Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures
Abstract
:1. Introduction
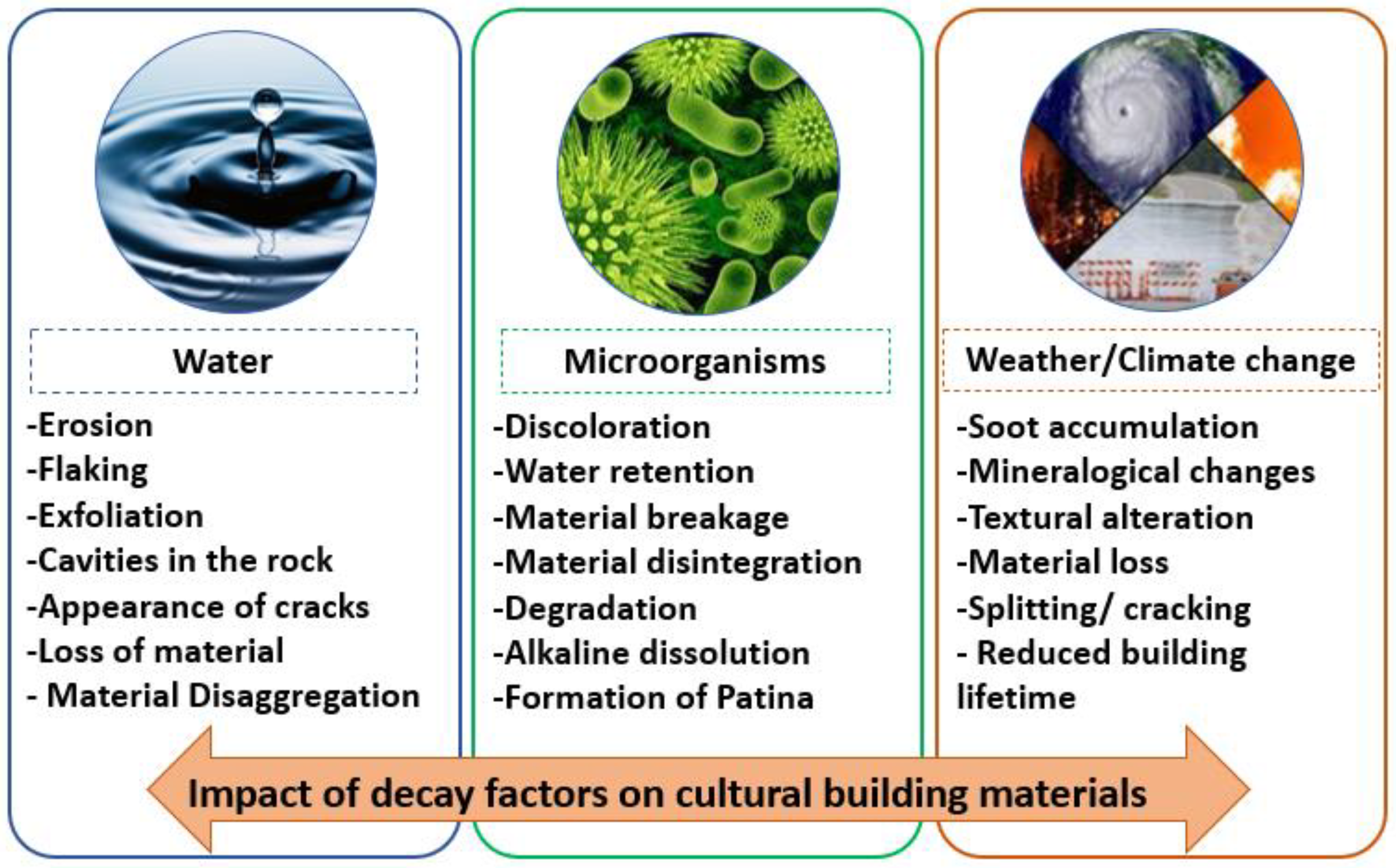
2. Decay Mechanisms of Stone Materials
- Intrinsic factors: depending on the nature of the material, orientation, manufacturing technique, and procedures used to affect the work.
- Extrinsic factors: due to external sources such as environmental factors (temperature, air pollutants, relative humidity, and light), anthropogenic factors (handling, misuse, vandalism, tourism, etc.), catastrophic factors (earthquakes, fires, floods, etc.), and not less important, biological factors such as macro- and micro-organisms [1].
2.1. Intrinsic Properties of Stone Materials
2.2. Water
2.3. Microorganism Colonization
2.4. Weather and Climate Changes
3. Metal Oxide Nanomaterials: Properties and Applications
4. Application of Metal Oxide-Based Nanocomposite Coatings on Stone Building Materials
5. Metal Oxide NPs with Enhanced Photo-Response Activity
6. Risk of Toxicity and Preventive Measures for Use of Nanomaterials in Art Conservation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Maalej, R.; Licchelli, M. Silver Nanoparticles in the Cultural Heritage Conservation. In Self-Assembly of Materials and Their Applications; Rathnayake, H., Pathiraja, G., Sharmin, E., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Bouaziz, J.; Licchelli, M. Enhanced Gd Doped TiO2 NPs-PDMS Nanocomposites as Protective Coatings for Bio-Calcarenite Stone: Preliminarily Analysis. In Design and Modeling of Mechanical Systems—V; Walha, L., Jarraya, A., Djemal, F., Chouchane, M., Aifaoui, N., Chaari, F., Abdennadher, M., Benamara, A., Haddar, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 885–893. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Anti-graffiti nanocomposite materials for surface protection of a very porous stone. Appl. Phys. A 2014, 116, 1525–1539. [Google Scholar] [CrossRef]
- Weththimuni, M.; Canevari, C.; Legnani, A.; Licchelli, M.; Malagodi, M.; Ricca, M.; Zeffiro, A. Experimental characterization of oil-colophony varnishes: A preliminary study. Int. J. Conserv. Sci. 2016, 7, 813–826. [Google Scholar]
- Angeli, M.; Bigas, J.-P.; Benavente, D.; Menéndez, B.; Hébert, R.; David, C. Salt crystallization in pores: Quantification and estimation of damage. Environ. Geol. 2007, 52, 205–213. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Fiocco, G.; Milanese, C.; Spinella, A.; Saladino, M.L.; Malagodi, M.; Licchelli, M. Stradivari’s Varnish Revisited: Feature Improvements Using Chemical Modification. Polymers 2023, 15, 3652. [Google Scholar] [CrossRef]
- Spinella, A.; Malagodi, M.; Saladino, M.L.; Weththimuni, M.L.; Caponetti, E.; Licchelli, M. A step forward in disclosing the secret of stradivari’s varnish by NMR spectroscopy. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3949–3954. [Google Scholar] [CrossRef]
- Fichera, G.V.; Malagodi, M.; Cofrancesco, P.; Weththimuni, M.L.; Guglieri, C.; Olivi, L.; Ruffolo, S.; Licchelli, M. Study of the copper effect in iron-gall inks after artificial ageing. Chem. Pap. 2018, 72, 1905–1915. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D. How Science Can Contribute to the Remedial Conservation of Cultural Heritage. Chemistry 2021, 27, 10798–10806. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.E.C.; Ramos, A.P.; Sánchez, J.I.C.; Serrano, M.E.D. Origin and Control Strategies of Biofilms in the Cultural Heritage. In Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods; Kırmusaoğlu, S., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M. Heritage Conservation and Restoration: Surface Characterization, Cleaning and Treatments. Coatings 2023, 13, 457. [Google Scholar] [CrossRef]
- Vinçotte, A.; Beauvoit, E.; Boyard, N.; Guilminot, E. Effect of solvent on PARALOID® B72 and B44 acrylic resins used as adhesives in conservation. Herit. Sci. 2019, 7, 42. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Chatzigrigoriou, A.; Manoudis, P.N. Chapter 10—Waterborne superhydrophobic coatings for the conservation of the cultural heritage: A case study for the protection of mortar, ceramic, and wood. In Handbook of Waterborne Coatings; Zarras, P., Soucek, M.D., Tiwari, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 229–247. [Google Scholar] [CrossRef]
- Weththimuni, M.; Crivelli, F.; Galimberti, C.; Malagodi, M.; Licchelli, M. Evaluation of commercial consolidating agents on very porous biocalcarenite. Int. J. Conserv. Sci. 2020, 11, 251–260. [Google Scholar]
- Artesani, A.; Di Turo, F.; Zucchelli, M.; Traviglia, A. Recent Advances in Protective Coatings for Cultural Heritage–An Overview. Coatings 2020, 10, 217. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.; Li, Z.; Duan, W.; Han, H.; Xie, Q. A superhydrophobic fluorinated PDMS composite for wearable strain sensor with excellent mechanical robustness and liquid impalement resistance. J. Mater. Chem. A 2020, 8, 3509–3516. [Google Scholar] [CrossRef]
- Licchelli, M.; Marzolla, S.J.; Poggi, A.; Zanchi, C. Crosslinked fluorinated polyurethanes for the protection of stone surfaces from graffiti. J. Cult. Herit. 2011, 12, 34–43. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.L.; Zanchi, C. Water-repellent properties of fluoroelastomers on a very porous stone: Effect of the application procedure. Prog. Org. Coat. 2013, 76, 495–503. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; La Russa, M. Consolidation of bio-calcarenite stone by treatment based on diammonium hydrogenphosphate and calcium hydroxide nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Liu, Y.; Wei, G.; Zhang, H.; Chen, W.; Xu, Z. Biomimic conservation of weathered calcareous stones by apatite. N. J. Chem. 2011, 35, 887. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial Effect of Zinc Oxide-Based Nanomaterials on Environmental Biodeteriogens Affecting Historical Buildings. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Dei, L.; Salvadori, B. Nanotechnology in cultural heritage conservation: Nanometric slaked lime saves architectonic and artistic surfaces from decay. J. Cult. Herit. 2006, 7, 110–115. [Google Scholar] [CrossRef]
- Facio, D.S.; Mosquera, M.J. Simple Strategy for Producing Superhydrophobic Nanocomposite Coatings In Situ on a Building Substrate. ACS Appl. Mater. Interfaces 2013, 5, 7517–7526. [Google Scholar] [CrossRef] [PubMed]
- Pedna, A.; Pinho, L.; Frediani, P.; Mosquera, M.J. Obtaining SiO2–fluorinated PLA bionanocomposites with application as reversible and highly-hydrophobic coatings of buildings. Prog. Org. Coat. 2016, 90, 91–100. [Google Scholar] [CrossRef]
- Petcu, C.; Alexandrescu, E.; Bălan, A.; Tănase, M.A.; Cinteză, L.O. Synthesis and Characterisation of Organo-Modified Silica Nanostructured Films for the Water-Repellent Treatment of Historic Stone Buildings. Coatings 2020, 10, 1010. [Google Scholar] [CrossRef]
- Xie, Z.; Duan, Z.; Zhao, Z.; Li, R.; Zhou, B.; Yang, D.; Hu, Y. Nano-materials enhanced protectants for natural stone surfaces. Herit. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. Construcción 2017, 67, 107. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A review of the photocatalysis process used for wastewater treatment. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Chobba, M.B.; Messaoud, M.; Weththimuni, M.L.; Bouaziz, J.; Licchelli, M.; De Leo, F.; Urzì, C. Preparation and characterization of photocatalytic Gd-doped TiO2 nanoparticles for water treatment. Environ. Sci. Pollut. Res. 2019, 26, 32734–32745. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef]
- Meng, H.; Katayama, Y.; Gu, J.-D. More wide occurrence and dominance of ammonia-oxidizing archaea than bacteria at three Angkor sandstone temples of Bayon, Phnom Krom and Wat Athvea in Cambodia. Int. Biodeterior. Biodegrad. 2017, 117, 78–88. [Google Scholar] [CrossRef]
- Zammit, G.; Sánchez-Moral, S.; Albertano, P. Bacterially mediated mineralisation processes lead to biodeterioration of artworks in Maltese catacombs. Sci. Total Environ. 2011, 409, 2773–2782. [Google Scholar] [CrossRef]
- Urzì, C.; Realini, M. Colour changes of Notos calcareous sandstone as related to its colonisation by microorganisms. Int. Biodeterior. Biodegrad. 1998, 42, 45–54. [Google Scholar] [CrossRef]
- Cappitelli, F.; Principi, P.; Pedrazzani, R.; Toniolo, L.; Sorlini, C. Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Sci. Total Environ. 2007, 385, 172–181. [Google Scholar] [CrossRef]
- Rosado, T.; Reis, A.; Mirão, J.; Candeias, A.; Vandenabeele, P.; Caldeira, A.T. Pink! Why not? On the unusual colour of Évora Cathedral. Int. Biodeterior. Biodegrad. 2014, 94, 121–127. [Google Scholar] [CrossRef]
- Schiavon, N.; De Caro, T.; Kiros, A.; Caldeira, A.T.; Parisi, I.E.; Riccucci, C.; Gigante, G.E. A multianalytical approach to investigate stone biodeterioration at a UNESCO world heritage site: The volcanic rock-hewn churches of Lalibela, Northern Ethiopia. Appl. Phys. A 2013, 113, 843–854. [Google Scholar] [CrossRef]
- Ahmed, I.; Basharat, M.; Sousa, L.; Mughal, M.S. Evaluation of building and dimension stone using physico-mechanical and petrographic properties: A case study from the Kohistan and Ladakh batholith, Northern Pakistan. Environ. Earth Sci. 2021, 80, 759. [Google Scholar] [CrossRef]
- Martínez-García, R.; de Rojas, M.S.; Jagadesh, P.; López-Gayarre, F.; Morán-Del-Pozo, J.M.; Juan-Valdes, A. Effect of pores on the mechanical and durability properties on high strength recycled fine aggregate mortar. Case Stud. Constr. Mater. 2022, 16, e01050. [Google Scholar] [CrossRef]
- García-Del-Cura, M.; Benavente, D.; Martínez-Martínez, J.; Cueto, N. Sedimentary structures and physical properties of travertine and carbonate tufa building stone. Constr. Build. Mater. 2012, 28, 456–467. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; An, M.; Sun, Y.; Yu, Z.; Huang, H. Factors Influencing the Capillary Water Absorption Characteristics of Concrete and Their Relationship to Pore Structure. Appl. Sci. 2022, 12, 2211. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F.; Rovella, N.; Ricca, M. The Impact of Air Pollution on Stone Materials. Environments 2023, 10, 119. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E. Consolidation of porous carbonate stones by an innovative phosphate treatment: Mechanical strengthening and physical-microstructural compatibility in comparison with TEOS-based treatments. Herit. Sci. 2015, 3, 1. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Jiang, X.; Mu, S.; Liu, J. Influence of chlorides and salt concentration on salt crystallization damage of cement-based materials. J. Build. Eng. 2022, 61, 105260. [Google Scholar] [CrossRef]
- Menéndez, B. Estimation of salt mixture damage on built cultural heritage from environmental conditions using ECOS-RUNSALT model. J. Cult. Herit. 2017, 24, 22–30. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L. An experimental investigation into the salt-weathering susceptibility of building limestones. Rock Mech. Rock Eng. 2020, 53, 5329–5343. [Google Scholar] [CrossRef]
- Urzì, C.; Krumbein, W.E. Microbiological Impacts on the Cultural Heritage. In Durability and Change: The Science, Responsibility, and Cost of Sustaining Cultural Heritage—Chapter 10; Krumbein, W.E., Brimblecombe, P., Cosgrove, D.E., Staniforth, S., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1994. [Google Scholar] [CrossRef]
- Zhang, X.; Hoff, I.; Saba, R.G. Response and Deterioration Mechanism of Bitumen under Acid Rain Erosion. Materials 2021, 14, 4911. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, L.; Li, W.; Zhang, Q. Effect of acid rain on economic loss of concrete structures in Hangzhou, China. Int. J. Low Carbon Technol. 2019, 14, 89–94. [Google Scholar] [CrossRef]
- Peng, N.; Hong, J.; Zhu, Y.; Dong, Y.; Sun, B.; Huang, J. Experimental Investigation of the Influence of Freeze–Thaw Mode on Damage Characteristics of Sandstone. Appl. Sci. 2022, 12, 12395. [Google Scholar] [CrossRef]
- Huang, S.; Cai, Y.; Liu, Y.; Liu, G. Experimental and Theoretical Study on Frost Deformation and Damage of Red Sandstones with Different Water Contents. Rock Mech. Rock Eng. 2021, 54, 4163–4181. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, X.; Yang, G. A study on mechanical properties and damage model of rock subjected to freeze-thaw cycles and confining pressure. Cold Reg. Sci. Technol. 2020, 174, 103056. [Google Scholar] [CrossRef]
- McNamara, C.; Mitchell, R. Microbial deterioration of historic stone. Front. Ecol. Environ. 2005, 3, 445–451. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.-D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good—Microorganisms in Cultural Heritage Environments—An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef]
- Ciferri, O. The role of microorganisms in the degradation of cultural heritage. Stud. Conserv. 2002, 47, 35–45. [Google Scholar] [CrossRef]
- Lo Schiavo, S.; De Leo, F.; Urzì, C. Present and Future Perspectives for Biocides and Antifouling Products for Stone-Built Cultural Heritage: Ionic Liquids as a Challenging Alternative. Appl. Sci. 2020, 10, 6568. [Google Scholar] [CrossRef]
- Miller, A.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Roig, P.B.; Ros, J.L.R.; Estellés, R.M. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef]
- Abdelhafez, A.; El-Wekeel, F.M.; Ramadan, E.; Abed-Allah, A. Microbial deterioration of archaeological marble: Identification and treatment. Ann. Agric. Sci. 2012, 57, 137–144. [Google Scholar] [CrossRef]
- De Leo, F.; Marchetta, A.; Urzì, C. Black Fungi on Stone-Built Heritage: Current Knowledge and Future Outlook. Appl. Sci. 2022, 12, 3969. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [PubMed]
- Krakova, L.; De Leo, F.; Bruno, L.; Pangallo, D.; Urzì, C. Complex bacterial diversity in the white biofilms of the Catacombs of St. Callixtus in Rome evidenced by different investigation strategies. Environ. Microbiol. 2015, 17, 1738–1752. [Google Scholar] [CrossRef] [PubMed]
- Urzì, C.; Bruno, L.; De Leo, F. Biodeterioration of Paintings in Caves, Catacombs and other Hypogean Sites. In Biodeterioration and Preservation in Art, Archaeology and Architecture; Archetype Publications: London, UK, 2018; pp. 114–129. [Google Scholar]
- Liang, X.; Meng, S.; He, Z.; Zeng, X.; Peng, T.; Huang, T.; Wang, J.; Gu, J.-D.; Hu, Z. Higher abundance of ammonia-oxidizing bacteria than ammonia-oxidizing archaea in biofilms and the microbial community composition of Kaiping Diaolou of China. Int. Biodeterior. Biodegrad. 2023, 184, 105647. [Google Scholar] [CrossRef]
- Joseph, E. Microorganisms in the Deterioration and Preservation of Cultural Heritage; Springer Science and Business Media LLC.: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Urzi, C. Microbial Deterioration of Rocks and Marble Monuments of the Mediterranean Basin: A Review. Corros. Rev. 2004, 22, 441–458. [Google Scholar] [CrossRef]
- Lamp, J.L.; Marchant, D.R.; Mackay, S.L.; Head, J.W. Thermal stress weathering and the spalling of Antarctic rocks. J. Geophys. Res. Earth Surf. 2016, 122, 3–24. [Google Scholar] [CrossRef]
- Sesana, E.; Gagnon, A.S.; Ciantelli, C.; Cassar, J.; Hughes, J.J. Climate change impacts on cultural heritage: A literature review. WIREs Clim. Chang. 2021, 12, e710. [Google Scholar] [CrossRef]
- Kapsomenakis, J.; Douvis, C.; Poupkou, A.; Zerefos, S.; Solomos, S.; Stavraka, T.; Melis, N.S.; Kyriakidis, E.; Kremlis, G.; Zerefos, C. Climate change threats to cultural and natural heritage UNESCO sites in the Mediterranean. Environ. Dev. Sustain. 2023, 25, 14519–14544. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Grøntoft, T.; Cassar, J. An assessment of the contribution of air pollution to the weathering of limestone heritage in Malta. Environ. Earth Sci. 2020, 79, 288. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, Y.; Li, H. Study of internal moisture condensation for the conservation of stone cultural heritage. J. Cult. Herit. 2022, 56, 1–9. [Google Scholar] [CrossRef]
- Vyshkvarkova, E.; Sukhonos, O. Climate Change Impact on the Cultural Heritage Sites in the European Part of Russia over the Past 60 Years. Climate 2023, 11, 50. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar]
- UNESCO World Heritage Centre. Climate Change and World Heritage: Report on Predicting and Managing the Impacts of Climate Change on World Heritage and Strategy to Assist States Parties to Implement Appropriate Management Responses; UNESCO World Heritage Centre: Paris, France, 2007. [Google Scholar]
- Ricca, M.; Le Pera, E.; Licchelli, M.; Macchia, A.; Malagodi, M.; Randazzo, L.; Rovella, N.; Ruffolo, S.A.; Weththimuni, M.L.; La Russa, M.F. The CRATI Project: New Insights on the Consolidation of Salt Weathered Stone and the Case Study of San Domenico Church in Cosenza (South Calabria, Italy). Coatings 2019, 9, 330. [Google Scholar] [CrossRef]
- Ravankhah, M.; de Wit, R.; Argyriou, A.V.; Chliaoutakis, A.; Revez, M.J.; Birkmann, J.; Žuvela-Aloise, M.; Sarris, A.; Tzigounaki, A.; Giapitsoglou, K. Integrated Assessment of Natural Hazards, Including Climate Change’s Influences, for Cultural Heritage Sites: The Case of the Historic Centre of Rethymno in Greece. Int. J. Disaster Risk Sci. 2019, 10, 343–361. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chobba, M.B.; Messaoud, M.; Bouaziz, J.; De Leo, F.; Urzì, C. The Effect of Heat Treatment on Photocatalytic Performance and Antibacterial Activity of TiO2 Nanoparticles Prepared by Sol-Gel Method. In Advances in Materials, Mechanics and Manufacturing; Chaari, F., Barkallah, M., Bouguecha, A., Zouari, B., Khabou, M.T., Kchaou, M., Haddar, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 71–79. [Google Scholar] [CrossRef]
- Abdelraheem, W.H.; Patil, M.K.; Nadagouda, M.N.; Dionysiou, D.D. Hydrothermal synthesis of photoactive nitrogen- and boron- codoped TiO2 nanoparticles for the treatment of bisphenol A in wastewater: Synthesis, photocatalytic activity, degradation byproducts and reaction pathways. Appl. Catal. B Environ. 2018, 241, 598–611. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular Toxicity of TiO2 Nanoparticles in Anatase and Rutile Crystal Phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Henningham, A.; Döhrmann, S.; Nizet, V.; Cole, J.N. Mechanisms of group A Streptococcus resistance to reactive oxygen species. FEMS Microbiol. Rev. 2015, 39, 488–508. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.-S.; Watanabe, A. Green synthesis of zinc oxide nanoparticles using aqueous extract of Garcinia mangostana fruit pericarp and their photocatalytic activity. Bull. Mater. Sci. 2018, 41, 50. [Google Scholar] [CrossRef]
- Raha, S. Ahmaruzzaman ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Milanese, C.; Licchelli, M. Shellac/nanoparticles dispersions as protective materials for wood. Appl. Phys. A 2016, 122, 1058. [Google Scholar] [CrossRef]
- Cinteză, L.O.; Tănase, M.A. Multifunctional ZnO Nanoparticle: Based Coatings for Cultural Heritage Preventive Conservation. In Thin Films; Ares, A.E., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Ben Chobba, M.; Tredici, I.; Licchelli, M. ZrO2-doped ZnO-PDMS nanocomposites as protective coatings for the stone materials. Acta IMEKO 2022, 11, 5. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Ben Chobba, M.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Milanese, C.; Licchelli, M.; Malagodi, M. Improving the Protective Properties of Shellac-Based Varnishes by Functionalized Nanoparticles. Coatings 2021, 11, 419. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Capsoni, D.; Malagodi, M.; Licchelli, M. Improving Wood Resistance to Decay by Nanostructured ZnO-Based Treatments. J. Nanomater. 2019, 2019, 6715756. [Google Scholar] [CrossRef]
- Mokammel, M.A.; Islam, M.J.; Hasanuzzaman, M.; Hashmi, S. Nanoscale Materials for Self-Cleaning and Antibacterial Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Ji, B.; Fang, X.; Jin, K.; Qian, J. The role of nanotechnology-based approaches for clinical infectious diseases and public health. Front. Bioeng. Biotechnol. 2023, 11, 1146252. [Google Scholar] [CrossRef]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar] [CrossRef]
- Busi, S.; Rajkumari, J. Chapter 15—Microbially synthesized nanoparticles as next generation antimicrobials: Scope and applications. In Nanoparticles in Pharmacotherapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 485–524. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Aziz, B.K.; Karim, M.A.H. Efficient catalytic photodegradation of methylene blue from medical lab wastewater using MgO nanoparticles synthesized by direct precipitation method. React. Kinet. Mech. Catal. 2019, 128, 1127–1139. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Gobinath, C.; Prabukumar, S.; Sivaramakrishnan, S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019, 190, 8–20. [Google Scholar] [CrossRef]
- Dobrucka, R. Synthesis of MgO Nanoparticles Using Artemisia abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 547–555. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Wetteland, C.L.; Nguyen, N.-Y.T.; Liu, H. Concentration-dependent behaviors of bone marrow derived mesenchymal stem cells and infectious bacteria toward magnesium oxide nanoparticles. Acta Biomater. 2016, 35, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Fruth, V.; Todan, L.; Codrea, C.I.; Poenaru, I.; Petrescu, S.; Aricov, L.; Ciobanu, M.; Jecu, L.; Ion, R.M.; Predoana, L. Multifunctional Composite Coatings Based on Photoactive Metal-Oxide Nanopowders (MgO/TiO2) in Hydrophobic Polymer Matrix for Stone Heritage Conservation. Nanomaterials 2021, 11, 2586. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P., Jr.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnol. 2016, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Negrescu, A.M.; Killian, M.S.; Raghu, S.N.V.; Schmuki, P.; Mazare, A.; Cimpean, A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. J. Funct. Biomater. 2022, 13, 274. [Google Scholar] [CrossRef]
- D’orazio, L.; Grippo, A. A water dispersed Titanium dioxide/poly(carbonate urethane) nanocomposite for protecting cultural heritage: Preparation and properties. Prog. Org. Coat. 2015, 79, 1–7. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; de Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Tavares, M.; Santos, A.; Santos, I.; Silva, M.; Bomio, M.; Longo, E.; Paskocimas, C.; Motta, F. TiO2/PDMS nanocomposites for use on self-cleaning surfaces. Surf. Coat. Technol. 2014, 239, 16–19. [Google Scholar] [CrossRef]
- Kapridaki, C.; Verganelaki, A.; Dimitriadou, P.; Maravelaki-Kalaitzaki, P. Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO2 Hydrophobic/Photoactive Hybrid Nanomaterials. Materials 2018, 11, 684. [Google Scholar] [CrossRef]
- Corcione, C.E.; Ingrosso, C.; Petronella, F.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Frigione, M.; Curri, M.L. A designed UV–vis light curable coating nanocomposite based on colloidal TiO2 NRs in a hybrid resin for stone protection. Prog. Org. Coat. 2018, 122, 290–301. [Google Scholar] [CrossRef]
- Azadi, N.; Parsimehr, H.; Ershad-Langroudi, A. Cultural heritage protection via hybrid nanocomposite coating. Plast. Rubber Compos. 2020, 49, 414–424. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; La Russa, M.F. Nanostructured Coatings for Stone Protection: An Overview. Front. Mater. 2019, 6, 147. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Guo, L.; Guo, J.; Zheng, L.; Yang, F.; Ma, Z.; Zhao, X. A Durable Nano-SiO2-TiO2/Dodecyltrimethoxysilane Superhydrophobic Coating for Stone Protection. Coatings 2022, 12, 1397. [Google Scholar] [CrossRef]
- Goffredo, G.B.; Quagliarini, E.; Bondioli, F.; Munafò, P. TiO2 nanocoatings for architectural heritage: Self-cleaning treatments on historical stone surfaces. Proc. Inst. Mech. Eng. Part N J. Nanomater. Nanoeng. Nanosyst. 2014, 228, 2–10. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Bergamonti, L.; Alfieri, I.; Franzò, M.; Lorenzi, A.; Montenero, A.; Predieri, G.; Raganato, M.; Calia, A.; Lazzarini, L.; Bersani, D.; et al. Synthesis and characterization of nanocrystalline TiO2 with application as photoactive coating on stones. Environ. Sci. Pollut. Res. 2014, 21, 13264–13277. [Google Scholar] [CrossRef]
- Gherardi, F.; Roveri, M.; Goidanich, S.; Toniolo, L. Photocatalytic Nanocomposites for the Protection of European Architectural Heritage. Materials 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Crupi, V.; Fazio, B.; Gessini, A.; Kis, Z.; La Russa, M.F.; Majolino, D.; Masciovecchio, C.; Ricca, M.; Rossi, B.; Ruffolo, S.A.; et al. TiO2–SiO2–PDMS nanocomposite coating with self-cleaning effect for stone material: Finding the optimal amount of TiO2. Constr. Build. Mater. 2018, 166, 464–471. [Google Scholar] [CrossRef]
- Colangiuli, D.; Lettieri, M.; Masieri, M.; Calia, A. Field study in an urban environment of simultaneous self-cleaning and hydrophobic nanosized TiO2-based coatings on stone for the protection of building surface. Sci. Total Environ. 2019, 650, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hou, P.; Stephan, D.; Huang, S.; Zhang, L.; Yang, P.; Cheng, X. SiO2/TiO2 composite powders deposited on cement-based materials: Rhodamine B removal and the bonding mechanism. Constr. Build. Mater. 2020, 241, 118124. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.; Hou, P.; Yang, P.; Cheng, X.; Huang, S. Rhodamine B Removal of TiO2@SiO2 Core-Shell Nanocomposites Coated to Buildings. Crystals 2020, 10, 80. [Google Scholar] [CrossRef]
- Luna, M.; Delgado, J.; Romero, I.; Montini, T.; Gil, M.A.; Martínez-López, J.; Fornasiero, P.; Mosquera, M. Photocatalytic TiO2 nanosheets-SiO2 coatings on concrete and limestone: An enhancement of de-polluting and self-cleaning properties by nanoparticle design. Constr. Build. Mater. 2022, 338, 127349. [Google Scholar] [CrossRef]
- Khannyra, S.; Luna, M.; Gil, M.A.; Addou, M.; Mosquera, M.J. Self-cleaning durability assessment of TiO2/SiO2 photocatalysts coated concrete: Effect of indoor and outdoor conditions on the photocatalytic activity. Build. Environ. 2022, 211, 108743. [Google Scholar] [CrossRef]
- Xia, X.; Liu, J.; Liu, Y.; Lei, Z.; Han, Y.; Zheng, Z.; Yin, J. Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings 2023, 13, 224. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Macchia, A.; La Russa, M.F.; Mazza, L.; Urzì, C.; De Leo, F.; Barberio, M.; Crisci, G.M. Marine Antifouling for Underwater Archaeological Sites: TiO2 and Ag-Doped TiO2. Int. J. Photoenergy 2013, 2013, 251647. [Google Scholar] [CrossRef]
- Roveri, M.; Gherardi, F.; Goidanich, S.; Gulotta, D.; Castelvetro, V.; Fischer, R.; Winandy, L.; Weber, J.; Toniolo, L. Self-cleaning and antifouling nanocomposites for stone protection: Properties and performances of stone-nanomaterial systems. IOP Conf. Ser. Mater. Sci. Eng. 2018, 364, 012070. [Google Scholar] [CrossRef]
- Van der Werf, I.D.; Ditaranto, N.; Picca, R.A.; Sportelli, M.C.; Sabbatini, L. Development of a novel conservation treatment of stone monuments with bioactive nanocomposites. Herit. Sci. 2015, 3, 29. [Google Scholar] [CrossRef]
- Aldosari, M.A.; Darwish, S.S.; Adam, M.A.; Elmarzugi, N.A.; Ahmed, S.M. Using ZnO nanoparticles in fungal inhibition and self-protection of exposed marble columns in historic sites. Archaeol. Anthr. Sci. 2019, 11, 3407–3422. [Google Scholar] [CrossRef]
- Dakal, T.C.; Cameotra, S.S. Microbially induced deterioration of architectural heritages: Routes and mechanisms involved. Environ. Sci. Eur. 2012, 24, 36. [Google Scholar] [CrossRef]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, M.A.; Cantoral, J.M.; Mosquera, M.J. CuO/SiO2 nanocomposites: A multifunctional coating for application on building stone. Mater. Des. 2017, 114, 364–372. [Google Scholar] [CrossRef]
- Zarzuela, R.; Gil, M.A.; Carretero, J.; Carbú, M.; Cantoral, J.M.; Mosquera, M.J. Development of a novel engineered stone containing a CuO/SiO2 nanocomposite matrix with biocidal properties. Constr. Build. Mater. 2021, 303, 124459. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, S.; Dehsheikh, H.G.; Boroujerdnia, M. MgO–CaO–Cr2O3 composition as a novel refractory brick: Use of Cr2O3 nanoparticles. Boletín Soc. Española Cerámica Vidr. 2017, 56, 83–89. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Bahadur, J.; Agrawal, S.; Panwar, V.; Parveen, A.; Pal, K. Antibacterial properties of silver doped TiO2 nanoparticles synthesized via sol-gel technique. Macromol. Res. 2016, 24, 488–493. [Google Scholar] [CrossRef]
- Chobba, M.B.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Bouaziz, J.; De Leo, F.; Licchelli, M. Ag-TiO2/PDMS nanocomposite protective coatings: Synthesis, characterization, and use as a self-cleaning and antimicrobial agent. Prog. Org. Coat. 2021, 158, 106342. [Google Scholar] [CrossRef]
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Sacchi, D.; Bouaziz, J.; De Leo, F.; Urzi, C.; Licchelli, M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability 2021, 13, 11033. [Google Scholar] [CrossRef]
- NORMAL 20/85, Interventi Conservativi: Progettazione Esecuzione e Valutazione Preventiva, Italian Standard, 1–20 Pages, CNR, Rome, Italy. 1985. Available online: https://moodle2.units.it/pluginfile.php/230506/mod_resource/content/1/Interventi%20di%20conservazione.pdf (accessed on 26 December 2023).
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Bouaziz, J.; Salhi, R.; De Leo, F.; Urzì, C.; Licchelli, M. Silver-Doped TiO2-PDMS Nanocomposite as a Possible Coating for the Preservation of Serena Stone: Searching for Optimal Application Conditions. Heritage 2022, 5, 3411–3426. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Rojas, M.; Mosquera, M.J. Ag–SiO2–TiO2 nanocomposite coatings with enhanced photoactivity for self-cleaning application on building materials. Appl. Catal. B Environ. 2015, 178, 144–154. [Google Scholar] [CrossRef]
- Luna, M.; Mosquera, M.J.; Vidal, H.; Gatica, J.M. Au-TiO2/SiO2 photocatalysts for building materials: Self-cleaning and de-polluting performance. Build. Environ. 2019, 164, 106347. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral Properties and Relaxation Dynamics of Surface Plasmon Electronic Oscillations in Gold and Silver Nanodots and Nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Zarzuela, R.; Moreno-Garrido, I.; Gil, M.A.; Mosquera, M.J. Effects of surface functionalization with alkylalkoxysilanes on the structure, visible light photoactivity and biocidal performance of Ag-TiO2 nanoparticles. Powder Technol. 2021, 383, 381–395. [Google Scholar] [CrossRef]
- Khannyra, S.; Mosquera, M.; Addou, M.; Gil, M. Cu-TiO2/SiO2 photocatalysts for concrete-based building materials: Self-cleaning and air de-pollution performance. Constr. Build. Mater. 2021, 313, 125419. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.; Ortiz, P. Silver/dioxide titanium nanocomposites as biocidal treatments on limestones. Ge Conserv. 2017, 1, 141–148. [Google Scholar]
- Luna, M.; Delgado, J.J.; Gil, M.L.A.; Mosquera, M.J. TiO2-SiO2 Coatings with a Low Content of AuNPs for Producing Self-Cleaning Building Materials. Nanomaterials 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Zaderenko, A.; Sayagués, M.; Ortiz, R.; Ortiz, P. Synergy achieved in silver-TiO2 nanocomposites for the inhibition of biofouling on limestone. Build. Environ. 2018, 141, 80–90. [Google Scholar] [CrossRef]
- Truppi, A.; Luna, M.; Petronella, F.; Falcicchio, A.; Giannini, C.; Comparelli, R.; Mosquera, M.J. Photocatalytic Activity of TiO2/AuNRs–SiO2 Nanocomposites Applied to Building Materials. Coatings 2018, 8, 296. [Google Scholar] [CrossRef]
- Kapridaki, C.; Xynidis, N.; Vazgiouraki, E.; Kallithrakas-Kontos, N.; Maravelaki-Kalaitzaki, P. Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials 2019, 12, 1847. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Use of Au/N-TiO2/SiO2 photocatalysts in building materials with NO depolluting activity. J. Clean. Prod. 2020, 243, 118633. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, A.; Cantoral, J.; Mosquera, M.J. Incorporation of functionalized Ag-TiO2NPs to ormosil-based coatings as multifunctional biocide, superhydrophobic and photocatalytic surface treatments for porous ceramic materials. Surf. Interfaces 2021, 25, 101257. [Google Scholar] [CrossRef]
- Khannyra, S.; Gil, M.L.A.; Addou, M.; Mosquera, M.J. Dye decomposition and air de-pollution performance of TiO2/SiO2 and N-TiO2/SiO2 photocatalysts coated on Portland cement mortar substates. Environ. Sci. Pollut. Res. 2022, 29, 63112–63125. [Google Scholar] [CrossRef]
- Mu, B.; Ying, X.; Petropoulos, E.; He, S. Preparation of AgCl/ZnO nano-composite for effective antimicrobial protection of stone-made building elements. Mater. Lett. 2021, 285, 129143. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, Photocatalytic, and Antifungal Properties of MgO, ZnO and Zn/Mg Oxide Nanoparticles for the Protection of Calcareous Stone Heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef]
- Gómez-Cornelio, S.; Ortega-Morales, O.; Morón-Ríos, A.; Reyes-Estebanez, M.; De la Rosa-García, S. Changes in fungal community composition of biofilms on limestone across a chronosequence in Campeche, Mexico. Acta Bot. Mex. 2016, 117, 59–77. [Google Scholar] [CrossRef]
- Weththimuni, M.; Chobba, M.B.; Tredici, I.; Licchelli, M. Polydimethylsiloxane (PDMS)/ZrO2-Doped ZnO Nanocomposites as Protective Coatings for Stone Materials. In Proceedings of the 2020 IMEKO TC-4 International Conference on Metrology for Archaeology and Cultural Heritage, Tento, Italy, 16 October 2020. [Google Scholar]
- Yang, Y.-F.; Wang, W.-M.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Assessing human exposure risk and lung disease burden posed by airborne silver nanoparticles emitted by consumer spray products. Int. J. Nanomed. 2019, 14, 1687–1703. [Google Scholar] [CrossRef]
- Simko, M.; Fiedeler, U.; Gazsó, A.; Nentwich, M. How Nanoparticles Enter the Human Body and Their Effects There. 2011. Available online: http://hw.oeaw.ac.at/nanotrust-dossier (accessed on 26 December 2023).
- European Commission. Guidance on the Protection of the Health and Safety of Workers from the Potential Risks Related to the Nanomaterials at Work. Guidance for Employers and Health and Safety Practitioners. 2013. Available online: https://ec.europa.eu/social/BlobServlet?docId=13087&langId=en%2 (accessed on 26 December 2023).
- Bian, Y.; Kim, K.; Ngo, T.; Kim, I.; Bae, O.-N.; Lim, K.-M.; Chung, J.-H. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part. Fibre Toxicol. 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Auclair, J.; Gagné, F. Shape-Dependent Toxicity of Silver Nanoparticles on Freshwater Cnidarians. Nanomaterials 2022, 12, 3107. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.; Lee, H.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.Y.; Ng, K.W.; Loo, S.C.J. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Al-Mutairi, E.M.; De, S.K. Impact of Nanomaterials on Health and Environment. Arab. J. Sci. Eng. 2013, 38, 457–477. [Google Scholar] [CrossRef]
- Löndahl, J.; Möller, W.; Pagels, J.H.; Kreyling, W.G.; Swietlicki, E.; Schmid, O. Measurement Techniques for Respiratory Tract Deposition of Airborne Nanoparticles: A Critical Review. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 229–254. [Google Scholar] [CrossRef]
- Sonwani, S.; Madaan, S.; Arora, J.; Suryanarayan, S.; Rangra, D.; Mongia, N.; Vats, T.; Saxena, P. Inhalation Exposure to Atmospheric Nanoparticles and Its Associated Impacts on Human Health: A Review. Front. Sustain. Cities 2021, 3, 690444. [Google Scholar] [CrossRef]
- Zhu, S.; Gong, L.; Li, Y.; Xu, H.; Gu, Z.; Zhao, Y. Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.w.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, M.; Grigoriadis, N.; Lagoudaki, R.; Tascos, N.; Milonas, I.; Lopez-Campos, J.L.; Calero-Acuña, C.; Lopez-Ramirez, C.; Abad-Arranz, M.; et al. The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system. Nanomedicine 2017, 13, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-Based Nanoparticles: Role of Reactive Oxygen Species in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Jayvadan, P.; Champavat, V. Toxicity of Nanomaterials on the Gastrointestinal Tract. In Biointeractions of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2014; pp. 259–284. [Google Scholar] [CrossRef]
- Gautam, A.; Singh, D.; Vijayaraghavan, R. Dermal exposure of nanoparticles: An understanding. J. Cell Tissue Res. 2011, 11, 2703–2708. [Google Scholar]
- Rafique, T.; Naseem, S.; Usmani, T.H.; Bashir, E.; Khan, F.A.; Bhanger, M.I. Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J. Hazard. Mater. 2009, 171, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.M.; Murugan, K.; Madhiyazhagan, P.; Kovendan, K.; Amerasan, D.; Chandramohan, B.; Dinesh, D.; Suresh, U.; Nicoletti, M.; Alsalhi, M.S.; et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol. Res. 2016, 115, 751–759. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Kim, S.-H.; Chung, I.M. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: Physiological and molecular level responses of in vitro grown plants. Acta Physiol. Plant. 2014, 36, 2947–2958. [Google Scholar] [CrossRef]
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyen, C.; Camacho-Chab, J.C.; Pereañez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Herit. Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- Suman, T.Y.; Li, W.-G.; Pei, D.-S. Chapter 5—Toxicity of metal oxide nanoparticles. In Nanotoxicity; Rajendran, S., Mukherjee, A., Nguyen, T.A., Godugu, C., Shukla, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–123. [Google Scholar] [CrossRef]
- Girigoswami, K. Toxicity of Metal Oxide Nanoparticles. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 99–122. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.-E.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoScience 2021, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.T.; Jacobsen, N.R.; Mortensen, A.; Szarek, J.; Jackson, P.; Madsen, A.M.; Jensen, K.; Koponen, I.K.; Brunborg, G.; Gützkow, K.B.; et al. Nanotitanium dioxide toxicity in mouse lung is reduced in sanding dust from paint. Part. Fibre Toxicol. 2012, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Element Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Hilal, S.M.; Mohmed, A.S.; Barry, N.M.; Ibrahim, M.H. Entomotoxicity of TiO2 and ZnO Nanoparticles Against Adults Tribolium Castaneum (Herbest) (Coleoptera: Tenebrionidae). IOP Conf. Ser. Earth Environ. Sci. 2021, 910, 012088. [Google Scholar] [CrossRef]
- Amara, S.; Ben Slama, I.; Omri, K.; EL Ghoul, J.; EL Mir, L.; Ben Rhouma, K.; Abdelmelek, H.; Sakly, M. Effects of nanoparticle zinc oxide on emotional behavior and trace elements homeostasis in rat brain. Toxicol. Ind. Health 2013, 31, 1202–1209. [Google Scholar] [CrossRef]
- Shrivastava, R.; Raza, S.; Yadav, A.; Kushwaha, P.; Flora, S.J.S. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem. Toxicol. 2014, 37, 336–347. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Y.; Yin, S.; Song, B.; Wei, L.; Chen, L.; Shao, L. Zinc oxide nanoparticles induce toxic responses in human neuroblastoma SHSY5Y cells in a size-dependent manner. Int. J. Nanomed. 2017, 12, 8085–8099. [Google Scholar] [CrossRef]
- Chen, T.-H.; Lin, C.-C.; Meng, P.-J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140. [Google Scholar] [CrossRef]
- Mansouri, E.; Khorsandi, L.; Orazizadeh, M.; Jozi, Z. Dose-dependent hepatotoxicity effects of Zinc oxide nanoparticles. Nanomed. J. 2015, 2, 273–282. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper Oxide Nanoparticles Stimulate the Immune Response and Decrease Antioxidant Defense in Mice After Six-Week Inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper Oxide Nanoparticles Induce Oxidative DNA Damage and Cell Death via Copper Ion-Mediated P38 MAPK Activation in Vascular Endothelial Cells. Int. J. Nanomed. 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Adefarati, T.; Bansal, R. Integration of renewable distributed generators into the distribution system: A review. IET Renew. Power Gener. 2016, 10, 873–884. [Google Scholar] [CrossRef]
- Bugata, L.S.P.; Venkata, P.P.; Gundu, A.R.; Fazlur, R.M.; Reddy, U.A.; Kumar, J.M.; Mekala, V.R.; Bojja, S.; Mahboob, M. Acute and subacute oral toxicity of copper oxide nanoparticles in female albino Wistar rats. J. Appl. Toxicol. 2019, 39, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.M.; Abdel-Rehiem, E.S.; Farghali, A.A.; Khidr, F.K.; Abdul-Hamid, M. Ameliorative role of nanocurcumin against the toxicological effects of novel forms of Cuo as nanopesticides: A comparative study. Environ. Sci. Pollut. Res. 2023, 30, 26270–26291. [Google Scholar] [CrossRef]
- De Jong, W.H.; De Rijk, E.; Bonetto, A.; Wohlleben, W.; Stone, V.; Brunelli, A.; Badetti, E.; Marcomini, A.; Gosens, I.; Cassee, F.R. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology 2018, 13, 50–72. [Google Scholar] [CrossRef]
- Gomes, S.I.; Murphy, M.; Nielsen, M.T.; Kristiansen, S.M.; Amorim, M.J.; Scott-Fordsmand, J.J. Cu-nanoparticles ecotoxicity—Explored and explained? Chemosphere 2015, 139, 240–245. [Google Scholar] [CrossRef]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Obando, V.; Rubio, L.; Marcos, R. Genotoxicity of copper oxide nanoparticles in Drosophila melanogaster. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 791, 1–11. [Google Scholar] [CrossRef]
- Gosens, I.; Cassee, F.R.; Zanella, M.; Manodori, L.; Brunelli, A.; Costa, A.L.; Bokkers, B.G.H.; De Jong, W.H.; Brown, D.; Hristozov, D.; et al. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 2016, 10, 1084–1095. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants: Phytotoxicity of copper oxide nanoparticles and arsenic. Environ. Toxicol. Chem. 2017, 37, 11–20. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Rempel, S.; Ogliari, A.J.; Bonfim, E.; Duarte, G.W.; Riella, H.G.; Silva, L.L.; Mello, J.M.M.; Baretta, C.R.D.M.; Fiori, M.A. Toxicity effects of magnesium oxide nanoparticles: A brief report. Matéria 2020, 25, e-1287. [Google Scholar] [CrossRef]
- Mangalampalli, B.; Dumala, N.; Grover, P. Acute oral toxicity study of magnesium oxide nanoparticles and microparticles in female albino Wistar rats. Regul. Toxicol. Pharmacol. 2017, 90, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Zhang, X.; Fan, G.-J.; Que, Y.; Xue, F.; Liu, Y.-H. Toxicity Effects and Mechanisms of MgO Nanoparticles on the Oomycete Pathogen Phytophthora infestans and Its Host Solanum tuberosum. Toxics 2022, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, N.; Naghsh, N.; Karimi, A.; Salavati, H. In vivo Toxicity Investigation of Magnesium Oxide Nanoparticles in Rat for Environmental and Biomedical Applications. Iran. J. Biotechnol. 2019, 17, e1543. [Google Scholar] [CrossRef] [PubMed]
- Vadiraj, K.T.; Shivaraju, H.P. Metal Oxide-Based Nanomaterials for the Treatment of Industrial Dyes and Colorants. In Advanced Oxidation Processes in Dye-Containing Wastewater; Springer Nature: Singapore, 2022; pp. 233–251. [Google Scholar] [CrossRef]
- Cruz-Yusta, M.; Sánchez, M.; Sánchez, L. Metal Oxide Nanomaterials for Nitrogen Oxides Removal in Urban Environments. In Tailored Functional Oxide Nanomaterials: From Design to Multi-Purpose Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 229–276. [Google Scholar] [CrossRef]
- Labanni, A.; Nasir, M.; Arief, S. Research progress and prospect of copper oxide nanoparticles with controllable nanostructure, morphology, and function via green synthesis. Mater. Today Sustain. 2023, 24, 100526. [Google Scholar] [CrossRef]
- Farani, M.R.; Farsadrooh, M.; Zare, I.; Gholami, A.; Akhavan, O. Green Synthesis of Magnesium Oxide Nanoparticles and Nanocomposites for Photocatalytic Antimicrobial, Antibiofilm and Antifungal Applications. Catalysts 2023, 13, 642. [Google Scholar] [CrossRef]














| Nanomaterials Composition | Material Size | Substrate | Experiment Conditions | Obtained Results | Ref. |
|---|---|---|---|---|---|
| TiO2 | - | Travertine, a natural limestone |
|
| [124] |
| SiO2-TiO2 | 40–100 nm | Greek marbles from Naxo |
|
| [125] |
| TiO2 | Anatase: 3–6 nm Brookit: 5–10 nm | Pietra di Lecce |
|
| [126] |
| TiO2 | 10–15 nm | -Apuan marble (AM) -Ajarte limestone (AL) |
|
| [127] |
| SiO2-TiO2-PDMS | 25 nm | Modica stone |
|
| [128] |
| TiO2 | - | Trani stone: Low porosity (2%) |
|
| [129] |
| SiO2-TiO2 | 20 nm | Portland cement (WC) paste |
|
| [130] |
| TiO2@SiO2 Core-Shell | Shell thicknesses: 1.95–6.13 nm | White Portland cement past |
|
| [131] |
| MgO/TiO2 | 24 to 56 nm | -Red bricks -Gypsum mortars |
|
| [111] |
| TiO2 nanosheets-SiO2 | Thickness ≈6.5 nm | Capri limestone (open porosity: 9%–12%) |
|
| [132] |
| TiO2-SiO2 | 100 nm | Concrete |
|
| [133] |
| TiO2-SiO2-PDMS | - | Cement mortar |
|
| [134] |
| Nanomaterials Composition | Particles Size | Substrate | Obtained Results | Ref. |
|---|---|---|---|---|
| Ag-TiO2 | 0.1–1 μm | Limestone slabs from quarry of Utrera (Seville, Spain) |
| [156] |
| Au-SiO2-TiO2 | 10–30 nm | Limestone with an open porosity of around 12% |
| [157] |
| Ag-TiO2 | 94–234 nm | Carbonate stone mainly composed from calcite (95%–98%) and quartz (2%–5%) with open porosity of 10% |
| [158] |
| TiO2/Au-SiO2 | 20–25 nm | Fossiliferous limestone (calcite 98.5%, α-quartz 1.5%) |
| [159] |
| Fe-TiO2 | 13–21 nm | Limestone |
| [160] |
| Au/N-TiO2/SiO2 | 5.2 nm |
|
| [161] |
| Ag-TiO2 | 12.5 ± 4.3 nm | Fossiliferous limestone, extracted from Cabra (Cordoba, Spain), composed of calcite (~100%) and with a 6% porosity |
| [162] |
| N-TiO2/SiO2 | 50–200 nm | Portland cement mortar |
| [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Urzi, C.; Licchelli, M. Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures. Coatings 2024, 14, 203. https://doi.org/10.3390/coatings14020203
Ben Chobba M, Weththimuni ML, Messaoud M, Urzi C, Licchelli M. Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures. Coatings. 2024; 14(2):203. https://doi.org/10.3390/coatings14020203
Chicago/Turabian StyleBen Chobba, Marwa, Maduka L. Weththimuni, Mouna Messaoud, Clara Urzi, and Maurizio Licchelli. 2024. "Recent Advances in the Application of Metal Oxide Nanomaterials for the Conservation of Stone Artefacts, Ecotoxicological Impact and Preventive Measures" Coatings 14, no. 2: 203. https://doi.org/10.3390/coatings14020203









