Microstructure, Hardness and Corrosion Resistance of Al-TiC MMC Prepared by Laser Cladding on AZ31B Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Equipment and Process Preparation
2.3. Microstructure Characterization
2.4. Microhardness and Corrosion Properties
3. Results and Discussion
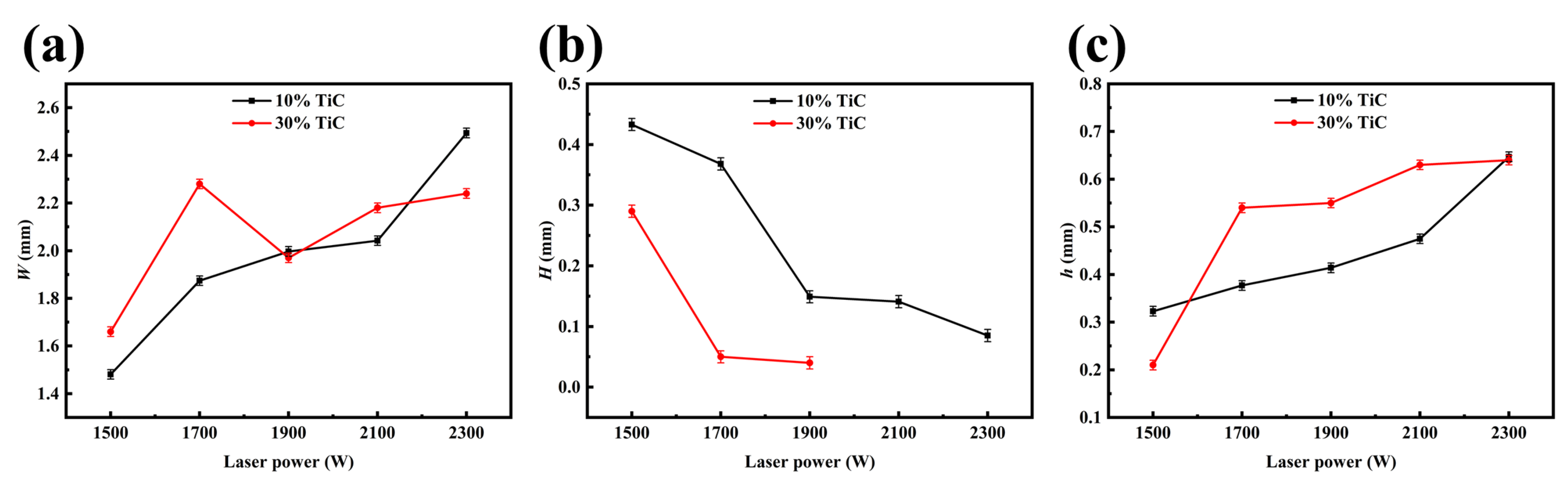
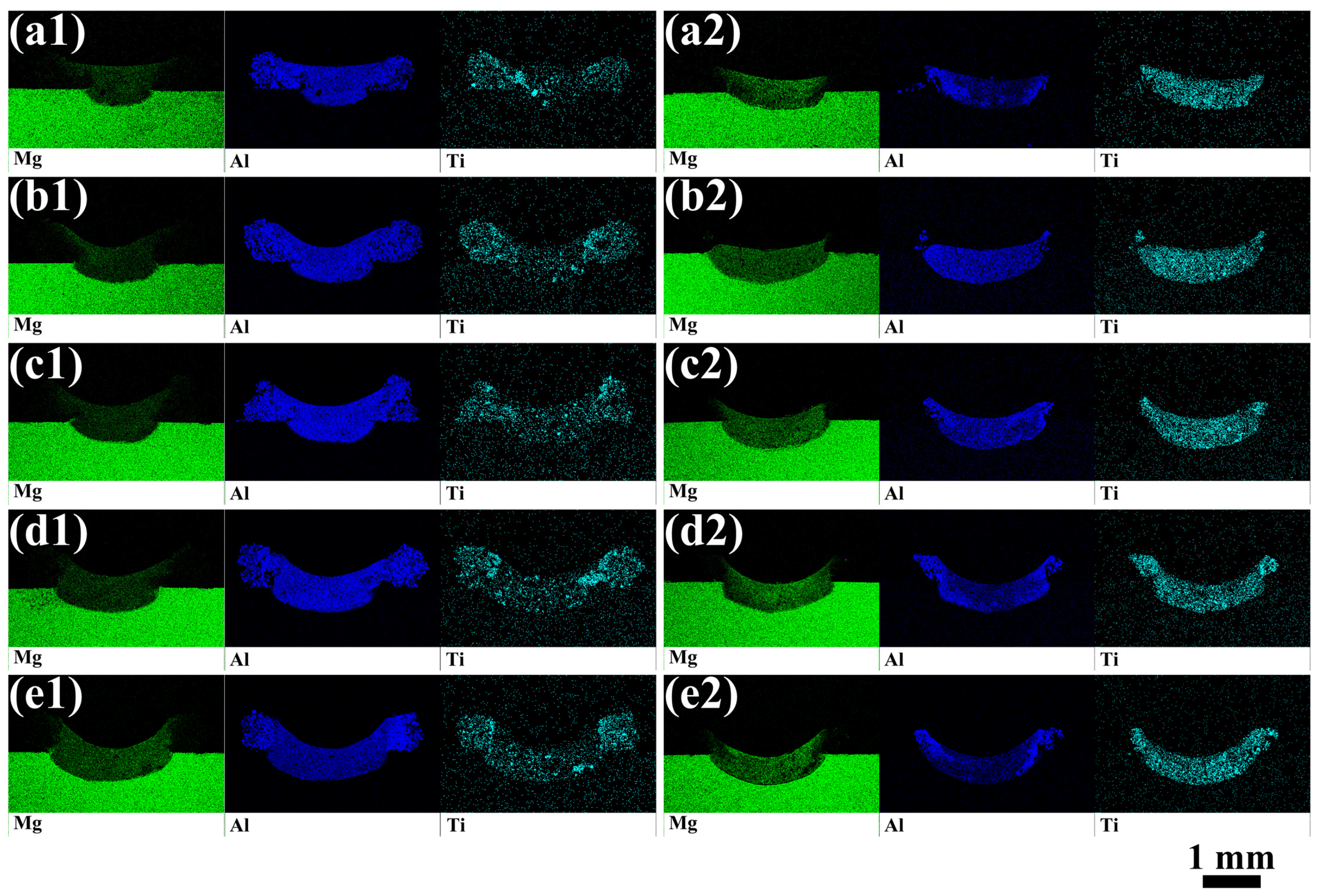
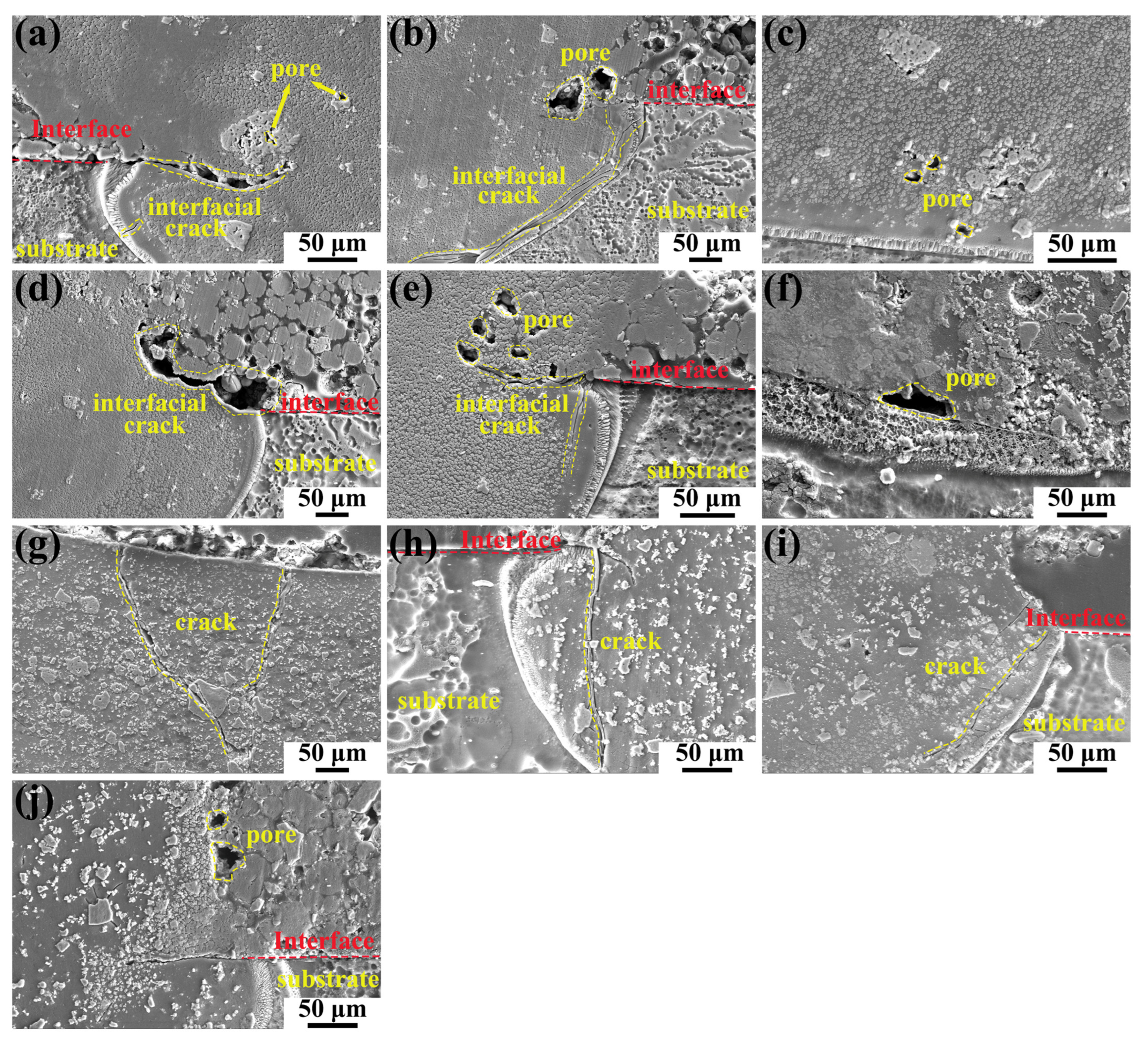
3.1. Cross-Sectional Morphology of Single-Track Coating
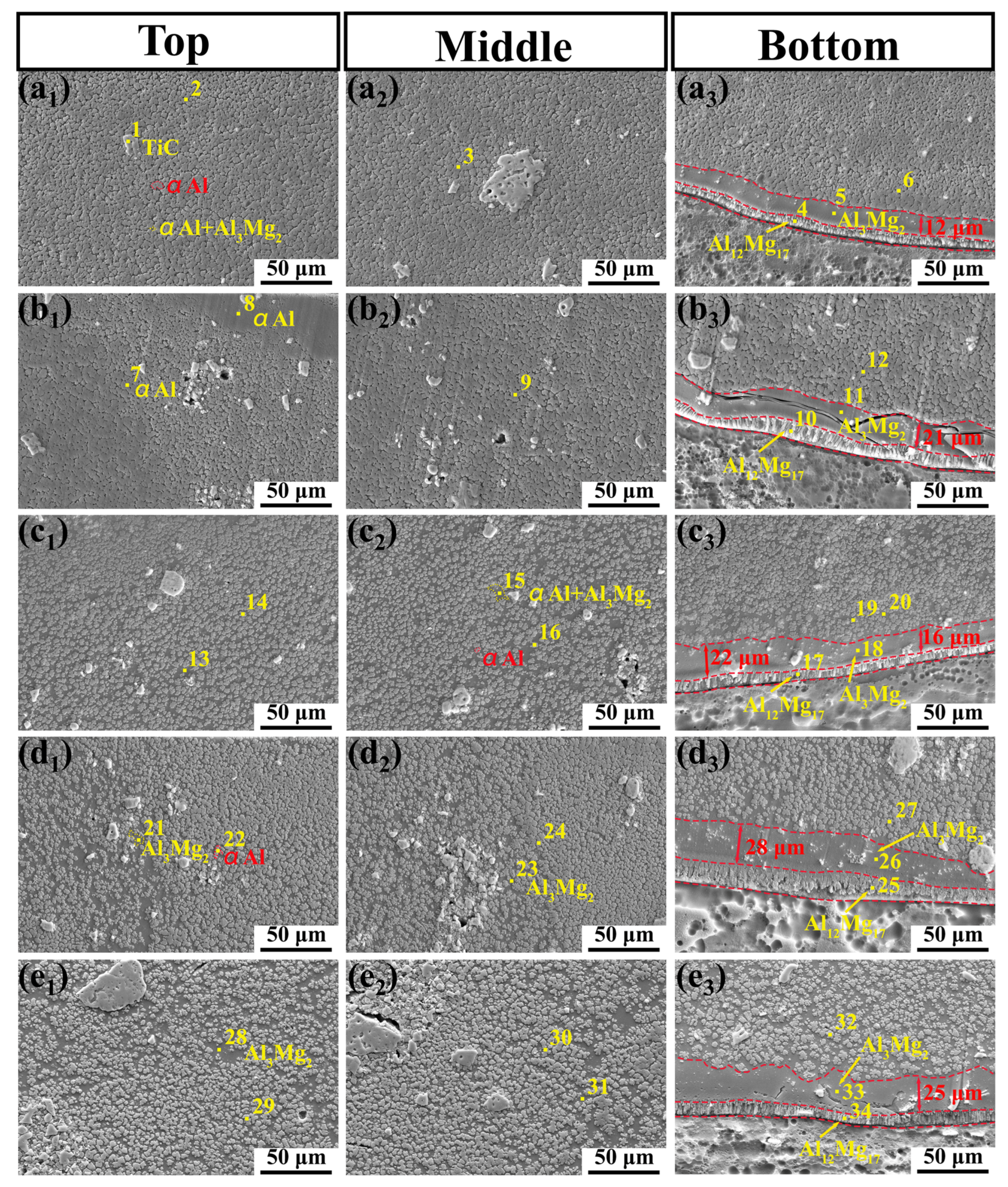
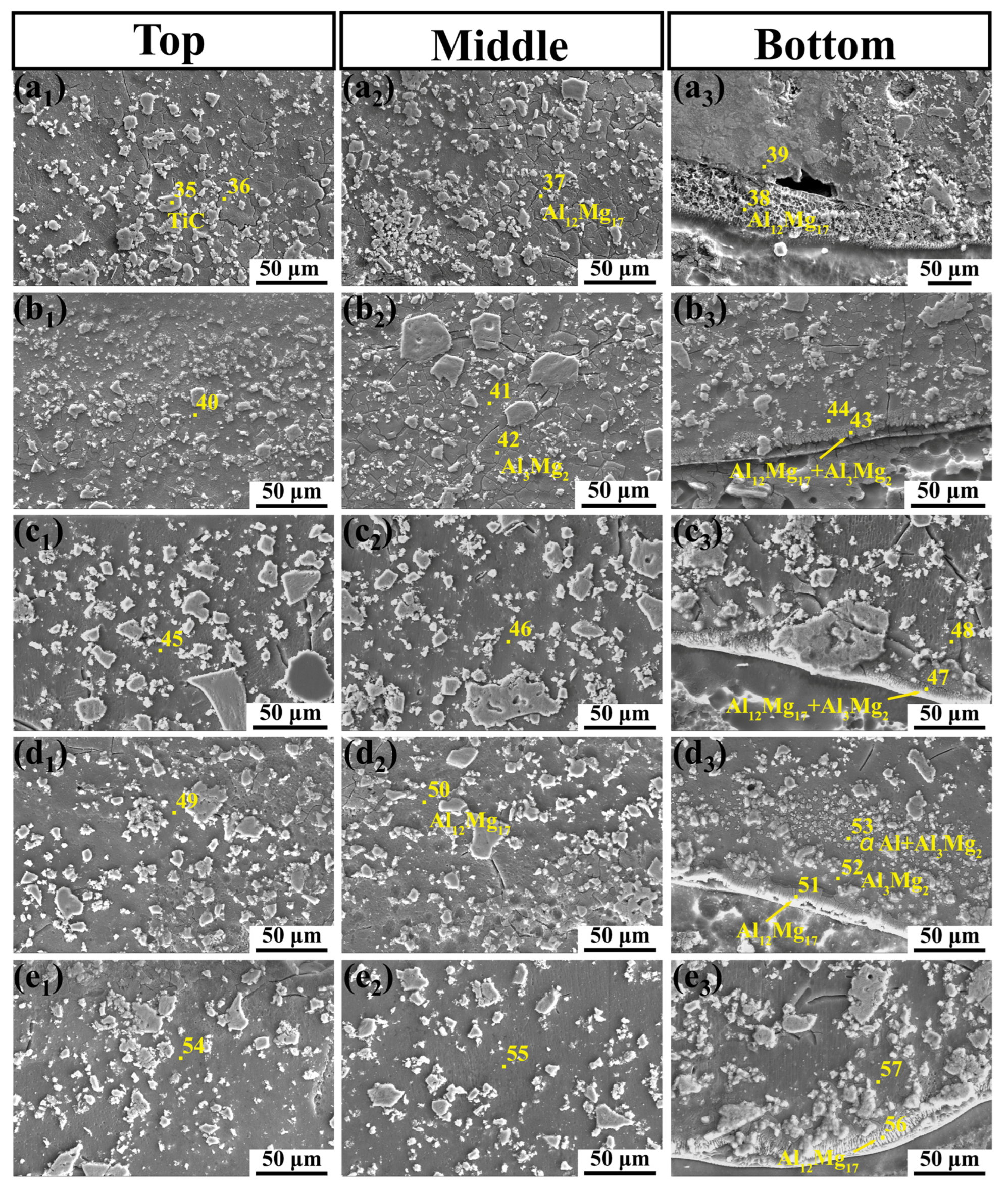
3.2. Microstructural Characteristic
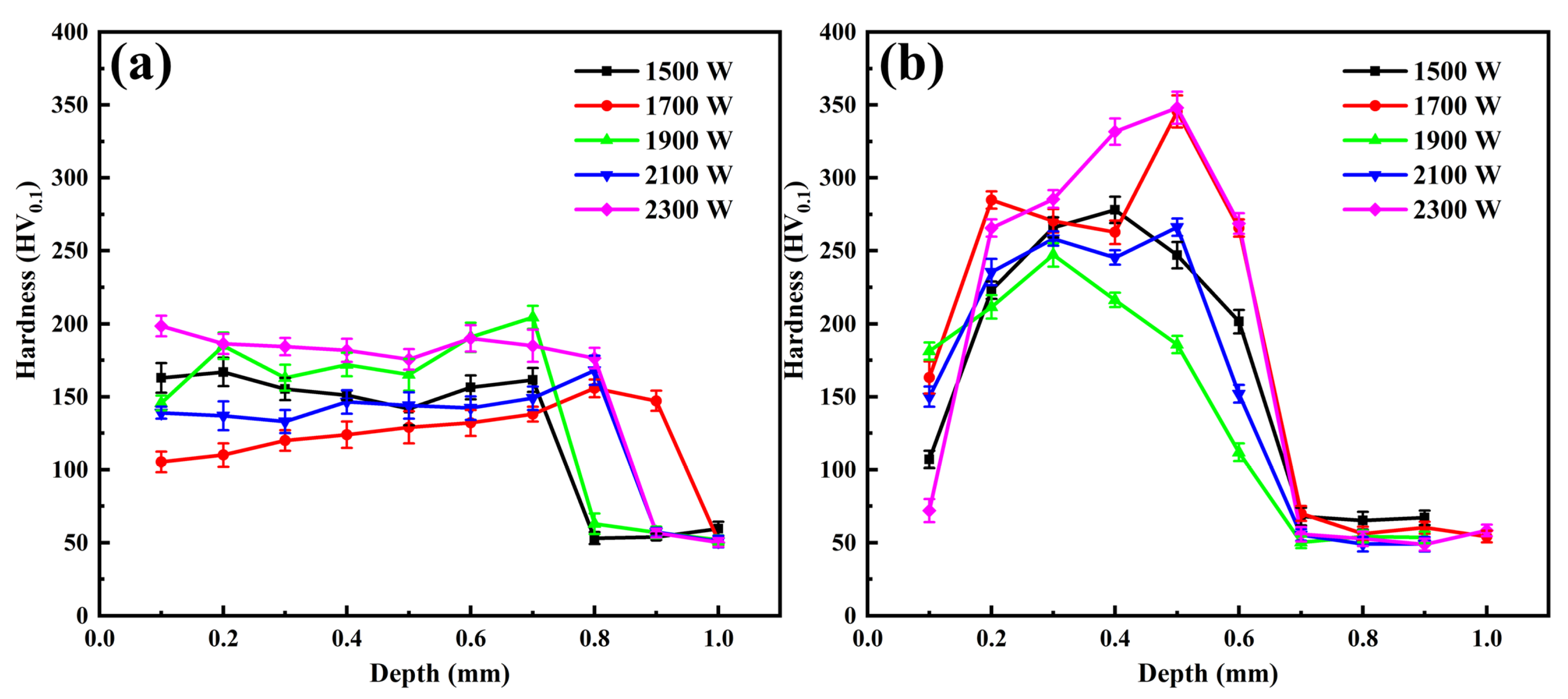
3.3. Microhardness
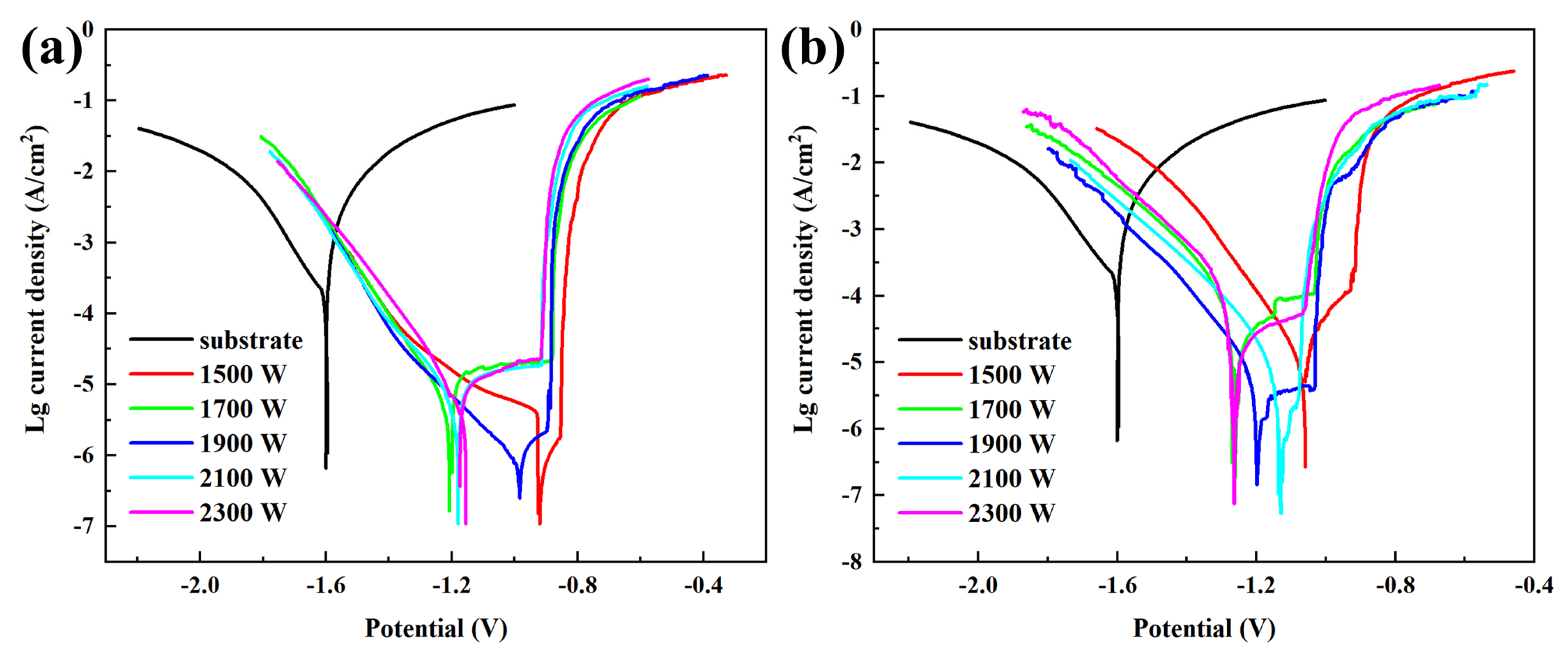
3.4. Corrosion Resistance
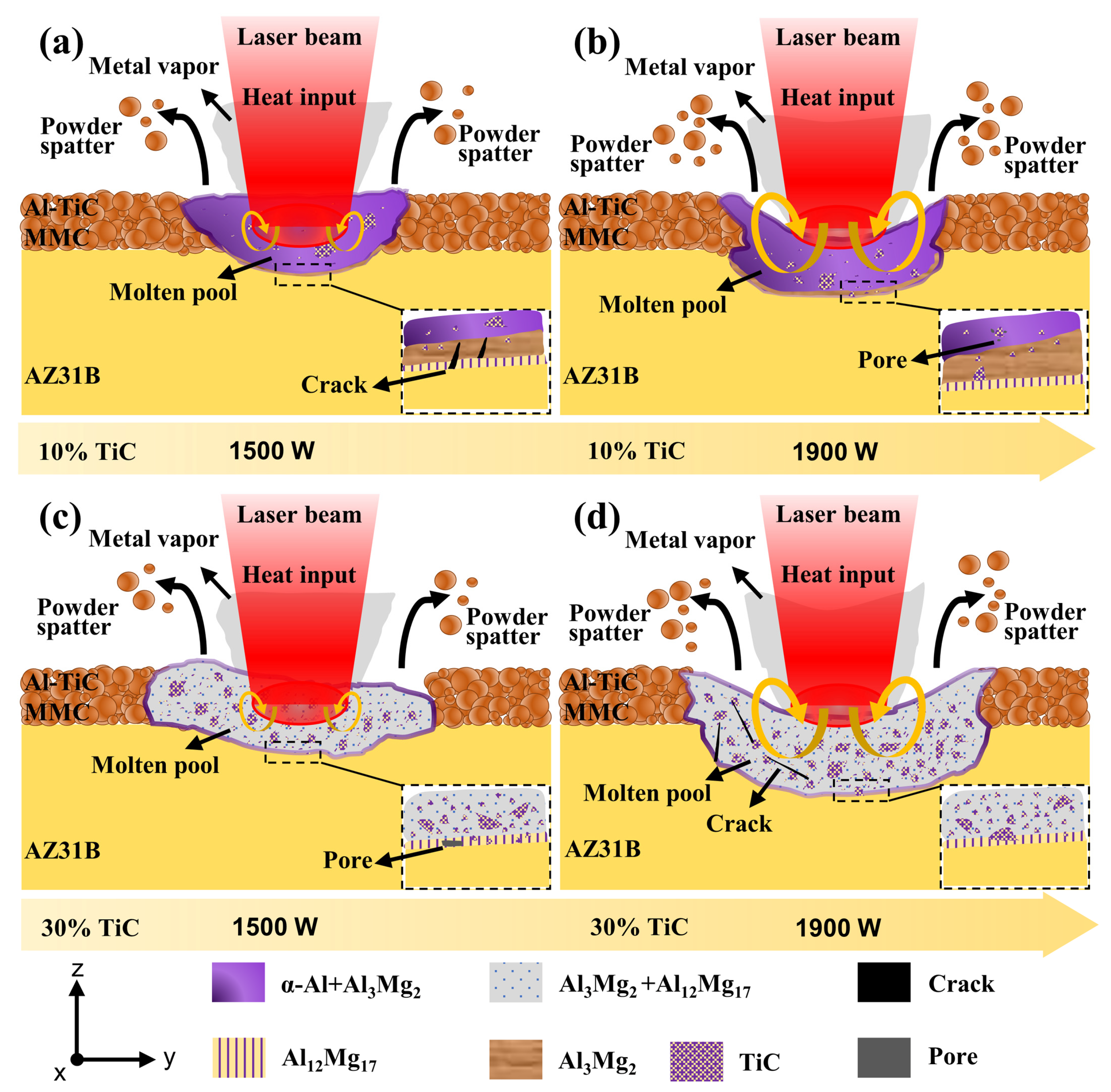
3.5. Microstructure Evolution
4. Conclusions
- (1)
- The Al-TiC MMC coating was prepared on the AZ31B magnesium alloy via laser cladding. A good metallurgical bonding between the coating and substrate was obtained. The shape of the coating is not the typical up-convex shape but rather a concave crescent shape.
- (2)
- The hardness of the MMC was significantly improved compared to the AZ31B magnesium alloy. The average hardness of the MMC with 10% TiC is 184 HV0.1, which is 3.5 times higher than that of the AZ31B magnesium alloy (52 HV0.1). Because the formation of more IMCs, TiC particles and a few α-Al phases in the MMC. Thus, the MMC with 30% TiC has better hardness than the MMC with 10%.
- (3)
- The corrosion resistance of the MMC was significantly improved compared to that if the AZ31B magnesium alloy. The current density of the MMC with 10% TiC is 3.90 × 10−7 A/cm2, which is three orders of magnitude lower than that of the AZ31B magnesium alloy (5.45 × 10−4 A/cm2). At the same laser power, the corrosion resistance of the MMC with 30% TiC is lower than that of the MMC with 10% TiC.
- (4)
- The microstructural evolution of the MMC with different laser power and TiC content levels was analyzed. The increase in laser power cannot change the phase composition of the MMC, but it can contribute to the formation of a concave crescent shape and can induce the formation of a thicker Al3Mg2 transition layer. The change in TiC content has a significant effect on the microstructure of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.L.; Yu, H.J.; Chen, C.Z.; Weng, F.; Dai, J.J. Research and development status of laser cladding on magnesium alloys: A review. Opt. Lasers Eng. 2017, 93, 195–210. [Google Scholar] [CrossRef]
- Li, N.; Ling, N.; Fan, H.Y.; Wang, L.; Zhang, J.L. Self-healing and superhydrophobic dual-function composite coating for active protection of magnesium alloys. Surf. Coat. Technol. 2023, 454, 11. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.Q.; Li, Z.Y.; Wei, S.Z.; Zhang, T.; Yang, L.Q.; Liu, S.Y. Microstructure and properties of in-situ synthesized ZrC-Al3Zr reinforced composite coating on AZ91D magnesium alloy by laser cladding. Surf. Coat. Technol. 2018, 334, 471–478. [Google Scholar] [CrossRef]
- Zhang, C.H.; Li, Z.; Zhang, J.K.; Tang, H.B.; Wang, H.M. Additive manufacturing of magnesium matrix composites: Comprehensive review of recent progress and research perspectives. J. Magnes. Alloys 2023, 11, 425–461. [Google Scholar] [CrossRef]
- Pak, S.N.; Jiang, Z.H.; Yao, Z.P.; Ju, J.M.; Ju, K.S.; Pak, U.J. Fabrication of environmentally friendly anti-corrosive composite coatings on AZ31B Mg alloy by plasma electrolytic oxidation and phytic acid/3-aminopropyltrimethoxysilane post treatment. Surf. Coat. Technol. 2017, 325, 579–587. [Google Scholar] [CrossRef]
- Taha, M.A.; El-Mahallawy, N.A.; Hammouda, R.M.; Nassef, S.I. PVD Coating of Mg-AZ31 by Thin Layer of Al and Al-Si. J. Coat. Technol. Res. 2010, 7, 793–800. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Morelli, S.; Rombola, G.; Bolelli, G.; Lopresti, M.; Puddu, P.; Boccaleri, E.; Seralessandri, L.; Palin, L.; Testa, V.; Milanesio, M.; et al. Hard ultralight systems by thermal spray deposition of WC-CoCr onto AZ31 magnesium alloy. Surf. Coat. Technol. 2022, 451, 26. [Google Scholar] [CrossRef]
- Pei, X.L.; Li, Z.Y.; Zhang, Y.Q.; Wei, S.Z.; Yang, L.Q.; Wang, Y.J. A gradient Al/Ni-Cr-Al layer formed by direct current pulse metal inert gas welding combined laser cladding on AZ91D magnesium alloy. Vacuu 2019, 165, 86–95. [Google Scholar] [CrossRef]
- Jiang, L.P.; Cui, X.F.; Jin, G.; Tian, Z.M.; Wen, X.; Tian, H.L.; Liu, E. Design and characterization of a novel Cu 2.3 Al 1.3 Ni 1.7 SnCr 0.3 multi-principal element alloy coating on magnesium alloy by laser cladding. J. Mater. Sci. Technol. 2023, 152, 220–236. [Google Scholar] [CrossRef]
- Jiang, L.P.; Cui, X.F.; Jin, G.; Tian, Z.M.; Wen, X.; Tian, H.L.; Wang, S. Design and characterization of in-situ TiB reinforced TiB/Ti50Zr25Al15Cu10 non-equiatomic medium-entropy alloy composite coating on magnesium alloy by laser cladding. Opt. Laser Technol. 2022, 156, 15. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Guo, J.; Xu, G.D.; Li, Z.Y.; Wei, S.Z. Effect of Nd2O3 on microstructure, corrosion and wear properties of laser cladding Zr-based amorphous composite coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2023, 611, 11. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Magham, H.S.; Mullick, S.; Gopinath, M. Evaluation of microstructure and thermal history for TiC/Inconel 625 MMC deposition through pre-placed laser cladding method with and without the application of ultrasonic vibration. CIRP J. Manuf. Sci. Technol. 2023, 41, 453–464. [Google Scholar] [CrossRef]
- Lin, P.Y.; Zhang, Z.H.; Ren, L.Q. The mechanical properties and microstructures of AZ91D magnesium alloy processed by selective laser cladding with Al powder. Opt. Laser Technol. 2014, 60, 61–68. [Google Scholar] [CrossRef]
- Zheng, B.J.; Chen, X.M.; Lian, J.S. Microstructure and wear property of laser cladding Al plus SiC powders on AZ91D magnesium alloy. Opt. Lasers Eng. 2010, 48, 526–532. [Google Scholar] [CrossRef]
- Ignat, S.; Sallamand, P.; Grevey, D.; Lambertin, M. Magnesium alloys laser (Nd:YAG) cladding and alloying with side injection of aluminium powder. Appl. Surf. Sci. 2004, 225, 124–134. [Google Scholar] [CrossRef]
- Zhao, D.C.; Kong, D.C.; Huang, J.; Wang, M.L.; Yamaguchi, T.; Wang, H.W. Achieving the lightweight wear-resistant TiC reinforced AlFeCrCo medium-entropy alloy coating on Mg alloy via resistance seam processing. Scr. Mater. 2022, 210, 5. [Google Scholar] [CrossRef]
- Das, A.K. Recent trends in laser cladding and alloying on magnesium alloys: A review. In Proceedings of the 1st International Conference on Computations in Materials and Applied Engineering (CMAE), Uttarakhand, India, 1–2 May 2021; pp. 723–727. [Google Scholar]
- Yang, L.; Li, Z.; Zhang, Y.; Wei, S.; Liu, F. Al-TiC in situ composite coating fabricated by low power pulsed laser cladding on AZ91D magnesium alloy. Appl. Surf. Sci. 2018, 435, 1187–1198. [Google Scholar] [CrossRef]
- Liu, F.J.; Li, A.D.; Shen, Z.K.; Chen, H.Y.; Ji, Y. Microstructure and corrosion behavior of Al-Ti-TiC-CNTs/AZ31 magnesium matrix composites prepared using laser cladding and high speed friction stir processing. Opt. Laser Technol. 2022, 152, 11. [Google Scholar] [CrossRef]
- Fang, Y.; Kim, M.K.; Zhang, Y.; Duan, Z.; Yuan, Q.; Suhr, J. Particulate-reinforced iron-based metal matrix composites fabricated by selective laser melting: A systematic review. J. Manuf. Process. 2022, 74, 592–639. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Jenn, M. Selective laser melting of TiC reinforced 316L stainless steel matrix nanocomposites: Influence of starting TiC particle size and volume content. Mater. Des. 2016, 104, 141–151. [Google Scholar] [CrossRef]
- Wei, W.H.; Zhang, Q.; Wu, W.J.; Cao, H.Z.; Shen, J.; Fan, S.Q.; Duan, X.M. Agglomeration-free nanoscale TiC reinforced titanium matrix composites achieved by in-situ laser additive manufacturing. Scr. Mater. 2020, 187, 310–316. [Google Scholar] [CrossRef]
- Gao, Z.A.N.; Wang, L.L.; Wang, Y.N.; Lyu, F.Y.; Zhan, X.H. Crack defects and formation mechanism of FeCoCrNi high entropy alloy coating on TC4 titanium alloy prepared by laser cladding. J. Alloys Compd. 2022, 903, 19. [Google Scholar] [CrossRef]
- Cai, Y.C.; Luo, Z.; Chen, Y.; Ma, C.Y.; Song, R.F. Influence of coating thickness on the morphology of TiC particles. Surf. Eng. 2017, 33, 521–528. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.M.; Zhang, S.Q.; He, B. Optimization of the dilution parameters to improve wear resistance of laser cladding 15-5PH steel coating on U75V pearlitic steel. Surf. Coat. Technol. 2023, 465, 12. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Z.Y.; Zhang, Y.Q.; Wei, S.Z.; Yang, L.Q.; Yang, K. Segregation in the magnesium/aluminum composites formed by cold arc cladding. MCP 2021, 266, 14. [Google Scholar] [CrossRef]
- Ao, S.S.; Wang, T.; Huang, Y.Z.; Dai, Y.; Cai, Y.C.; Luo, Z. The Wear Properties of TiC/Al-Based Composite Coating Applied by Laser Cladding. Metals 2018, 8, 975. [Google Scholar] [CrossRef]
- Ya, W.; Pathiraj, B.; Matthew, D.T.A.; Bright, M.; Melzer, S. Cladding of Tribaloy T400 on steel substrates using a high power Nd:YAG laser. Surf. Coat. Technol. 2018, 350, 323–333. [Google Scholar] [CrossRef]
- Cen, L.; Du, W.B.; Gong, M.C.; Lu, Y.; Zhang, C.; Gao, M. Effect of high-frequency beam oscillation on microstructures and cracks in laser cladding of Al-Cu-Mg alloys. Surf. Coat. Technol. 2022, 447, 10. [Google Scholar] [CrossRef]
- Chu, F.Z.; Li, E.L.; Shen, H.P.; Chen, Z.E.; Li, Y.X.; Liu, H.; Min, S.L.; Tian, X.N.; Zhang, K.; Zhou, Z.Y.; et al. Influence of powder size on defect generation in laser powder bed fusion of AlSi10Mg alloy. J. Manuf. Process. 2023, 94, 183–195. [Google Scholar] [CrossRef]
- Qi, Z.W.; Chen, C.Y.; Wang, C.Y.; Zhou, Z.K.; Zhou, J.; Long, Y. Effect of different laser wavelengths on laser cladding of pure copper. Surf. Coat. Technol. 2023, 454, 11. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, C.Y.; Zhou, Z.K.; Long, H.Y.; Jia, J.; He, L.G.; Long, Y. Dissimilar laser lap welding of Mg and Al alloys using a CoCrFeNi medium-entropy alloy interlayer. Opt. Laser Technol. 2023, 157, 16. [Google Scholar] [CrossRef]
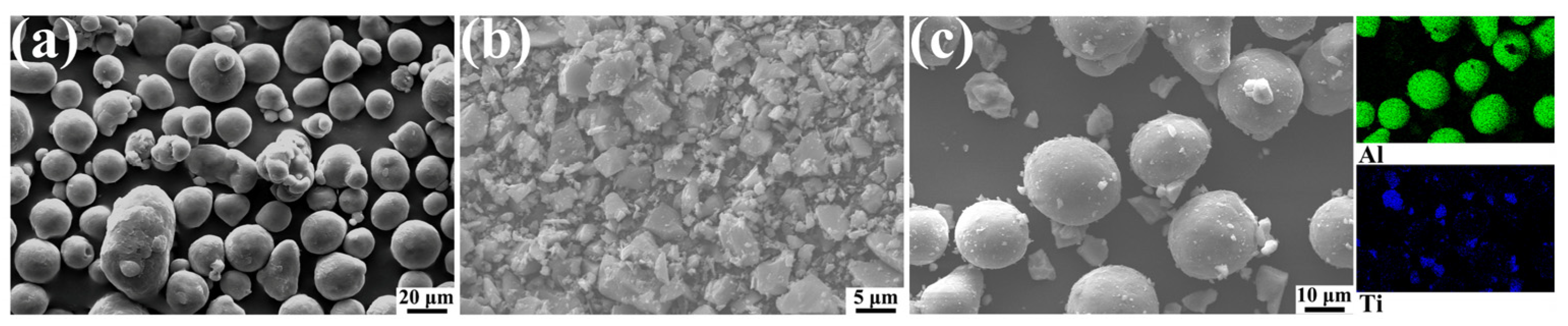
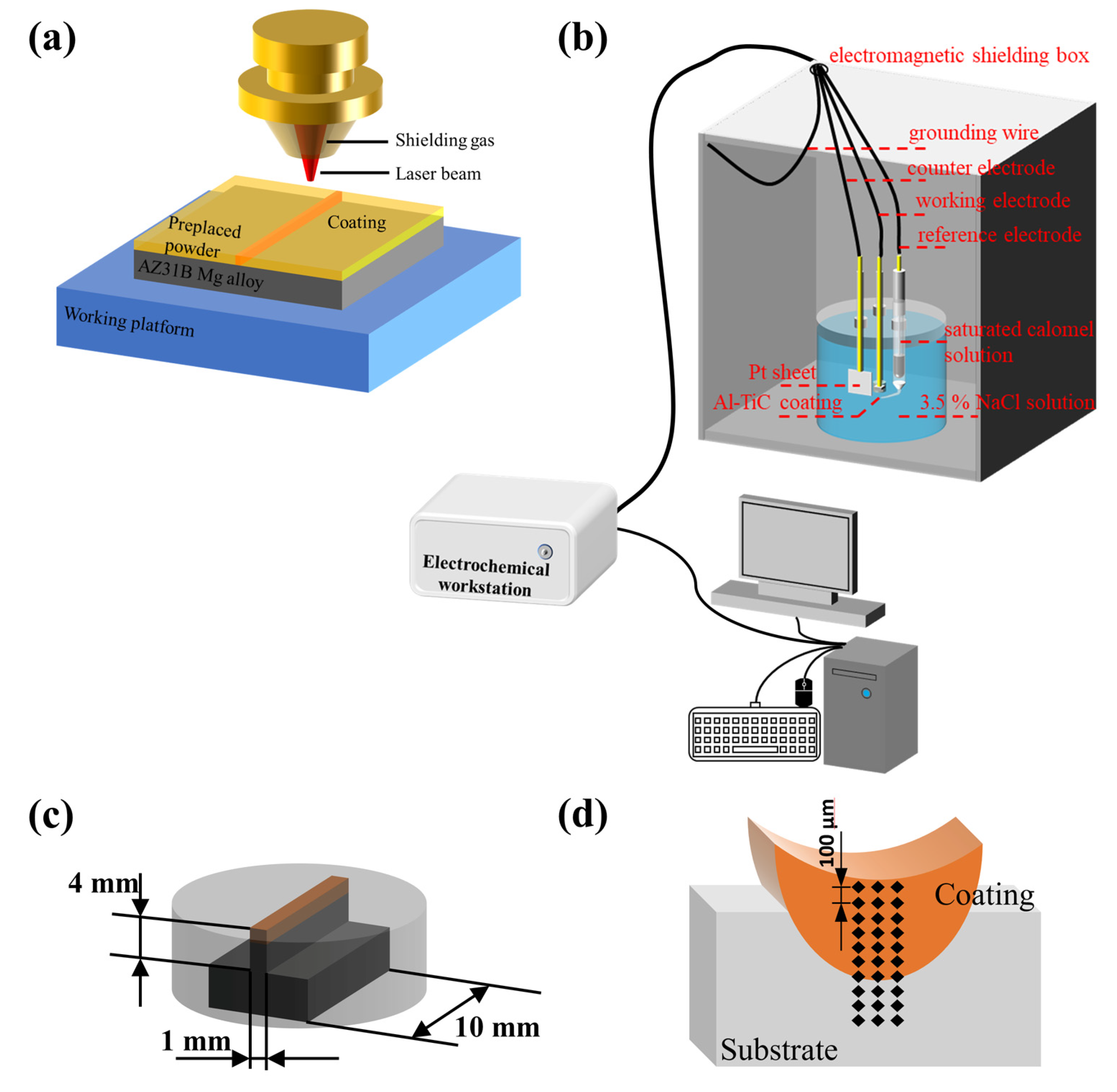
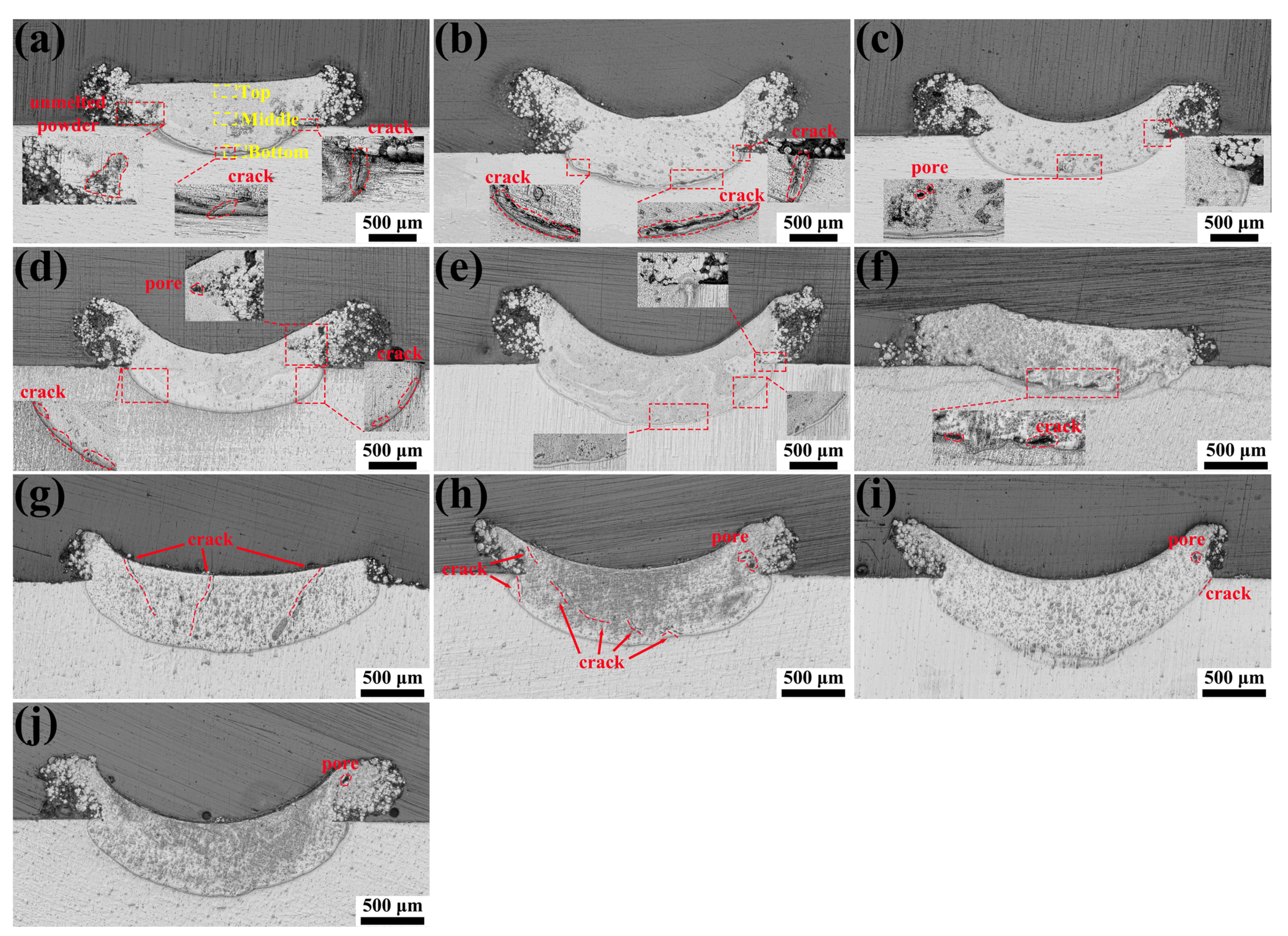
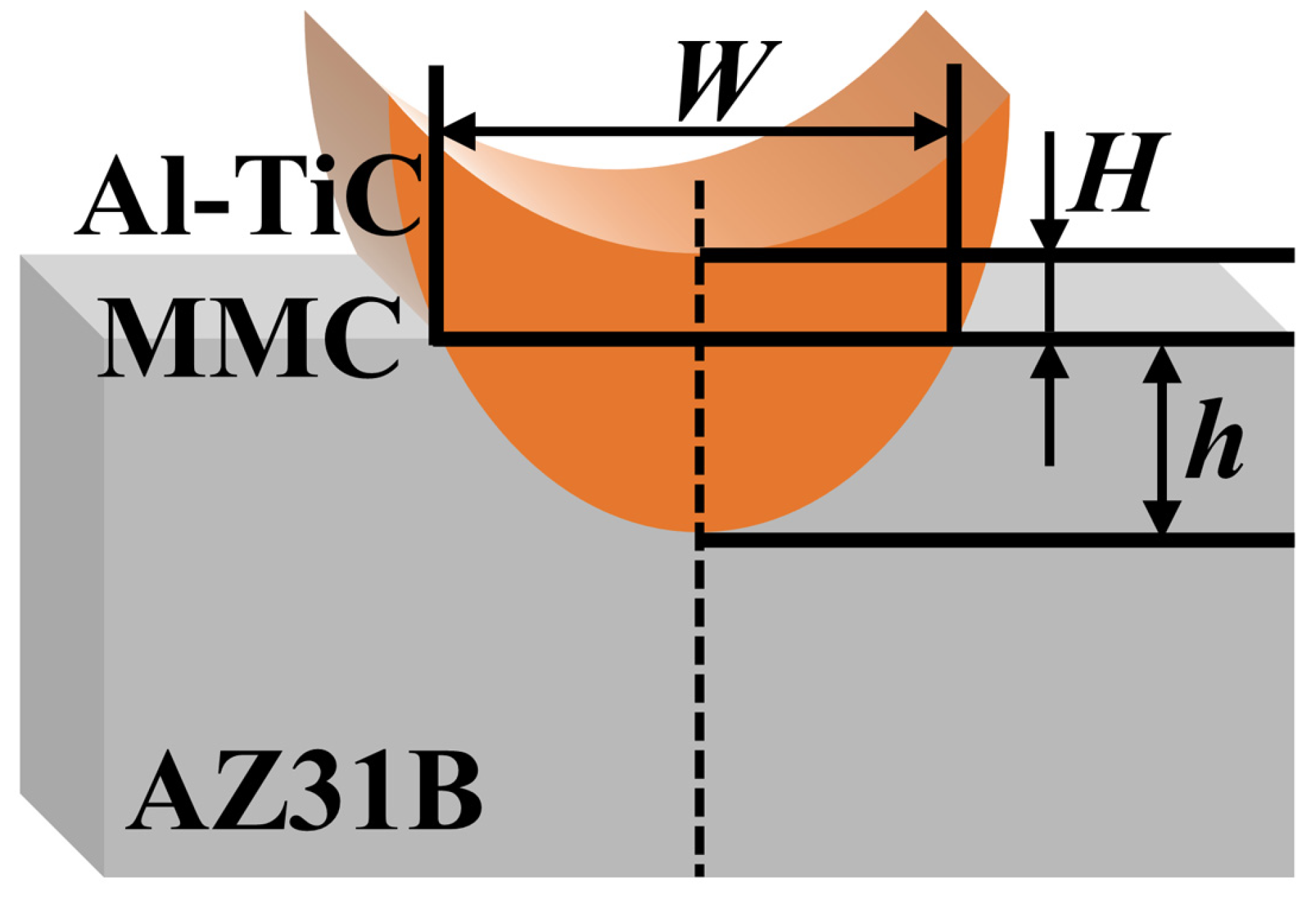


| Al | Zn | Mn | Si | Fe | Cu | Ni | Mg |
|---|---|---|---|---|---|---|---|
| 3.02 | 0.65 | 0.35 | 0.16 | 0.003 | 0.01 | 0.001 | Bal. |
| Site | Mg | Al | Ti | Site | Mg | Al | Ti |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 100 | 18 | 49.57 | 50.39 | 0.04 |
| 2 | 13.94 | 85.85 | 0.21 | 19 | 26.07 | 73.75 | 0.17 |
| 3 | 13.17 | 86.67 | 0.16 | 20 | 33.91 | 67.93 | 0.16 |
| 4 | 60.72 | 39.24 | 0.04 | 21 | 38.52 | 61.42 | 0.06 |
| 5 | 44.68 | 54.93 | 0.38 | 22 | 22.31 | 77.59 | 0.10 |
| 6 | 18.25 | 81.60 | 0.15 | 23 | 36.98 | 62.80 | 0.22 |
| 7 | 9.36 | 90.42 | 0.22 | 24 | 12.87 | 86.79 | 0.34 |
| 8 | 10.67 | 89.05 | 0.28 | 25 | 63.60 | 36.39 | 0.01 |
| 9 | 16.70 | 82.18 | 0.12 | 26 | 46.34 | 53.63 | 0.03 |
| 10 | 62.52 | 37.48 | 0 | 27 | 23.89 | 76.02 | 0.09 |
| 11 | 45.59 | 54.38 | 0.03 | 28 | 40.51 | 59.43 | 0.06 |
| 12 | 13.71 | 86.11 | 0.18 | 29 | 20.17 | 79.55 | 0.28 |
| 13 | 33.69 | 66.27 | 0.04 | 30 | 20.37 | 79.40 | 0.23 |
| 14 | 22.39 | 77.51 | 0.10 | 31 | 23.81 | 76.06 | 0.13 |
| 15 | 28.64 | 68.89 | 2.47 | 32 | 25.04 | 74.91 | 0.05 |
| 16 | 24.77 | 75.10 | 0.13 | 33 | 46.42 | 53.54 | 0.04 |
| 17 | 60.92 | 39.05 | 0.03 | 34 | 64.05 | 35.93 | 0.02 |
| Site | Mg | Al | Ti | Site | Mg | Al | Ti |
|---|---|---|---|---|---|---|---|
| 35 | 0 | 0 | 100 | 47 | 49.83 | 50.08 | 0.09 |
| 36 | 46.24 | 50.66 | 3.10 | 48 | 53.77 | 46.00 | 0.23 |
| 37 | 55.45 | 44.22 | 0.33 | 49 | 50.83 | 48.83 | 0.34 |
| 38 | 67.31 | 32.42 | 0.27 | 50 | 56.81 | 42.95 | 0.24 |
| 39 | 52.48 | 45.65 | 1.87 | 51 | 55.19 | 44.60 | 0.21 |
| 40 | 48.18 | 51.50 | 0.32 | 52 | 42.18 | 57.52 | 0.30 |
| 41 | 47.13 | 52.65 | 0.47 | 53 | 20.73 | 76.66 | 2.61 |
| 42 | 43.88 | 55.65 | 0.47 | 54 | 44.65 | 55.03 | 0.31 |
| 43 | 51.69 | 47.97 | 0.34 | 55 | 53.99 | 45.82 | 0.19 |
| 44 | 47.44 | 52.34 | 0.22 | 56 | 64.83 | 35.07 | 0.10 |
| 45 | 49.66 | 49.93 | 0.41 | 57 | 53.97 | 45.78 | 0.25 |
| 46 | 47.07 | 52.68 | 0.25 |
| 10% TiC | Icorr (A/cm2) | Ecorr (V) | Epit (V) | 30% TiC | Icorr (A/cm2) | Ecorr (V) | Epit (V) |
|---|---|---|---|---|---|---|---|
| substrate | 5.45 × 10−4 | −1.68 | - | substrate | 5.45 × 10−4 | −1.68 | - |
| 1500 W | 4.02 × 10−6 | −0.89 | −0.85 | 1500 W | 1.90 × 10−5 | −1.07 | −0.92 |
| 1700 W | 1.00 × 10−5 | −1.25 | −0.88 | 1700 W | 2.88 × 10−5 | −1.24 | −1.03 |
| 1900 W | 3.90 × 10−7 | −0.90 | −0.89 | 1900 W | 3.23 × 10−6 | −1.16 | −1.04 |
| 2100 W | 9.33 × 10−6 | −1.22 | −0.91 | 2100 W | 1.09 × 10−5 | −1.14 | −1.08 |
| 2300 W | 8.12 × 10−6 | −1.20 | −0.92 | 2300 W | 1.99 × 10−5 | −1.26 | −1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi, H.; Zhi, G.; Chen, C.; Li, C.; Zhou, J.; Long, Y. Microstructure, Hardness and Corrosion Resistance of Al-TiC MMC Prepared by Laser Cladding on AZ31B Magnesium Alloy. Coatings 2024, 14, 211. https://doi.org/10.3390/coatings14020211
Pi H, Zhi G, Chen C, Li C, Zhou J, Long Y. Microstructure, Hardness and Corrosion Resistance of Al-TiC MMC Prepared by Laser Cladding on AZ31B Magnesium Alloy. Coatings. 2024; 14(2):211. https://doi.org/10.3390/coatings14020211
Chicago/Turabian StylePi, Haiwen, Geng Zhi, Canyang Chen, Chen Li, Jun Zhou, and Yu Long. 2024. "Microstructure, Hardness and Corrosion Resistance of Al-TiC MMC Prepared by Laser Cladding on AZ31B Magnesium Alloy" Coatings 14, no. 2: 211. https://doi.org/10.3390/coatings14020211




