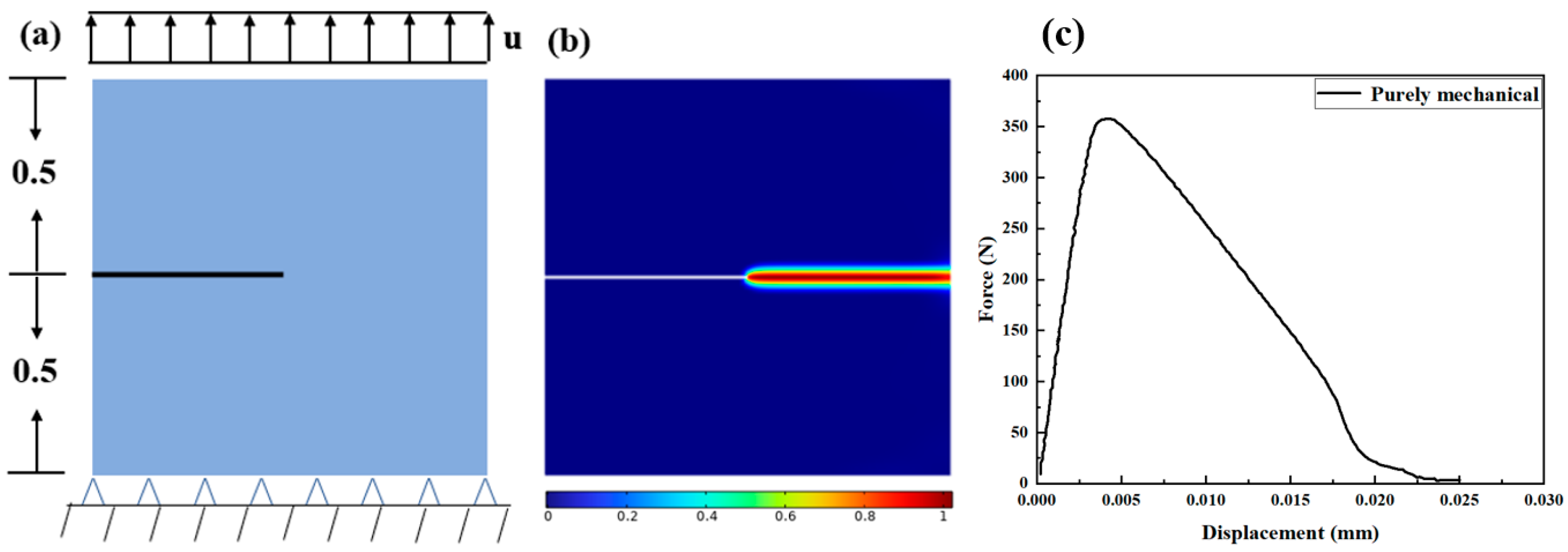
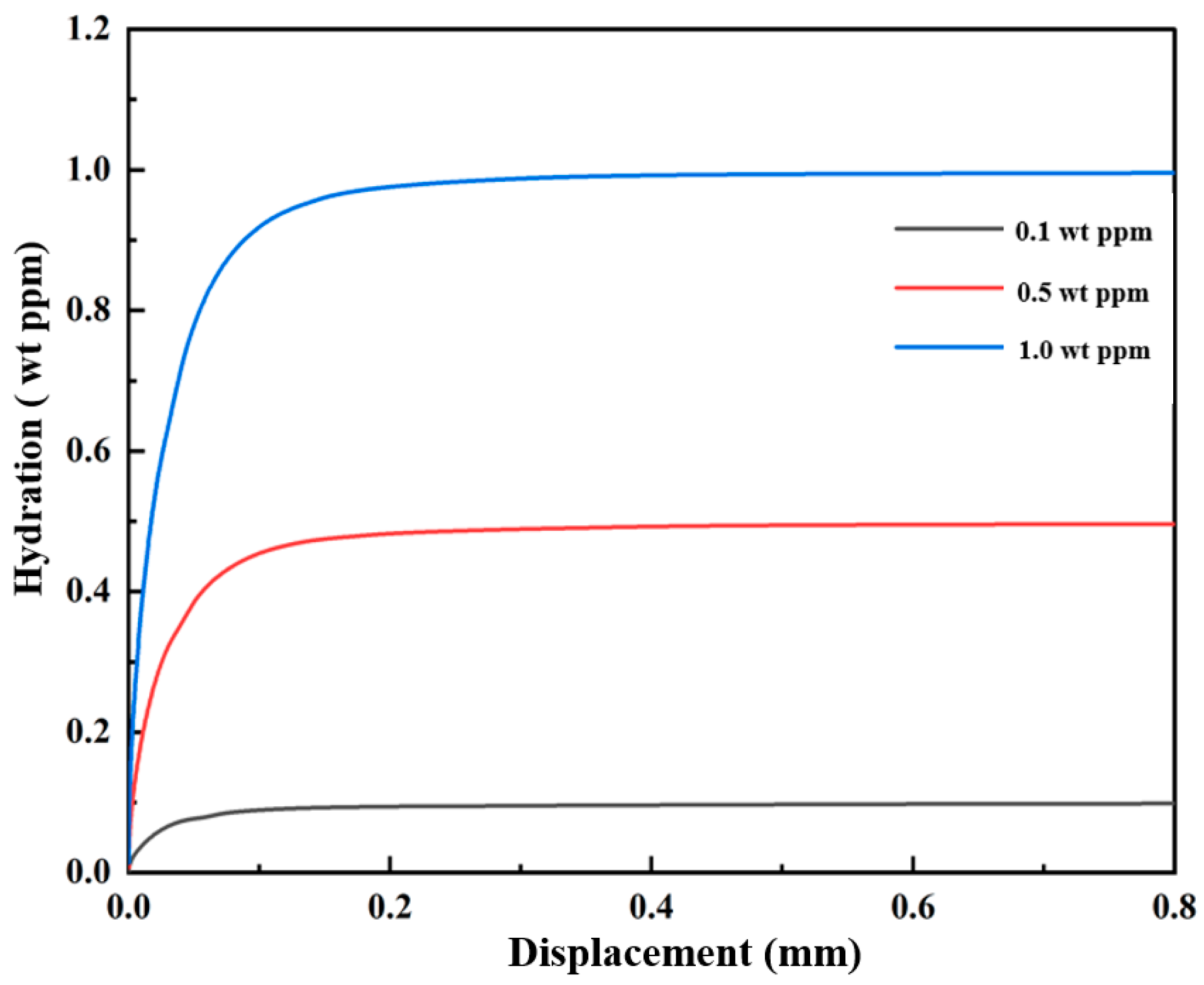
3.1. Cracked Square Plate Subjected to Tensions at Hydrogen Concentration
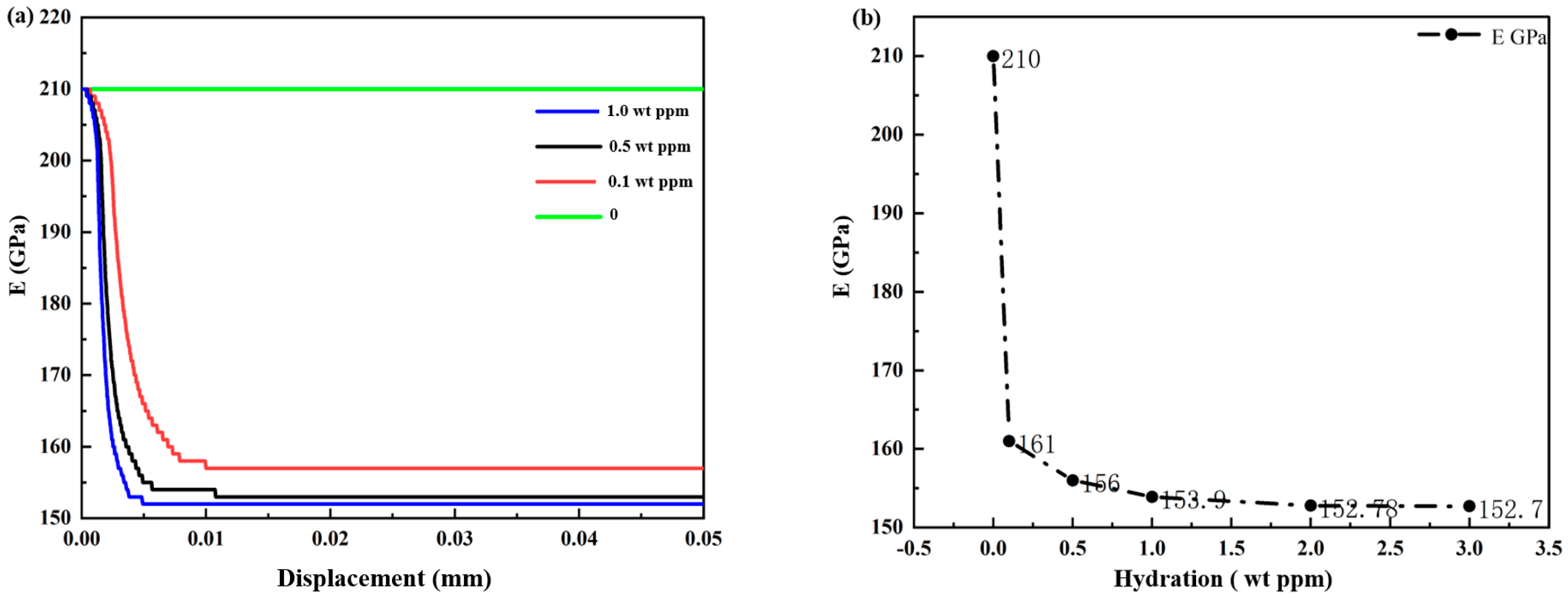
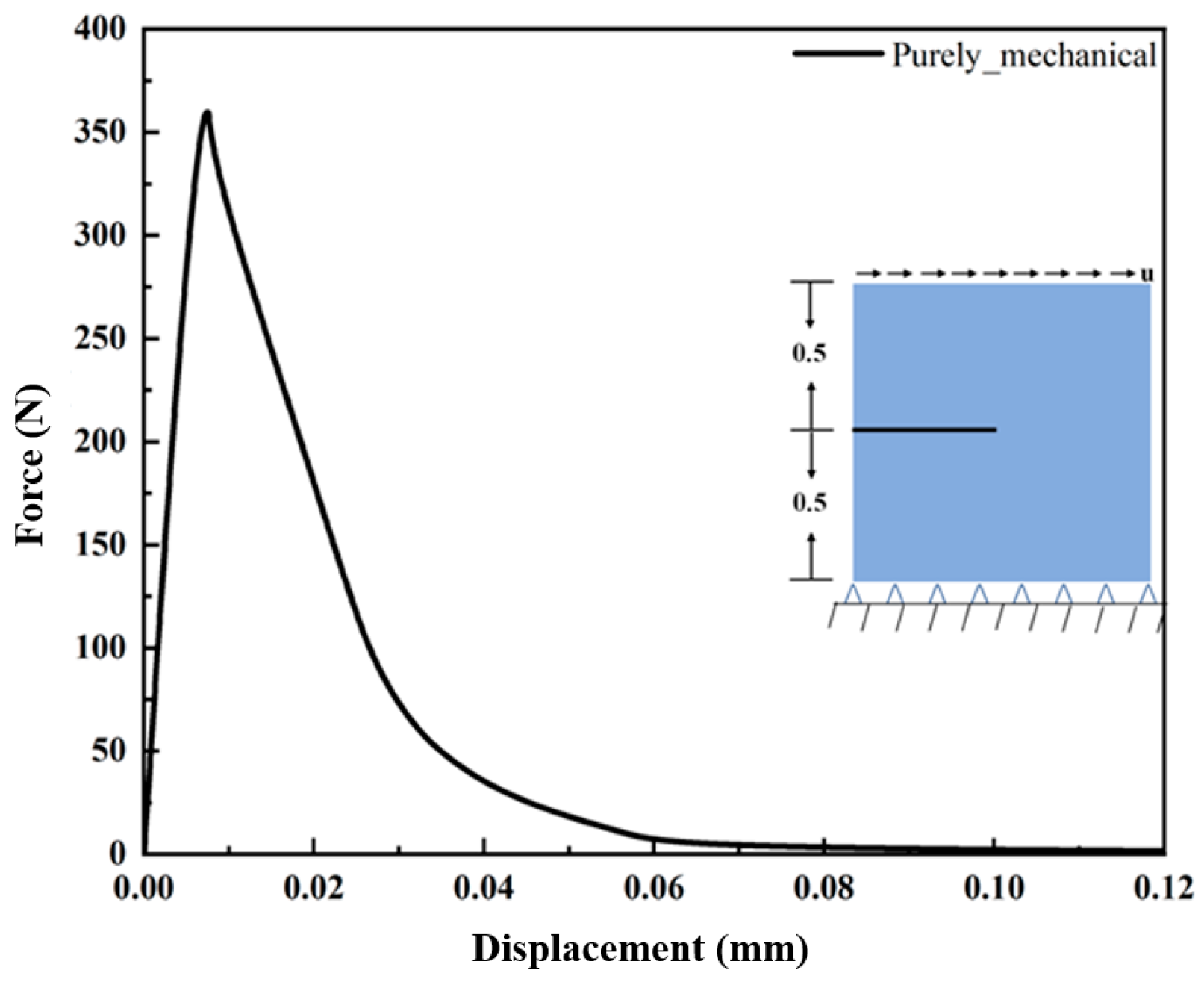
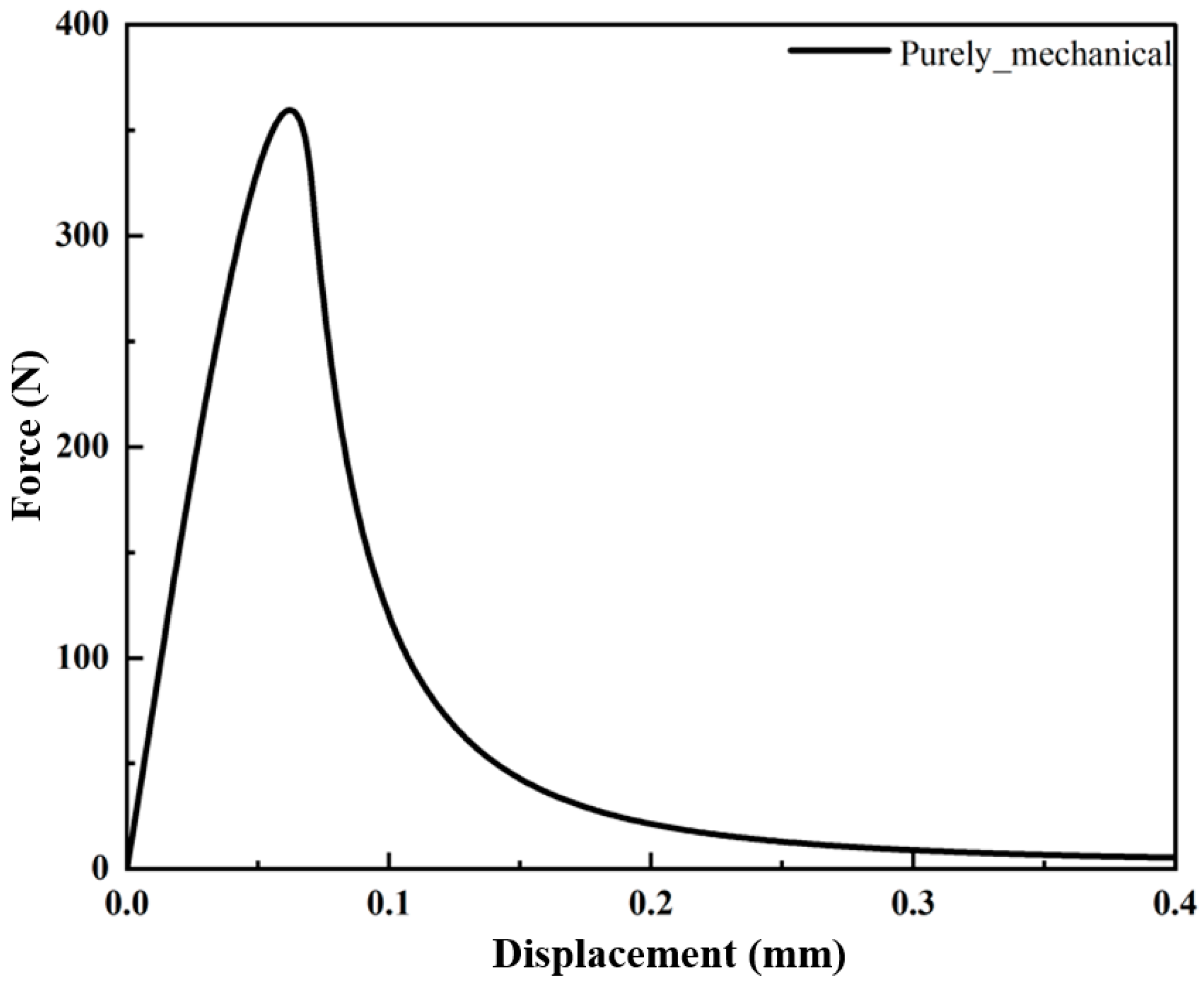
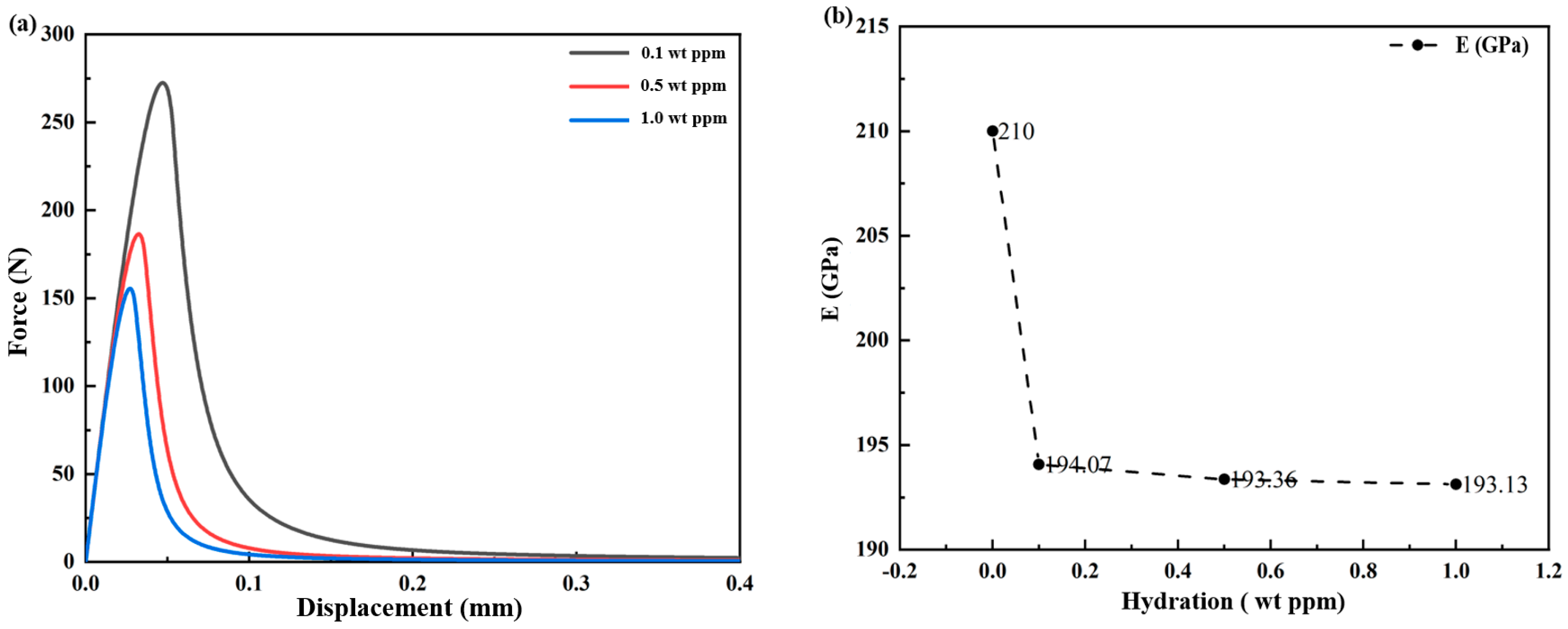
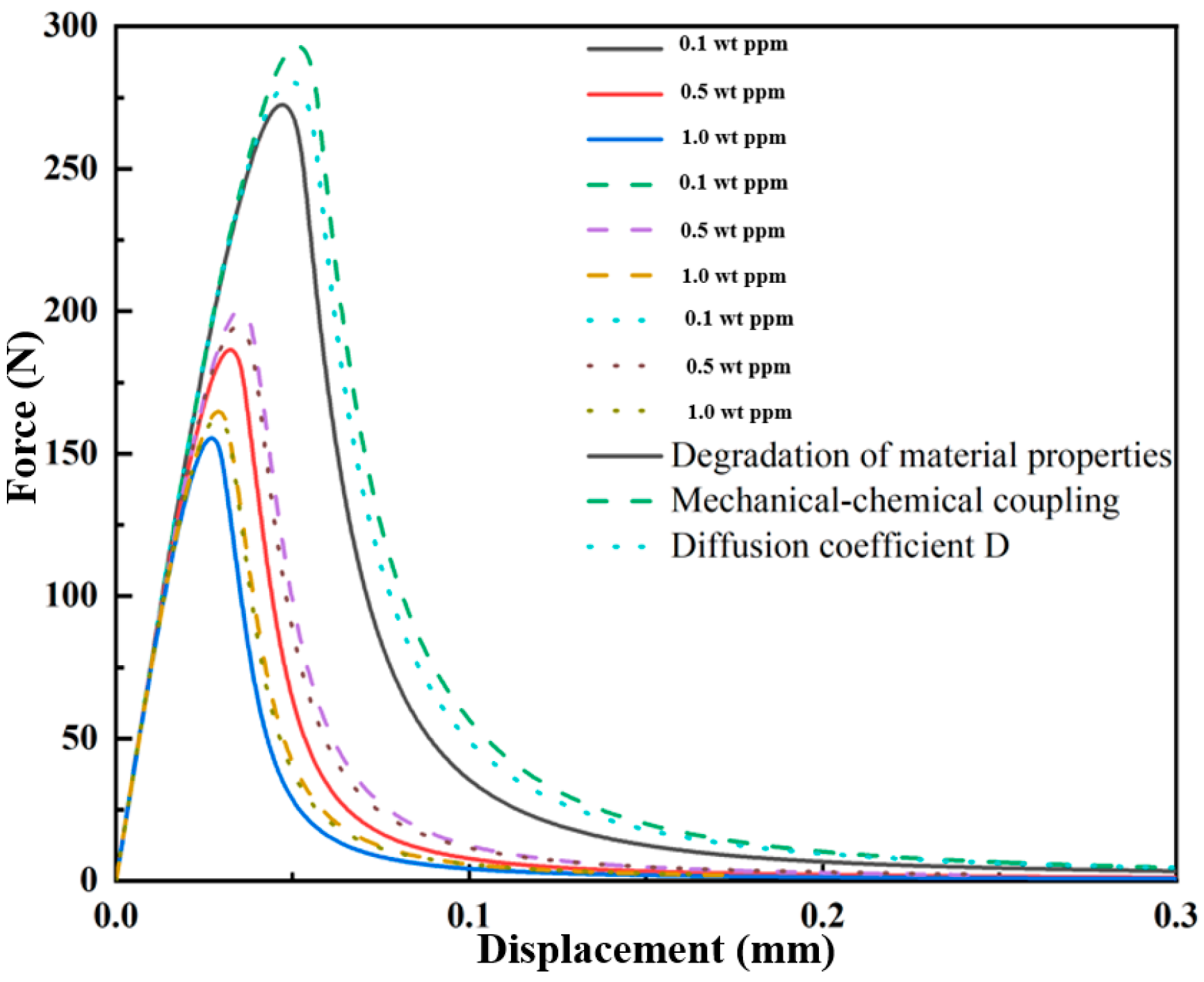
3.1.1. Hydrogen-Dependent Fracture Energy Degradation
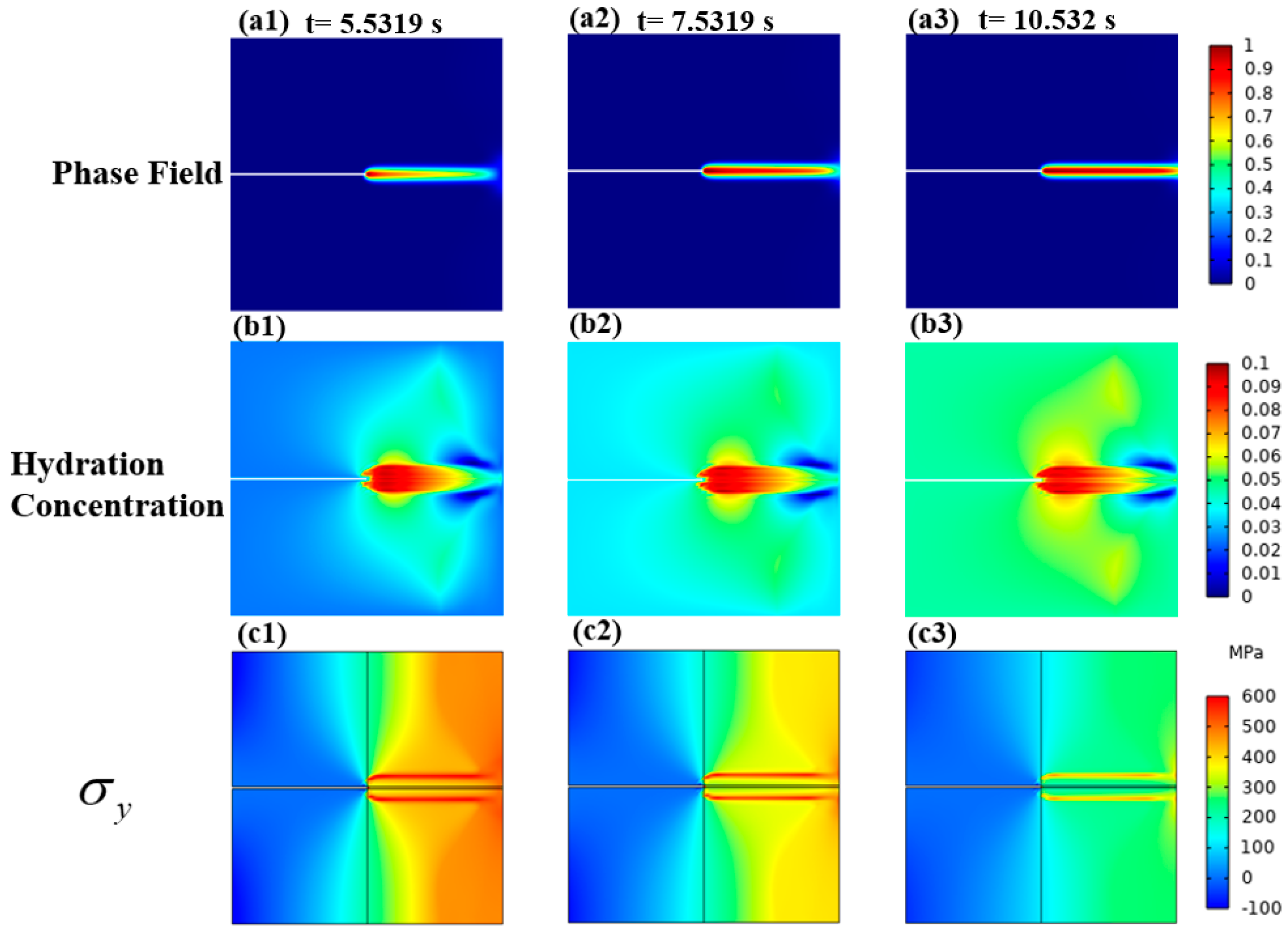
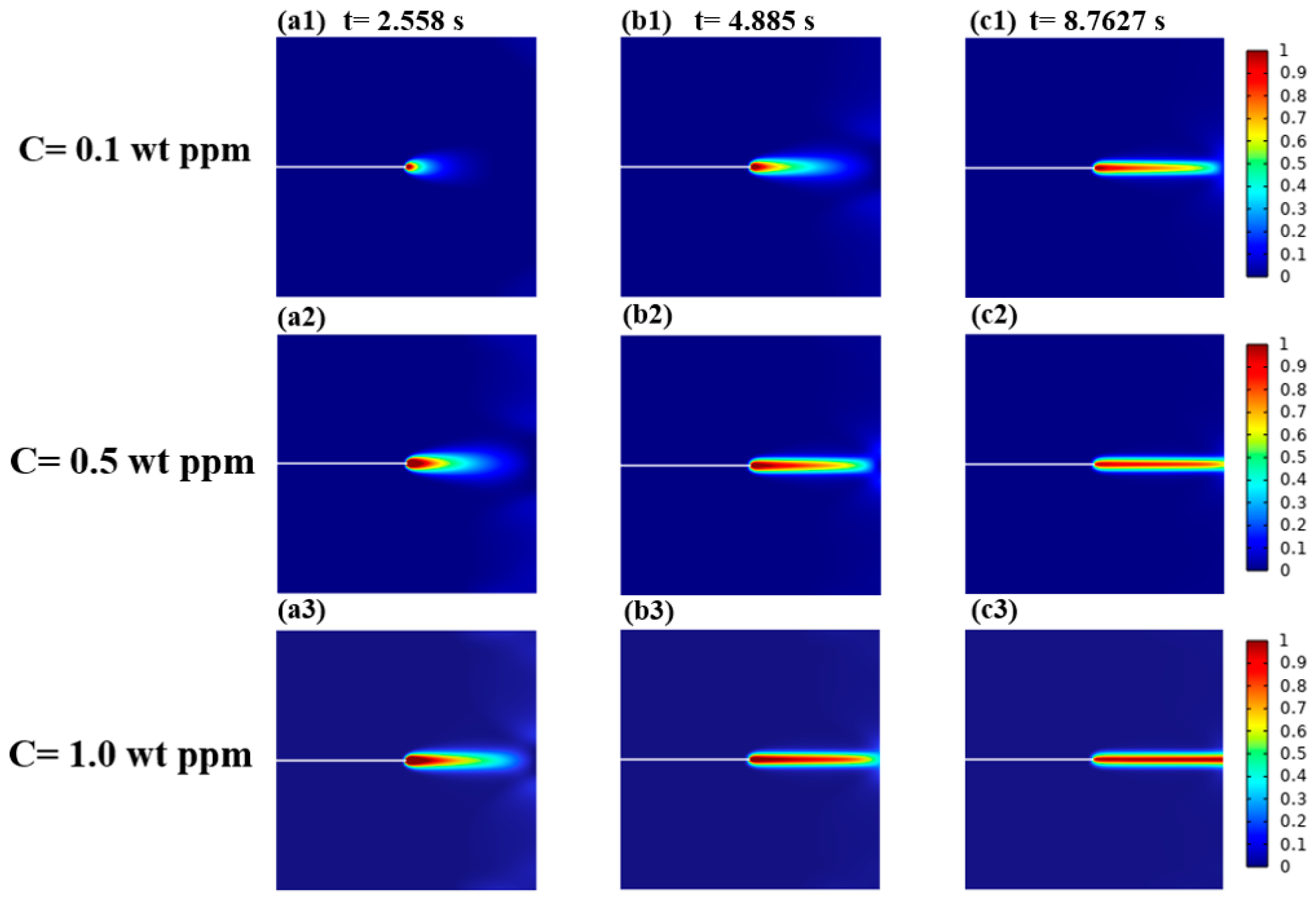
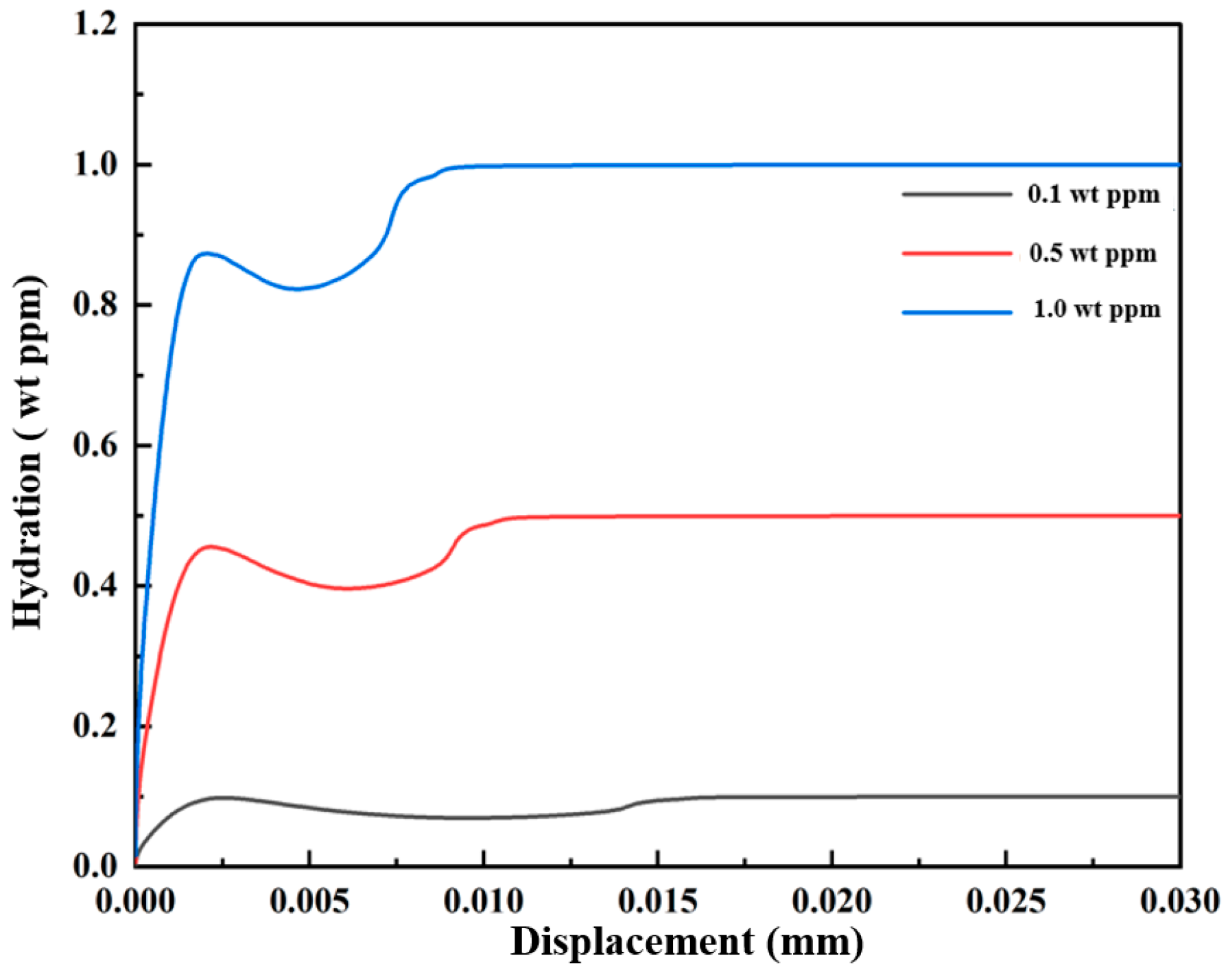
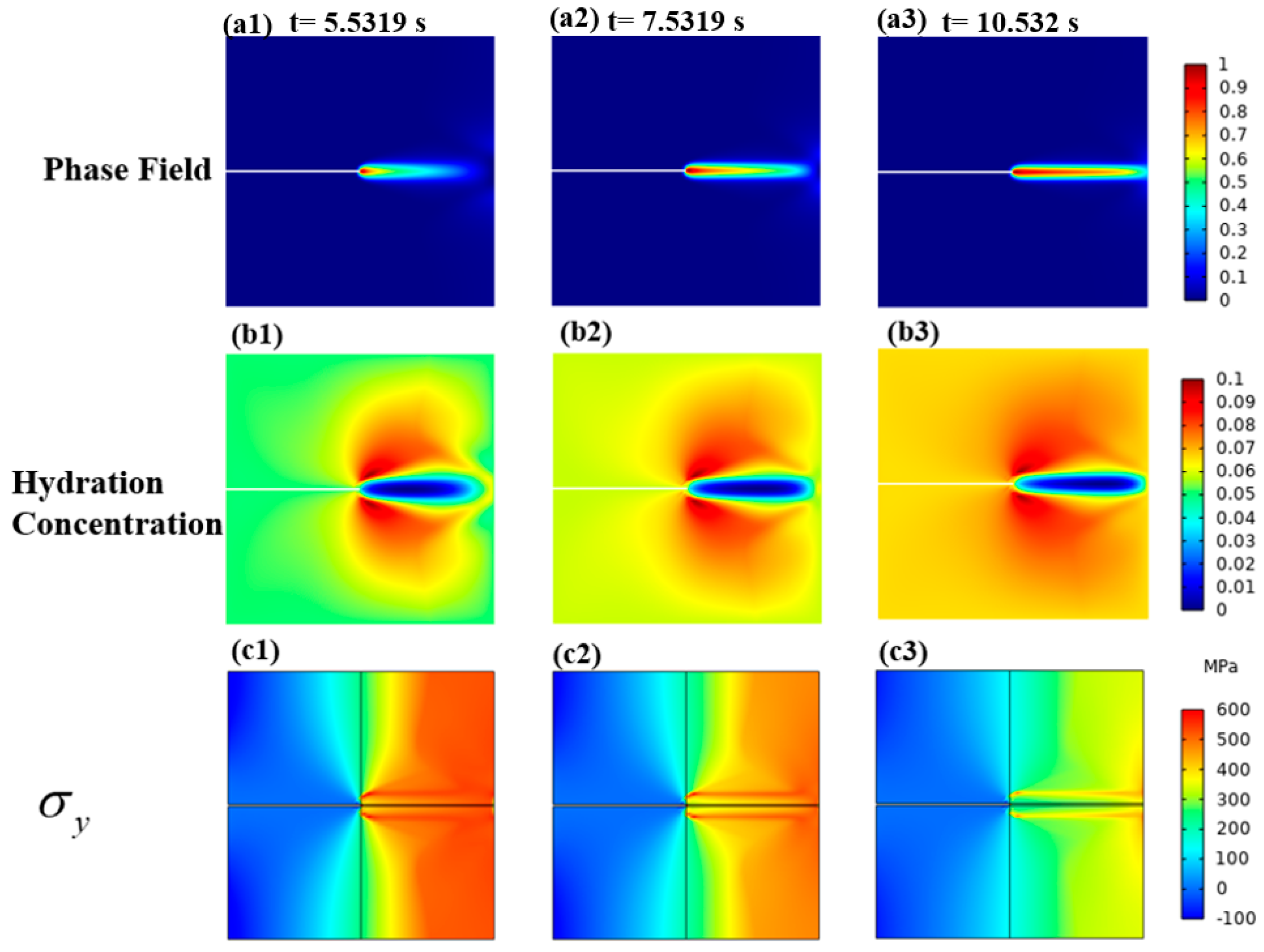
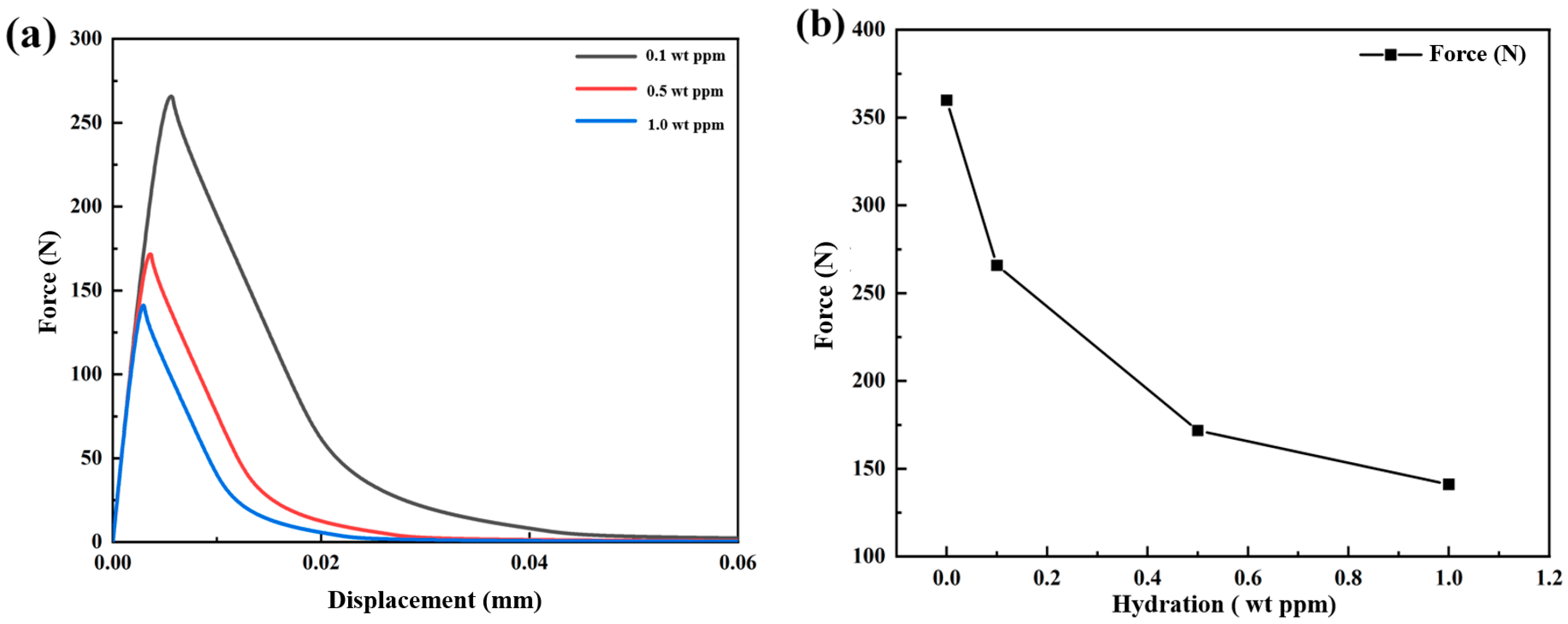
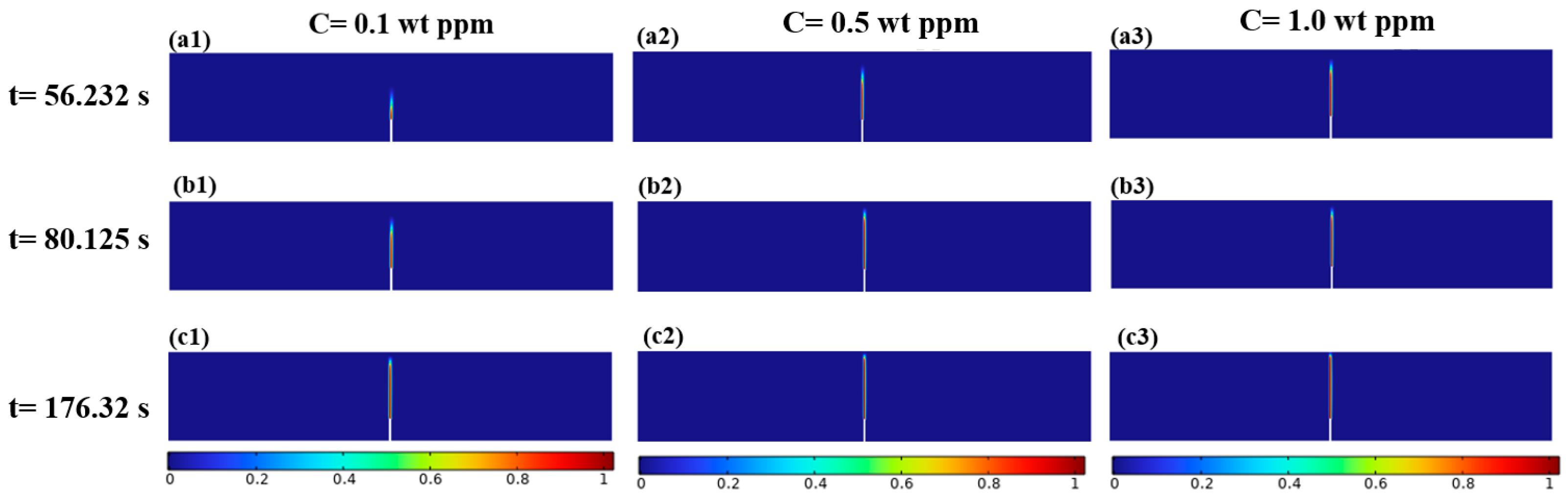
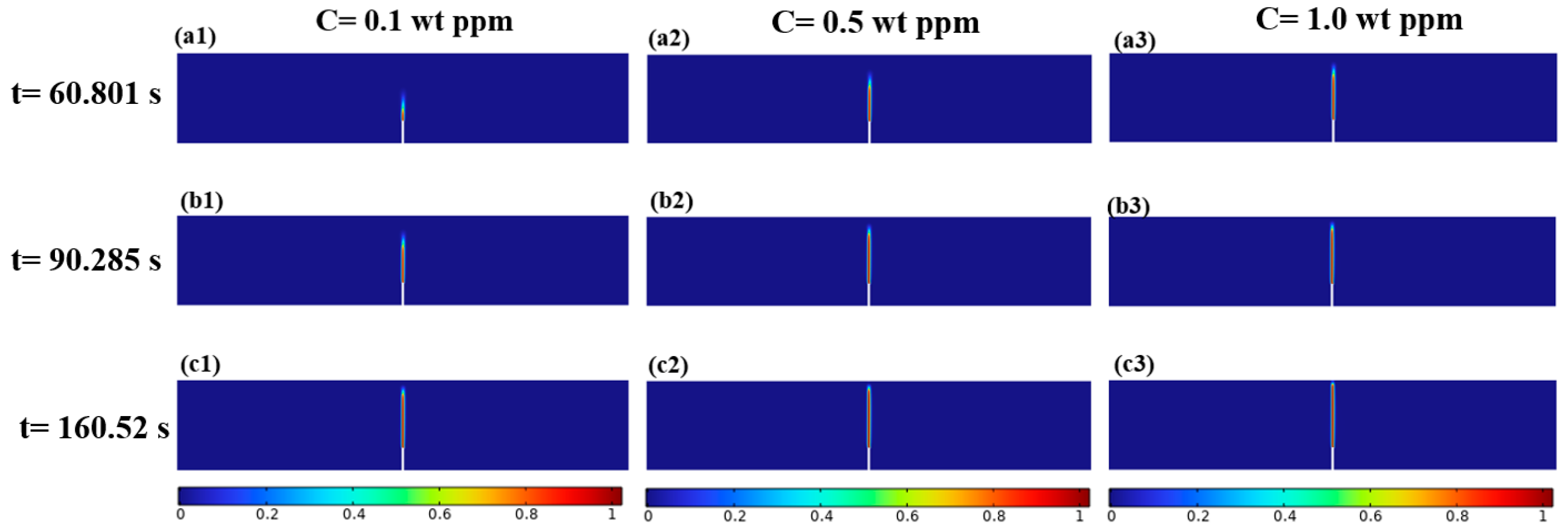
3.1.2. Mechanical–Chemical Coupling
3.1.3. Effect of the Diffusion Coefficient D on the Crack Extension
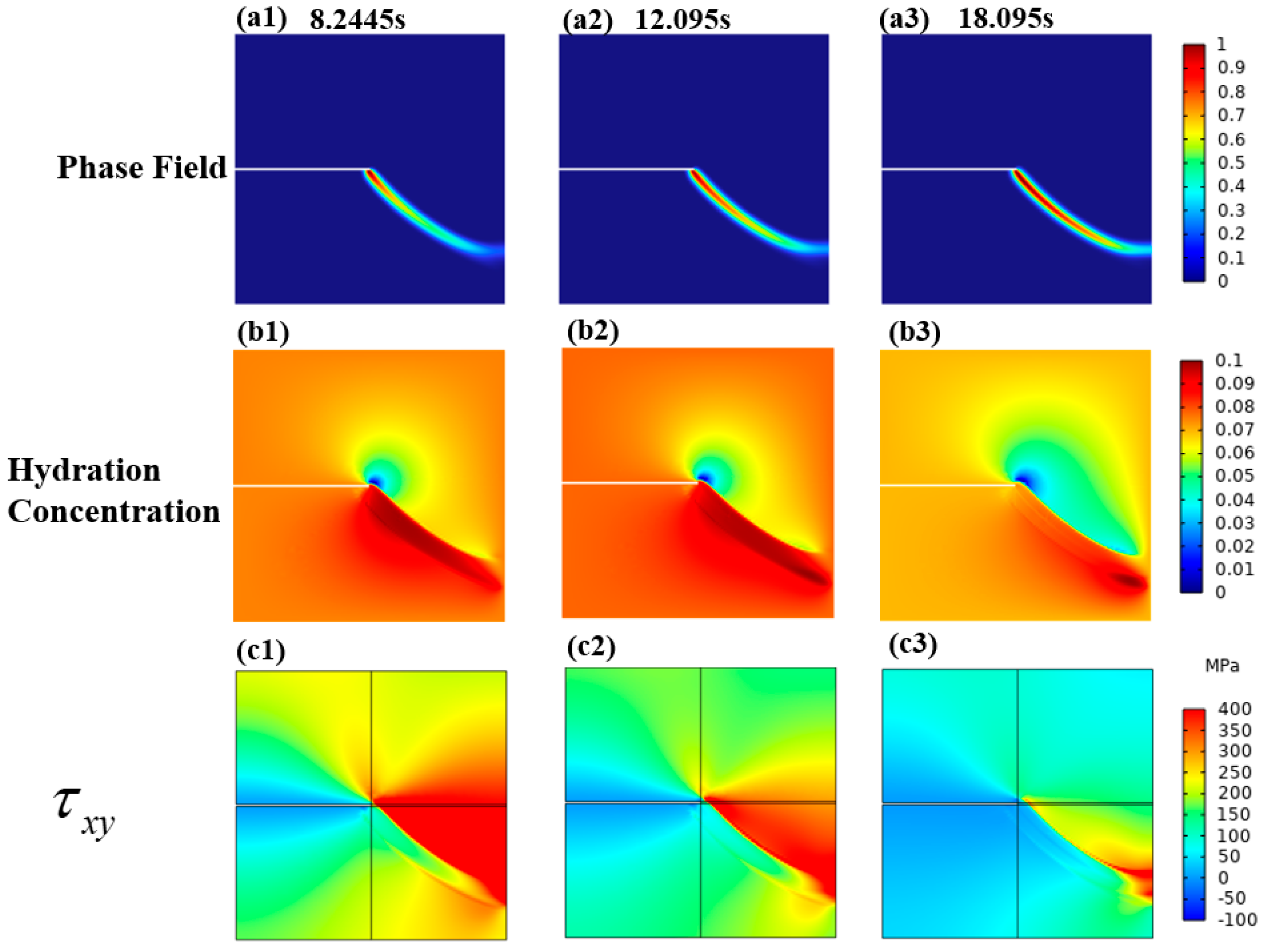
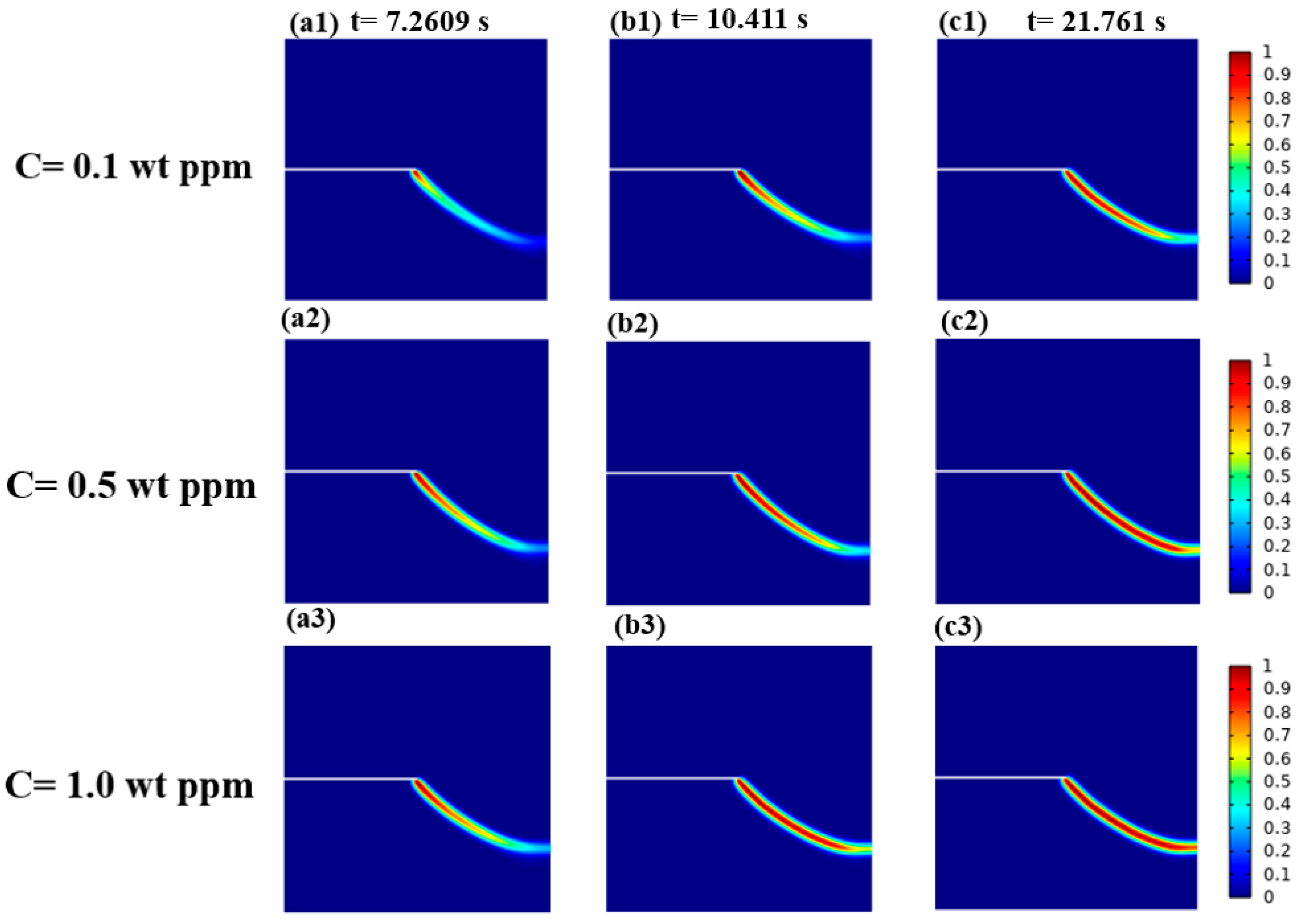
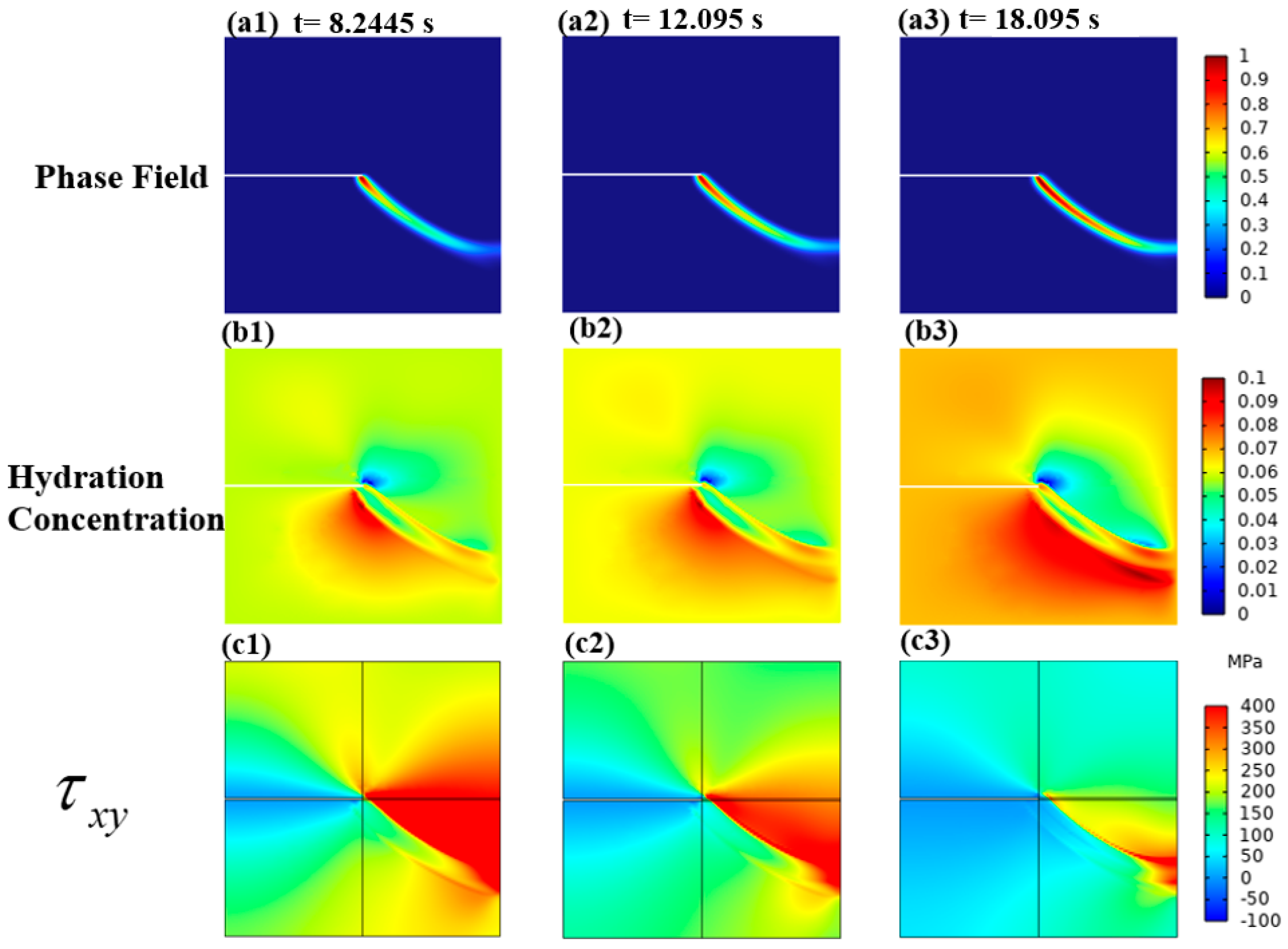
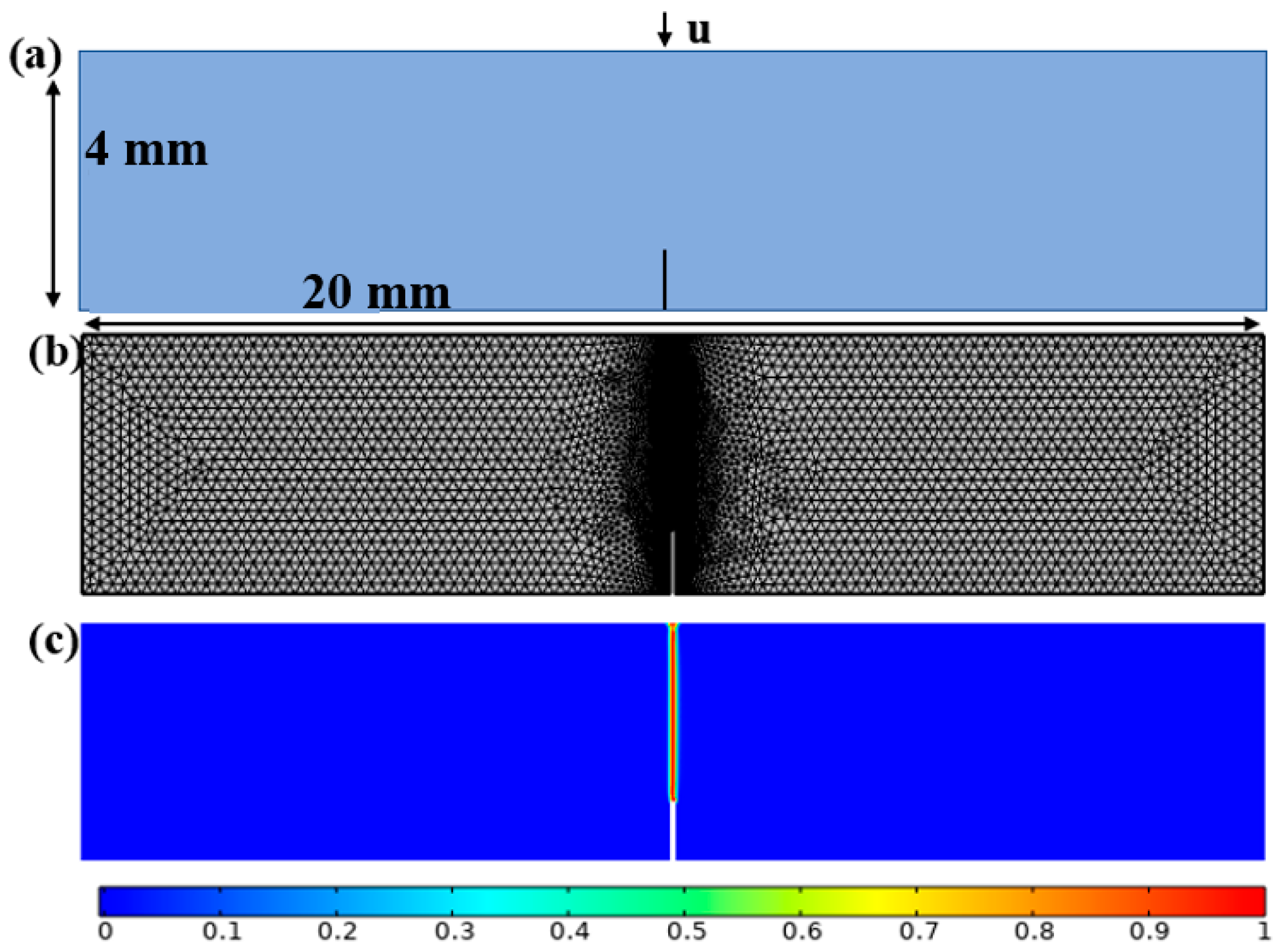
3.2. A Cracked Square Plate Subjected to Shear at Hydrogen Concentration
3.2.1. Hydrogen-Dependent Fracture Energy Degradation
3.2.2. Mechanical–Chemical Coupling
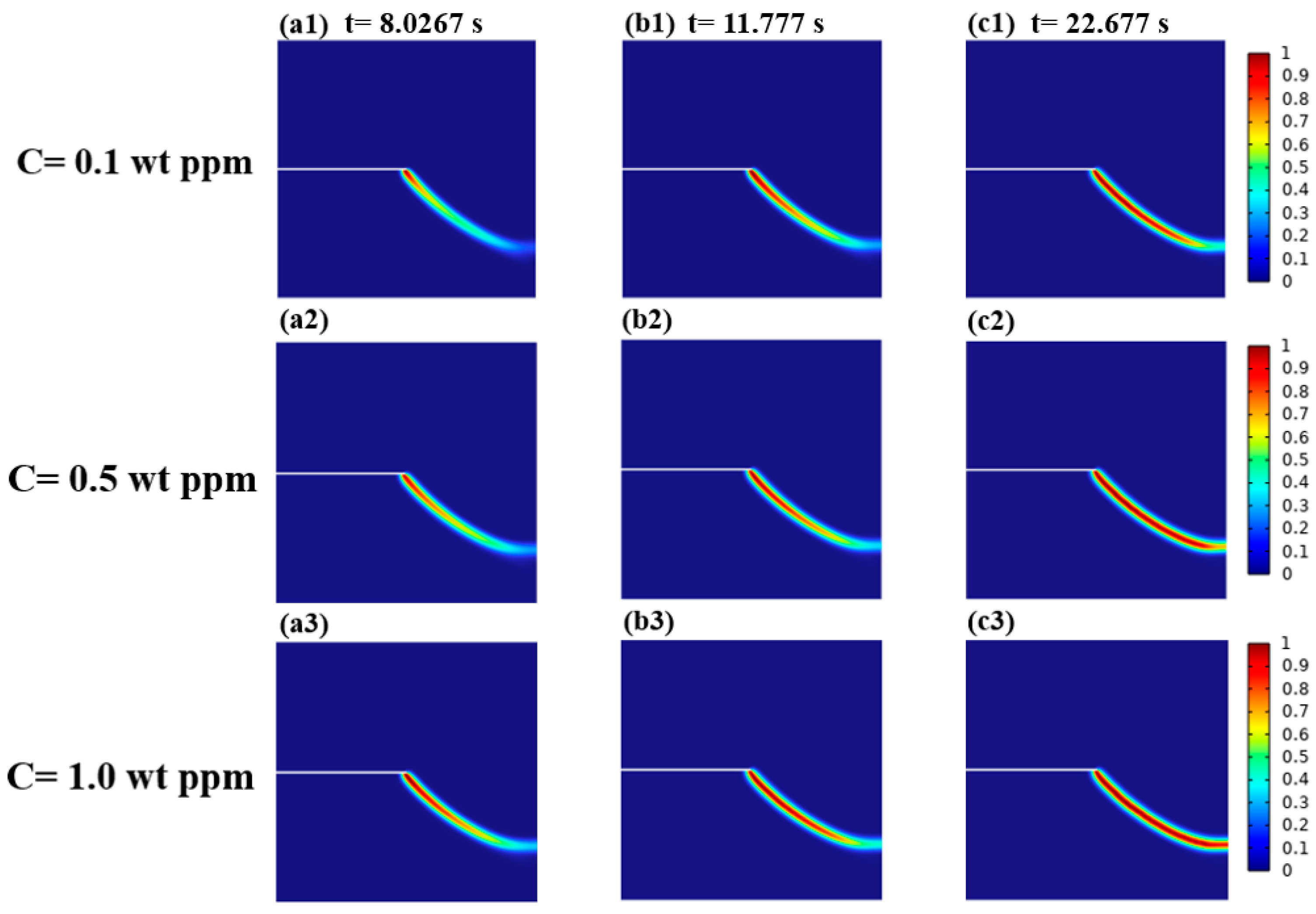
3.2.3. Effects of the Diffusion Coefficient D on the Crack Extension
3.3. Three-Point Bending Beams under the Influence of Hydrogen Concentration
3.3.1. Hydrogen-Dependent Fracture Energy Degradation
3.3.2. Mechanical–Chemical Coupling
3.3.3. Effect of the Diffusion Coefficient D on the Crack Extension
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Yang, L.; Liu, Z.; Li, H.; Zhang, J.; Lian, H. Phase Field Simulation of Hydrogen-Assisted Cracking with Length-Scale Insensitive Degradation Function. Comput. Mater. Sci. 2023, 228, 112309. [Google Scholar] [CrossRef]
- Mandal, T.K.; Nguyen, V.P.; Wu, J.Y. Comparative Study of Phase-Field Damage Models for Hydrogen Assisted Cracking. Theor. Appl. Fract. Mech. 2021, 111, 102840. [Google Scholar] [CrossRef]
- Wu, J.Y.; Mandal, T.K.; Nguyen, V.P. A Phase-Field Regularized Cohesive Zone Model for Hydrogen Assisted Cracking. Comput. Methods Appl. Mech. Eng. 2020, 358, 112614. [Google Scholar] [CrossRef]
- Watson, J.W.; Meshii, M.; Shen, Y.Z. Effect of Cathodic Charging on the Mechanical Properties of Aluminum. Metall. Trans. A 1988, 19, 2299–2304. [Google Scholar]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-Enhanced Localized Plasticity—A Mechanism for Hydrogen-Related Fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Del Busto, S.; Niordson, C.F.; Betegón, C. Strain Gradient Plasticity Modeling of Hydrogen Diffusion to the Crack Tip. Int. J. Hydrogen Energy 2016, 41, 10265–10274. [Google Scholar] [CrossRef]
- Barenblatt, G.I. The Formation of Equilibrium Cracks during Brittle Fracture. General Ideas and Hypotheses. Axially-Symmetric Cracks. J. Appl. Math. Mech. 1959, 23, 622–636. [Google Scholar] [CrossRef]
- Scheider, I.; Pfuff, M.; Dietzel, W. Simulation of Hydrogen Assisted Stress Corrosion Cracking Using the Cohesive Model. Eng. Fract. Mech. 2008, 75, 4283–4291. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R.; Østby, E.; Berstad, T. Influence of Hydrogen from Cathodic Protection on the Fracture Susceptibility of 25%Cr Duplex Stainless Steel–Constant Load SENT Testing and FE-Modelling Using Hydrogen Influenced Cohesive Zone Elements. Eng. Fract. Mech. 2009, 76, 827–844. [Google Scholar] [CrossRef]
- Moriconi, C.; Hénaff, G.; Halm, D. Cohesive Zone Modeling of Fatigue Crack Propagation Assisted by Gaseous Hydrogen in Metals. Int. J. Fatigue 2014, 68, 56–66. [Google Scholar] [CrossRef]
- Alvaro, A.; Jensen, I.T.; Kheradmand, N.; Løvvik, O.M.; Olden, V. Hydrogen Embrittlement in Nickel, Visited by First Principles Modeling, Cohesive Zone Simulation and Nanomechanical Testing. Int. J. Hydrogen Energy 2015, 40, 16892–16900. [Google Scholar] [CrossRef]
- Xu, X.P.; Needleman, A. Numerical Simulations of Fast Crack Growth in Brittle Solids. J. Mech. Phys. Solids 1994, 42, 1397–1434. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Nguyen, C.T.; Bordas, S.; Heidarpour, A. Modelling Interfacial Cracking with Non-Matching Cohesive Interface Elements. Comput. Mech. 2016, 58, 731–746. [Google Scholar] [CrossRef]
- Wells, G.N.; Sluys, L. A New Method for Modelling Cohesive Cracks Using Finite Elements. Int. J. Numer. Methods Eng. 2001, 50, 2667–2682. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, F.B. An Improved Stable XFEM (Is-XFEM) with a Novel Enrichment Function for the Computational Modeling of Cohesive Cracks. Comput. Methods Appl. Mech. Eng. 2015, 295, 77–107. [Google Scholar] [CrossRef]
- Novak, P.; Yuan, R.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. A Statistical, Physical-Based, Micro-Mechanical Model of Hydrogen-Induced Intergranular Fracture in Steel. J. Mech. Phys. Solids 2010, 58, 206–226. [Google Scholar] [CrossRef]
- Ayas, C.; Deshpande, V.S.; Fleck, N.A. A Fracture Criterion for the Notch Strength of High Strength Steels in the Presence of Hydrogen. J. Mech. Phys. Solids 2014, 63, 80–93. [Google Scholar] [CrossRef]
- Jirásek, M. Comparative Study on finite Elements with Embedded Discontinuities. Comput. Methods Appl. Mech. Eng. 2000, 188, 307–330. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, F.B.; Xu, S.L. Extended Embedded Finite Elements with Continuous Displacement Jumps for the Modeling of Localized Failure in Solids. Comput. Methods Appl. Mech. Eng. 2015, 285, 346–378. [Google Scholar] [CrossRef]
- Bourdin, B.; Francfort, G.A.; Marigo, J.J. The Variational Approach to Fracture. J. Elast. 2008, 91, 5–148. [Google Scholar] [CrossRef]
- Francfort, G.A.; Marigo, J.J. Revisiting Brittle Fracture as an Energy Minimization Problem. J. Mech. Phys. Solids 1998, 46, 1319–1342. [Google Scholar] [CrossRef]
- Bourdin, B.; Francfort, G.A.; Marigo, J.-J. Numerical Experiments in Revisited Brittle Fracture. J. Mech. Phys. Solids 2000, 48, 797–826. [Google Scholar] [CrossRef]
- Pham, K.H.; Ravi-Chandar, K. The Formation and Growth of Echelon Cracks in Brittle Materials. Int. J. Fract. 2017, 206, 229–244. [Google Scholar] [CrossRef]
- Tanné, E.; Li, T.; Bourdin, B.; Marigo, J.J.; Maurini, C. Crack Nucleation in Variational Phase-Field Models of Brittle Fracture. J. Mech. Phys. Solids 2018, 110, 80–99. [Google Scholar] [CrossRef]
- Wu, J.Y.; Nguyen, V.P. A Length Scale Insensitive Phase-Field Damage Model for Brittle Fracture. J. Mech. Phys. Solids 2018, 119, 20–42. [Google Scholar] [CrossRef]
- Borden, M.J.; Hughes, T.J.; Landis, C.M.; Anvari, A.; Lee, I.J. A Phase-Field Formulation for Fracture in Ductile Materials: Finite Deformation Balance Law Derivation, Plastic Degradation, and Stress Triaxiality Effects. Comput. Methods Appl. Mech. Eng. 2016, 312, 130–166. [Google Scholar] [CrossRef]
- Borden, M.J.; Verhoosel, C.V.; Scott, M.A.; Hughes, T.J.; Landis, C.M. A Phase-Field Description of Dynamic Brittle Fracture. Comput. Methods Appl. Mech. Eng. 2012, 217–220, 77–95. [Google Scholar] [CrossRef]
- Schlüter, A.; Willenbücher, A.; Kuhn, C.; Müller, R. Phase Field Approximation of Dynamic Brittle Fracture. Comput. Mech. 2014, 5, 1141–1161. [Google Scholar] [CrossRef]
- Wu, J.-Y. A Geometrically Regularized Gradient-Damage Model with Energetic Equivalence. Comput. Methods Appl. Mech. Eng. 2018, 328, 612–637. [Google Scholar] [CrossRef]
- Wu, J.-Y. Robust Numerical Implementation of Non-Standard Phase-Field Damage Models for Failure in Solids. Comput. Methods Appl. Mech. Eng. 2018, 340, 767–797. [Google Scholar] [CrossRef]
- Feng, D.C.; Wu, J.Y. Phase-Field Regularized Cohesive Zone Model (CZM) and Size Effect of Concrete. Eng. Fract. Mech. 2018, 197, 66–79. [Google Scholar] [CrossRef]
- Lee, S.; Wheeler, M.F.; Wick, T. Pressure and Fluid-Driven Fracture Propagation in Porous Media Using an Adaptive Finite Element Phase Field Model. Comput. Methods Appl. Mech. Eng. 2016, 305, 111–132. [Google Scholar] [CrossRef]
- Miehe, C.; Mauthe, S. Phase Field Modeling of Fracture in Multi-Physics Problems. Part III. Crack Driving Forces in Hydro-Poro-Elasticity and Hydraulic Fracturing of Fluid-Saturated Porous Media. Comput. Methods Appl. Mech. Eng. 2016, 304, 619–655. [Google Scholar] [CrossRef]
- Areias, P.; Rabczuk, T.; Msekh, M.A. Phase-Field Analysis of Finite-Strain Plates and Shells Including Element Subdivision. Comput. Methods Appl. Mech. Eng. 2016, 312, 322–350. [Google Scholar] [CrossRef]
- Mandal, T.K.; Nguyen, V.P.; Wu, J.Y. A Length Scale Insensitive Anisotropic Phase Field Fracture Model for Hyperelastic Composites. Int. J. Mech. Sci. 2020, 188, 105941. [Google Scholar] [CrossRef]
- Miehe, C.; Schänzel, L.M. Phase Field Modeling of Fracture in Rubbery Polymers. Part I. Finite Elasticity Coupled with Brittle Failure. J. Mech. Phys. Solids 2014, 65, 93–113. [Google Scholar] [CrossRef]
- Falkenberg, R. A Phase Field Approach to Fracture with Mass Transport Extension for the Simulation of Environmentally-Assisted Cracking. Key Eng. Mater. 2017, 754, 153–156. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bolivar, J.; Réthoré, J.; Baietto, M.C.; Fregonese, M. A Phase Field Method for Modeling Stress Corrosion Crack Propagation in a Nickel Base Alloy. Int. J. Solids Struct. 2017, 112, 65–82. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Alireza, G.; Christian, F.N. A Phase Field Formulation for Hydrogen Assisted Cracking. Comput. Methods Appl. Mech. Eng. 2018, 342, 742–761. [Google Scholar] [CrossRef]
- Falkenberg, R. Modelling of Environmentally Assisted Material Degradation in the Crack Phase-Field Framework. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 5–12. [Google Scholar] [CrossRef]
- Miehe, C.; Dal, H.; Schänzel, L.-M.; Raina, A. A Phase-Field Model for Chemo-Mechanical Induced Fracture in Lithium-Ion Battery Electrode Particles: Phase-field model of fracture in li-ion battery electrodes. Int. J. Numer. Methods Eng. 2016, 106, 683–711. [Google Scholar] [CrossRef]
- Serebrinsky, S. A Quantum-Mechanically Informed Continuum Model of Hydrogen Embrittlement. J. Mech. Phys. Solids 2004, 52, 2403–2430. [Google Scholar] [CrossRef]
- Jiang, D.E.; Carter, E.A. First Principles Assessment of Ideal Fracture Energies of Materials with Mobile Impurities: Implications for Hydrogen Embrittlement of Metals. Acta Mater. 2004, 52, 4801–4807. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Miehe, C.; Hofacker, M.; Welschinger, F. A phase field model for rate-independent crack propagation: Robust algorithmic implementation based on operator splits. Comput. Methods Appl. Mech. Eng. 2010, 199, 2765–2778. [Google Scholar] [CrossRef]












| Mechanical Properties | Chemical Properties | Finite Element |
|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Shen, Q. A Phase-Field Regularized Cohesion Model for Hydrogen-Assisted Cracking. Coatings 2024, 14, 202. https://doi.org/10.3390/coatings14020202
Zhang S, Shen Q. A Phase-Field Regularized Cohesion Model for Hydrogen-Assisted Cracking. Coatings. 2024; 14(2):202. https://doi.org/10.3390/coatings14020202
Chicago/Turabian StyleZhang, Shiyi, and Qiang Shen. 2024. "A Phase-Field Regularized Cohesion Model for Hydrogen-Assisted Cracking" Coatings 14, no. 2: 202. https://doi.org/10.3390/coatings14020202
APA StyleZhang, S., & Shen, Q. (2024). A Phase-Field Regularized Cohesion Model for Hydrogen-Assisted Cracking. Coatings, 14(2), 202. https://doi.org/10.3390/coatings14020202






