Aluminum Guefoams Coated with Reduced Graphene Oxide: A Corrosion Protection Study
Abstract
1. Introduction
- The charge transfer between metal surfaces and graphene induces a potential barrier at the graphene/metal interface. This polarization impedes the electron transfer processes that are necessary for corrosion.
- The conductivity of graphene materials provides an alternative pathway for transporting electrons generated at the anode away from cathodic sites; in this way, corrosion reactions can be reduced.
- The structure of graphene materials provides a physical barrier to the diffusion of gases, vapors, and ions.
2. Materials and Methods
2.1. Reagents
2.2. Aluminum Guefoam Fabrication
2.3. Electrochemical Synthesis of RGO Coatings
2.4. FESEM Characterization
2.5. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR)
2.6. Corrosion Tests and Electrochemical Measurements
2.7. Determination of Al Concentration after Corrosion Tests
2.8. Adsorption–Desorption Isotherms
3. Results
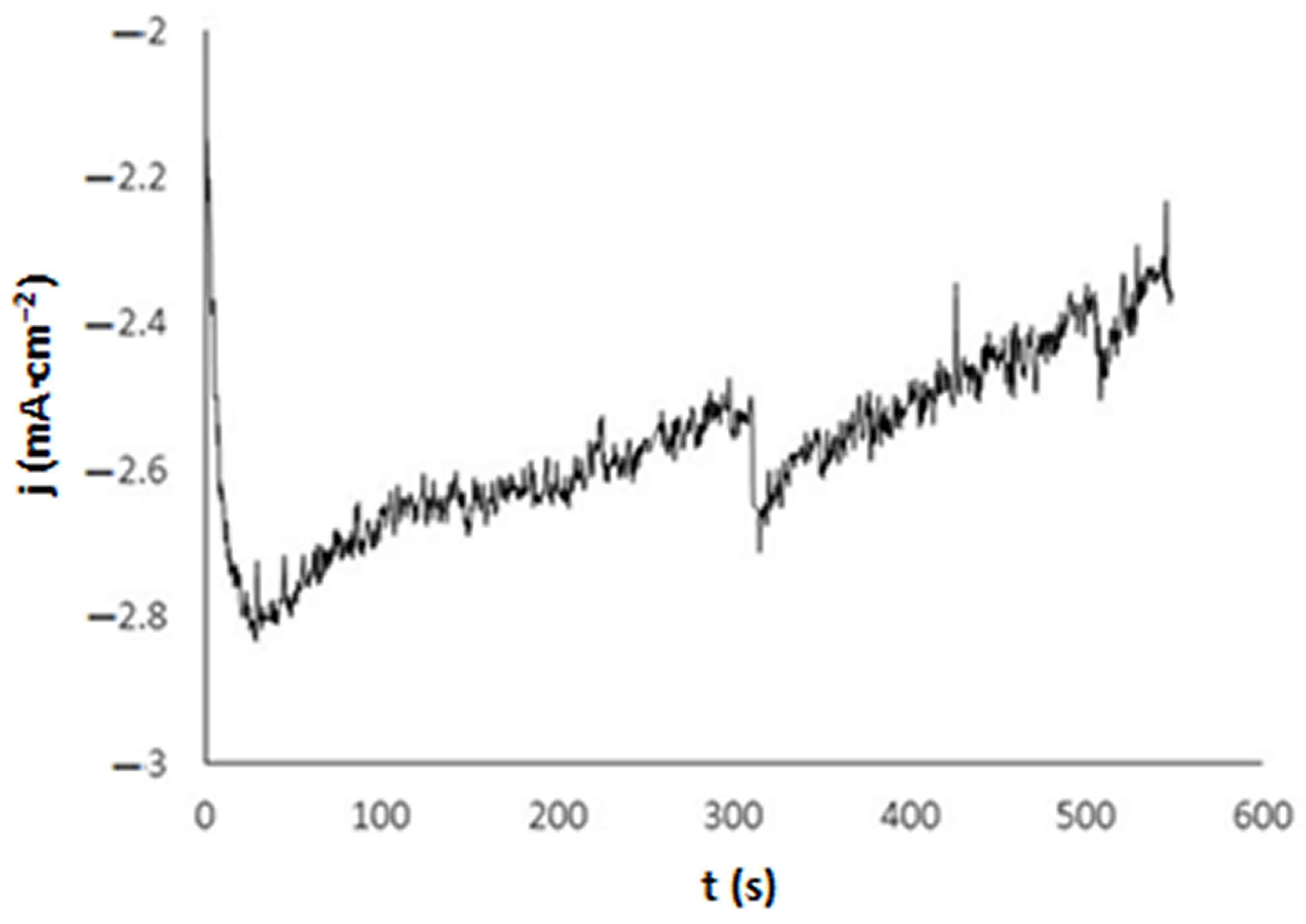
3.1. Electrochemical Synthesis
3.2. FESEM Characterization
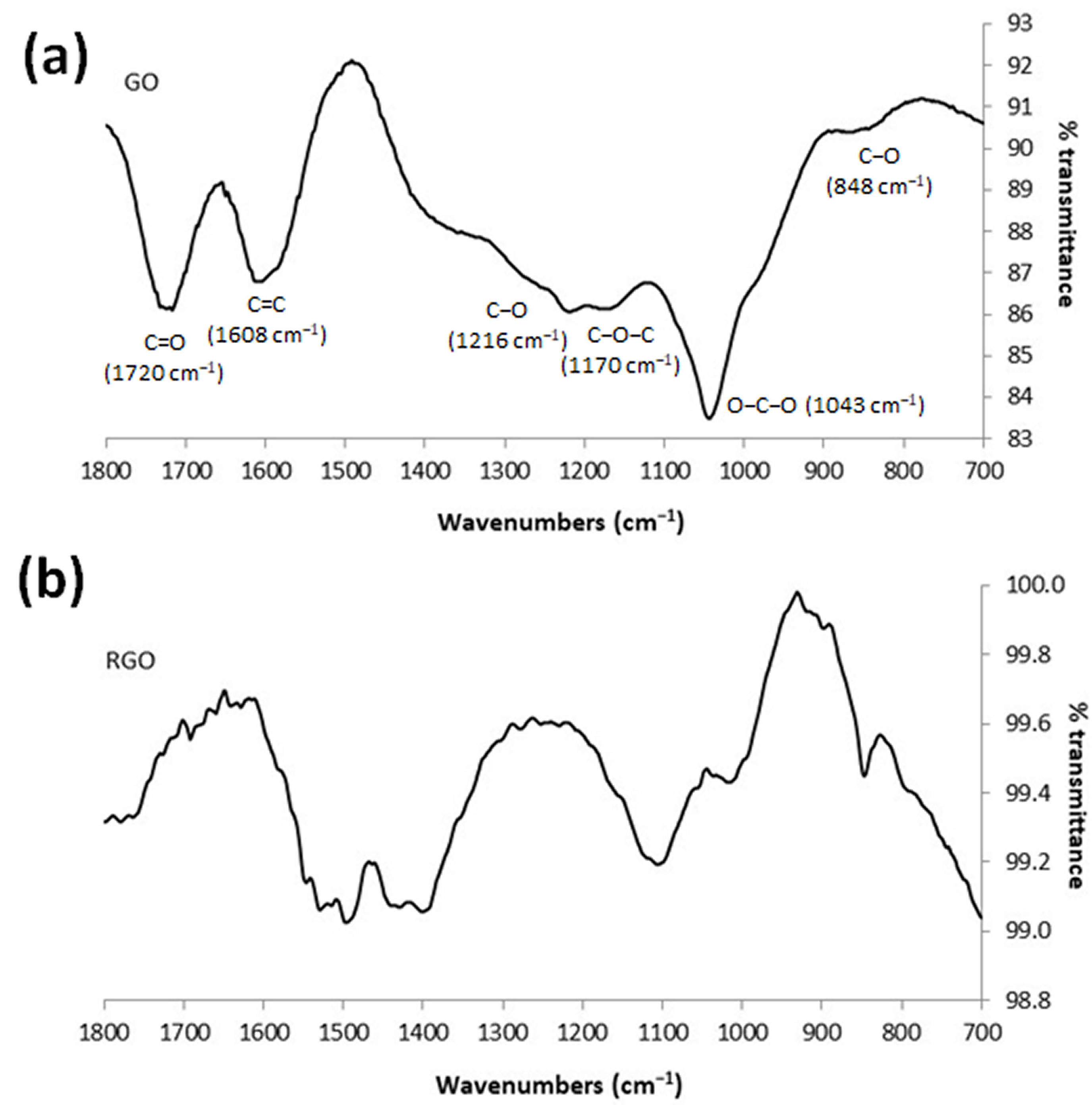
3.3. FTIR-ATR Characterization
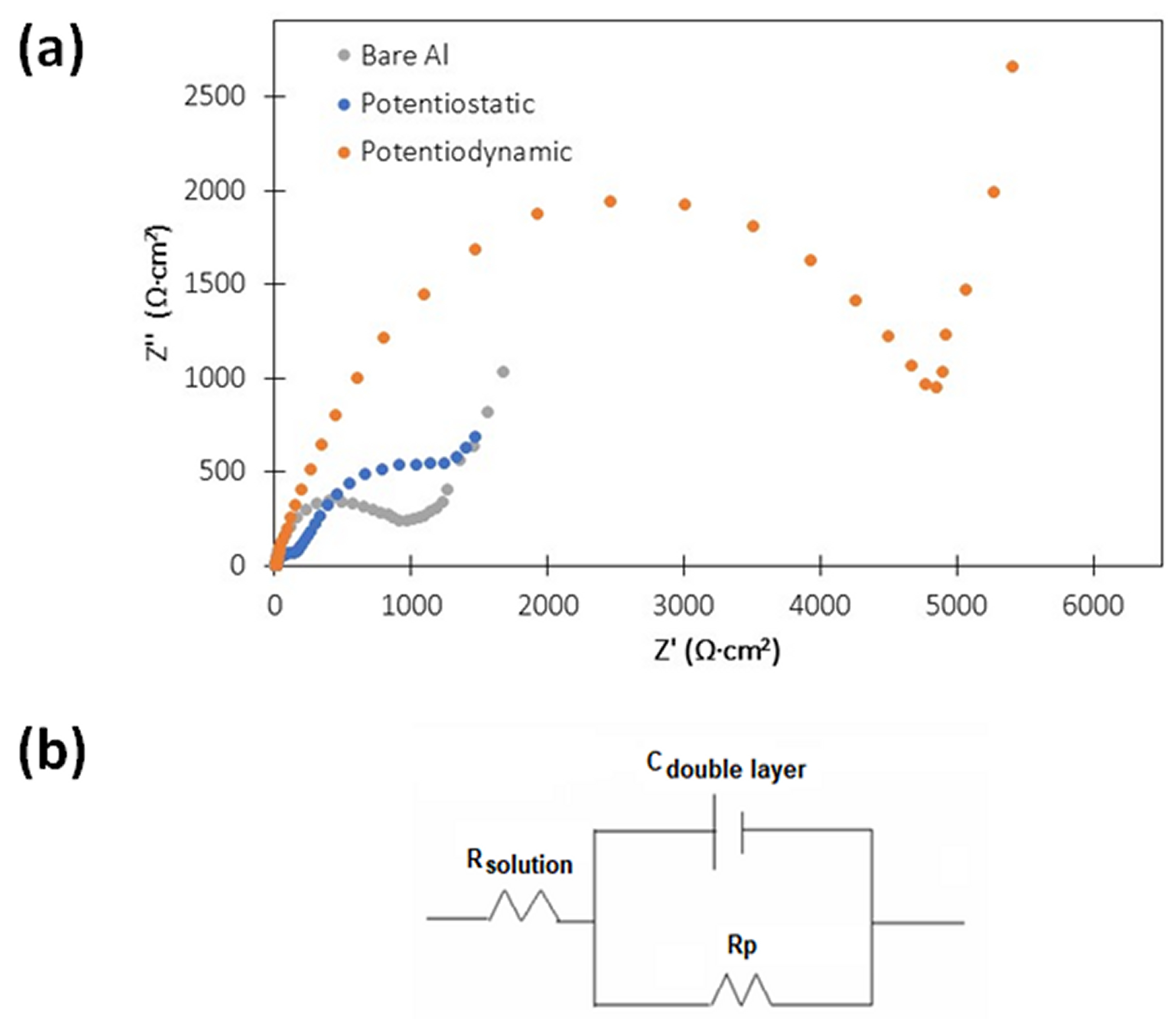
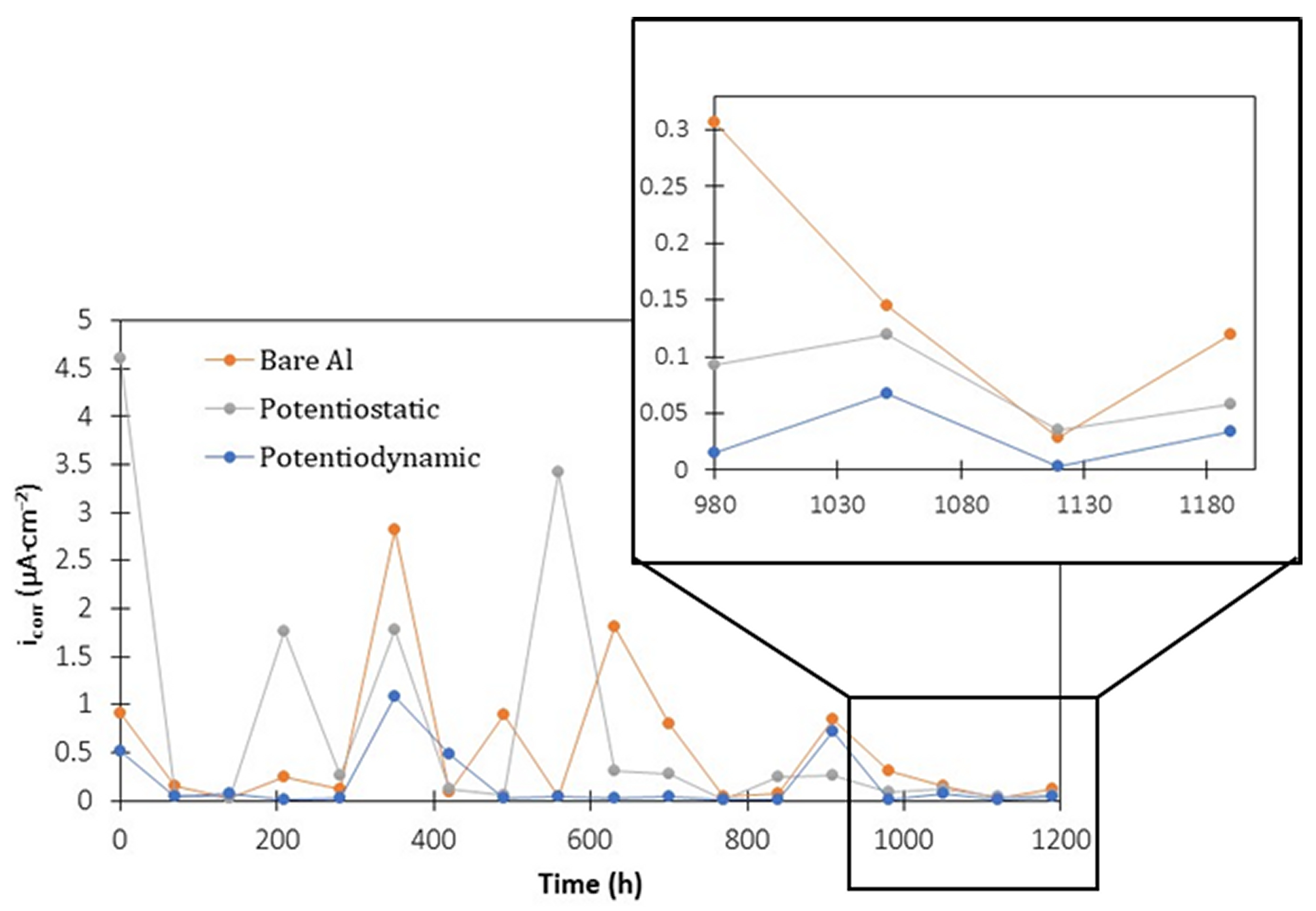
3.4. Electrochemical Measurements during Corrosion Tests
3.5. FESEM and EDX Characterization after Corrosion Tests
3.6. Measurement of Al Concentration
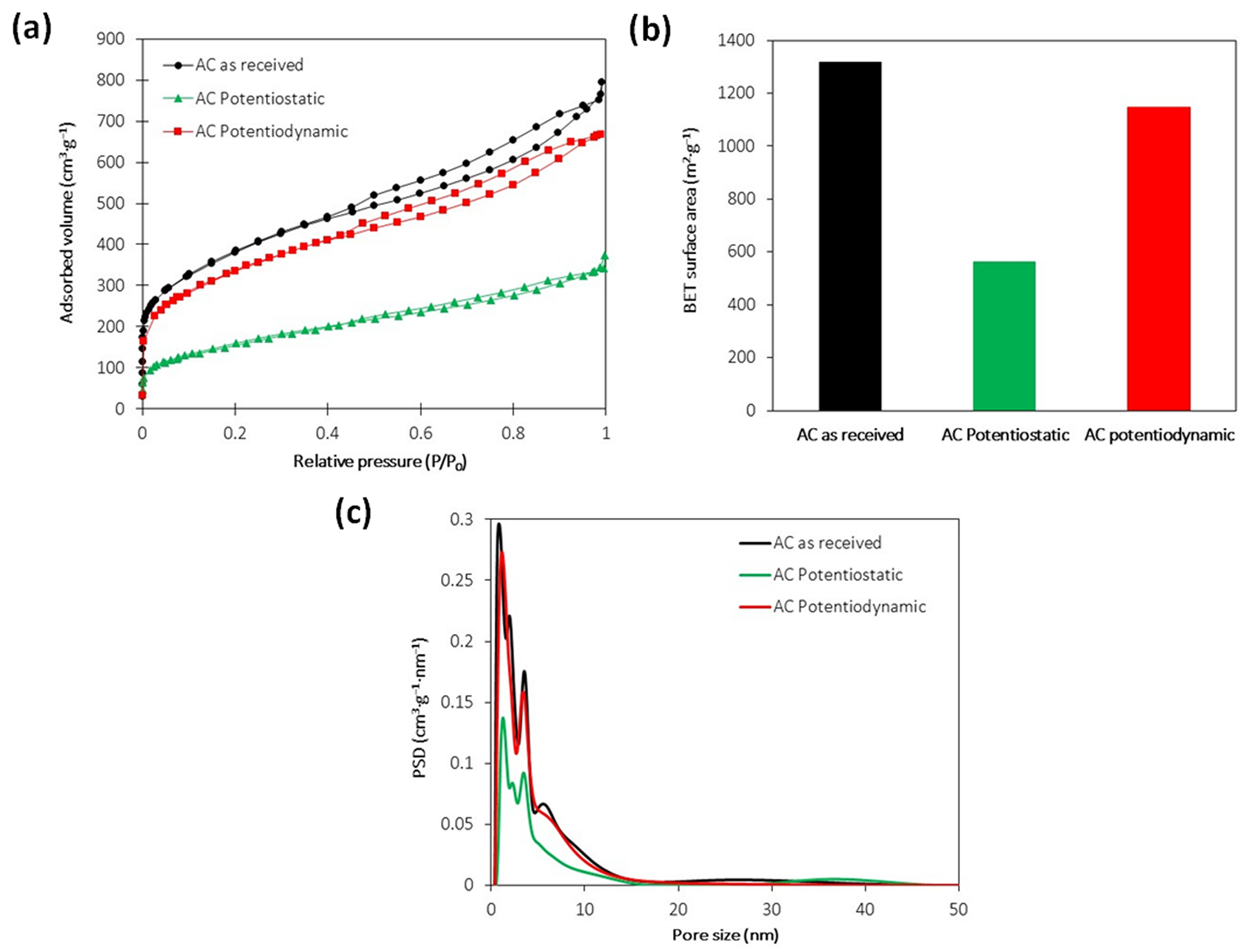
3.7. Surface Characterization of the Guest-Phase Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina-Jordá, J.M. Materiales Espumados de Poro Interconectado con Fases Huésped, Procedimiento Para la Preparación de Dichos Materiales y usos de los Mismos. Spanish Patent P201730890, 6 August 2019. [Google Scholar]
- Molina-Jordá, J.M. Open-pore foam materials with guest phases, procedure for the preparation of these materials and uses thereof. US Patent US11623277B2, 11 April 2023. [Google Scholar]
- Molina-Jordá, J.M. Highly adsorptive and magneto-inductive guefoams (multifunctional guest-containing foams) for enhanced energy-efficient preconcentration and management of VOCs. ACS Appl. Mat. Interfaces 2020, 12, 11702–11712. [Google Scholar] [CrossRef] [PubMed]
- Molina-Jordá, J.M. Magneto-inductive open-cell cellular carbon composites with activated carbon as guest phase: Guefoams for energy-efficient VOCs management. Ceram. Int. 2022, 48, 17440–17448. [Google Scholar] [CrossRef]
- Maiorano, L.P.; Chaparro-Garnica, C.Y.; García, E.B.; Lozano-Castello, D.; Bueno-Lopez, A.; Molina-Jorda, J.M. Guefoams (guest-containing foams) as novel heterogeneous catalysts: Preparation, characterization and proof-of-concept testing for CO2 methanation. Mater. Des. 2022, 217, 110619. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fatemi, V.; Fang, S.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Jarillo-Herrero, P. Unconventional superconductivity in magic-angle graphene superlattices. Nature 2018, 556, 43–50. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 598–4810. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, M.; Pumera, M. Graphene and its electrochemistry–an update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Yuan, B.; Jahan, S.; Saleh, M.S.; Guo, Z.; Guellman, A.J.; Panat, R. Breaking the barrier to biomolecule limit-of-detection via 3D printed multi-length-scale graphene-coated electrodes. Nat. Commun. 2021, 12, 7077. [Google Scholar] [CrossRef]
- Burkholder, M.B.; Rahman, F.B.A.; Chandler Jr, E.H.; Regalbuto, J.R.; Gupton, B.F.; Tengco, J.M.M. Metal supported graphene catalysis: A review on the benefits of nanoparticular supported specialty sp2 carbon catalysts on enhancing the activities of multiple chemical transformations. Carbon Trends 2022, 9, 100196. [Google Scholar] [CrossRef]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A review of graphene-based semiconductors for photocatalytic degradation of pollutants in wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef]
- Fei, H.; Dong, J.; Chen, D.; Hu, T.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241. [Google Scholar] [CrossRef]
- Seelajaroen, H.; Bakandritsos, A.; Otyepka, M.; Zboril, R.; Sariciftci, N.S. Immobilized enzymes on graphene as nanobiocatalyst. ACS App. Mater. Interfaces 2020, 12, 250–259. [Google Scholar] [CrossRef]
- Molina, J.; Cases, F.; Moretto, L.M. Graphene-based materials for the electrochemical determination of hazardous ions. Anal. Chim. Acta 2016, 946, 9–39. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Umar, K.; Ibrahim, M.N.M. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide–A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.V.; Fleer, N.A.; Davidson, R.D.; Banerjee, S. Graphene Coatings for the Corrosion Protection of Base Metals. In Graphene Technology: From Laboratory to Fabrication, 1st ed.; Soroush, N., Stephen, R.W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 155–176. [Google Scholar] [CrossRef]
- Yu, F.; Camilli, L.; Wang, T.; Mackenzie, D.M.; Curioni, M.; Akid, R.; Bøggild, P. Complete long-term corrosion protection with chemical vapor deposited graphene. Carbon 2018, 132, 78–84. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Schiller, T.; Medhekar, N.; Birbilis, N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 2012, 56, 1–4. [Google Scholar] [CrossRef]
- Othman, N.H.; Ismail, M.C.; Mustapha, M.; Sallih, N.; Kee, K.E.; Jaal, R.A. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Progr. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- López-Oyama, A.B.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Onofre-Bustamante, E.; Gámez-Corrales, R.; Cayetano-Castro, N. Electrochemical alternative to obtain reduced graphene oxide by pulse potential: Effect of synthesis parameters and study of corrosion properties. Diam. Relat. Mater. 2018, 88, 167–188. [Google Scholar] [CrossRef]
- Zhang, H.H.; Liu, Y.W.; Bian, H.; Zhang, Y.; Yang, Z.N.; Zhang, Z.; Chen, Y. Electrodeposition of silane/reduced graphene oxide nanocomposite on AA2024-T3 alloy with enhanced corrosion protection, chemical and mechanical stability. J. Alloys Compd. 2022, 911, 165058. [Google Scholar] [CrossRef]
- Clavilier, J. The role of anion on the electrochemical behaviour of a {111} platinum surface; an unusual splitting of the voltammogram in the hydrogen region. J. Electroanal. Chem. 1979, 107, 211–216. [Google Scholar] [CrossRef]
- Hong, B.J.; Compton, O.C.; An, Z.; Eryazici, I.; Nguyen, S.T. Successful stabilization of graphene oxide in electrolyte solutions: Enhancement of biofunctionalization and cellular uptake. ACS Nano 2012, 6, 63–73. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X.; Zhu, G.; Yuan, A.; Zhang, J.; Ji, Z.; Qiu, D. The influence of wrinkling in reduced graphene oxide on their adsorption and catalytic properties. Carbon 2013, 60, 157–168. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, B.; Yang, Y.; Wu, N.; Sui, Z.; Ban, Q.; Wu, L.; Liu, W.; Liu, J.; Zeng, Z. Ultralight aerogel sphere composed of nanocellulose-derived carbon nanofiber and graphene for excellent electromagnetic wave absorption. Nano Res. 2023, 16, 7931–7940. [Google Scholar] [CrossRef]
- Garrote de Barros, N.; Cardoso Gonzaga Neto, A.; Bitencourt Vaccioli, K.; Vallejo Angulo, H.R.; Gondim de Andrade e Silva, L.; Toffoli, S.M.; Sanches Valera, T. Graphene Oxide: A Comparison of Reduction Methods. J. Carbon Res. 2023, 9, 73. [Google Scholar] [CrossRef]
- Khurshid, F.; Jeyavelan, M.; Sterlin Leo Hudson, M.; Nagarajan, S. Organic semiconductor/graphene oxide composites as a photo-anode for photo-electrochemical applications. RSC Adv. 2018, 8, 35959–35965. [Google Scholar] [CrossRef]
- An, S.; Zeng, Q.; Li, W.; Fortner, J. A graphene oxide Cookbook: Exploring chemical and colloidal properties as a function of synthesis parameters. J. Colloid Interface Sci. 2021, 588, 725–736. [Google Scholar] [CrossRef]
- Noell, P.J.; Schindelholz, E.J.; Melia, M.A. Revealing the growth kinetics of atmospheric corrosion pitting in aluminum via in situ microtomography. NPJ Mater. Degrad. 2020, 4, 32. [Google Scholar] [CrossRef]
- Energy Table for EDS Analysis. Available online: https://www.unamur.be/services/microscopie/sme-documents/Energy-20table-20for-20EDS-20analysis-1.pdf (accessed on 29 May 2023).






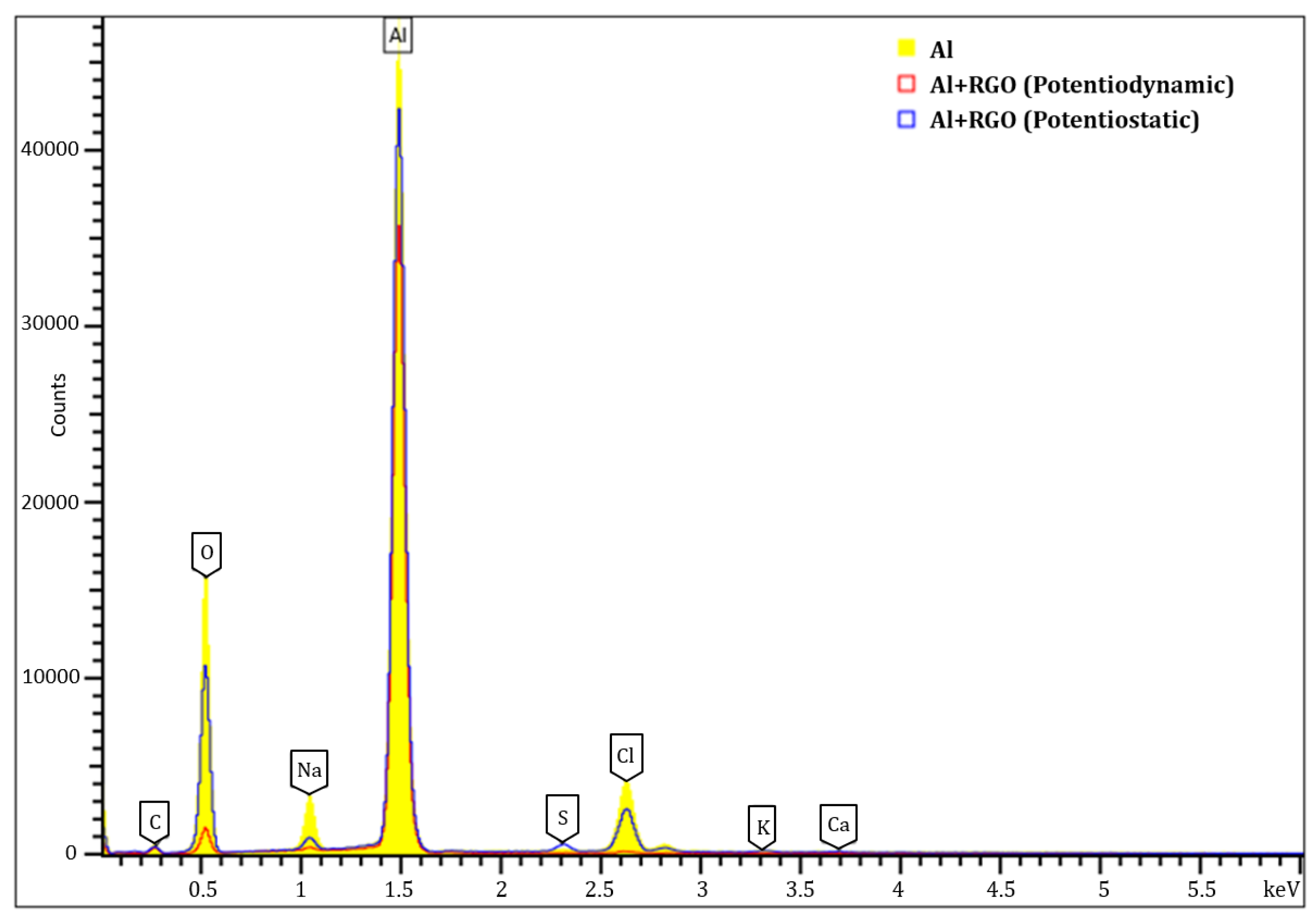
| Bare Al | Potentiostatic | Potentiodynamic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | RP (Ω·cm2) | icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance | RP (Ω·cm2) | icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance | RP (Ω·cm2) | icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance |
| 0 | 4956 | 0.91 | 3138 | 1367 | 4.61 | 1307 | 8680 | 0.51 | 7565 |
| 24 | 6982 | 0.64 | 6851 | 1856 | 3.40 | 2329 | 12,723 | 0.35 | 7174 |
| 48 | 952 | 4.72 | 779 | 6732 | 0.94 | 4337 | 6530 | 0.67 | 7225 |
| 72 | 1175 | 3.83 | 1227 | 22,661 | 0.28 | 3807 | 5945 | 0.74 | 6953 |
| 96 | 1754 | 2.56 | 1238 | 3193 | 1.97 | 6005 | 7793 | 0.56 | 6613 |
| 168 | 1359 | 3.31 | 1408 | 1414 | 4.46 | 2324 | 7268 | 0.60 | 6256 |
| 216 | 1509 | 2.98 | 1248 | 74,426 | 0.08 | 2371 | 10,129 | 0.43 | 6579 |
| 336 | 1359 | 3.31 | 1217 | 785 | 8.03 | 2392 | 5583 | 0.79 | 6460 |
| 504 | 1518 | 2.96 | 1139 | 1504 | 4.19 | 2292 | 5736 | 0.77 | 5916 |
| 672 | 1459 | 3.08 | 1239 | 1214 | 5.19 | 2262 | 5314 | 0.83 | 5374 |
| Bare Al | Potentiostatic | Potentiodynamic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | RP (Ω·cm2) | Icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance | RP (Ω·cm2) | Icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance | RP (Ω·cm2) | Icorr (µA·cm−2) | RP (Ω·cm2) Calculated by Impedance |
| 0 | 4956 | 0.91 | 3138 | 1367 | 4.61 | 1307 | 8680 | 0.51 | 7565 |
| 70 | 29,181 | 0.15 | 22,757 | 106,656 | 0.06 | 20,548 | 120,187 | 0.04 | 52,895 |
| 140 | 144,084 | 0.03 | 4390 | 259,920 | 0.02 | 13,668 | 60,982 | 0.07 | 80,413 |
| 210 | 18,438 | 0.24 | 252,858 | 3570 | 1.77 | 26,448 | 1,152,668 | <0.01 | 141,236 |
| 280 | 40,298 | 0.11 | 54,148 | 24,511 | 0.26 | 28,088 | 259,692 | 0.02 | 758,574 |
| 350 | 1590 | 2.83 | 4788 | 3545 | 1.78 | 3955 | 4055 | 1.08 | 17,131 |
| 420 | 49,752 | 0.09 | 8474 | 52,235 | 0.12 | 80,641 | 9080 | 0.48 | 41,346 |
| 490 | 5078 | 0.89 | 88,614 | 118,456 | 0.05 | 39,956 | 189,530 | 0.02 | 38,134 |
| 560 | 123,582 | 0.04 | 265,159 | 1841 | 3.42 | 7071 | 123,855 | 0.04 | 119,139 |
| 630 | 2481 | 1.81 | 4930 | 20,554 | 0.31 | 84,969 | 182,235 | 0.02 | 156,499 |
| 700 | 5618 | 0.80 | 9682 | 22,240 | 0.28 | 111,394 | 109,207 | 0.04 | 79,958 |
| 770 | 104,970 | 0.04 | 168,100 | 518,245 | 0.01 | 209,262 | 1,993,478 | <0.01 | 808,269 |
| 840 | 62,531 | 0.07 | 85,425 | 26,356 | 0.24 | 11,777 | 359,013 | 0.01 | 389,538 |
| 910 | 5353 | 0.84 | 6121 | 24,762 | 0.25 | 20,183 | 6137 | 0.72 | 10,615 |
| 980 | 14,629 | 0.31 | 14,556 | 68,499 | 0.09 | 82,236 | 302,063 | 0.01 | 213,676 |
| 1050 | 31,026 | 0.14 | 31,528 | 52,986 | 0.12 | 33,714 | 65,287 | 0.07 | 77,680 |
| 1120 | 155,587 | 0.03 | 156,499 | 179,598 | 0.04 | 267,665 | 1,822,628 | <0.01 | 1,264,290 |
| 1190 | 37,519 | 0.12 | 61,734 | 108,251 | 0.06 | 116,178 | 130,643 | 0.03 | 107,066 |
| Aluminum Concentration (mg·L−1) | |
|---|---|
| Bare Al | 145 |
| Al + RGO (potentiostatic) | 70 |
| Al + RGO (potentiodynamic) | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, R.; Molina, J.; Bonastre, J.; Maiorano, L.P.; Molina, J.M.; Cases, F. Aluminum Guefoams Coated with Reduced Graphene Oxide: A Corrosion Protection Study. Coatings 2024, 14, 201. https://doi.org/10.3390/coatings14020201
Rodrigo R, Molina J, Bonastre J, Maiorano LP, Molina JM, Cases F. Aluminum Guefoams Coated with Reduced Graphene Oxide: A Corrosion Protection Study. Coatings. 2024; 14(2):201. https://doi.org/10.3390/coatings14020201
Chicago/Turabian StyleRodrigo, Rubén, Javier Molina, José Bonastre, Lucila P. Maiorano, José M. Molina, and Francisco Cases. 2024. "Aluminum Guefoams Coated with Reduced Graphene Oxide: A Corrosion Protection Study" Coatings 14, no. 2: 201. https://doi.org/10.3390/coatings14020201
APA StyleRodrigo, R., Molina, J., Bonastre, J., Maiorano, L. P., Molina, J. M., & Cases, F. (2024). Aluminum Guefoams Coated with Reduced Graphene Oxide: A Corrosion Protection Study. Coatings, 14(2), 201. https://doi.org/10.3390/coatings14020201








