Influence of a UVA-Activated TiO2 Coating on Bacterial Surface Colonization in Water-Bearing Systems
Abstract
:1. Introduction
2. Materials and Methods
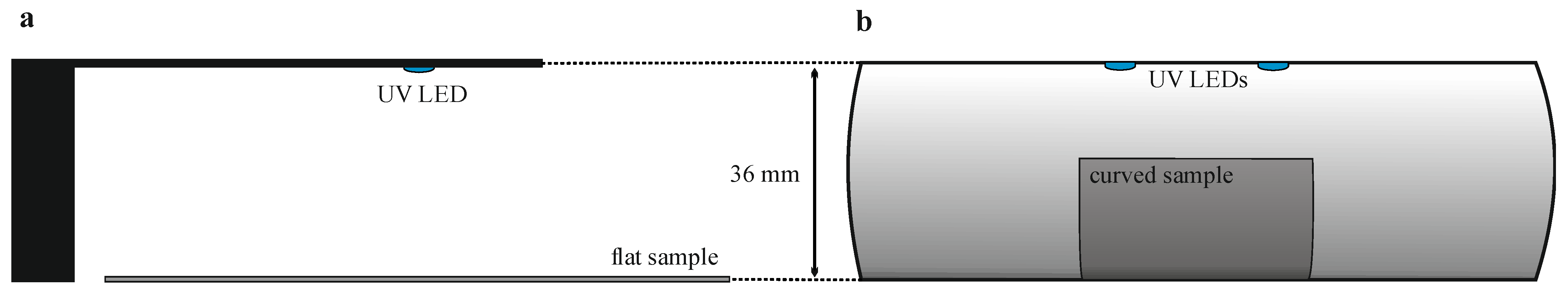
2.1. UV Irradiation Setup for Surface Activation
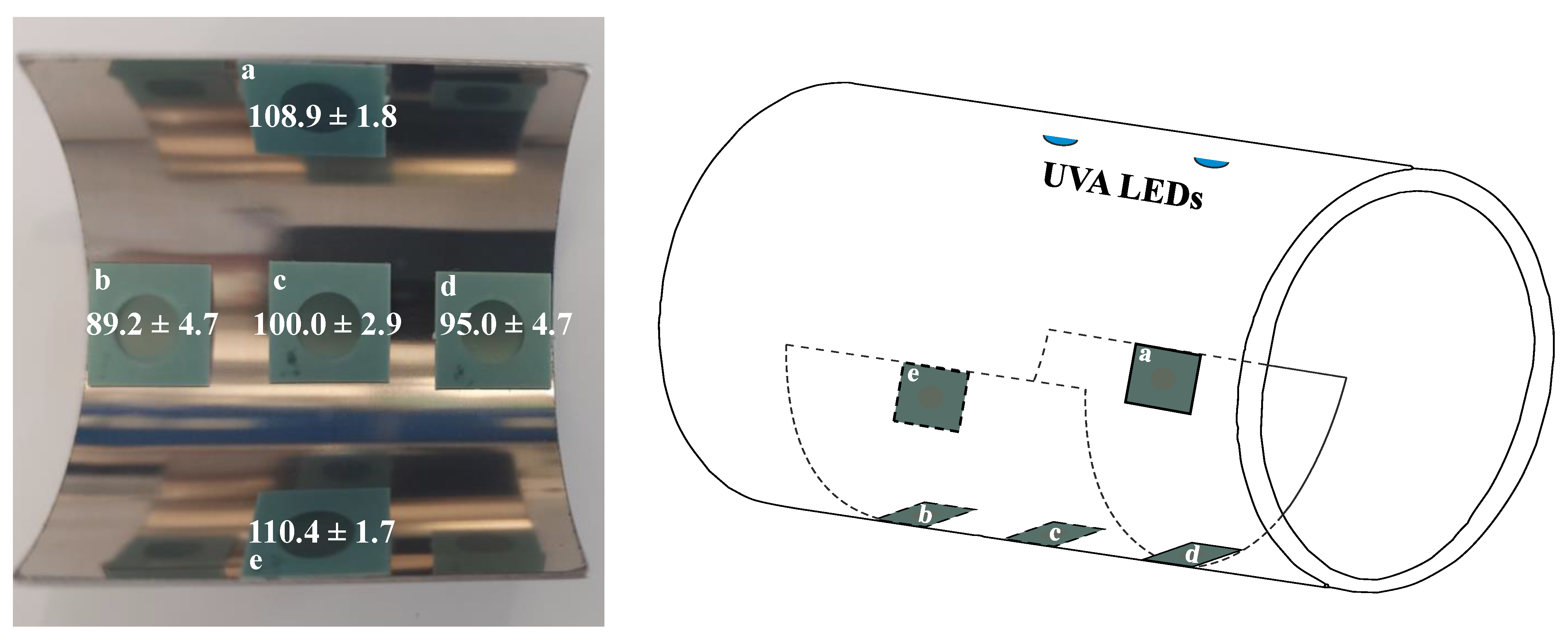
2.2. Determination of UV Dose Distribution
2.3. Sample Coating, Conditioning, and Activation
2.4. Sample Characterization
2.4.1. Determination of Layer Thickness
2.4.2. Atomic Force Microscopy (AFM), Scanning Electron Microscopy (SEM), and X-ray Diffraction (XRD) Measurement
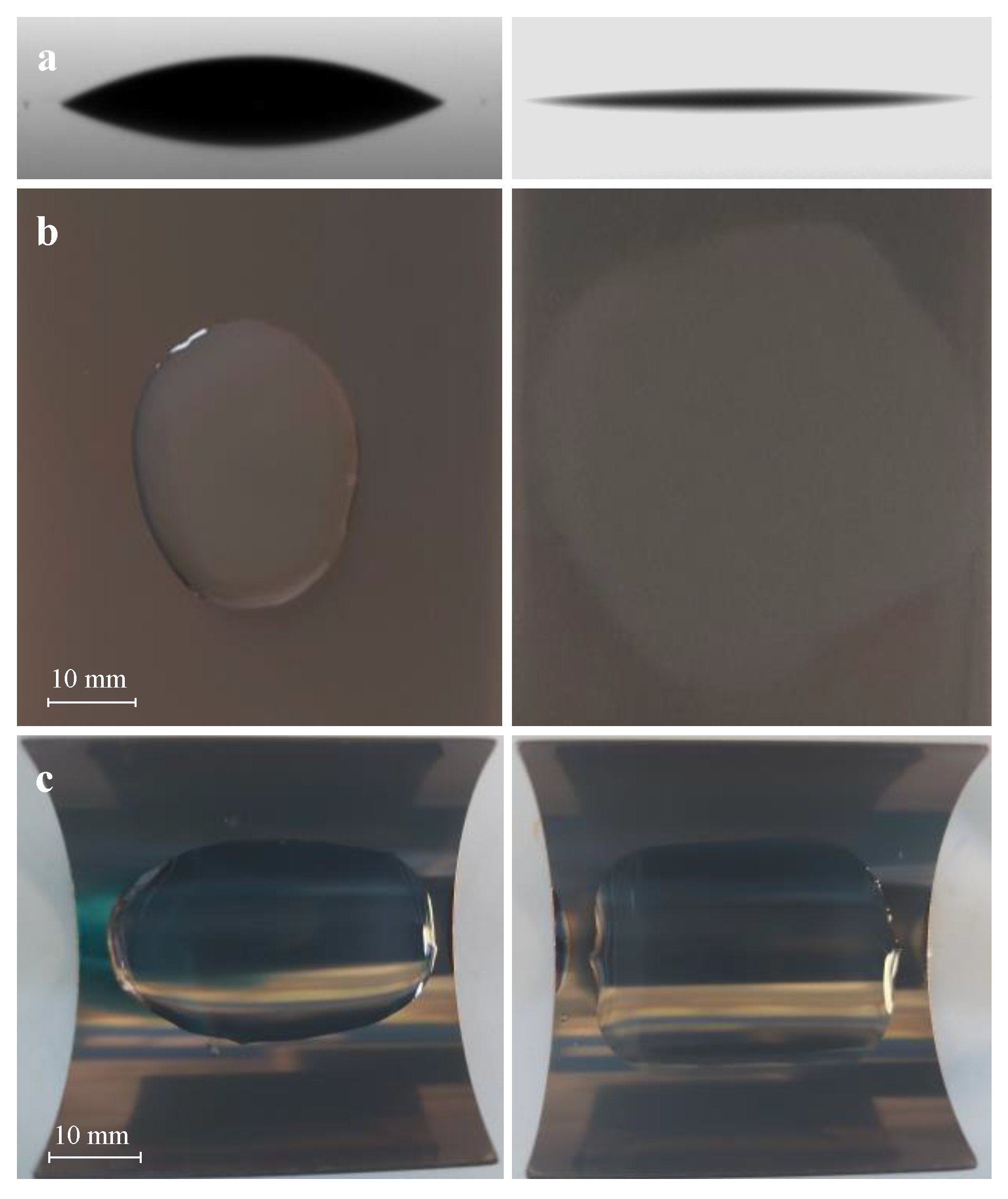
2.4.3. Contact Angle Measurement, Superhydrophilicity, and Stability
2.5. Microbiological Evaluation
2.5.1. Stock Culture and Sample Inoculation
2.5.2. Impedance Measurement
2.5.3. Plate Count Method
2.6. Statistics
3. Results and Discussion
3.1. Dose Distribution and Influence of UV Wavelength on Sample Activation
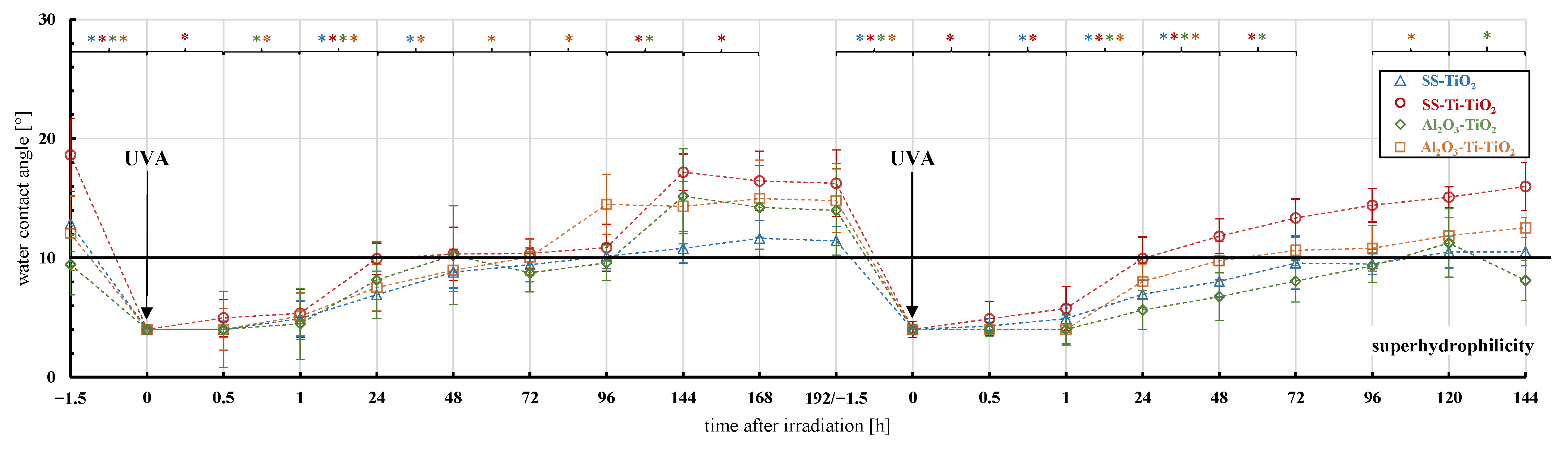
3.2. Surface Characterization and Superhydrophilicity
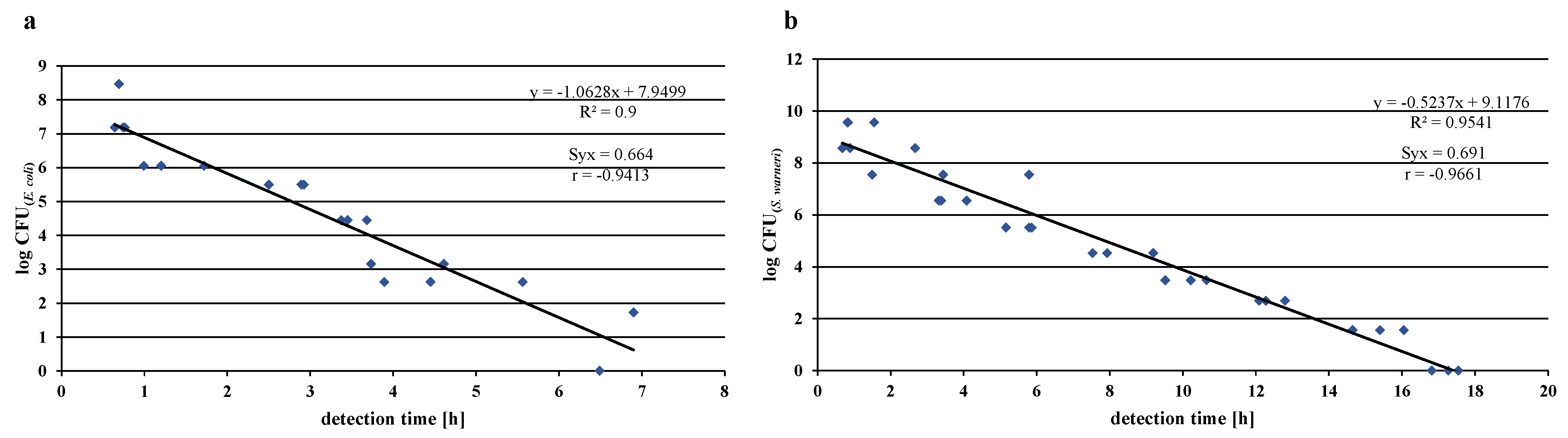
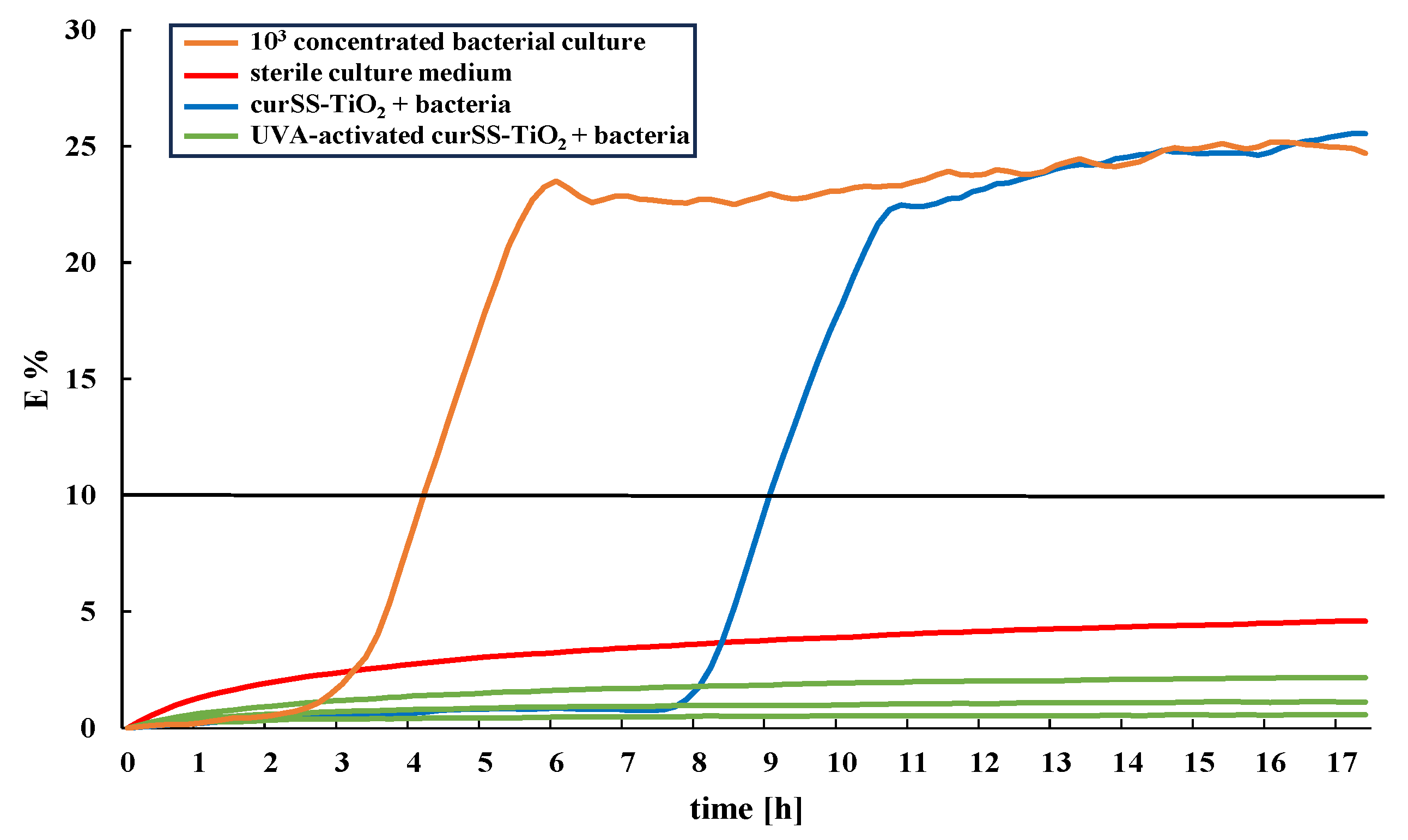
3.3. Development of the Microbiological Test Regime for the Application-Oriented Evaluation of Bacterial Colonization in Water-Bearing Pipes
3.4. Investigation of Bacterial Colonization on TiO2 Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frach, P.; Glöß, D.; Metzner, C.; Modes, T.; Scheffel, B.; Zywitzki, O. Deposition of photocatalytic TiO2 layers by pulse magnetron sputtering and by plasma-activated evaporation. Vacuum 2006, 80, 679–683. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Films Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Permpoon, S.; Fallet, M.; Berthomé, G.; Baroux, B.; Joud, J.C.; Langlet, M. Photo-Induced Hydrophilicity of TiO2 Films Deposited on Stainless Steel via Sol-Gel Technique. J. Sol.-Gel. Sci. Technol. 2005, 35, 127–136. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V. Photoinduced Hydrophilicity of Surfaces of Thin Films. Colloid J. 2021, 83, 20–48. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2018, 15, 2009–2032. [Google Scholar] [CrossRef]
- Achinas, S.; Charalampogiannis, N.; Euverink, G.J.W. A Brief Recap of Microbial Adhesion and Biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Brooks, J.D.; Flint, S.H. Biofilms in the food industry: Problems and potential solutions. Int. J. Food Sci. Technol. 2008, 43, 2163–2176. [Google Scholar] [CrossRef]
- Satpathy, S.; Sen, S.K.; Pattanaik, S.; Raut, S. Review on bacterial biofilm: An universal cause of contamination. Biocatal. Agric. Biotechnol. 2016, 7, 56–66. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, Y.F.; Sharma, M.; Voordouw, G. Effect of fluid flow on biofilm formation and microbiologically influenced corrosion of pipelines in oilfield produced water. J. Pet. Sci. Eng. 2017, 156, 451–459. [Google Scholar] [CrossRef]
- Parkes, L.O.; Hota, S.S. Sink-Related Outbreaks and Mitigation Strategies in Healthcare Facilities. Curr. Infect. Dis. Rep. 2018, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Geyter, D.D.; Blommaert, L.; Verbraeken, N.; Sevenois, M.; Huyghens, L.; Martini, H.; Covens, L.; Piérard, D.; Wybo, I. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob. Resist. Infect. Control 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Haapasalo, M. Biofilm models and methods of biofilm assessment. Endod Top. 2010, 22, 58–78. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces—A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Hofmaenner, D.A.; Wendel Garcia, P.D.; Duvnjak, B.; Chakrakodi, B.; Maier, J.D.; Huber, M.; Huder, J.; Wolfensberger, A.; Schreiber, P.W.; Schuepbach, R.A.; et al. Bacterial but no SARS-CoV-2 contamination after terminal disinfection of tertiary care intensive care units treating COVID-19 patients. Antimicrob. Resist. Infect. Control 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Krzymińska, S.; Kaznowski, A. Clonality, virulence and the occurrence of genes encoding antibiotic resistance among Staphylococcus warneri isolates from bloodstream infections. J. Med. Microbiol. 2016, 65, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- DIN EN 12056-2; Gravity Drainage Systems Inside Buildings—Part 2: Sanitary Pipework, Layout and Calculation. Deutsches Institut für Normung: Berlin, Germany, 2001.
- Schiller, S.; Goedicke, K.; Reschke, J.; Kirchhoff, V.; Schneider, S.; Milde, F. Pulsed magnetron sputter technology. Surf. Coat. Technol. 1993, 61, 331–337. [Google Scholar] [CrossRef]
- Fietzke, F.; Zywitzki, O. Structure and properties of magnetron-sputtered manganese ferrite films. Thin Solid Film. 2017, 644, 138–145. [Google Scholar] [CrossRef]
- Anderson, O.; Ottermann, C.R.; Kuschnereit, R.; Hess, P.; Bange, K. Density and Young’s modulus of thin TiO2 films. Fresenius’ J. Anal. Chem. 1997, 358, 315–318. [Google Scholar] [CrossRef]
- ISO 4287; Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface Texture Parameters. ISO Publishing: Geneve, Switzerland, 2010.
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Standard X-ray Diffraction Powder Patterns: Monograph 25, Section 7; U.S. Department of Commerce National Bureau of Standards: Washington, DC, USA, 1969; pp. 82–83.
- Silley, P.; Forsythe, S. Impedance microbiology—A rapid change for microbiologists. J. Appl. Bacteriol. 1996, 80, 233–243. [Google Scholar] [CrossRef]
- Futschik, K.; Pfutzner, H. Electrode and media impedance for detection and characterization of microorganisms. In Proceedings of the IEEE Engineering in Medicine and Biology Society and 14th Conference of the Biomedical Engineering Society of India, New Delhi, India, 15–18 February 1995; IEEE: Piscataway, NJ, USA, 1995; pp. 1/75–1/76, ISBN 0-7803-2711-X. [Google Scholar]
- Yang, L.; Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Schopf, S.; Gotzmann, G.; Dietze, M.; Gerschke, S.; Kenner, L.; König, U. Investigations Into the Suitability of Bacterial Suspensions as Biological Indicators for Low-Energy Electron Irradiation. Front. Immunol. 2022, 13, 814767. [Google Scholar] [CrossRef] [PubMed]
- DIN 10115; Fundamentals for Detection and Determination of Microorganisms in Foodstuffs with Impedance-Method. Deutsches Institut für Normung: Berlin, Germany, 2020.
- DIN 10122; Analysis of Foodstuffs—Enumeration of Microorganisms by Means of Impedance-Method—Determination of Aerobic Mesophile Bacterial Count. Deutsches Institut für Normung: Berlin, Germany, 2018.
- Nguyen, V.-H.; Lin, S.D.; Wu, J.C.-S.; Bai, H. Artificial sunlight and ultraviolet light induced photo-epoxidation of propylene over V-Ti/MCM-41 photocatalyst. Beilstein J. Nanotechnol. 2014, 5, 566–576. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol–gel preparation and characterization of alkaline earth metal doped nano TiO2: Efficient photocatalytic degradation of 4-chlorophenol. J. Mol. Catal. A Chem. 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Lv, L.; Li, K.; Xie, Y.; Cao, Y.; Zheng, X. Enhanced osteogenic activity of anatase TiO2 film: Surface hydroxyl groups induce conformational changes in fibronectin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 96–104. [Google Scholar] [CrossRef]
- Vrakatseli, V.; Farsari, E.; Mataras, D. Wetting Properties of Transparent Anatase/Rutile Mixed Phase Glancing Angle Magnetron Sputtered Nano-TiO2 Films. Micromachines 2020, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, J.C.; Ho, W.; Jiang, Z. Effects of calcination temperature on the photocatalytic activity and photo-induced super-hydrophilicity of mesoporous TiO2 thin films. N. J. Chem. 2002, 26, 607–613. [Google Scholar] [CrossRef]
- Kang, H.; Lai, H.; Cheng, Z.; Liu, Y.; Jiang, L. Restoration of superwetting switching on TiO2 coated shape memory polymer arrays. Chem. Eng. J. 2020, 394, 124996. [Google Scholar] [CrossRef]
- Xu, Q.F.; Liu, Y.; Lin, F.-J.; Mondal, B.; Lyons, A.M. Superhydrophobic TiO2-polymer nanocomposite surface with UV-induced reversible wettability and self-cleaning properties. ACS Appl. Mater. Interfaces 2013, 5, 8915–8924. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nakajima, A.; Wang, R.; Minabe, M.; Koizumi, S.; Fujishima, A.; Hashimoto, K. Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass. Thin Solid Film. 1999, 351, 260–263. [Google Scholar] [CrossRef]
- Gan, W.Y.; Lam, S.W.; Chiang, K.; Amal, R.; Zhao, H.; Brungs, M.P. Novel TiO2 thin film with non-UV activated superwetting and antifogging behaviours. J. Mater. Chem. 2007, 17, 952. [Google Scholar] [CrossRef]
- Selloni, A. Crystal growth: Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Ma, M.; Wang, Q.; Yan, T.; Zhao, B.; Guo, S.; Tong, S. Advances in the superhydrophilicity-modified titanium surfaces with antibacterial and pro-osteogenesis properties: A review. Front. Bioeng. Biotechnol. 2022, 10, 1000401. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Madeira, M.D.P.; Gusmão, S.B.; Lima, I.S.D.; Lemos, G.M.; Barreto, H.M.; Abi-chacra, É.D.A.; Vega, M.L.; Hidalgo, A.A.; Santos, F.E.; Silva-Filho, E.C.; et al. Depositation of sodium titanate nanotubes: Superhydrophilic surface and antibacterial approach. J. Mater. Res. Technol. 2022, 19, 2104–2114. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Heo, J.; Lee, S.-E.; Shin, J.-W.; Chang, M.; Hong, J. Polysaccharide-based superhydrophilic coatings with antibacterial and anti-inflammatory agent-delivering capabilities for ophthalmic applications. J. Ind. Eng. Chem. 2018, 68, 229–237. [Google Scholar] [CrossRef]
- Hwangbo, S.; Jeong, H.; Heo, J.; Lin, X.; Kim, Y.; Chang, M.; Hong, J. Antibacterial nanofilm coatings based on organosilicate and nanoparticles. React. Funct. Polym. 2016, 102, 27–32. [Google Scholar] [CrossRef]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. Antimicrobial surfaces: A review of synthetic approaches, applicability and outlook. J. Mater. Sci. 2021, 56, 17915–17941. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Stobnicka-Kupiec, A.; Ławniczek-Wałczyk, A.; Bakal-Kijek, A.; Gołofit-Szymczak, M.; Górny, R.L. Anaerobic bacteria in wastewater treatment plant. Int. Arch. Occup. Environ. Health 2018, 91, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kjølen, H.; Andersen, B.M. Handwashing and disinfection of heavily contaminated hands—Effective or ineffective? J. Hosp. Infect. 1992, 21, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Löffler, H.; Gastmeier, P. Hand hygiene for the prevention of nosocomial infections. Dtsch. Arztebl. Int. 2009, 106, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Oughton, M.T.; Loo, V.G.; Dendukuri, N.; Fenn, S.; Libman, M.D. Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile. Infect. Control Hosp. Epidemiol. 2009, 30, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Eggers, M.; Terletskaia-Ladwig, E.; Enders, M. How effective is hand washing against influenza virus? Hyg. Med. 2009, 34, 492–498. (In German) [Google Scholar]
- Crosby, H.A.; Kwiecinski, J.; Horswill, A.R. Staphylococcus aureus Aggregation and Coagulation Mechanisms, and Their Function in Host-Pathogen Interactions. Adv. Appl. Microbiol. 2016, 96, 1–41. [Google Scholar]
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Ed Engl. 2015, 54, 3387–3399. [Google Scholar] [CrossRef]
- Koubali, H.; EL Louali, M.; Zahir, H.; Soufiani, S.; Mabrouki, M.; Latrache, H. Physicochemical characterization of glass and polyethylene surfaces treated with different surfactants and their effects on bacterial adhesion. Int. J. Adhes. Adhes. 2021, 104, 102754. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.-Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired Hierarchical Surface Structures with Tunable Wettability for Regulating Bacteria Adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Samonin, V.V.; Elikova, E.E. A study of the adsorption of bacterial cells on porous materials. Microbiology 2004, 73, 696–701. [Google Scholar] [CrossRef]
- Prasad, A.; Gänzle, M.; Roopesh, M.S. Inactivation of Escherichia Coli and Salmonella Using 365 and 395 nm High Intensity Pulsed Light Emitting Diodes. Foods 2019, 8, 679. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. Development of a new water sterilization device with a 365 nm UV-LED. Med. Biol. Eng. Comput. 2007, 45, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, A.; Mori, M.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Akutagawa, M.; Ikehara, T.; Nakaya, Y.; Kinouchi, Y. New water disinfection system using UVA light-emitting diodes. J. Appl. Microbiol. 2007, 103, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Amariei, G.; Valenzuela, L.; Iglesias-Juez, A.; Rosal, R.; Visa, M. ZnO-functionalized fly-ash based zeolite for ciprofloxacin antibiotic degradation and pathogen inactivation. J. Environ. Chem. Eng. 2022, 10, 107603. [Google Scholar] [CrossRef]
- Tyrrell, R.M.; Peak, M.J. Interactions between uv radiation of different energies in the inactivation of bacteria. J. Bacteriol. 1978, 136, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]


| Sample Type | Substrate | T Heater [°C] | Pulse Pattern [µs] | ΓAr [sccm] | ΓO2 [sccm] | p [Pa] | Coating Rate [nm/min] | Coating |
|---|---|---|---|---|---|---|---|---|
| SS-TiO2 | stainless steel | 250 | 16:10 | 100 | 38–39 | 1.12 | 47–51 | pure TiO2 |
| SS-Ti-TiO2 | stainless steel | 250 | 16:10 | 100 | 40 | 1.03 | 50–51 | Ti interlayer |
| 39 | 1.13 | 200–210 | TiO2 coating | |||||
| Al2O3-TiO2 | Al2O3 ceramic | 250 | 16:10 | 100 | 38–39 | 1.12–1.13 | 47–51 | pure TiO2 |
| Al2O3-Ti-TiO2 | Al2O3 ceramic | 250 | 16:10 | 100 | 40 | 1.03 | 50–51 | Ti interlayer |
| 1.13 | 200–210 | TiO2 coating | ||||||
| curSS-TiO2 | stainless steel | 250 | 15.5:10: 16.5:10 | 100 | 38 | 1.12 | 53 | pure TiO2 on curved samples |
| Sample Type | Coating Thickness [nm] | Roughness Ra [nm] | Lateral Size TiO2 Crystallites [nm] | |
|---|---|---|---|---|
| SS substrate | - | 5.0 | - | |
| Al2O3 substrate | - | 120.0 | - | |
| SS-TiO2 | 2600 | 21.3 | ≈150 | |
| SS-Ti-TiO2 | 1200 | (Ti interlayer) | 44.5 | ≈500 |
| 2800 | (TiO2 coating) | |||
| Al2O3-TiO2 | 2600 | 56.4 | ≈200 | |
| Al2O3-Ti-TiO2 | 1200 | (Ti interlayer) | 67.4 | ≈350 |
| 2800 | (TiO2 coating) | |||
| curSS-TiO2 | 3200 | - | ≈200 | |
| Sample Type | Microorganism | UVA Dose [J/cm2] | Viable Bacteria [CFU/mL] |
|---|---|---|---|
| curSS-TiO2 | E. coli | 17.6 ± 1.2 | 0.000 ± 0.000 |
| no activation | 0.015 ± 0.002 | ||
| S. warneri | 17.6 ± 1.2 | 0.000 ± 0.000 | |
| no activation | 0.939 ± 1.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhäußer, L.; König, U.; Fietzke, F.; Gotzmann, G. Influence of a UVA-Activated TiO2 Coating on Bacterial Surface Colonization in Water-Bearing Systems. Coatings 2024, 14, 454. https://doi.org/10.3390/coatings14040454
Steinhäußer L, König U, Fietzke F, Gotzmann G. Influence of a UVA-Activated TiO2 Coating on Bacterial Surface Colonization in Water-Bearing Systems. Coatings. 2024; 14(4):454. https://doi.org/10.3390/coatings14040454
Chicago/Turabian StyleSteinhäußer, Linda, Ulla König, Fred Fietzke, and Gaby Gotzmann. 2024. "Influence of a UVA-Activated TiO2 Coating on Bacterial Surface Colonization in Water-Bearing Systems" Coatings 14, no. 4: 454. https://doi.org/10.3390/coatings14040454





