Microstructure, Mechanical Properties, and Lead–Bismuth Eutectic Corrosion Behaviors of FeCrAlY-Al2O3 Nanoceramic Composite Coatings
Abstract
:1. Introduction
2. Experiment
2.1. Material Preparation
2.2. Coating Characterization
2.3. Static LBE Corrosion Test
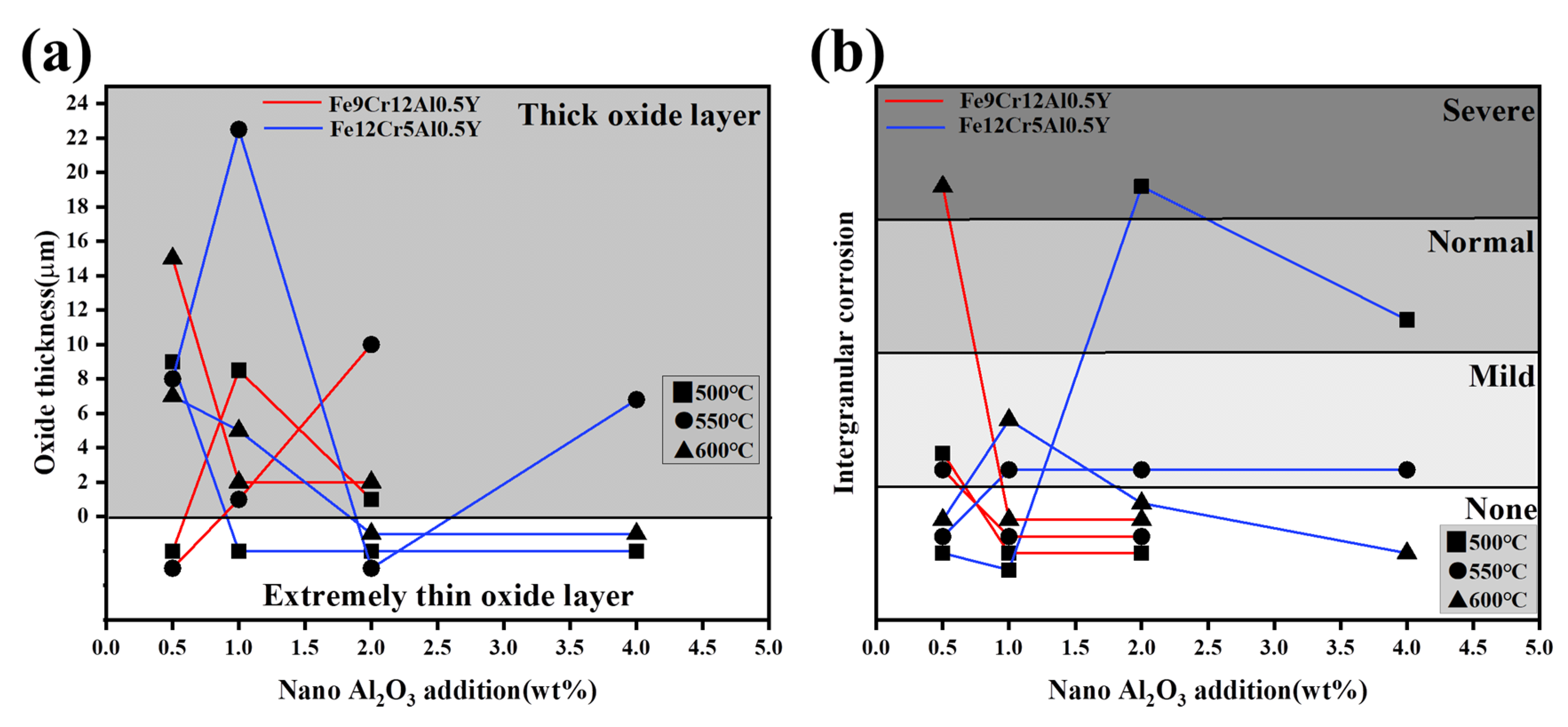
3. Results and Discussion
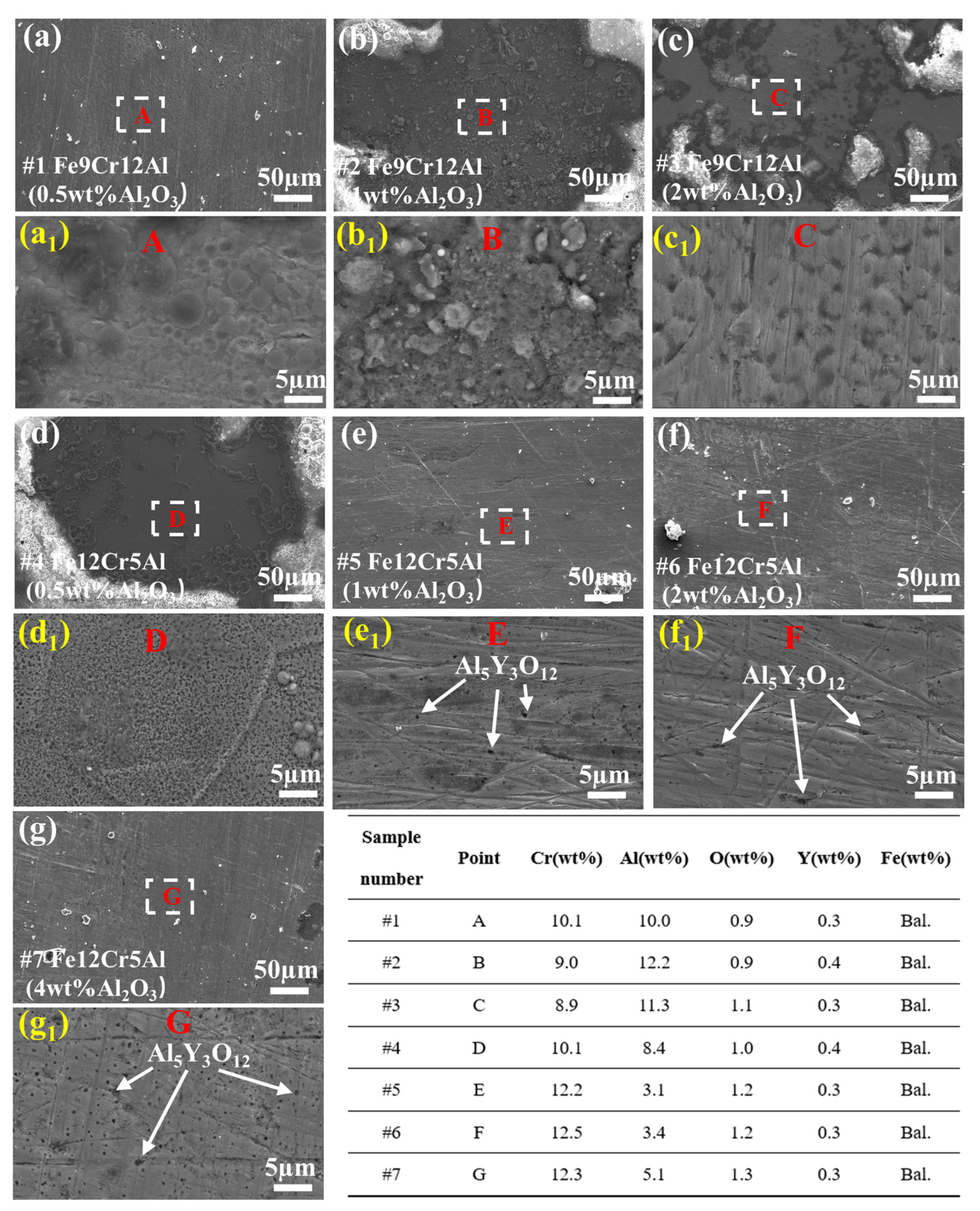
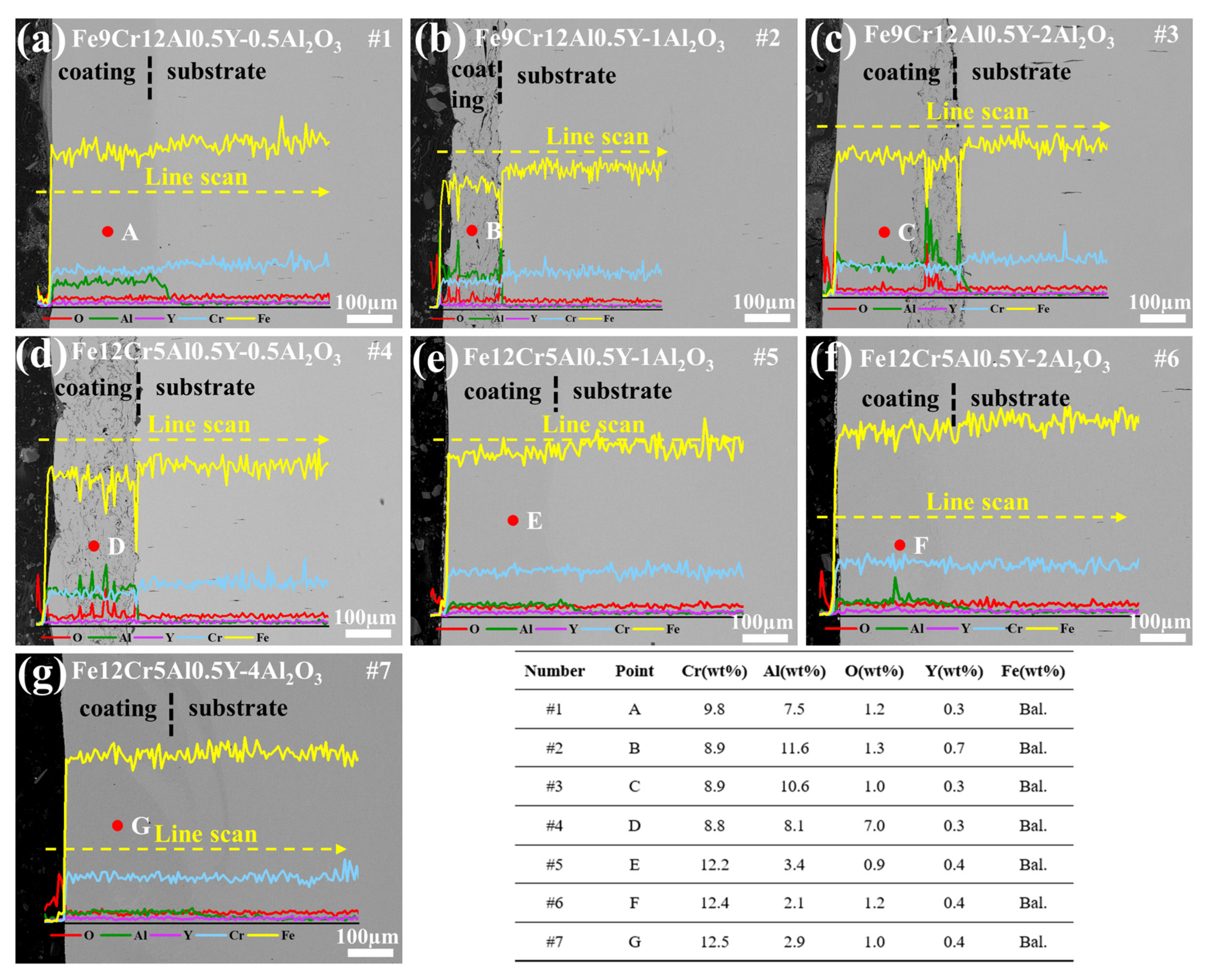
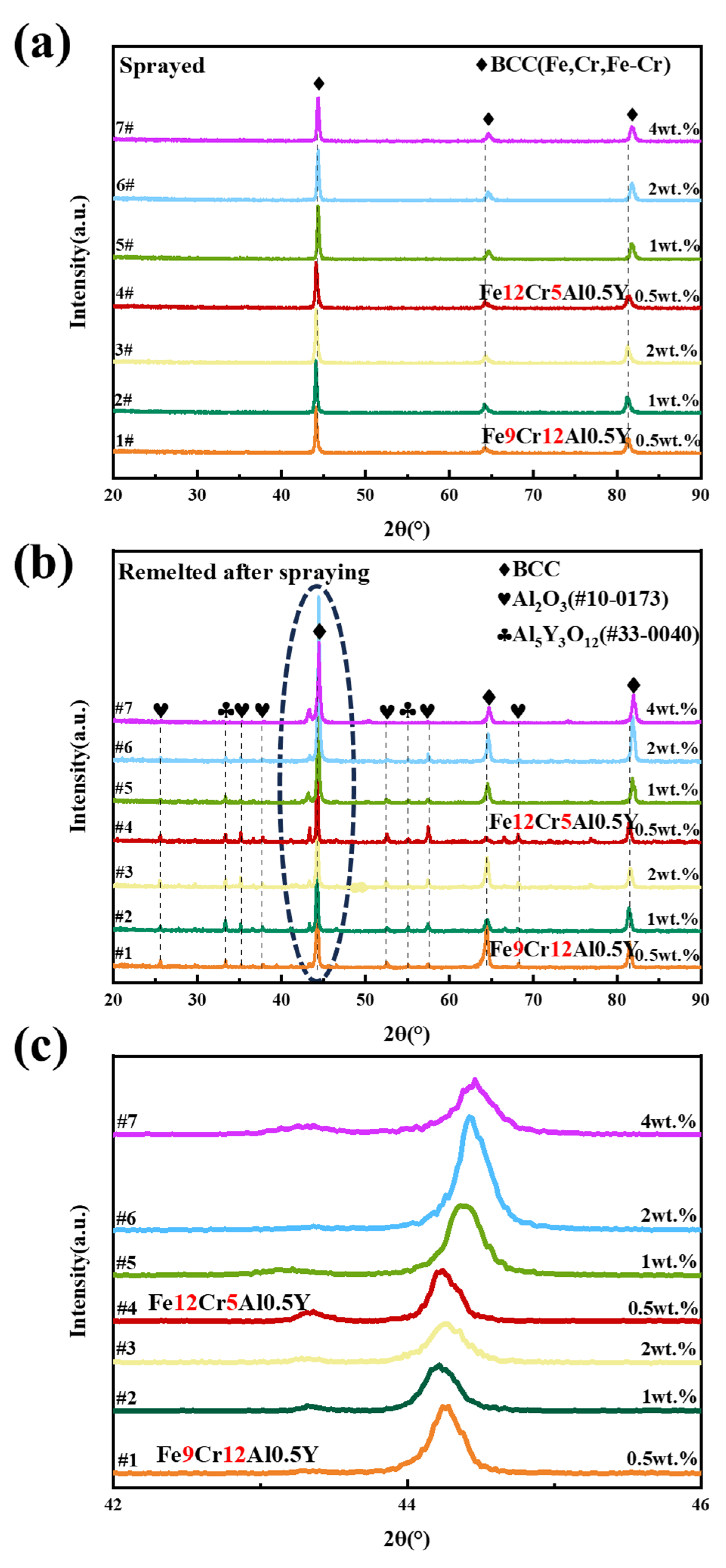
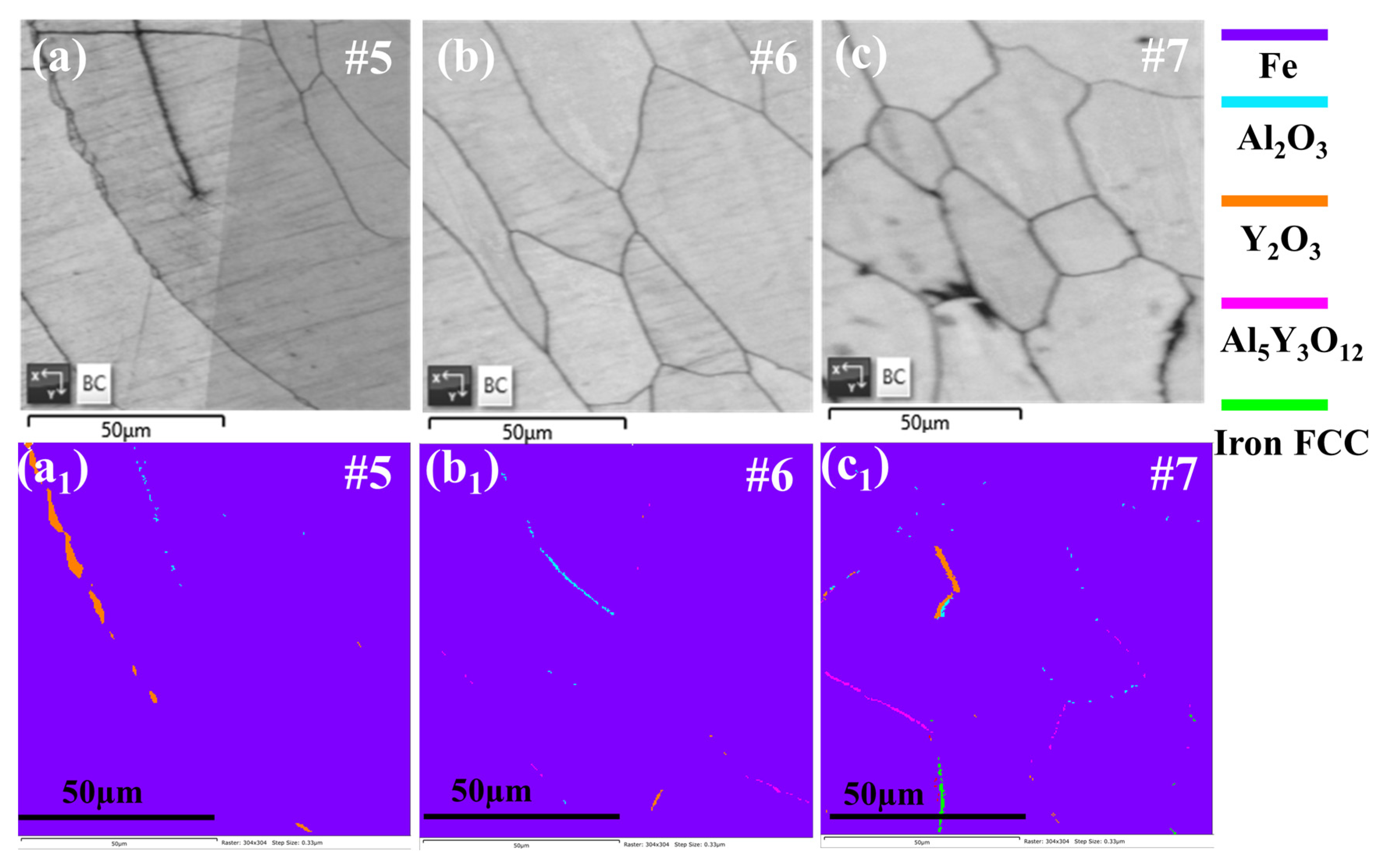
3.1. Microstructure
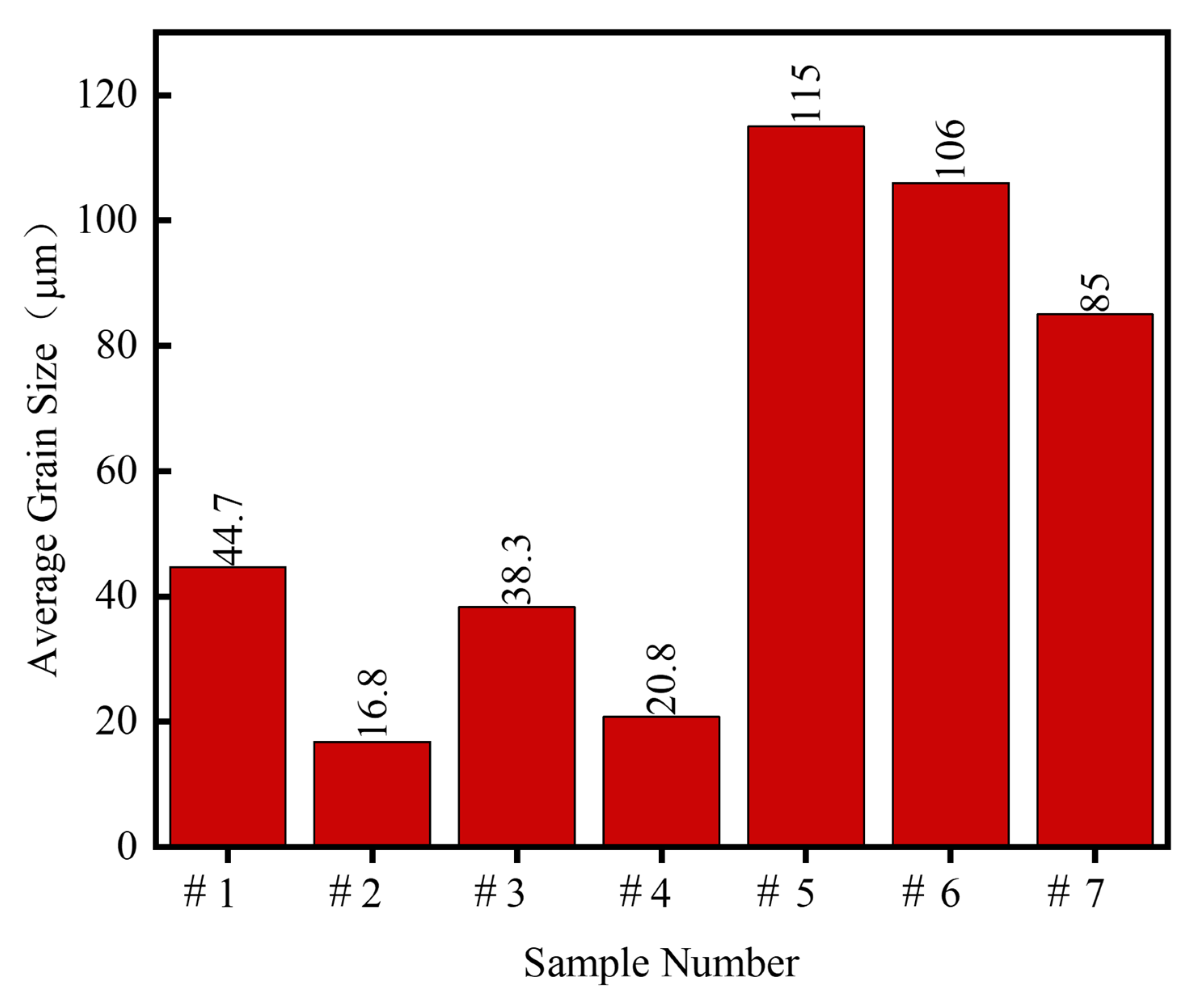
3.2. Mechanical Properties
3.3. LBE Corrosion Experiments
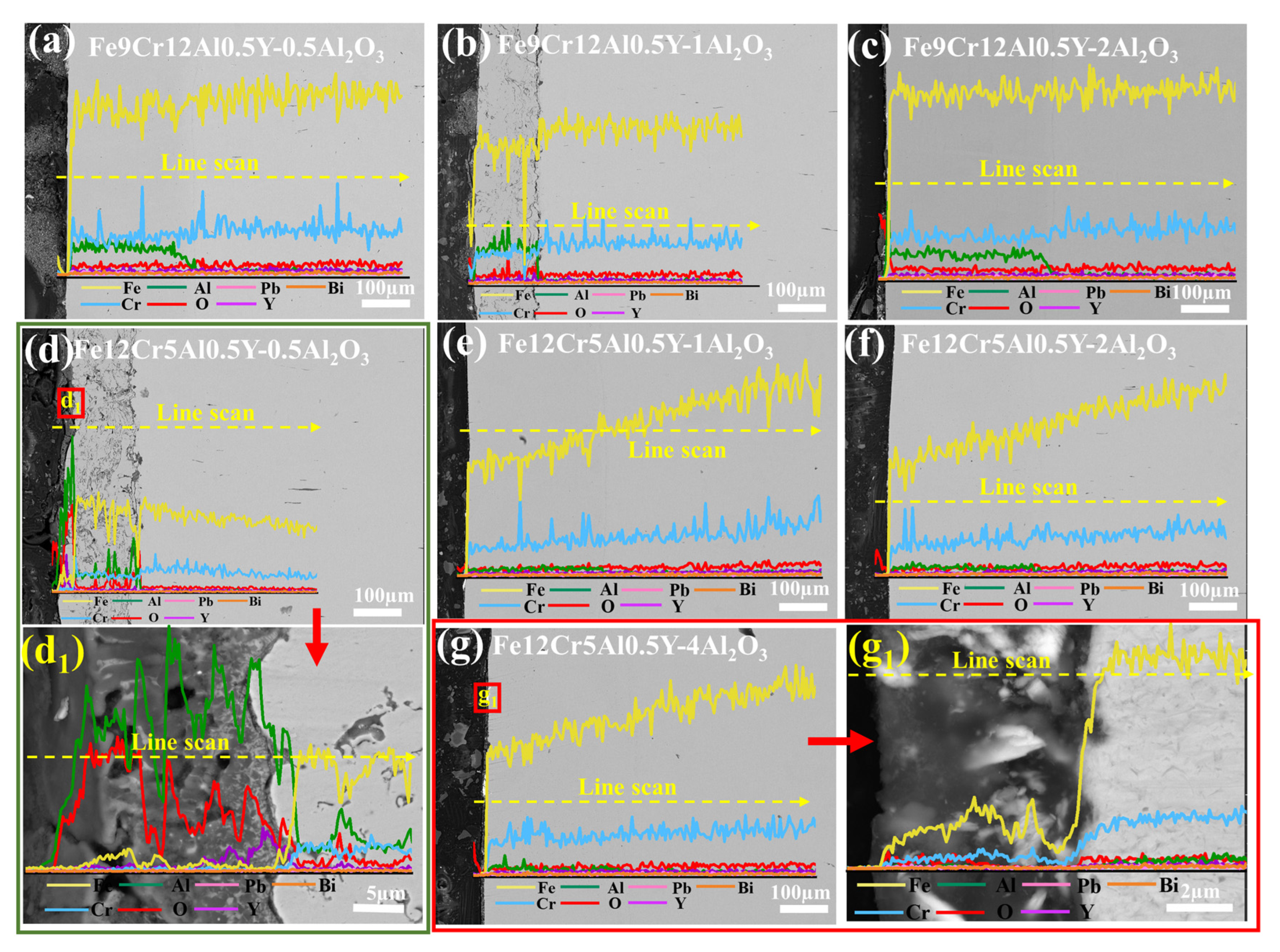
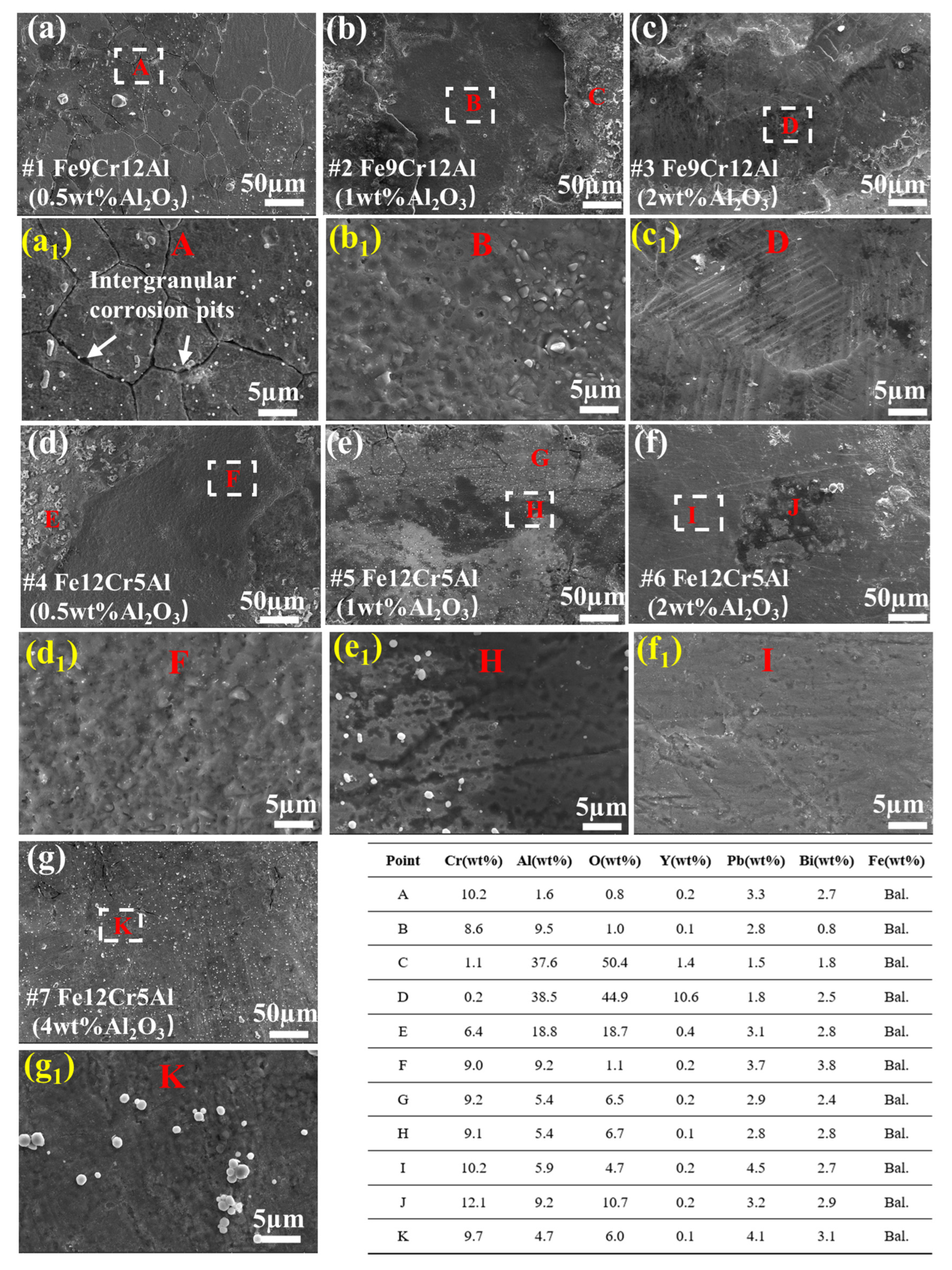
3.3.1. Coatings Corroded at 500 °C for 1000 h
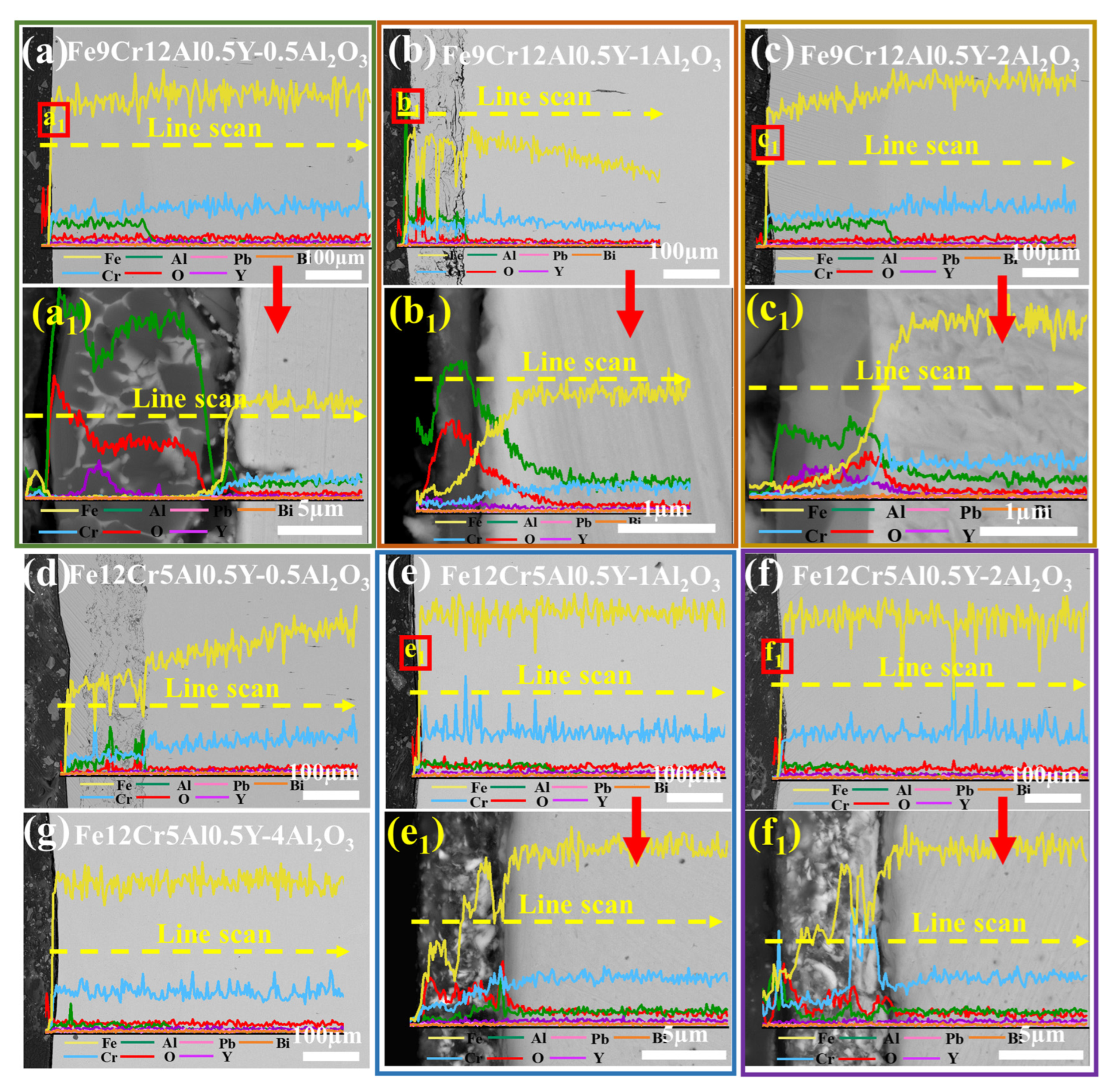
3.3.2. Coatings Corroded at 550 °C for 1000 h
3.3.3. Coatings Corroded at 600 °C for 1000 h
4. Discussion
4.1. Microstructure of FeCrAlY-Al2O3 Composite Coatings
4.2. Mechanical Properties of FeCrAlY-Al2O3 Composite Coatings
4.3. LBE Corrosion Behavior of FeCrAlY-Al2O3 Composite Coatings
5. Conclusions
- (1)
- With the increase in Al content, the grains in the coatings are gradually refined. The added nano-Al2O3 exacerbates the coatings elemental segregation and the second-phase precipitation;
- (2)
- The addition of nano-Al2O3 results in second-phase reinforcement and grain refinement can improve the mechanical properties of FeCrAlY coatings, especially in terms of hardness;
- (3)
- Intergranular corrosion is observed after LBE corrosion tests. This phenomenon, which is sensitive to the corrosion temperature, is closely related to the Cr and Al content of the coating.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abram, T.; Ion, S. Generation-IV nuclear power: A review of the state of the science. Energy Policy 2008, 36, 4323–4330. [Google Scholar] [CrossRef]
- Lorusso, P.; Bassini, S.; Del Nevo, A.; Di Piazza, I.; Giannetti, F.; Tarantino, M.; Utili, M. GEN-IV LFR development: Status & perspectives. Prog. Nucl. Energy 2018, 105, 318–331. [Google Scholar] [CrossRef]
- Grasso, G.; Petrovich, C.; Mattioli, D.; Artioli, C.; Sciora, P.; Gugiu, D.; Bandini, G.; Bubelis, E.; Mikityuk, K. The core design of ALFRED, a demonstrator for the European lead-cooled reactors. Nucl. Eng. Des. 2014, 278, 287–301. [Google Scholar] [CrossRef]
- Alemberti, A.; Smirnov, V.; Smith, C.F.; Takahashi, M. Overview of lead-cooled fast reactor activities. Prog. Nucl. Energy 2014, 77, 300–307. [Google Scholar] [CrossRef]
- Weisenburger, A.; Schroer, C.; Jianu, A.; Heinzel, A.; Konys, J.; Steiner, H.; Müller, G.; Fazio, C.; Gessi, A.; Babayan, S.; et al. Long term corrosion on T91 and AISI1 316L steel in flowing lead alloy and corrosion protection barrier development: Experiments and models. J. Nucl. Mater. 2011, 415, 260–269. [Google Scholar] [CrossRef]
- Chen, G.; Lei, Y.; Zhu, Q.; Ju, N.; Li, T.; Wang, D. Corrosion behavior of CLAM steel weld bead in flowing Pb-Bi at 550 °C. J. Nucl. Mater. 2019, 515, 187–198. [Google Scholar] [CrossRef]
- Gong, X.; Short, M.P.; Auger, T.; Charalampopoulou, E.; Lambrinou, K. Environmental degradation of structural materials in liquid lead- and lead-bismuth eutectic-cooled reactors. Prog. Mater. Sci. 2022, 126, 100920. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Martín-Muñoz, F.J.; Soler-Crespo, L.; Gómez-Briceño, D. Corrosion behaviour of martensitic and austenitic steels in flowing lead–bismuth eutectic. J. Nucl. Mater. 2011, 416, 87–93. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Martinelli, L.; Jean-Louis, C.; Fanny, B.-C. Oxidation of steels in liquid lead bismuth: Oxygen control to achieve efficient corrosion protection. Nucl. Eng. Des. 2011, 241, 1288–1294. [Google Scholar] [CrossRef]
- Ma, H.; Gong, X.; Zhu, J.; Chen, H.; Li, J.; Liu, Y.; Ren, Q.; Zhang, F.; Pang, Z. Liquid metal embrittlement susceptibility of an oxide-dispersion strengthened FeCrAl alloy in contact with liquid lead-bismuth eutectic at 350 °C. Corros. Sci. 2022, 207, 110603. [Google Scholar] [CrossRef]
- Tan, L.; Machut, M.T.; Sridharan, K.; Allen, T.R. Corrosion behavior of a ferritic/martensitic steel HCM12A exposed to harsh environments. J. Nucl. Mater. 2007, 371, 161–170. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S.; Kaito, T.; de Carlan, Y.; Ribis, J.; Malaplate, J. Oxide dispersion-strengthened/ferrite-martensite steels as core materials for Generation IV nuclear reactors. In Structural Materials for Generation IV Nuclear Reactors; Woodhead Publishing: Cambridge, UK, 2017; pp. 357–414. [Google Scholar]
- Serag, E.; Caers, B.; Schuurmans, P.; Lucas, S.; Haye, E. Challenges and coating solutions for wear and corrosion inside Lead Bismuth Eutectic: A review. Surf. Coat. Technol. 2022, 441, 128542. [Google Scholar] [CrossRef]
- Ferré, F.G.; Mairov, A.; Iadicicco, D.; Vanazzi, M.; Bassini, S.; Utili, M.; Tarantino, M.; Bragaglia, M.; Lamastra, F.R.; Nanni, F.; et al. Corrosion and radiation resistant nanoceramic coatings for lead fast reactors. Corros. Sci. 2017, 124, 80–92. [Google Scholar] [CrossRef]
- Utili, M.; Agostini, M.; Coccoluto, G.; Lorenzini, E. Ti3SiC2 as a candidate material for lead cooled fast reactor. Nucl. Eng. Des. 2011, 241, 1295–1300. [Google Scholar] [CrossRef]
- Glasbrenner, H.; Gröschel, F. Exposure of pre-stressed T91 coated with TiN, CrN and DLC to Pb–55.5Bi. J. Nucl. Mater. 2006, 356, 213–221. [Google Scholar] [CrossRef]
- Heinzel, A.; Weisenburger, A.; Müller, G. Long-term corrosion tests of Ti3SiC2 and Ti2AlC in oxygen containing LBE at temperatures up to 700 °C. J. Nucl. Mater. 2016, 482, 114–123. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhao, X.; Liu, Y.; Cai, Y.; Li, J.Y.; Chen, H.; Wan, Q.; Yang, D.; Tan, J.; Liu, H.D.; et al. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- García Ferré, F.; Ormellese, M.; Di Fonzo, F.; Beghi, M.G. Advanced Al2O3 coatings for high temperature operation of steels in heavy liquid metals: A preliminary study. Corros. Sci. 2013, 77, 375–378. [Google Scholar] [CrossRef]
- Wan, Q.; Wu, Z.Y.; Liu, Y.; Yang, B.; Liu, H.D.; Ren, F.; Wang, P.; Xiao, Y.Y.; Zhang, J.; Zhang, G.D. Lead-bismuth eutectic (LBE) corrosion mechanism of nano-amorphous composite TiSiN coatings synthesized by cathodic arc ion plating. Corros. Sci. 2021, 183, 109264. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, W.; Chen, Q.; Yang, J.; Zhu, C.; Li, Q.; Yang, J.; Liu, N.; Yang, J. Effect of LBE corrosion on microstructure of amorphous Al2O3 coating by magnetron sputtering. Surf. Coat. Technol. 2022, 443, 128598. [Google Scholar] [CrossRef]
- Lapauw, T.; Tunca, B.; Joris, J.; Jianu, A.; Fetzer, R.; Weisenburger, A.; Vleugels, J.; Lambrinou, K. Interaction of Mn+1AXn phases with oxygen-poor, static and fast-flowing liquid lead-bismuth eutectic. J. Nucl. Mater. 2019, 520, 258–272. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, C.; Zhang, W.; Yang, J.; Deng, J.; Li, Q.; Liu, H.; Zhou, Y.; Yue, H.; Qiu, X.; et al. Exploring the application potential of FexAl/Al2O3 coatings for lead-cooled fast reactors. J. Nucl. Mater. 2024, 588, 154807. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Q.; Zhao, Y.; Zhou, Y.; Zhang, W.; Yang, J.; Zhu, C.; Deng, J.; Chen, Q.; Zhao, S.; et al. Irradiation effects on microstructure, mechanical properties, and lead-bismuth eutectic corrosion resistance of alumina coating. J. Mater. Res. Technol. 2023, 25, 2014–2028. [Google Scholar] [CrossRef]
- Fetzer, R.; Weisenburger, A.; Jianu, A.; Müller, G. Oxide scale formation of modified FeCrAl coatings exposed to liquid lead. Corros. Sci. 2012, 55, 213–218. [Google Scholar] [CrossRef]
- Weisenburger, A.; Heinzel, A.; Müller, G.; Muscher, H.; Rousanov, A. T91 cladding tubes with and without modified FeCrAlY coatings exposed in LBE at different flow, stress and temperature conditions. J. Nucl. Mater. 2008, 376, 274–281. [Google Scholar] [CrossRef]
- Kurata, Y.; Yokota, H.; Suzuki, T. Development of aluminum-alloy coating on type 316SS for nuclear systems using liquid lead–bismuth. J. Nucl. Mater. 2012, 424, 237–246. [Google Scholar] [CrossRef]
- Dai, Y.; Boutellier, V.; Gavillet, D.; Glasbrenner, H.; Weisenburger, A.; Wagner, W. FeCrAlY and TiN coatings on T91 steel after irradiation with 72MeV protons in flowing LBE. J. Nucl. Mater. 2012, 431, 66–76. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Xiao, J.; Sun, Y.; Zhao, K.; Wu, J.; Sui, X.; Wang, H.; Chen, Y. A high-entropy alloy nitride protective coating for fuel cladding in high temperature lead-bismuth eutectic alloy. J. Nucl. Mater. 2022, 568, 153888. [Google Scholar] [CrossRef]
- Deng, J.; Yang, J.; Lv, L.; Zhang, W.; Chen, Q.; Zhou, M.; Zhu, C.; Liu, N.; Yang, J. Corrosion behavior of refractory TiNbZrMoV high-entropy alloy coating in static lead-bismuth eutectic alloy: A novel design strategy of LBE corrosion-resistant coating? Surf. Coat. Technol. 2022, 448, 128884. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, Y.; Qiu, X.; Li, Q.; Yue, H.; Zhou, Y.; Deng, J.; Yang, J.; Liu, H.; Li, Q.; et al. Screening of the FeCrAl LBE corrosion-resistant coatings: The effect of Cr and Al contents. Surf. Coat. Technol. 2023, 462, 129477. [Google Scholar] [CrossRef]
- Short, M.P.; Ballinger, R.G.; Hänninen, H.E. Corrosion resistance of alloys F91 and Fe–12Cr–2Si in lead–bismuth eutectic up to 715 °C. J. Nucl. Mater. 2013, 434, 259–281. [Google Scholar] [CrossRef]
- Hussain, T.; Simms, N.J.; Nicholls, J.R.; Oakey, J.E. Fireside corrosion degradation of HVOF thermal sprayed FeCrAl coating at 700–800 °C. Surf. Coat. Technol. 2015, 268, 165–172. [Google Scholar] [CrossRef]
- Wang, H.; Liang, G.; Meng, C.; An, X.; Wang, Y.; He, X. A comparative study on the corrosion performance of four FeCrAl alloys with different Cr contents in contact with oxygen-saturated LBE. J. Mater. Res. Technol. 2023, 23, 3492–3504. [Google Scholar] [CrossRef]
- Edmondson, P.D.; Briggs, S.A.; Yamamoto, Y.; Howard, R.H.; Sridharan, K.; Terrani, K.A.; Field, K.G. Irradiation-enhanced α’ precipitation in model FeCrAl alloys. Scr. Mater. 2016, 116, 112–116. [Google Scholar] [CrossRef]
- Field, K.G.; Briggs, S.A.; Sridharan, K.; Howard, R.H.; Yamamoto, Y. Mechanical properties of neutron-irradiated model and commercial FeCrAl alloys. J. Nucl. Mater. 2017, 489, 118–128. [Google Scholar] [CrossRef]
- Rajput, A.; Ramkumar, J.; Mondal, K. Effect of addition of strong oxidizer and temperature on the cavitation erosion resistance of different microstructures made from a high carbon steel. Wear 2022, 494–495, 204245. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z.; Pang, S.; Li, X.; Dong, C.; Liaw, P.K. Coherent Precipitation and Strengthening in Compositionally Complex Alloys: A Review. Entropy 2018, 20, 878. [Google Scholar] [CrossRef]
- Müller, G.; Schumacher, G.; Strauß, D. Oxide scale growth on MCrAlY coatings after pulsed electron beam treatment. Surf. Coat. Technol. 1998, 108–109, 43–47. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Peng, Y.; Shen, G. In-situ synthesized novel eyeball-like Al2O3/TiC composite ceramics reinforced Fe-based alloy coating produced by laser cladding. Surf. Coat. Technol. 2020, 391, 125671. [Google Scholar] [CrossRef]
- Yue, J.; Liu, X.; Sui, Y.; Liu, C.; Sun, X.; Chen, W. Combined effect of Y2O3 nanoparticles and Si second-phase oxide on microstructure and wear resistance of plasma-clad steel coating. Surf. Coat. Technol. 2020, 403, 126348. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, W.; Su, J.; Luo, F.; Zhu, D.; Dong, Y. Plasma sprayed Al2O3/FeCrAl composite coatings for electromagnetic wave absorption application. Appl. Surf. Sci. 2012, 258, 2691–2696. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Zhang, Y.; Wei, S.; Wang, Y.; Kang, Y. In-situ TiC-Al3Ti reinforced Al-Mg composites with Y2O3 addition formed by laser cladding on AZ91D. Surf. Coat. Technol. 2020, 383, 125249. [Google Scholar] [CrossRef]
- Shen, J.; Chai, L.; Wang, H.; Wang, C.; Yuan, Q.; Guo, N.; Xiao, J.; Yin, X. Surface microstructures and properties of oxide-reinforced FeCrAl matrix composite coatings prepared by laser cladding on a ferritic-martensitic steel. J. Nucl. Mater. 2023, 578, 154345. [Google Scholar] [CrossRef]
- Ghosh, A.; Wu, W.; Sahu, B.P.; Wang, J.; Misra, A. Enabling plastic co-deformation of disparate phases in a laser rapid solidified Sr-modified Al–Si eutectic through partial-dislocation-mediated-plasticity in Si. Mater. Sci. Eng. A 2023, 885, 145648. [Google Scholar] [CrossRef]
- Hong, J.; Choi, H.; Lee, S.; Kim, J.K.; Koo, Y.m. Effect of Al content on magnetic properties of Fe-Al Non-oriented electrical steel. J. Magn. Magn. Mater. 2017, 439, 343–348. [Google Scholar] [CrossRef]
- Straumal, B.B.; Korneva, A.; Kuzmin, A.; Lopez, G.A.; Rabkin, E.; Straumal, A.B.; Gerstein, G.; Gornakova, A.S. The grain boundary wetting phenomena in the ti-containing high-entropy alloys: A review. Metals 2021, 11, 1881. [Google Scholar] [CrossRef]
- London, A.J.; Santra, S.; Amirthapandian, S.; Panigrahi, B.K.; Sarguna, R.M.; Balaji, S.; Vijay, R.; Sundar, C.S.; Lozano-Perez, S.; Grovenor, C.R.M. Effect of Ti and Cr on dispersion, structure and composition of oxide nano-particles in model ODS alloys. Acta Mater. 2015, 97, 223–233. [Google Scholar] [CrossRef]
- Karak, S.K.; Chudoba, T.; Witczak, Z.; Lojkowski, W.; Manna, I. Development of ultra high strength nano-Y2O3 dispersed ferritic steel by mechanical alloying and hot isostatic pressing. Mater. Sci. Eng. A 2011, 528, 7475–7483. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, J.; Guan, H.; Wang, M.; Cheng, X.; Liu, L.; Liu, X.; Wang, J.; Zhang, Y.; Zhang, Z.; et al. Study of pre-precipitated δ phase promoting deformation twinning and recrystallization behavior of Inconel 718 superalloy during hot compression. Mater. Des. 2023, 226, 111693. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; guan, F.; Li, G.; Fan, Z. Interface formation and strengthening mechanisms of Al/Mg bimetallic composite via compound casting with rare earth Ce introduction. Mater. Sci. Eng. A 2022, 854, 143830. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, H.; Lu, J.; Sun, G.; Du, L. Effect of grain size on mechanical property and corrosion behavior of a metastable austenitic stainless steel. Mater. Charact. 2022, 194, 112360. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, F.; Zhang, H.; Zhang, T.; Wang, H. Microstructure and element distribution of laser cladding TiCx-reinforced CrTi4-based composite coating with CeO2/Ce2O3. Mater. Lett. 2021, 283, 128772. [Google Scholar] [CrossRef]
- Gan, J.; Gong, Q.; Jiang, Y.; Chen, H.; Huang, Y.; Du, K.; Li, Y.; Zhao, M.; Lin, F.; Zhuang, D. Microstructure and high-temperature mechanical properties of second-phase enhanced Mo-La2O3-ZrC alloys post-treated by cross rolling. J. Alloys Compd. 2019, 796, 167–175. [Google Scholar] [CrossRef]
- Pint, B.A. Experimental observations in support of the dynamic-segregation theory to explain the reactive-element effect. Oxid. Met. 1996, 45, 1–37. [Google Scholar] [CrossRef]
- Pint, B.A. Optimization of reactive-element additions to improve oxidation performance of alumina-forming alloys. J. Am. Ceram. Soc. 2002, 86, 686–695. [Google Scholar]
- He, Y.; Liu, J.; Qiu, S.; Deng, Z.; Yang, Y.; McLean, A. Microstructure and high temperature mechanical properties of as-cast FeCrAl alloys. Mater. Sci. Eng. A 2018, 726, 56–63. [Google Scholar] [CrossRef]
- Dular, M.; Delgosha, O.C.; Petkovšek, M. Observations of cavitation erosion pit formation. Ultrason. Sonochem. 2013, 20, 1113–1120. [Google Scholar] [CrossRef]
- Gong, X.; Marmy, P.; Yin, Y. The role of oxide films in preventing liquid metal embrittlement of T91 steel exposed to liquid lead-bismuth eutectic. J. Nucl. Mater. 2018, 509, 401–407. [Google Scholar] [CrossRef]
- Popovic, M.P.; Chen, K.; Shen, H.; Stan, C.V.; Olmsted, D.L.; Tamura, N.; Asta, M.; Abad, M.D.; Hosemann, P. A study of deformation and strain induced in bulk by the oxide layers formation on a Fe-Cr-Al alloy in high-temperature liquid Pb-Bi eutectic. Acta Mater. 2018, 151, 301–309. [Google Scholar] [CrossRef]
- Weisenburger, A.; Jianu, A.; Doyle, S.; Bruns, M.; Fetzer, R.; Heinzel, A.; DelGiacco, M.; An, W.; Müller, G. Oxide scales formed on Fe–Cr–Al-based model alloys exposed to oxygen containing molten lead. J. Nucl. Mater. 2013, 437, 282–292. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gesmundo, F.; Hou, P.Y.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe–Al and Fe–Cr–Al alloys. Corros. Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Lim, J.; Nam, H.O.; Hwang, I.S.; Kim, J.H. A study of early corrosion behaviors of FeCrAl alloys in liquid lead–bismuth eutectic environments. J. Nucl. Mater. 2010, 407, 205–210. [Google Scholar] [CrossRef]
- Engelko, V.; Mueller, G.; Rusanov, A.; Markov, V.; Tkachenko, K.; Weisenburger, A.; Kashtanov, A.; Chikiryaka, A.; Jianu, A. Surface modification/alloying using intense pulsed electron beam as a tool for improving the corrosion resistance of steels exposed to heavy liquid metals. J. Nucl. Mater. 2011, 415, 270–275. [Google Scholar] [CrossRef]
- Fazio, C.; Balbaud, F. 2—Corrosion phenomena induced by liquid metals in Generation IV reactors. In Structural Materials for Generation IV Nuclear Reactors; Yvon, P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 23–74. [Google Scholar] [CrossRef]
- Xu, Z.; Song, L.; Zhao, Y.; Liu, S. The formation mechanism and effect of amorphous SiO2 on the corrosion behaviour of Fe-Cr-Si ODS alloy in LBE at 550 °C. Corros. Sci. 2021, 190, 109634. [Google Scholar] [CrossRef]






| Elements | Cr | W | V | Mn | Ta | Fe |
|---|---|---|---|---|---|---|
| wt% | 11.6 | 1.5 | 0.13 | 1.1 | 0.15 | Bal. |
| Spraying Distance (mm) | Electric Current (A) | Voltage (V) | Main Gas Flow (L/min) | Second Gas Flow (L/min) | Powder Feeding Speed (g/min) |
|---|---|---|---|---|---|
| 130 | 600 | 70 | 45 | 8 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhong, Y.; Zhang, W.; Liu, H.; Yang, J.; Zhu, C.; Deng, J.; Zhao, S.; Zhong, Y.; Zhou, M.; et al. Microstructure, Mechanical Properties, and Lead–Bismuth Eutectic Corrosion Behaviors of FeCrAlY-Al2O3 Nanoceramic Composite Coatings. Coatings 2024, 14, 393. https://doi.org/10.3390/coatings14040393
Li Q, Zhong Y, Zhang W, Liu H, Yang J, Zhu C, Deng J, Zhao S, Zhong Y, Zhou M, et al. Microstructure, Mechanical Properties, and Lead–Bismuth Eutectic Corrosion Behaviors of FeCrAlY-Al2O3 Nanoceramic Composite Coatings. Coatings. 2024; 14(4):393. https://doi.org/10.3390/coatings14040393
Chicago/Turabian StyleLi, Qingyu, Yilong Zhong, Wei Zhang, Hao Liu, Jian Yang, Changda Zhu, Jiuguo Deng, Sha Zhao, Yuxin Zhong, Mingyang Zhou, and et al. 2024. "Microstructure, Mechanical Properties, and Lead–Bismuth Eutectic Corrosion Behaviors of FeCrAlY-Al2O3 Nanoceramic Composite Coatings" Coatings 14, no. 4: 393. https://doi.org/10.3390/coatings14040393





