Removing Aged Polymer Coatings from Porous Stone Surfaces Using the Gel Cleaning Method
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Chemicals and Lithotypes
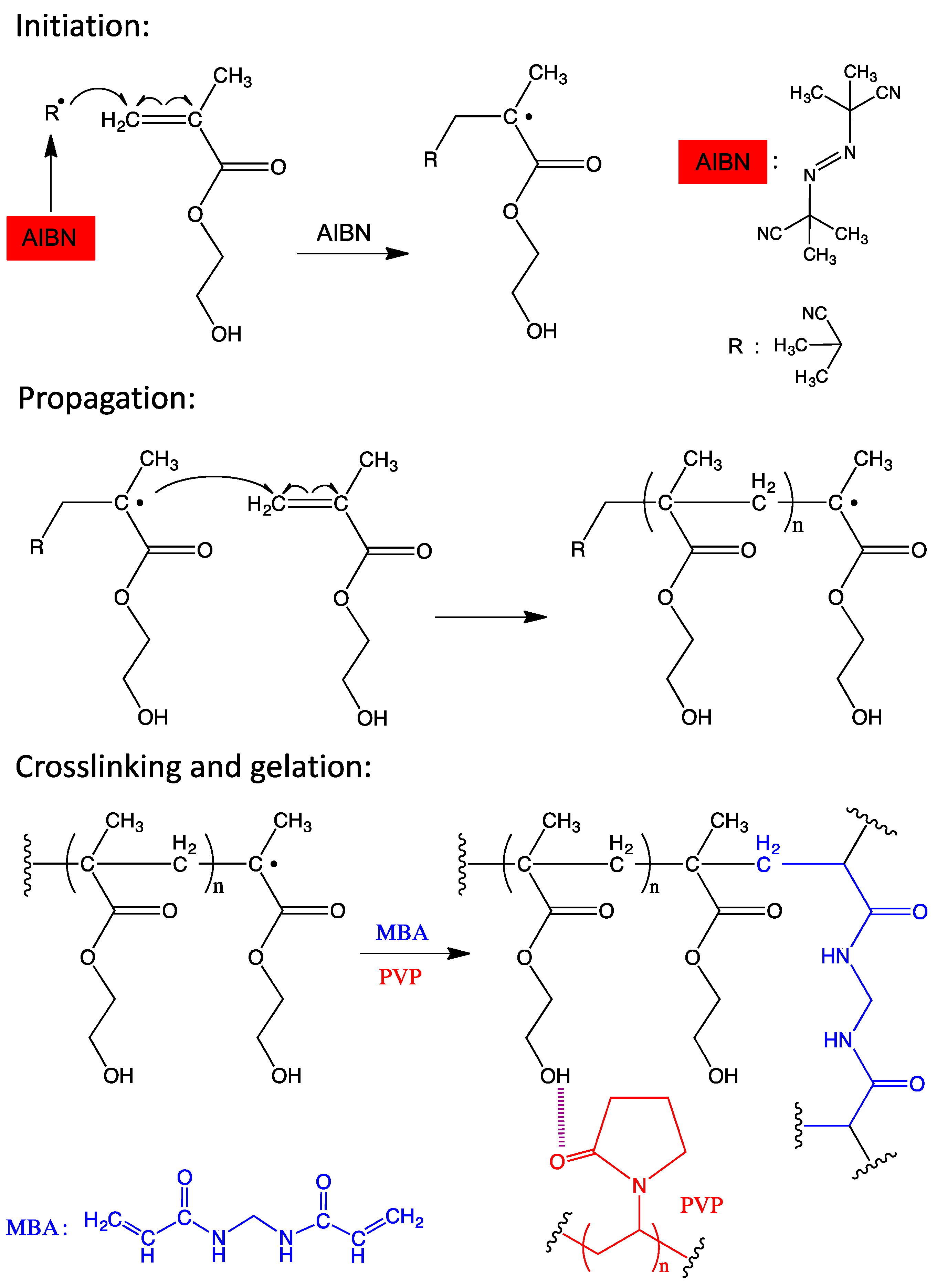
2.2. Synthesis of the Hydrogel
2.3. Characterization of the Synthesized Hydrogel
2.3.1. Gel Content
2.3.2. Water Content and Release Capacity
2.3.3. Rheological Properties
2.4. Preparation of Coated Stone Specimens
2.5. Preparation of Nano-Structured Emulsions and Application of the Emulsion-Loaded Gels
2.6. Instrumental Techniques
3. Results and Discussion
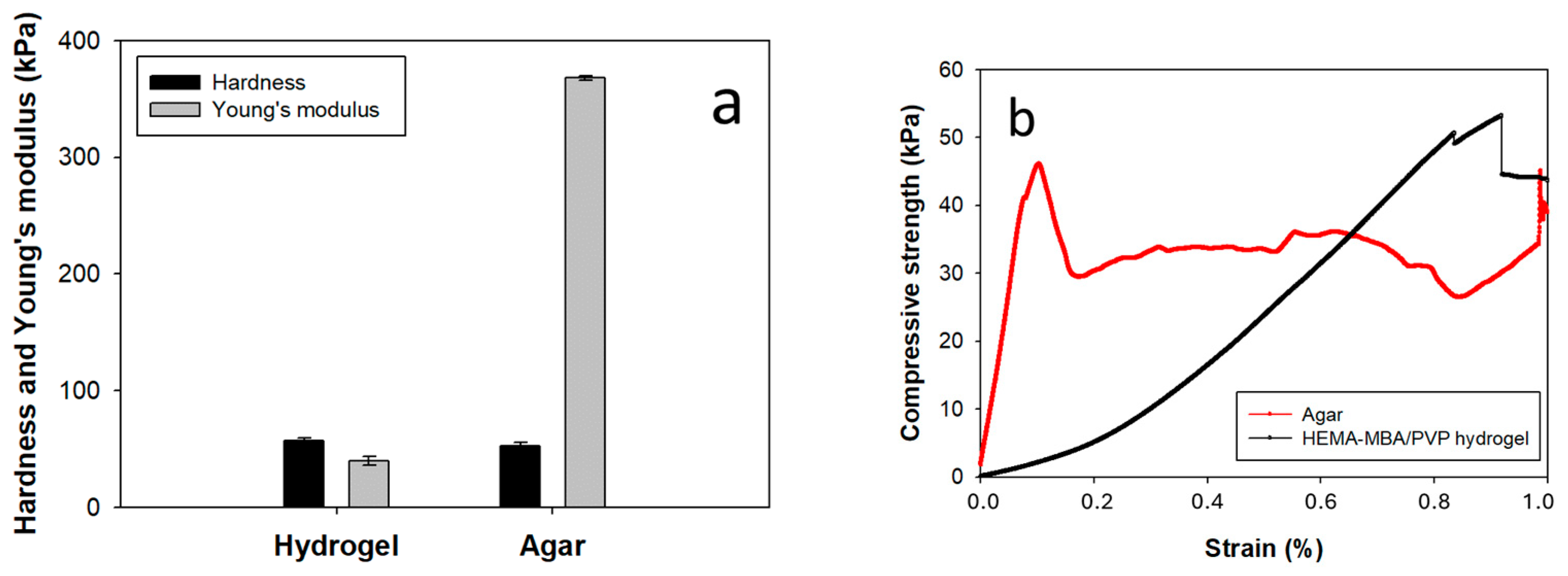
3.1. Characterization of the Synthesized Hydrogel
3.2. Characterization of the Aged Polymer (Paraloid B-72) Coating
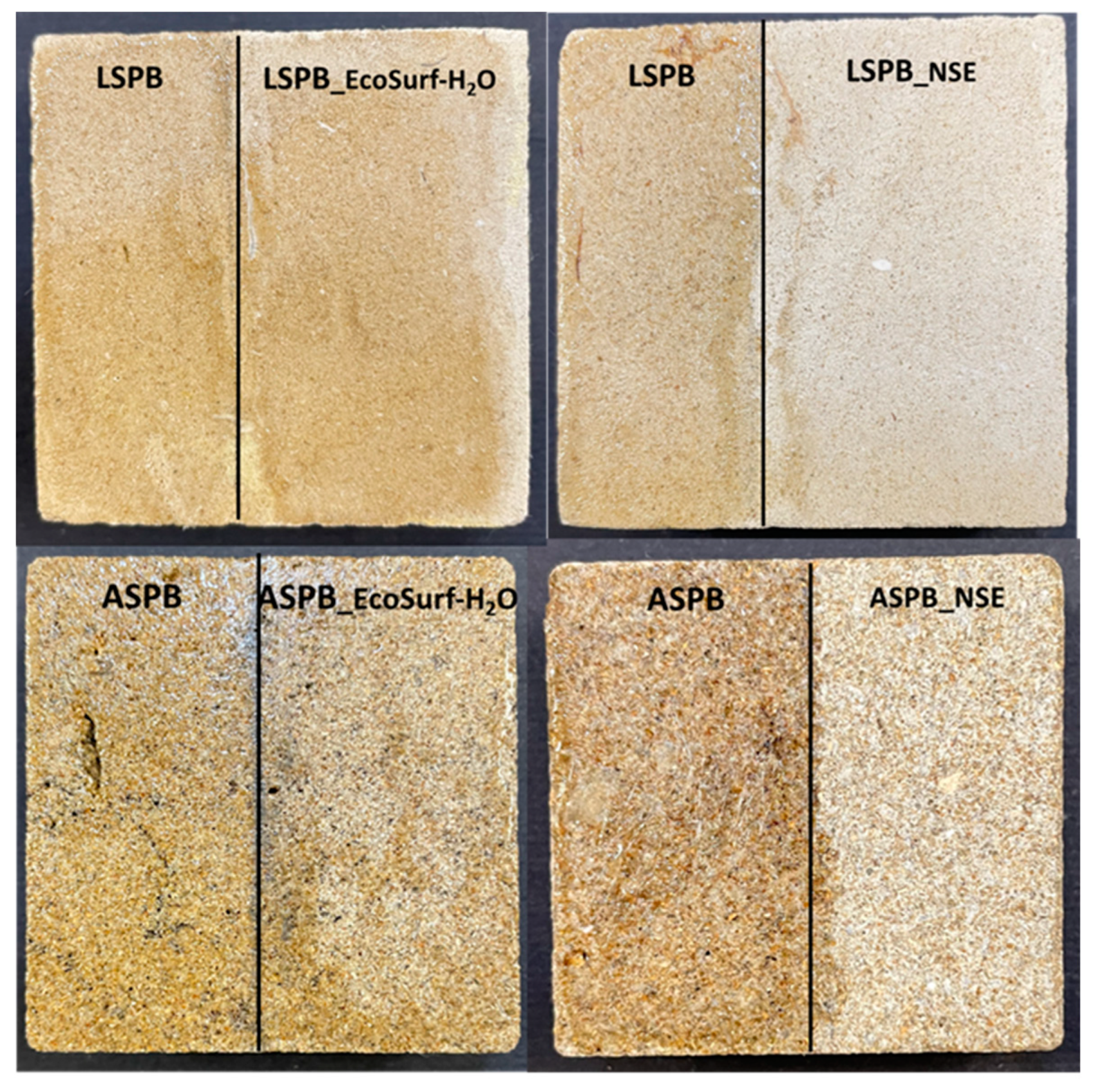
3.3. Cleaning Performances of the Loaded Hydrogels
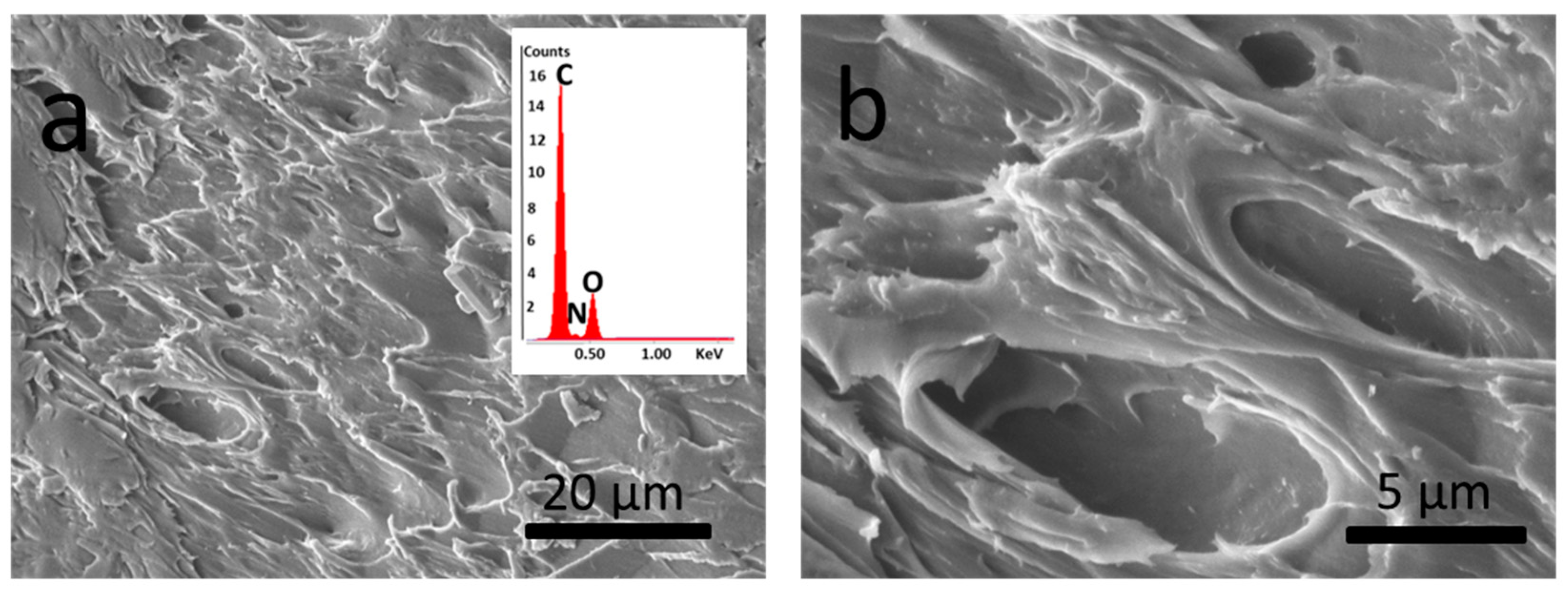
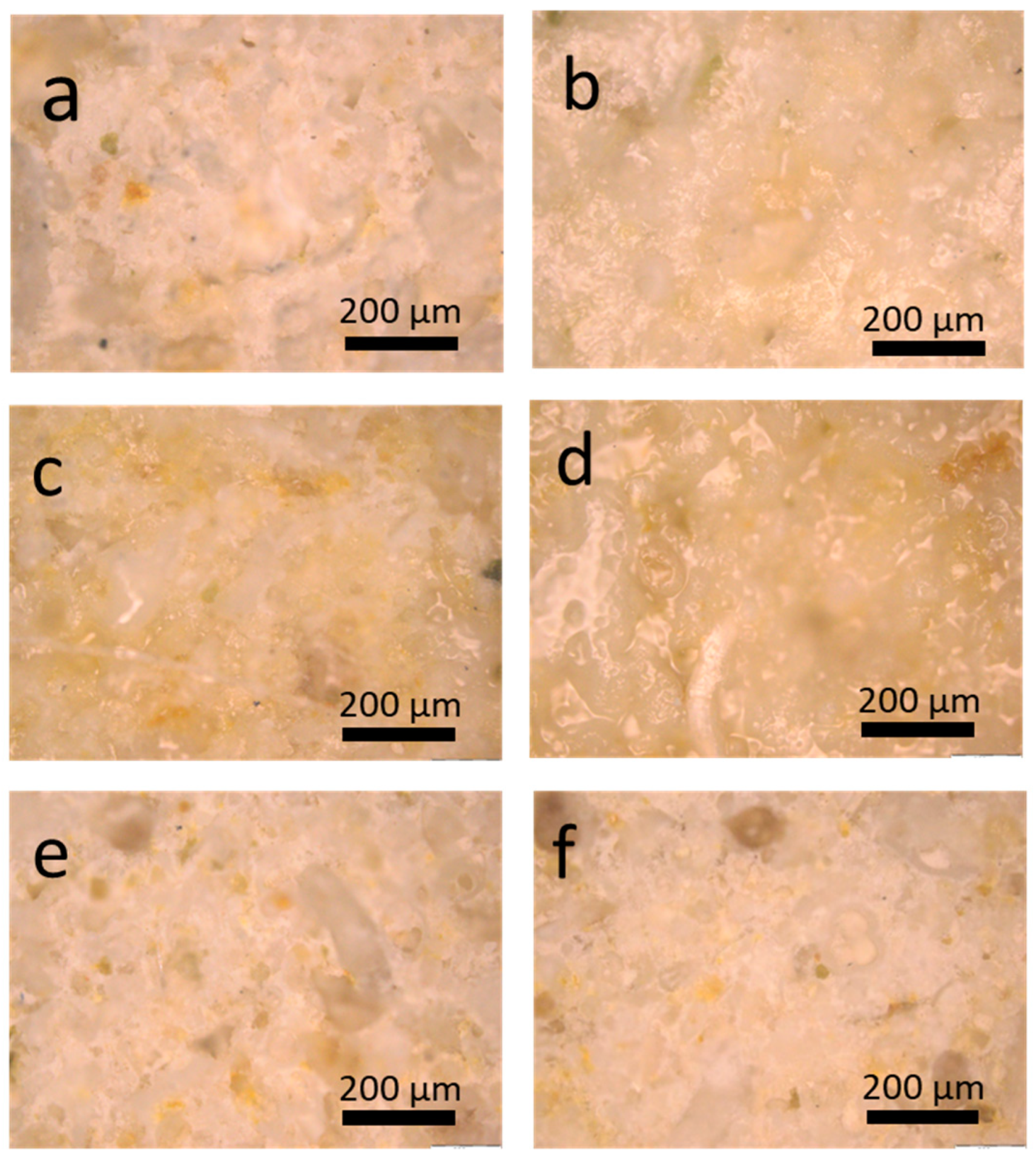
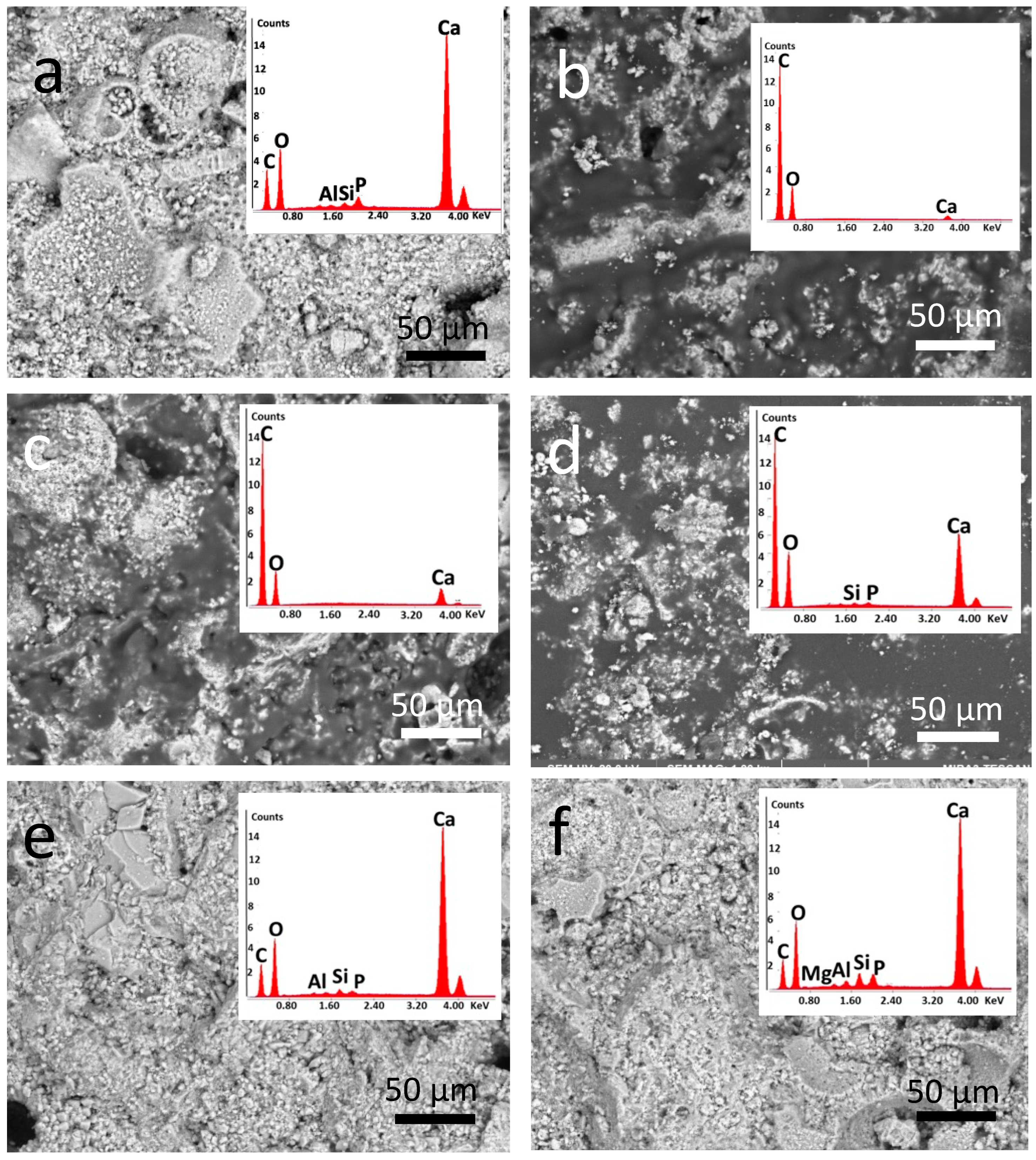
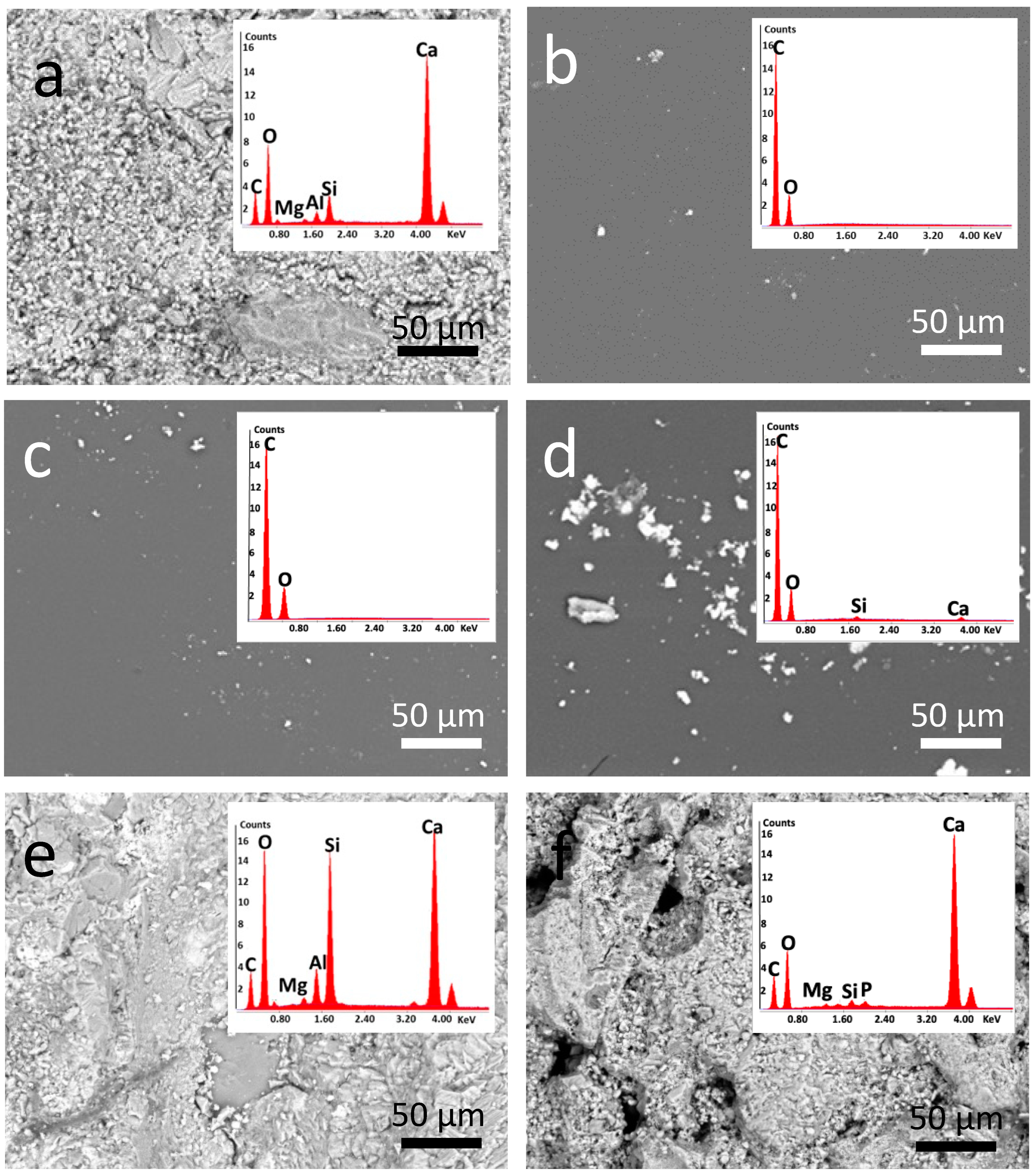
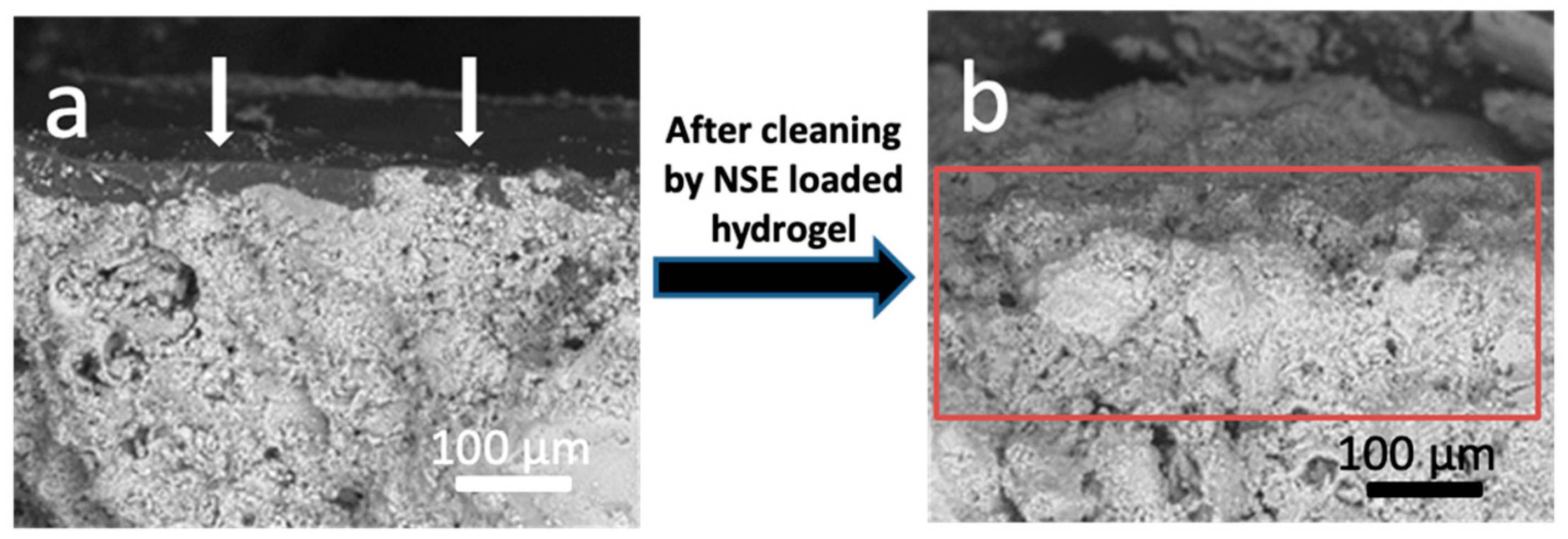
3.3.1. Optical and Electron Microscopy
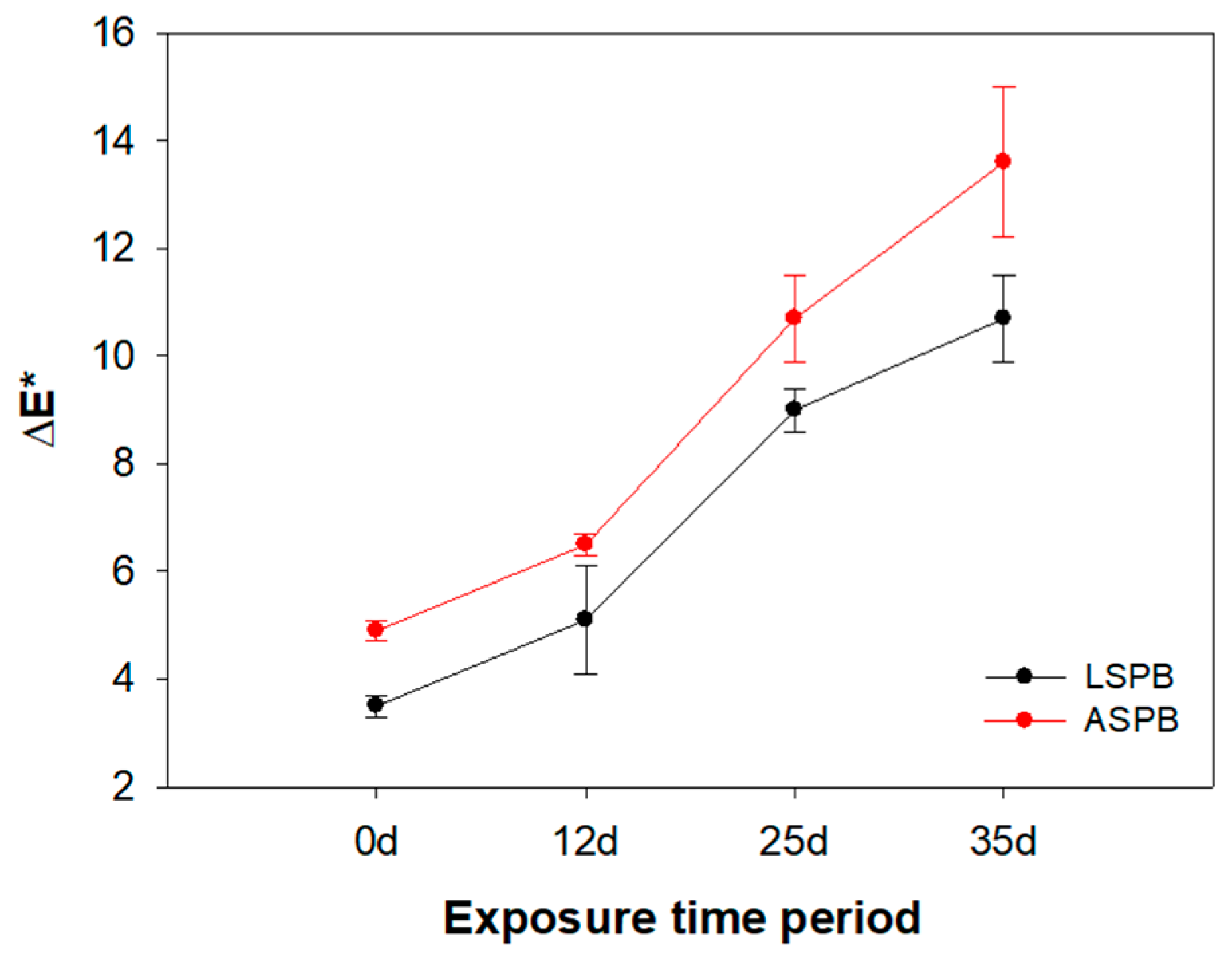
3.3.2. Chromatic and Wettability Measurements
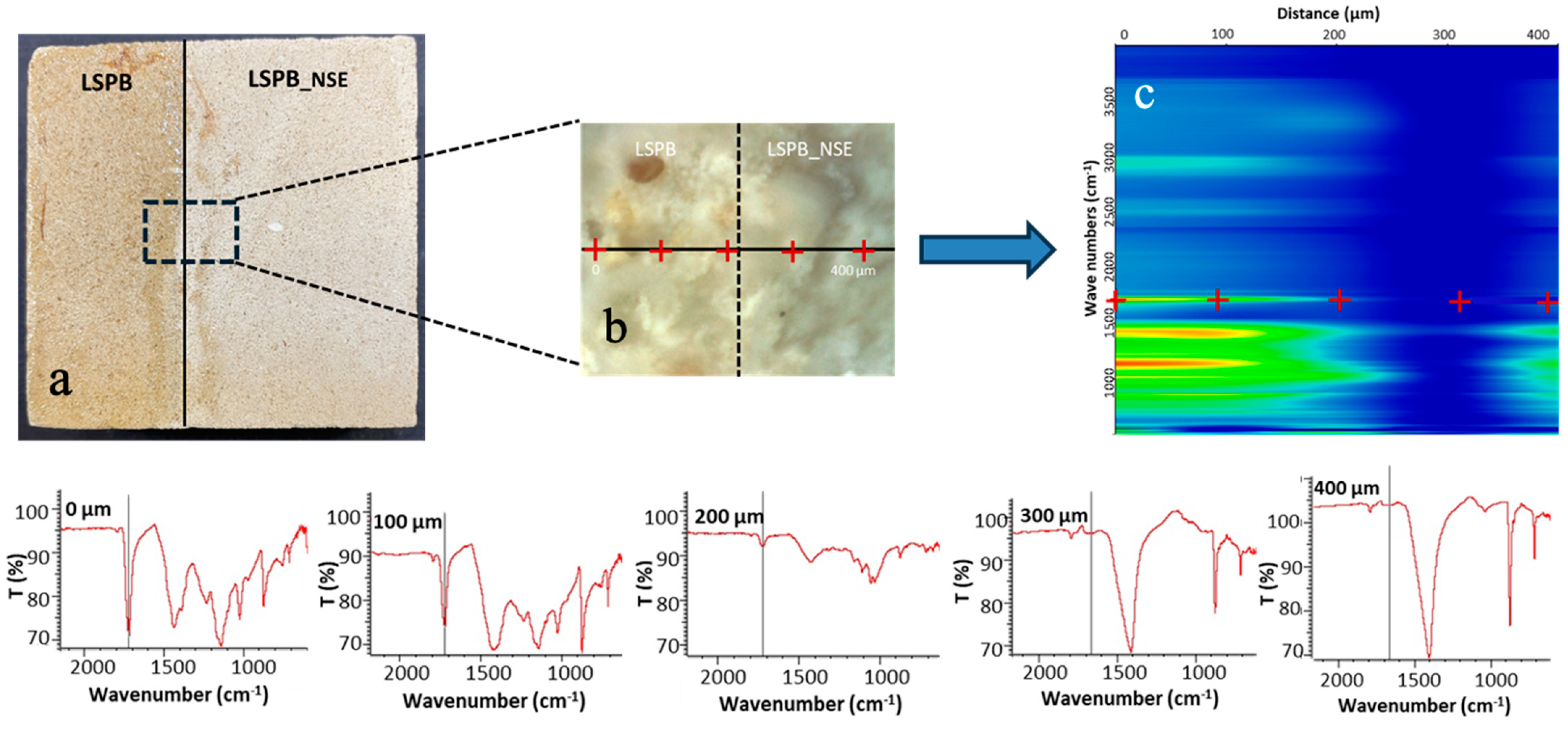
3.3.3. Micro-FTIR Mapping
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Nanoparticles for conservation of bio-calcarenite stone. Appl. Phys. A 2014, 114, 673–683. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Crivelli, F.; Galimberti, C.; Malagodi, M.; Licchelli, M. Evaluation of commercial consolidating agents on very porous biocalcarenite. Int. J. Conserv. Sci. 2020, 11, 251–260. [Google Scholar]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogel Based on Semi-Intrepenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Alterini, M.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Removing Polymeric Coatings with Nanostructured Fluids: Influence of Substrate, Nature of the Film, and Application Methodology. Front. Mater. 2019, 6, 311. [Google Scholar] [CrossRef]
- Brajer, I.; Rouzic, M.F.; Shashoua, Y.; Taube, M.; Chelazzi, D.; Baglioni, M.; Giorgi, R.; Baglioni, P. The Removal of Aged Acrylic Coatings from Wall Paintings using Microemulsions. In Proceedings of the ICOM-CC, 17th Triennial Conference 2014, Melbourne, Australia, 15–19 September 2014. [Google Scholar]
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced methodologies for the cleaning of works of art. Sci. China Technol. Sci. 2023, 66, 2162–2182. [Google Scholar] [CrossRef]
- Rosciardi, V.; Bandelli, D.; Bassu, G.; Casu, I.; Baglioni, P. Highly biocidal poly(vinyl alcohol)-hydantoin/starch hybrid gels: A “Trojan Horse” for Bacillus subtilis. J. Colloid Interface Sci. 2024, 657, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, R.; Baglioni, M.; Baglioni, P. Nanofluids and chemical highly retentive hydrogels for controlled and selective removal of overpainting and undesired graffiti from street art. Anal. Bioanal. Chem. 2017, 409, 3707–3712. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Girella, A.; Ferretti, M.; Sacchi, D.; Licchelli, M. Nanostructured Emulsions as Smart Cleaning Materials for Removing Aged Polymer Coatings from Stone Substrates. Sustainability 2023, 15, 8117. [Google Scholar] [CrossRef]
- Richteringa, W.; Saunders, B.R. Gel architectures and their complexity. Soft Matter 2014, 10, 3695–3702. [Google Scholar] [CrossRef]
- Lee, C.; Volpi, F.; Fiocco, G.; Weththimuni, M.L.; Licchelli, M.; Malagodi, M. Preliminary Cleaning Approach with Alginate and Konjac Glucomannan Polysaccharide Gel for the Surfaces of East Asian and Western String Musical Instruments. Materials 2022, 15, 1100. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Arotiba, O.A. Synthesis, swelling and adsorption studies of a responsive sodium alginate–poly(acrylic acid) superabsorbent hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Djabourov, M. Book Chapter; Chapter 1: Gels, Series: New Developments in NMR, NMR and MRI of Gels, Edited by Yves De Deener, ISBN: 978-1-78801-317-8, The Royal Society of Chemistry 2020, Published by the Royal Society of Chemistry. Available online: https://books.rsc.org/books/edited-volume/756/chapter/475969/Gels (accessed on 13 April 2024).
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Diferentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 3256. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, M.; Tsujimoto, M.; Ikkai, F. Static Inhomogeneities in Physical Gels: Comparison of Temperature-Induced and Concentration-Induced Sol-Gel Transition. Macromolecules 2000, 33, 7868–7876. [Google Scholar] [CrossRef]
- Baglioni, M.; Bartoletti, A.; Bozee, L.; Chelazzi, D.; Giorgi, R.; Odlyha, M.; Pianorsi, D.; Poggi, G.; Baglioni, P. Nanomaterials for the cleaning and pH adjustment of vegetable-tanned leather. Appl. Phys. A 2016, 122, 114–124. [Google Scholar] [CrossRef]
- Domingues, J.; Bonelli, N.; Giorgi, R.; Baglioni, P. Chemical semi-IPN hydrogels for the removal of adhesives from canvas paintings. Appl. Phys. A 2014, 114, 705–710. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.R. Poly(2-Hydroxyethyl methacrylate-co-N,Ndimethylacrylamide)-Coated Quartz Crystal Microbalance Sensor: Membrane Characterization and Proof of Concept. Gels 2021, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Huaman, M.A.L.; Vega-Chacòn, J.; Quispe, R.I.H.; Negròn, A.C.V. Synthesis and swelling behaviors of poly(2-hydroxyethyl methacrylate-co-itaconic acid) and poly(2-hydroxyethylmethacrylate-co-sodium itaconate) hydrogels as potential drug carriers. Results Chem. 2023, 5, 100917. [Google Scholar] [CrossRef]
- Buemi, L.P.; Petruzzellis, M.L.; Chelazzi, D.; Baglioni, M.; Mastrangelo, R.; Giorgi, R.; Baglioni, P. Twin-chain polymer networks loaded with nanostructured fluids for the selective removal of a non-original varnish from Picasso’s “L’Atelier” at the Peggy Guggenheim Collection, Venice. Herit. Sci. 2020, 8, 77. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Tighe, B.J. Hydrogels as Contact Lens Materials. Hydrogels Med. Pharm. 1987, 3, 53–82. [Google Scholar]
- Mack, E.J.; Okano, T.; Kim, S.W. Biomedical Applications of Poly(2-Hydroxyethyl Methacrylate) and its Copolymers. Hydrogels Med. Pharm. 1987, 2, 65–93. [Google Scholar]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef]
- Carretti, E.; Dei, L. Physicochemical characterization of acrylic polymeric resins coating porous materials of artistic interest. Prog. Org. Coat 2004, 49, 282–289. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000, 76, 962–977. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Fardia, T.; Pintus, V.; Kampasakalia, E.; Pavlidou, E.; Schreiner, M.; Kyriacou, G. Analytical characterization of artist’s paint systems based on emulsion polymers and synthetic organic pigments. J. Anal. Appl. Pyrolysis 2018, 135, 231–241. [Google Scholar] [CrossRef]
- Jablonski, E.; Hayes, J.; Learner, T.; Golden, M. Conservation concerns for acrylic emulsion paints. Rev. Conserv. 2003, 4, 3–12. [Google Scholar] [CrossRef]
- Learner, T.J.S.; Patricia Smithen, J.W.K.; Michael, R.S. (Eds.) Modern Paints Uncovered. In Proceedings of the Modern Paints Uncovered Symposium Organized by the Getty Conservation Institute, Tate and the National Gallery of Art, Tate Modern, London, UK, 16–19 May 2006; Getty Conservation Institute: Los Angeles, CA, USA, 2007. [Google Scholar]
- Stoye, D.; Freitag, W. (Eds.) Paints, Coatings and Solvents, 2nd ed.; WILEY–VCH: Weinheim, Germany, 1998; pp. 125–129. ISBN 9783527611867. [Google Scholar]
- Pintus, V.; Wei, S.; Schreiner, M. UV ageing studies: Evaluation of lightfastness declarations of commercial acrylic paints. Anal. Bioanal. Chem. 2012, 402, 1567–1584. [Google Scholar] [CrossRef]
- Silva, M.F.; Doménech–Carbó, M.T.; Osete–Cortina, L. Characterization of additives of PVAc and acrylic waterborne dispersions and paints by analytical pyrolysis–GC–MS and pyrolysis–silylation–GC–MS. J. Anal. Appl. Pyrol 2015, 113, 606–620. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; La Russa, M. Consolidation of Bio-Calcarenite Stone by Treatment Based on Diammonium Hydrogenphosphate and Calcium Hydroxide Nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Bergamonti, L.; Potenza, M.; Scigliuzzo, F.; Meli, S.; Casoli, A.; Lottici, P.P.; Graiff, C. Hydrophobic and Photocatalytic Treatment for the Conservation of Painted Lecce stone in Outdoor Conditions: A New Cleaning Approach. Appl. Sci. 2024, 14, 1261. [Google Scholar] [CrossRef]
- Ghio, F.; Stefanelli, E.M.; Ampolo, E. The Flight of Saint Mary Magdalene—A Case Study of the Dismantling, Repositioning and Restoration of a Votive Aedicule and Wall Painting in Nardò, Lecce, Italy. Heritage 2023, 6, 3429–3447. [Google Scholar] [CrossRef]
- Esposito, D.; Vitarelli, F.; Vita, L.; D’Onofrio, M. Conoscenza e progetto. Un caso di studio. Santa Maria di Cerrate. In RPR, Rilievo, Progetto, Riuso; Maggioli: Santarcangelo di Romagna, Italy, 2017; pp. 285–298. ISBN 9788891624833. [Google Scholar]
- De Pascalis, D.G.; Leucci, G.; De Giorgi, L.; Giuri, F.; Scardozzi, G. The Basilica of Santa Caterina d’Alessandria in Galatina (Lecce, Italy): NDT surveys for the conservation project. In Proceedings of the 2019 IMEKO TC-4 International Conference on Metrology for Archaeology and Cultural Heritage, Florence, Italy, 4–6 December 2019; pp. 369–371, ISBN 978-92-990084-5-4. [Google Scholar]
- Riganti, V.; Perotti, A.; Fiumara, A.; Veniale, F.; Zezza, U. Applicazione di tecniche strumentali al controllo del degrado delle pietre nei monumenti: Il caso della Basilica di S. Michele in Pavia. Atti. Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1981, 122, 109–138. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and Materials Science for the Conservation of Cultural Heritage: Cleaning, Consolidation, and Deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Shashoua, Y.R. Resins in the conservation of three-dimensional works of art. In Plastics and Resin Compositions; Simpson, W.G., Ed.; The Royal Society of Chemistry: London, UK, 1995; pp. 294–328. [Google Scholar]
- UNI 10921:2001; Beni Culturali—Materiali Lapidei Naturali Ed Artificiali—Prodotti Idrorepellenti—Applicazione Su Provini e Determinazione in Laboratorio Delle Loro Caratteristiche. UNI Ente Italiano di Normazione: Milan, Italy, 2001. Available online: https://www.biblio.units.it/SebinaOpac/resource/beni-culturali-materiali-lapidei-naturali-ed-artificiali-prodotti-idrorepellenti-applicazione-su-pro/TSA1388731?locale=eng (accessed on 28 February 2024).
- Weththimuni, M.L.; Ben Chobba, M.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, S.J.; Wang, Y.J. Properties of PVA-AA Cross-linked HEMA-based Hydrogels. J. Polym. Res. 1999, 6, 191–196. [Google Scholar]
- Vigani, B.; Valentino, C.; Sandri, G.; Carla, M.C.; Ferrari, F.; Rossi, S. Spermidine Crosslinked Gellan Gum-Based “Hydrogel Nanofibers” as Potential Tool for the Treatment of Nervous Tissue Injuries: A Formulation Study. Int. J. Nanomed. 2022, 17, 3421–3439. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Kermany, P.B.; Škalko-Basnet, N. Improved Texture Analysis for Hydrogel Characterization: Gel Cohesiveness, Adhesiveness, and Hardness. J. Appl. Poly. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Hackla, E.V.; Khutoryanskiy, V.V.; Ermolinaa, I. Hydrogels based on copolymers of 2-hydroxyethylmethacrylate and 2-hydroxyethylacrylate as a delivery system for proteins: Interactions with lysozyme. J. Appl. Poly. Sci. 2017, 134, 44768. [Google Scholar] [CrossRef]
- Smułek, W.; Grząbka-Zasadzi’nska, A.; Kilian, A.; Ciesielczyk, F.; Borysiak, S.; Baranowska, H.M.; Walkowiak, K.; Kaczorek, E.; Jarzębski, M. Design of vitamin-loaded emulsions in agar hydrogel matrix dispersed with plant surfactants. Food Biosci. 2023, 53, 102559. [Google Scholar] [CrossRef]
- UNI EN 15886:2010; Conservazione dei Beni Culturali, Metodi di Prova, Misura del Colore Delle Superfici. UNI Ente Italiano di Normazione: Milan, Italy, 2010.
- Ben Chobba, M.; Weththimuni, M.L.; Messaoud, M.; Sacchi, D.; Bouaziz, J.; De Leo, F.; Urzi, C.; Licchelli, M. Multifunctional and Durable Coatings for Stone Protection Based on Gd-Doped Nanocomposites. Sustainability 2021, 13, 11033. [Google Scholar] [CrossRef]
- UNI EN 15802:2010; Conservazione dei Beni Culturali—Metodi di Prova—Determinazione Dell’angolo di Contatto Statico. UNI: Milan, Italy, 2010.
- Weththimuni, M.L.; Milanese, C.; Licchelli, M.; Malagodi, M. Improving the Protective Properties of Shellac-Based Varnishes by Functionalized Nanoparticles. Coatings 2021, 11, 419. [Google Scholar] [CrossRef]
- Tamburini, G.; Canevali, C.; Ferrario, S.; Bianchi, A.; Sansonetti, A.; Simonutti, R. Optimized Semi-Interpenetrated p(HEMA)/PVP Hydrogels for Artistic Surface Cleaning. Materials 2022, 15, 6739. [Google Scholar] [CrossRef] [PubMed]
- Oyarce, E.; Pizarro, G.; Del, C.; Oyarzún, D.P.; Zúñiga, C.; Sánchez, J. Hydrogels based on 2-hydroxyethyl methacrylate: Synthesis, characterization and hydration capacity. J. Chil. Chem. Soc. 2020, 65, 4682–4685. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.H.; Amidon, G.L.; Lee, P.I. pH-Dependent Swelling and Solute Diffusion Characteristics of Poly (Hydroxyethyl Methacrylate–CO–Methacrylie Acid) Hydrogels. Pharm. Res 1988, 5, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, P.; Uddin, M.; Dip, T.M.; Islam, S.; Hoque, S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Lee, C.; Di Turo, F.; Vigani, B.; Weththimuni, M.L.; Rossi, S.; Beltram, F.; Pingue, P.; Licchelli, M.; Malagodi, M.; Fiocco, G.; et al. Biopolymer Gels as a Cleaning System for Different Featured Wooden Surfaces. Polymers 2023, 15, 36. [Google Scholar] [CrossRef]
- Passaretti, A.; Cuvillier, L.; Sciutto, G.; Guilminot, E.; Joseph, E. Biologically Derived Gels for the Cleaning of Historical and Artistic Metal Heritage. Appl. Sci. 2021, 11, 3405. [Google Scholar] [CrossRef]
- Giordano, A.; Caruso, M.R.; Lazzara, G. New tool for sustainable treatments: Agar spray—Research and practice. Herit. Sci. 2022, 10, 123. [Google Scholar] [CrossRef]
- Weththimuni, M.; Ben Chobba, M.; Tredici, I.; Licchelli, M. ZrO2-Doped ZnO-PDMS Nanocomposites as Protective Coatings for the Stone Materials. Acta IMEKO 2022, 11, 5. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectroscopy of Polymers X: Polyacrylates. Spectroscopy 2023, 38, 10–14. [Google Scholar] [CrossRef]
- Carretti, E.; Chelazzi, D.; Rocchigiani, G.; Baglioni, P.; Poggi, G.; Dei, L. Interactions between Nanostructured Calcium Hydroxide and Acrylate Copolymers: Implications in Cultural Heritage Conservation. Langmuir 2013, 29, 9881–9890. [Google Scholar] [CrossRef]
- Spathis, P.; Karagiannidou, E.; Magoula, A.E. Influence of Titanium Dioxide Pigments on the Photodegradation of Paraloid Acrylic Resin. Stud. Conserv. 2003, 48, 57–64. [Google Scholar] [CrossRef]
- Hwidi, R.S.; Izhar, T.N.T.; Saad, F.N.M. Characterization of Limestone as Raw Material to Hydrated Lime. E3S Web Conf. 2018, 34, 02042. [Google Scholar] [CrossRef]
- Cizer, Ö.; Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Elsen, J.; Gemert, D.V.; Balen, K.V. Phase and morphology evolution of calcium carbonate precipitated by carbonation of hydrated lime. J. Mater. Sci. 2012, 47, 6151–6165. [Google Scholar] [CrossRef]
- Taylor, D.R.; Crowther, R.S.; Cozart, J.C.; Sharrock, P.; Wu, J.; Soloway, R.D. Calcium carbonate in cholesterol gallstones: Polymorphism, distribution, and hypotheses about pathogenesis. Hepathology 1995, 22, 488–496. [Google Scholar]
- Kalbus, G.E.; Kalbus, L.H. Use of infrared spectrophotometry in the analysis of limestone. J. Chem. Educ. 1966, 43, 314–318. [Google Scholar] [CrossRef]
- Gunasekarana, S.; Anbalagan, G. Spectroscopic characterization of natural calcite minerals. Spectrochim. Acta. A 2007, 68, 656–664. [Google Scholar] [CrossRef]

| Samples | Gel Content (G, %) | Equilibrium Water Content (EWC, %) | Retention Capability (RC, Water Released, mg/cm2) |
|---|---|---|---|
| HEMA-MBA/PVP (a) | 76 ± 5 | 81 ± 2 | 13 ± 2 |
| Agar (a) | - | 95 ± 2 | 28 ± 2 |
| Acrylamide (soft) (b) | 88 | 97 | 56 |
| Kelcogel (b) | - | 97 | 33 |
| Stones | Carbon wt% | ||||||
|---|---|---|---|---|---|---|---|
| Uncoated | Coated | Cleaned—EcoSurf-H2O | Cleaned—NSE | ||||
| Unaged | 35 d Aged | Unaged | 35 d Aged | Unaged | 35 d Aged | ||
| LS | 11.3 ± 0.7 | 64.8 ± 0.9 | 46.8 ± 1.2 | 59.1 ± 0.7 | 43.7 ± 0.2 | 10.7 ± 0.5 | 10.2 ± 0.3 |
| AS | 10.5 ± 1.4 | 67.4 ± 0.7 | 67.4 ± 0.5 | 66.3 ± 0.2 | 65.2 ± 0.9 | 11.1 ± 0.2 | 11.7 ± 0.4 |
| Stones | Contact Angle, α (°) | |||||
|---|---|---|---|---|---|---|
| Coated | Cleaned—EcoSurf/H2O | Cleaned—NSE | ||||
| Unaged | 35 d Aged | Unaged | 35 d Aged | Unaged | 35 d Aged | |
| LS | 95 ± 2 | 80 ± 3 | 78 ± 1 | 72 ± 3 | n.d. | n.d. |
| AS | 115 ± 5 | 95 ± 3 | 106 ± 3 | 90 ± 1 | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weththimuni, M.L.; Fiocco, G.; Girella, A.; Vigani, B.; Sacchi, D.; Rossi, S.; Licchelli, M. Removing Aged Polymer Coatings from Porous Stone Surfaces Using the Gel Cleaning Method. Coatings 2024, 14, 482. https://doi.org/10.3390/coatings14040482
Weththimuni ML, Fiocco G, Girella A, Vigani B, Sacchi D, Rossi S, Licchelli M. Removing Aged Polymer Coatings from Porous Stone Surfaces Using the Gel Cleaning Method. Coatings. 2024; 14(4):482. https://doi.org/10.3390/coatings14040482
Chicago/Turabian StyleWeththimuni, Maduka L., Giacomo Fiocco, Alessandro Girella, Barbara Vigani, Donatella Sacchi, Silvia Rossi, and Maurizio Licchelli. 2024. "Removing Aged Polymer Coatings from Porous Stone Surfaces Using the Gel Cleaning Method" Coatings 14, no. 4: 482. https://doi.org/10.3390/coatings14040482
APA StyleWeththimuni, M. L., Fiocco, G., Girella, A., Vigani, B., Sacchi, D., Rossi, S., & Licchelli, M. (2024). Removing Aged Polymer Coatings from Porous Stone Surfaces Using the Gel Cleaning Method. Coatings, 14(4), 482. https://doi.org/10.3390/coatings14040482











