The Growth Behavior and Mechanisms of Pitting Corrosion on Super 13Cr Martensitic Stainless Steel Surfaces at Different Diameter-to-Depth Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Testing
2.2. The Finite Element Model
3. Results
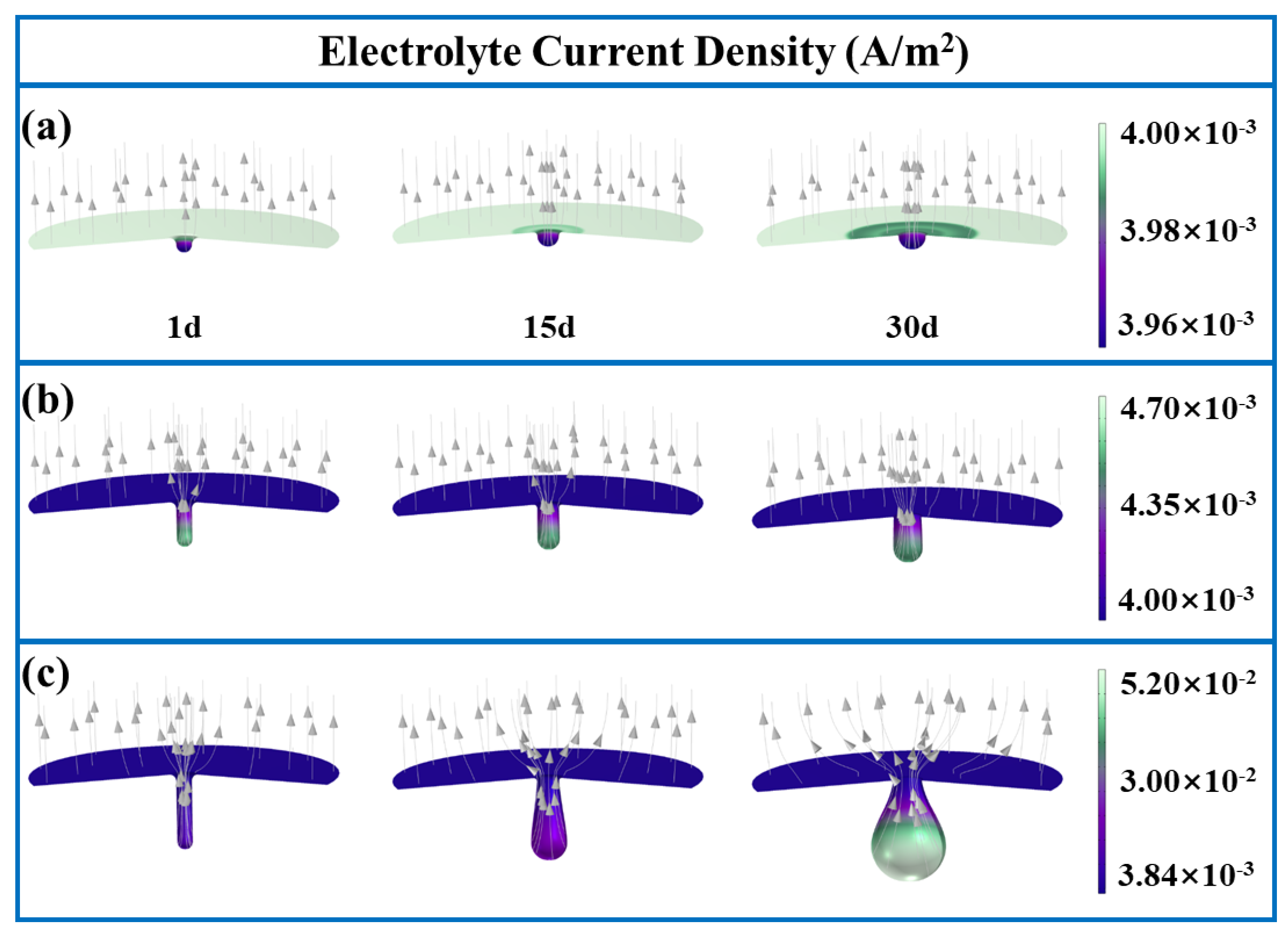
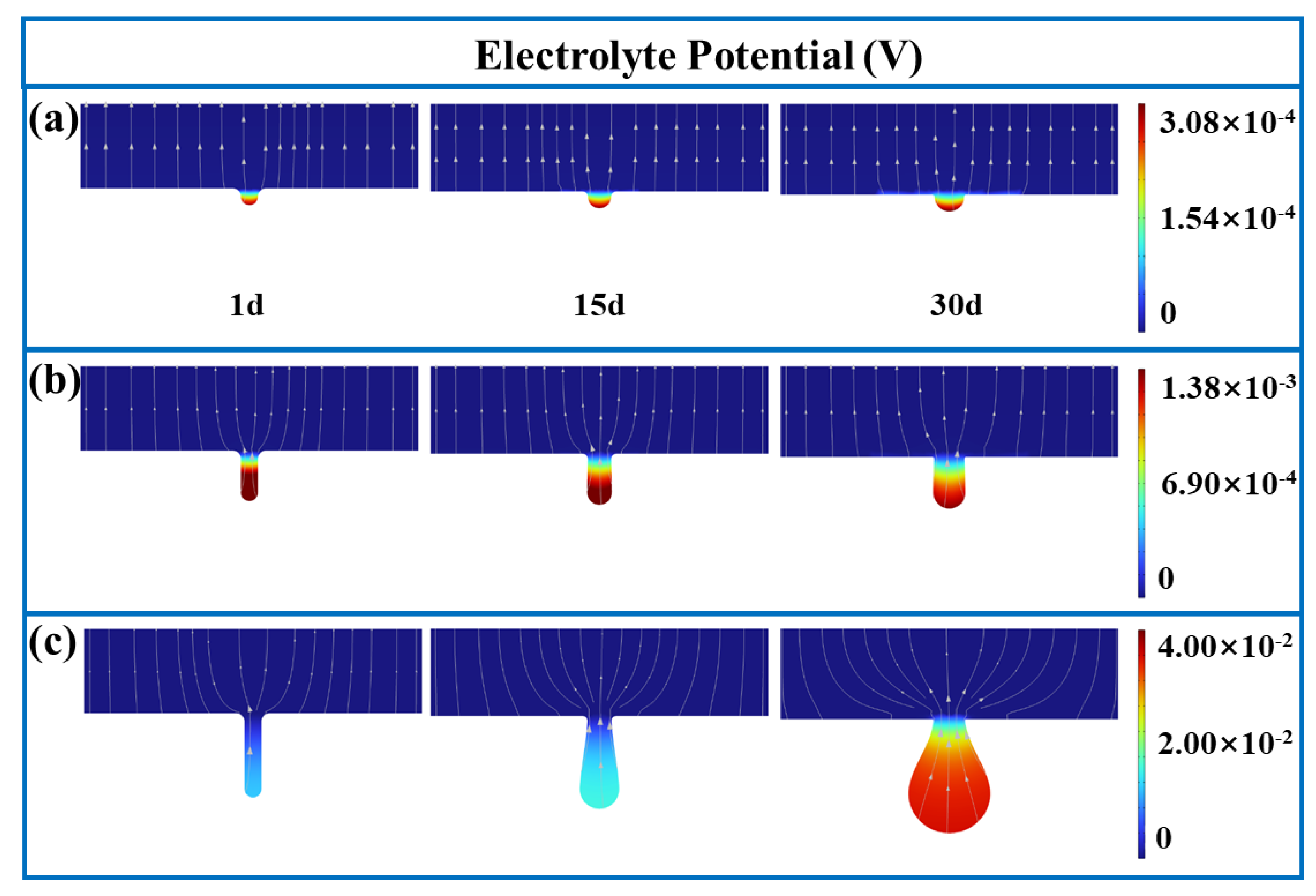
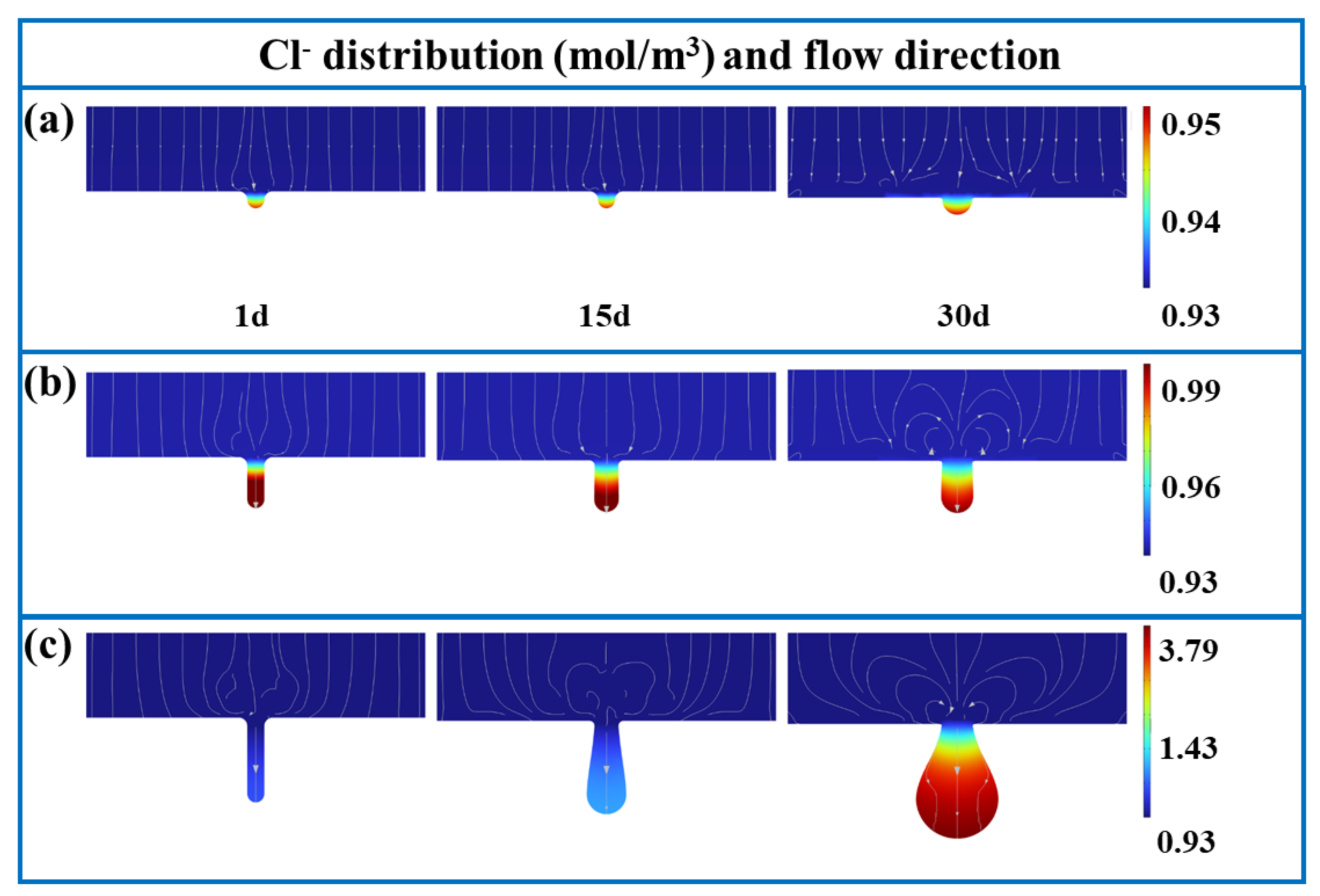
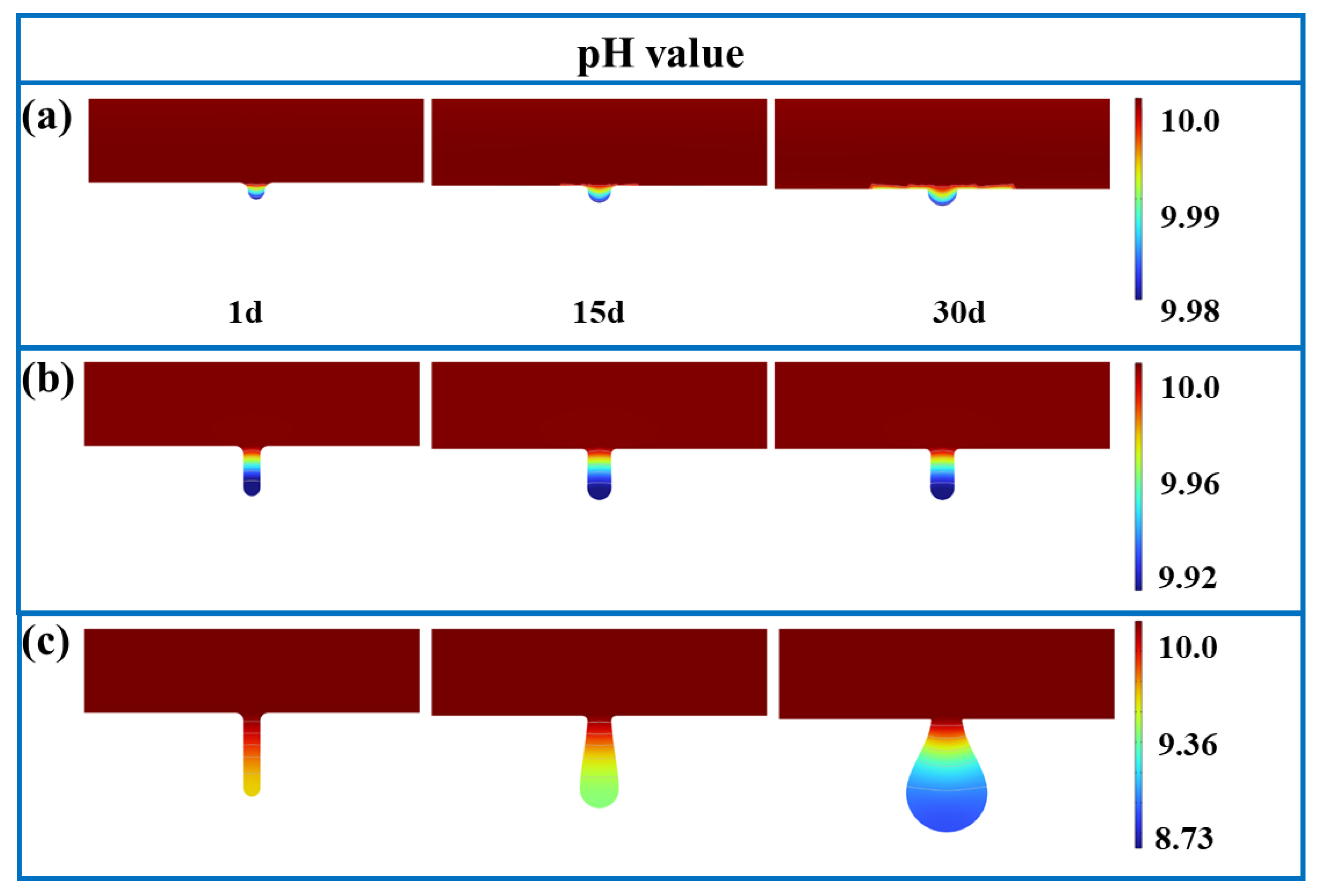
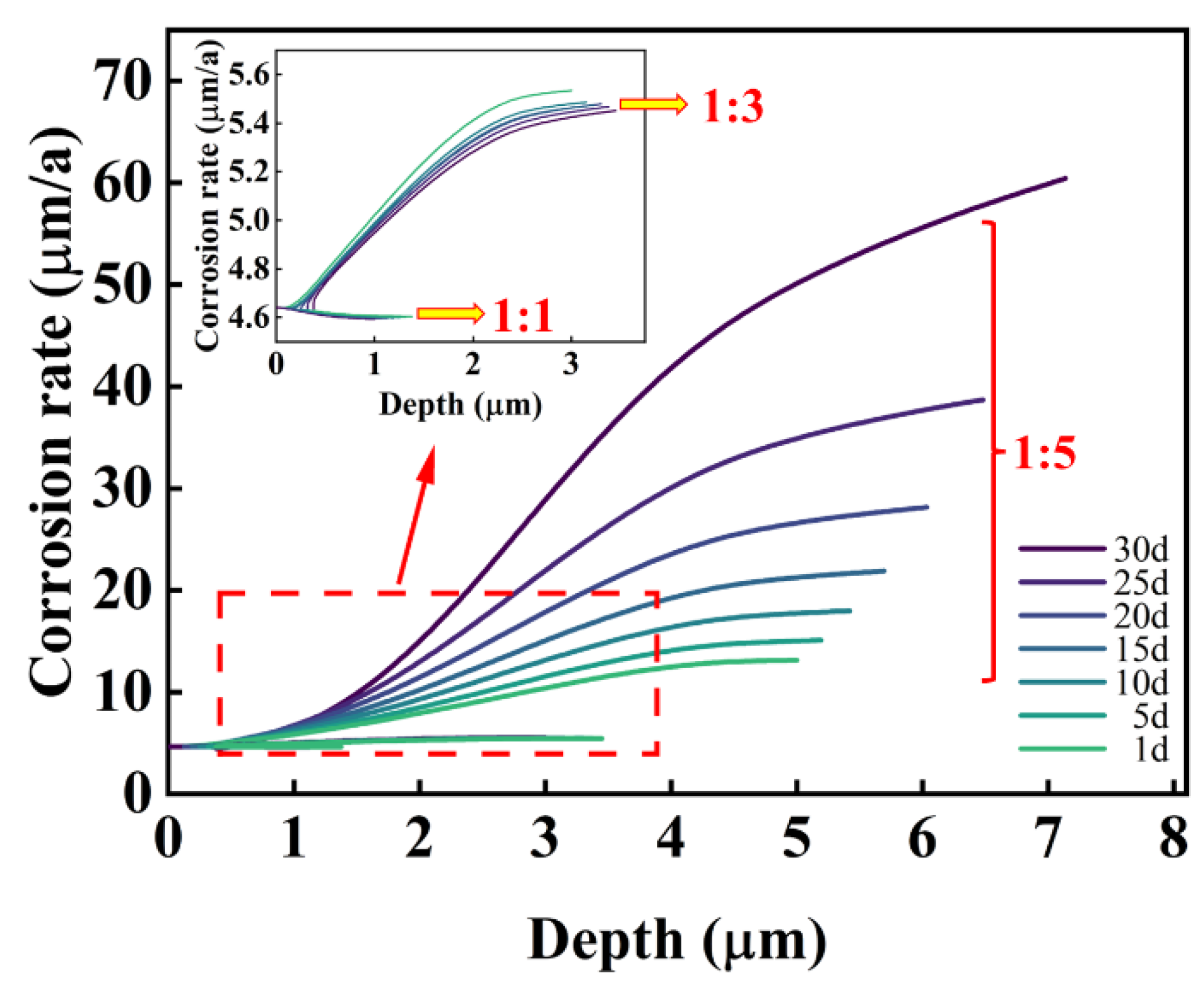
3.1. FE Analysis Results
3.2. Analytical Insights from Simulated Immersion Experiments
4. Conclusions
- (1)
- The corrosion rate is influenced by electrolyte current density, potential, and concentrations of Fe and Cl ions, as well as pH values. For pits with diameter-to-depth ratios below 1:3, growth rates are similar in all directions, leading to the uniform expansion of pitting. At a ratio of 1:5, the growth rate at the bottom of pits surpasses those at the middle and top, resulting in a droplet-shaped pit morphology.
- (2)
- The corrosion rate at the bottom of pits increases exponentially with the diameter-to-depth ratio. Fe and Cl ions accumulate within pits, creating a localized galvanic cell that accelerates corrosion through the pronounced cathode–small anode effect.
- (3)
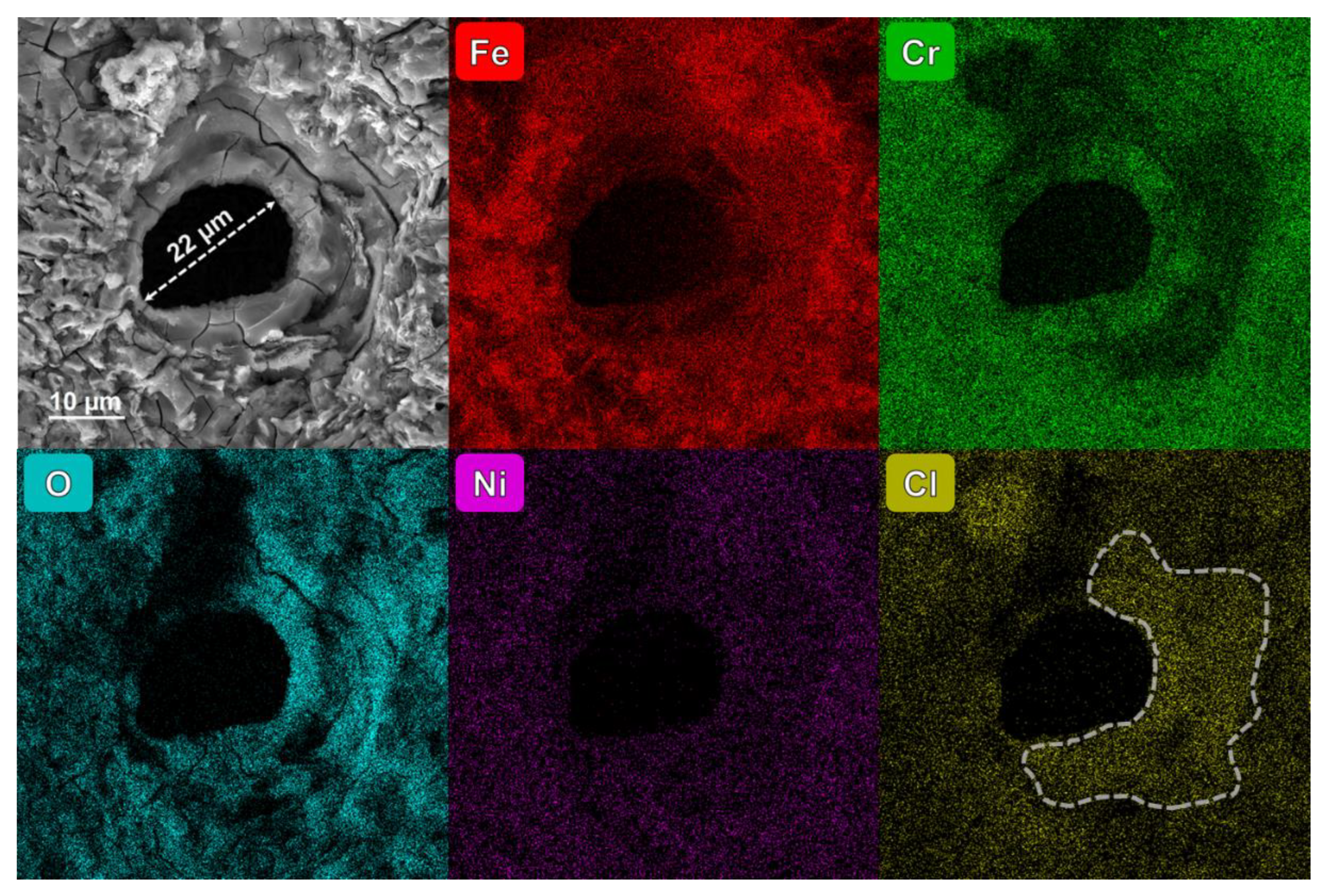
- The diffusion of Cl ions into pits causes Cr depletion at the edges, undermining the protective oxide layer around pits and lowering the local corrosion potential, which accelerates corrosion around the pits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, Y.; Wu, G.; Fu, A.Q.; Xie, J.F.; Zhao, M.F.; Bai, Z.Q.; Luo, J.H.; Feng, Y.R. Failure Analysis of the 13Cr Valve Cage of Tubing Pump Used in an Oilfield. Eng. Fail. Anal. 2018, 93, 330–339. [Google Scholar] [CrossRef]
- Fu, A.; Long, Y.; Liu, H.; Zhao, M.; Xie, J.; Su, H.; Li, X.; Yuan, J.; Lei, X.; Yin, C.; et al. Stress Corrosion Cracking Behavior of Super 13Cr Tubing in Phosphate Packer Fluid of High Pressure High Temperature Gas Well. Eng. Fail. Anal. 2022, 139, 106478. [Google Scholar] [CrossRef]
- Qi, W.; Wang, J.; Li, X.; Cui, Y.; Zhao, Y.; Xie, J.; Zeng, G.; Gao, Q.; Zhang, T.; Wang, F. Effect of Oxide Scale on Corrosion Behavior of HP-13Cr Stainless Steel during Well Completion Process. J. Mater. Sci. Technol. 2021, 64, 153. [Google Scholar] [CrossRef]
- Cho, H.-J.; Kim, S.-H.; Cho, Y.; Kim, S.-J. Effect of Copper Addition on Tensile Behavior in Fe-Cr-Ni Stable Austenitic Stainless Steel. Mater. Charact. 2022, 190, 112052. [Google Scholar] [CrossRef]
- Dai, J.; Li, H.-B.; Feng, H.; Jiang, Z.-H.; Qu, J.-D.; Zhao, Y.; Zhang, T. Revealing Significant Effect of Nitrogen on Prolonging Pitting Corrosion Lifetime of Martensitic Stainless Steel by Modelling Approach. Corros. Sci. 2022, 203, 110369. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, X.; Zhang, G.A. Corrosion Behaviour of 13Cr Stainless Steel under Stress and Crevice in 3.5 Wt.% NaCl Solution. Corros. Sci. 2020, 163, 108290. [Google Scholar] [CrossRef]
- Li, J.; Lin, B.; Zheng, H.; Wang, Y.; Zhang, H.; Zhang, Y.; Nie, Z.; Tang, J. Study on Pitting Corrosion Behavior and Semi In-Situ Pitting Corrosion Growth Model of 304 L SS with Elastic Stress in NaCl Corrosion Environment. Corros. Sci. 2023, 211, 110862. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Liu, T.; Kong, D.; Wang, D.; Li, X. The Enhancement of Microstructure on the Passive and Pitting Behaviors of Selective Laser Melting 316L SS in Simulated Body Fluid. Appl. Surf. Sci. 2019, 467–468, 193–205. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Bhuyan, P.; Bairi, L.R.; Mandal, S. Comprehending the Role of Individual Microstructural Features on Electrochemical Response and Passive Film Behaviour in Type 304 Austenitic Stainless Steel. Corros. Sci. 2021, 180, 109187. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kim, K.-W.; Park, S.-J.; Lee, T.-H.; Park, H.; Moon, J.; Hong, H.-U.; Lee, C.-H. Effects of Cr on Pitting Corrosion Resistance and Passive Film Properties of Austenitic Fe–19Mn–12Al–1.5 C Lightweight Steel. Corros. Sci. 2022, 206, 110529. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J.; Zhang, G.-H.; Fan, X.-H.; Zhang, L. Effect of Temperature on the Passive Film Structure and Corrosion Performance of CoCrFeMoNi High-Entropy Alloy. Corros. Sci. 2022, 208, 110661. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Nagaumi, H.; Wu, Z.; Li, X.; Zhang, H. Understanding the Corrosion Behavior by Passive Film Evolution in Zn-Containing Al-Si-Cu Cast Alloy. Corros. Sci. 2022, 205, 110468. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wang, X.Z.; Liu, L.; Wu, J.Q.; Zhang, Y.Q. Characterization of Corrosion Product Layers from CO2 Corrosion of 13Cr Stainless Steel in Simulated Oilfield Solution. J. Mater. Eng. Perform. 2011, 20, 1330–1335. [Google Scholar] [CrossRef]
- Xiao, G.; Tan, S.; Yu, Z.; Dong, B.; Yi, Y.; Tian, G.; Yu, H.; Shi, S. CO2 Corrosion Behaviors of 13Cr Steel in the High-Temperature Steam Environment. Petroleum 2020, 6, 106–113. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Sun, C.; Xu, S.; Wang, C.; Lu, M.; Neville, A.; Hua, Y. A Thermodynamic and Kinetic Study of the Formation and Evolution of Corrosion Product Scales on 13Cr Stainless Steel in a Geothermal Environment. Corros. Sci. 2020, 169, 108640. [Google Scholar] [CrossRef]
- Loable, C.; Viçosa, I.N.; Mesquita, T.J.; Mantel, M.; Nogueira, R.P.; Berthomé, G.; Chauveau, E.; Roche, V. Synergy between Molybdenum and Nitrogen on the Pitting Corrosion and Passive Film Resistance of Austenitic Stainless Steels as a pH-Dependent Effect. Mater. Chem. Phys. 2017, 186, 237–245. [Google Scholar] [CrossRef]
- Moon, J.; Ha, H.-Y.; Park, S.-J.; Lee, T.-H.; Jang, J.H.; Lee, C.-H.; Han, H.N.; Hong, H.-U. Effect of Mo and Cr Additions on the Microstructure, Mechanical Properties and Pitting Corrosion Resistance of Austenitic Fe-30Mn-10.5Al-1.1C Lightweight Steels. J. Alloys Compd. 2019, 775, 1136–1146. [Google Scholar] [CrossRef]
- Qi, X.; Mao, H.; Yang, Y. Corrosion Behavior of Nitrogen Alloyed Martensitic Stainless Steel in Chloride Containing Solutions. Corros. Sci. 2017, 120, 90–98. [Google Scholar] [CrossRef]
- Lei, X.; Feng, Y.; Zhang, J.; Fu, A.; Yin, C.; Macdonald, D.D. Impact of Reversed Austenite on the Pitting Corrosion Behavior of Super 13Cr Martensitic Stainless Steel. Electrochim. Acta 2016, 191, 640–650. [Google Scholar] [CrossRef]
- Turnbull, A. Modelling of Environment Assisted Cracking. Corros. Sci. 1993, 34, 921–960. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E. Progress and Opportunities in Modelling Environmentally Assisted Cracking. arXiv 2021, arXiv:2108.00816. [Google Scholar]
- Sharland, S.M.; Tasker, P.W. A Mathematical Model of Crevice and Pitting Corrosion—I. The Physical Model. Corros. Sci. 1988, 28, 603–620. [Google Scholar] [CrossRef]
- Sarkar, S.; Aquino, W. Electroneutrality and Ionic Interactions in the Modeling of Mass Transport in Dilute Electrochemical Systems. Electrochim. Acta 2011, 56, 8969–8978. [Google Scholar] [CrossRef]
- Sun, X.; Srinivasan, J.; Kelly, R.G.; Duddu, R. Numerical Investigation of Critical Electrochemical Factors for Pitting Corrosion Using a Multi-Species Reactive Transport Model. Corros. Sci. 2021, 179, 109130. [Google Scholar] [CrossRef]
- Ståhle, P.; Hansen, E. Phase Field Modelling of Stress Corrosion. Eng. Fail. Anal. 2015, 47, 241–251. [Google Scholar] [CrossRef]
- Abubakar, A.A.; Akhtar, S.S.; Arif, A.F.M. Phase Field Modeling of V2O5 Hot Corrosion Kinetics in Thermal Barrier Coatings. Comput. Mater. Sci. 2015, 99, 105–116. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S.; Buchheit, R.G. A Phase Field Model for Simulating the Pitting Corrosion. Corros. Sci. 2016, 110, 157–166. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S. A Phase Field Model for Simulating the Stress Corrosion Cracking Initiated from Pits. Corros. Sci. 2017, 125, 87–98. [Google Scholar] [CrossRef]
- Ansari, T.Q.; Xiao, Z.; Hu, S.; Li, Y.; Luo, J.-L.; Shi, S.-Q. Phase-Field Model of Pitting Corrosion Kinetics in Metallic Materials. Npj Comput. Mater. 2018, 4, 38. [Google Scholar] [CrossRef]
- Gutman, E.M. An Inconsistency in “Film Rupture Model” of Stress Corrosion Cracking. Corros. Sci. 2007, 49, 2289–2302. [Google Scholar] [CrossRef]
- Scully, J.C. The Interaction of Strain-Rate and Repassivation Rate in Stress Corrosion Crack Propagation. Corros. Sci. 1980, 20, 997–1016. [Google Scholar] [CrossRef]
- Xu, D.; Ling, G.; Wang, H. Effect of Current Density on the Mechanical Properties and Defects of Nanocrystalline Cr Al Solid Solution Alloy Coatings. Surf. Coat. Technol. 2023, 461, 129424. [Google Scholar] [CrossRef]
- Xu, L.Y.; Cheng, Y.F. Development of a Finite Element Model for Simulation and Prediction of Mechanoelectrochemical Effect of Pipeline Corrosion. Corros. Sci. 2013, 73, 150–160. [Google Scholar] [CrossRef]
- Ansari, T.Q.; Luo, J.-L.; Shi, S.-Q. Modeling the Effect of Insoluble Corrosion Products on Pitting Corrosion Kinetics of Metals. NPJ Mater. Degrad. 2019, 3, 28. [Google Scholar] [CrossRef]
- Cui, C.; Ma, R.; Martínez-Pañeda, E. Electro-Chemo-Mechanical Phase Field Modeling of Localized Corrosion: Theory and COMSOL Implementation. Eng. Comput. 2023, 39, 3877–3894. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 Inclusions on the Localized Corrosion of Q460NH Weathering Steel in Marine Environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y.; Hara, N. Passivity of (Mn,Cr)S Inclusions in Type 304 Stainless Steel: The Role of Cr and the Critical Concentration for Preventing Inclusion Dissolution in NaCl Solution. Corros. Sci. 2020, 176, 109060. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Zhao, X.; Tian, R.; Lei, Y.; Yu, P.; Peng, H.; Chen, L. Clarify the Effect of Reversed Austenite on the Pitting Corrosion Resistance of Super 13Cr Martensitic Stainless Steel. Corros. Sci. 2023, 213, 110992. [Google Scholar] [CrossRef]
- Sahu, S.; Frankel, G.S. Phase Field Modeling of Crystallographic Corrosion Pits. J. Electrochem. Soc. 2022, 169, 020557. [Google Scholar] [CrossRef]
- Wei, G.; Lu, S.; Li, S.; Yao, K.; Luan, H.; Fang, X. Unmasking of the Temperature Window and Mechanism for “Loss of Passivation” Effect of a Cr-13 Type Martensite Stainless Steel. Corros. Sci. 2020, 177, 108951. [Google Scholar] [CrossRef]
- Kayani, S.H.; Ha, H.-Y.; Cho, Y.-H.; Son, H.-W.; Lee, J.-M. Dislocation-Assisted Localised Pitting Corrosion Behaviour of Al Si Mg Cu Mn Alloy. Corros. Sci. 2023, 221, 111372. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Yu, Z.; Xie, Y.; Liu, F.; Jin, Y. A FEM Model for Simulating Trenching Process around a MnS Inclusion Embedded in Stainless Steel. J. Electroanal. Chem. 2021, 882, 114977. [Google Scholar] [CrossRef]
- Nohira, H.; Tsai, W.; Besling, W.; Young, E.; Petry, J.; Conard, T.; Vandervorst, W.; De Gendt, S.; Heyns, M.; Maes, J.; et al. Characterization of ALCVD-Al2O3 and ZrO2 Layer Using X-Ray Photoelectron Spectroscopy. J. Non-Cryst. Solids 2002, 303, 83–87. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Zhan, Z.; Xiao, K.; Fang, X.; Li, Z. Effect of Long-Term Pre-Oxidation on the Corrosion Rate of 316L Stainless Steel in a High-Temperature Water Environment. J. Mater. Eng. Perform. 2022, 31, 7935–7944. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, L.; Wang, Y.; Xu, S.; Wang, C.; Lu, M.; Neville, A.; Hua, Y. Evolution and Characterization of the Film Formed on Super 13Cr Stainless Steel in CO2-Saturated Formation Water at High Temperature. Corros. Sci. 2020, 163, 108277. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Shi, S.; Wang, M.; Li, J.; Xu, X.; Sun, J. Corrosion Product Film Formation Mechanism of 110H-13Cr Casing Steel in Simulated Ultra-High-Temperature Gas Environment for Shale Oil Production. Corros. Sci. 2024, 226, 111662. [Google Scholar] [CrossRef]
- Duan, X.; Han, T.; Guan, X.; Wang, Y.; Su, H.; Ming, K.; Wang, J.; Zheng, S. Cooperative Effect of Cr and Al Elements on Passivation Enhancement of Eutectic High-Entropy Alloy AlCoCrFeNi2.1 with Precipitates. J. Mater. Sci. Technol. 2023, 136, 97–108. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking Chloride Attack on the Passive Film of Metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | Ni | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.017 | 0.23 | 0.49 | 0.012 | 0.0043 | 13.3 | 5.01 | 1.85 | Bal. |
| Full-Life-Cycle Environment | Composition/mg·L−1 | pH | Temperature/°C | Time |
|---|---|---|---|---|
| Live acid (LA) | 10%HCl + 1.5%HF + 3%HAc + 5%TG201 corrosion inhibitor | 10%~20% acid solution | 120 | 4 h |
| Spent acid (SA) | K+/1597.32, Ca2+/11,693.00, Mg2+/3145.63, Cl−/62,738.01, Fe2+/52.40 | 2.6 | 180 | 5 d |
| Condensate water (CW) | K+/7380, Ca2+/4.745, Mg2+/2.463, Na+/1050, HCO3−/4310, Cl−/2990, SO42−/862 | 7.79 | 180 | 10 d |
| Formation water (FW) | K+/6620, Ca2+/8310, Mg2+/561, Na+/76,500, HCO3−/189, Cl−/128,000, SO42−/430 | 6.98 | 180 | 15, 30, 60 and 90 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Dong, H.; Han, Y.; Zhang, Y.; Li, P.; Feng, Y. The Growth Behavior and Mechanisms of Pitting Corrosion on Super 13Cr Martensitic Stainless Steel Surfaces at Different Diameter-to-Depth Ratios. Coatings 2024, 14, 435. https://doi.org/10.3390/coatings14040435
Yang Z, Dong H, Han Y, Zhang Y, Li P, Feng Y. The Growth Behavior and Mechanisms of Pitting Corrosion on Super 13Cr Martensitic Stainless Steel Surfaces at Different Diameter-to-Depth Ratios. Coatings. 2024; 14(4):435. https://doi.org/10.3390/coatings14040435
Chicago/Turabian StyleYang, Zichen, Hui Dong, Yan Han, Yongjie Zhang, Pengyu Li, and Yukun Feng. 2024. "The Growth Behavior and Mechanisms of Pitting Corrosion on Super 13Cr Martensitic Stainless Steel Surfaces at Different Diameter-to-Depth Ratios" Coatings 14, no. 4: 435. https://doi.org/10.3390/coatings14040435
APA StyleYang, Z., Dong, H., Han, Y., Zhang, Y., Li, P., & Feng, Y. (2024). The Growth Behavior and Mechanisms of Pitting Corrosion on Super 13Cr Martensitic Stainless Steel Surfaces at Different Diameter-to-Depth Ratios. Coatings, 14(4), 435. https://doi.org/10.3390/coatings14040435







