Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
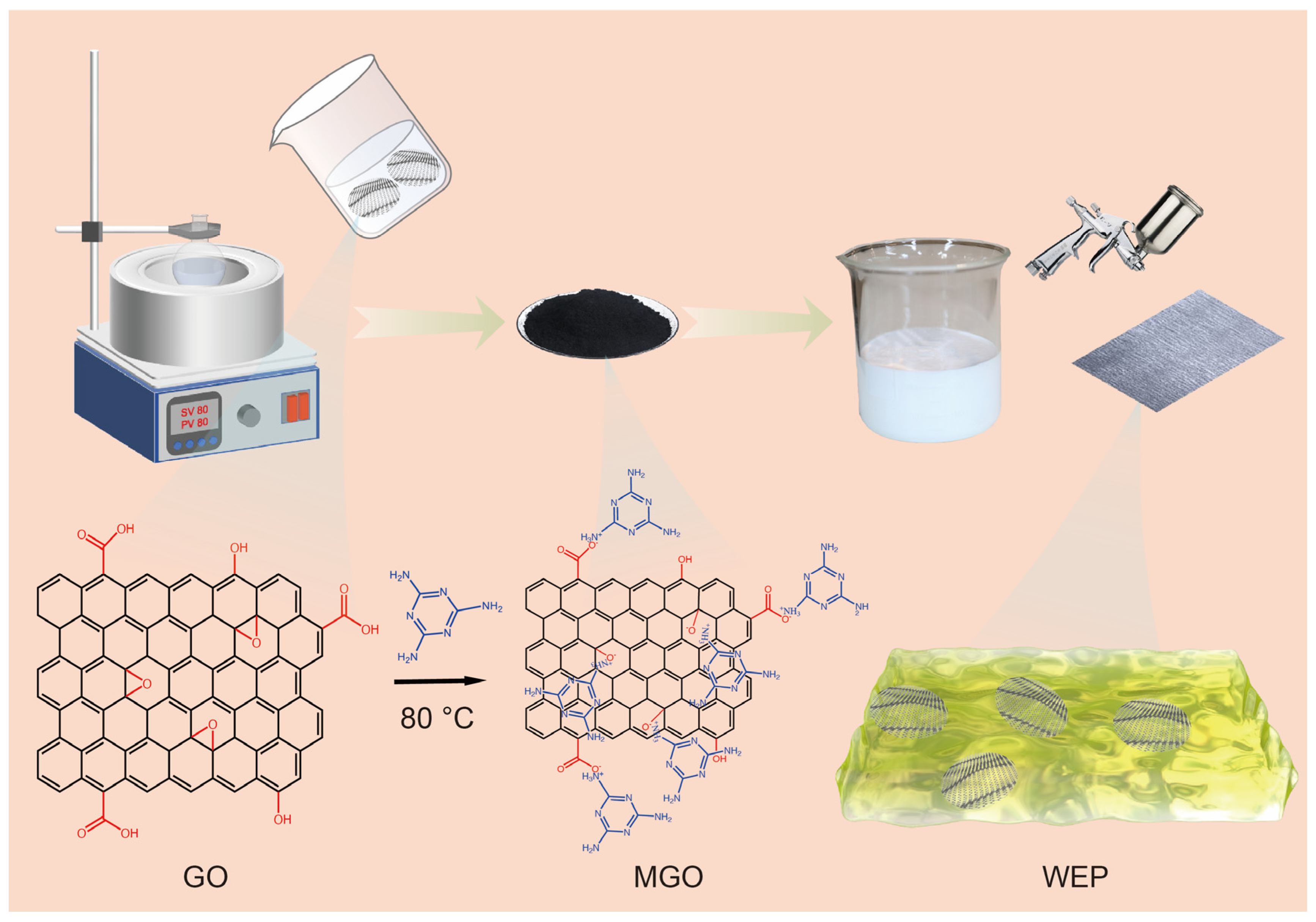
2.2. Preparation of Melamine-Modified Graphene Oxide
2.3. Preparation of WEP/MGO Composite Coatings
2.4. Characterization
3. Results and Discussion
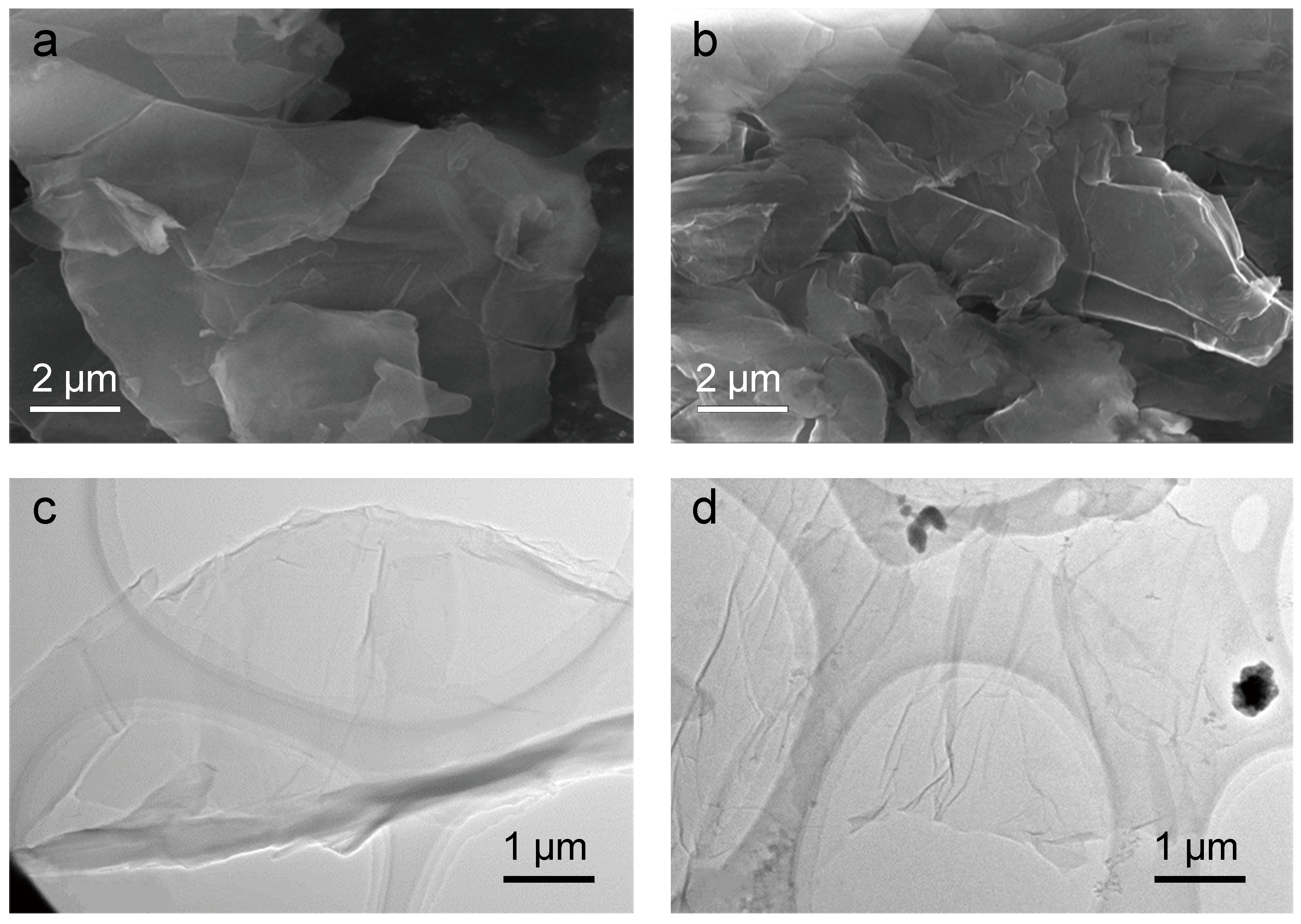
3.1. Characterization of GO and MGO
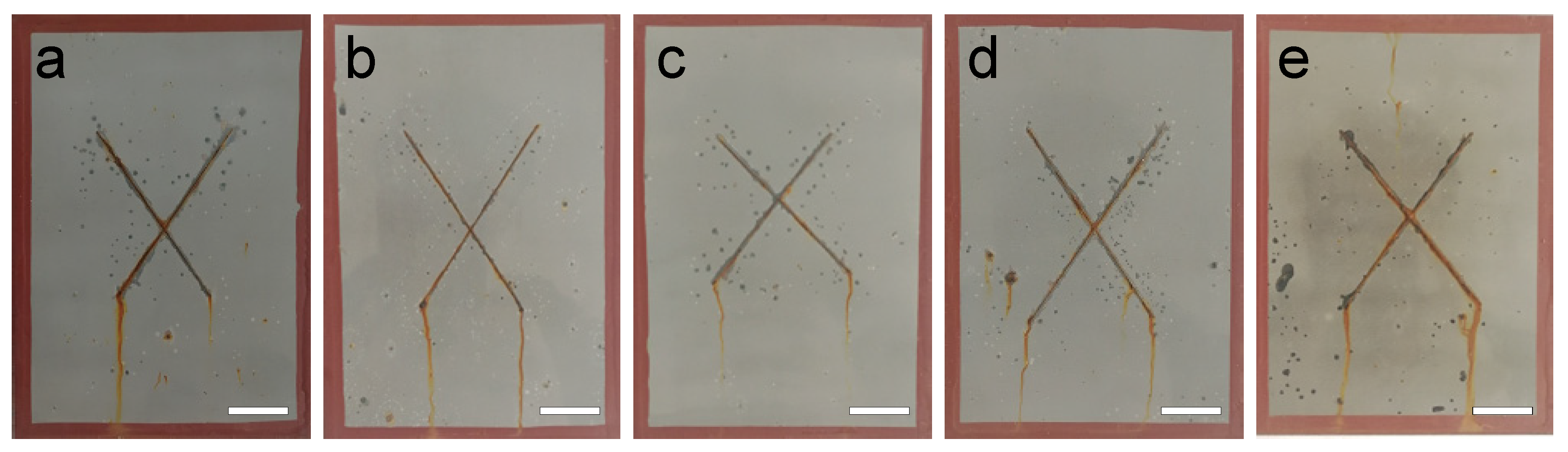
3.2. Corrosion Resistance of Epoxy Composite Coated Q235 Samples
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, J.; Zhao, H.; Shao, Z.; Yu, H. Bioinspired Smart Anticorrosive Coatings with an Emergency-Response Closing Function. ACS Appl. Mater. Interfaces 2019, 11, 42646–42653. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Rentería, J.A.; Cházaro-Ruiz, L.F.; Rangel-Mendez, J.R. Synthesis of reduced graphene oxide (rGO) films onto carbon steel by cathodic electrophoretic deposition: Anticorrosive coating. Carbon 2017, 122, 266–275. [Google Scholar] [CrossRef]
- Yimyai, T.; Crespy, D.; Rohwerder, M. Corrosion-Responsive Self-Healing Coatings. Adv. Mater. 2023, 35, e2300101. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Féron, D.; Mills, D.; Ritter, S.; Bäßler, R.; Bettge, D.; De Graeve, I.; Dugstad, A.; Grassini, S.; Hack, T.; et al. Corrosion challenges towards a sustainable society. Mater. Corros. 2022, 73, 1730–1751. [Google Scholar] [CrossRef]
- Liu, T.; Ma, L.; Wang, X.; Wang, J.; Qian, H.; Zhang, D.; Li, X. Self-healing corrosion protective coatings based on micro/nanocarriers: A review. Corros. Commun. 2021, 1, 18–25. [Google Scholar] [CrossRef]
- Pletincx, S.; Fockaert, L.L.I.; Mol, J.M.C.; Hauffman, T.; Terryn, H. Probing the formation and degradation of chemical interactions from model molecule/metal oxide to buried polymer/metal oxide interfaces. NPJ Mater. Degrad. 2019, 3, 23. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Tedim, J.; Ferreira, M. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Pei, L.; Lin, D.; Yuan, S.; Lu, R.; Bai, Z.; Sun, Y.; Zhu, Y.; Jiang, Y.; Zhu, J.; Wang, H. A multifunctional and long-term waterborne anti-corrosion coating with excellent ‘hexagonal warrior’ properties. Chem. Eng. J. 2023, 457, 141158. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Zhang, M.; Lin, D.; Yuan, S.; Xu, F.; Zhou, Y.; Wang, C.; Zhu, Y.; Wang, H. A novel waterborne epoxy coating with anti-corrosion performance under harsh oxygen environment. Chem. Eng. J. 2022, 430, 133156. [Google Scholar] [CrossRef]
- Ghosal, A.; Iqbal, S.; Ahmad, S. NiO nanofiller dispersed hybrid Soy epoxy anticorrosive coatings. Prog. Org. Coat. 2019, 133, 61–76. [Google Scholar] [CrossRef]
- Quites, D.; Somers, A.; Forsyth, M.; Paulis, M. Development of waterborne anticorrosive coatings by the incorporation of coumarate based corrosion inhibitors and phosphate functionalization. Prog. Org. Coat. 2023, 183, 107781. [Google Scholar] [CrossRef]
- Feng, Z.; Song, G.-L.; Xu, Y.; Zheng, D.; Chen, X. Micro-galvanic corrosion during formation of epoxy coating. Prog. Org. Coat. 2020, 147, 105799. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, Q. Relationship between ion transport and the failure behavior of epoxy resin coatings. Corros. Sci. 2014, 78, 22–28. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, H.; Prabhakar, M.; Rohwerder, M. Organic composite coatings containing mesoporous silica particles: Degradation of the SiO2 leading to self-healing of the delaminated interface. Corros. Sci. 2022, 200, 110252. [Google Scholar] [CrossRef]
- Yin, Y.; Schulz, M.; Rohwerder, M. Optimizing smart self-healing coatings: Investigating the transport of active agents from the coating towards the defect. Corros. Sci. 2021, 190, 109661. [Google Scholar] [CrossRef]
- Enríquez, E.; Fernández, J.; de la Rubia, M. Highly conductive coatings of carbon black/silica composites obtained by a sol–gel process. Carbon 2012, 50, 4409–4417. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Lamprakou, Z.; Bi, H.; Weinell, C.E.; Tortajada, S.; Dam-Johansen, K. Smart epoxy coating with mesoporous silica nanoparticles loaded with calcium phosphate for corrosion protection. Prog. Org. Coat. 2022, 165, 106740. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, W.; Yin, X.; Chen, Y.; Liu, Y.; Xu, B. Corrosion protection of 304 stainless steel from a smart conducting polypyrrole coating doped with pH-sensitive molybdate-loaded TiO2 nanocontainers. Prog. Org. Coat. 2020, 146, 105750. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Wang, W.; Lin, Z.; Li, X. Synthesis and characterisation of composite nanoparticles of mesoporous silica loaded with inhibitor for corrosion protection of Cu-Zn alloy. Corros. Sci. 2017, 120, 139–147. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, J.; Yang, B. Structural Fabrication and Functional Modulation of Nanoparticle–Polymer Composites. Adv. Funct. Mater. 2010, 20, 1533–1550. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H.; Zhou, H.; Graham, M.; Smith, J.; Sheng, X.; Chen, Y.; Zhang, L.; Zhang, X.; Shchukina, E.; et al. Superior anti-corrosion and self-healing bi-functional polymer composite coatings with polydopamine modified mesoporous silica/graphene oxide. J. Mater. Sci. Technol. 2021, 95, 95–104. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; Moghadam, M.M. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Ning, Y.-J.; Zhu, Z.-R.; Cao, W.-W.; Wu, L.; Jing, L.-C.; Wang, T.; Yuan, X.-T.; Teng, L.-H.; Bin, P.-S.; Geng, H.-Z. Anti-corrosion reinforcement of waterborne polyurethane coating with polymerized graphene oxide by the one-pot method. J. Mater. Sci. 2021, 56, 337–350. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Polyaniline-cerium oxide (PAni-CeO2) coated graphene oxide for enhancement of epoxy coating corrosion protection performance on mild steel. Corros. Sci. 2018, 137, 111–126. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Ahmadi, A.; Mahdavian, M.; Moghadam, M.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Li, F.; Ma, Y.; Chen, L.; Li, H.; Zhou, H.; Chen, J. In-situ polymerization of polyurethane/aniline oligomer functionalized graphene oxide composite coatings with enhanced mechanical, tribological and corrosion protection properties. Chem. Eng. J. 2021, 425, 130006. [Google Scholar] [CrossRef]
- Wu, Y.; He, Y.; Chen, C.; Zhong, F.; Li, H.; Chen, J.; Zhou, T. Non-covalently functionalized boron nitride by graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124337. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, W.; Zhong, L.; Jiang, X.; Zhang, X. Investigation of the anticorrosion properties of graphene oxide-modified waterborne epoxy coatings for AA6061. Prog. Org. Coat. 2022, 163, 106655. [Google Scholar] [CrossRef]
- Chai, Z.-L.; Chen, Y.-X.; Zhou, D.; Zhang, M.; Liu, J.-K. Excellent corrosion resistance of FGO/Zn2SiO4 composite material in epoxy coatings. Prog. Org. Coat. 2022, 170, 106992. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]

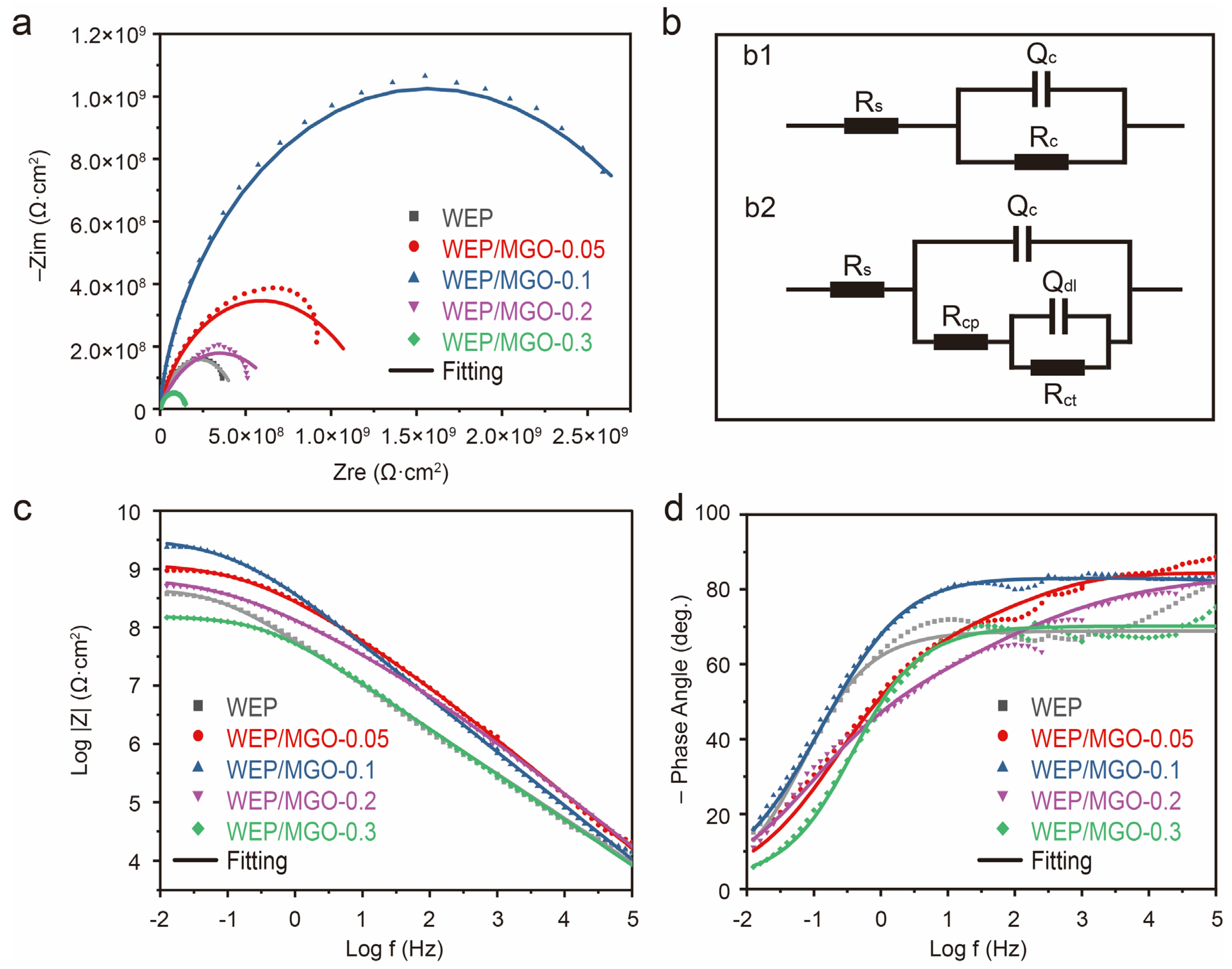

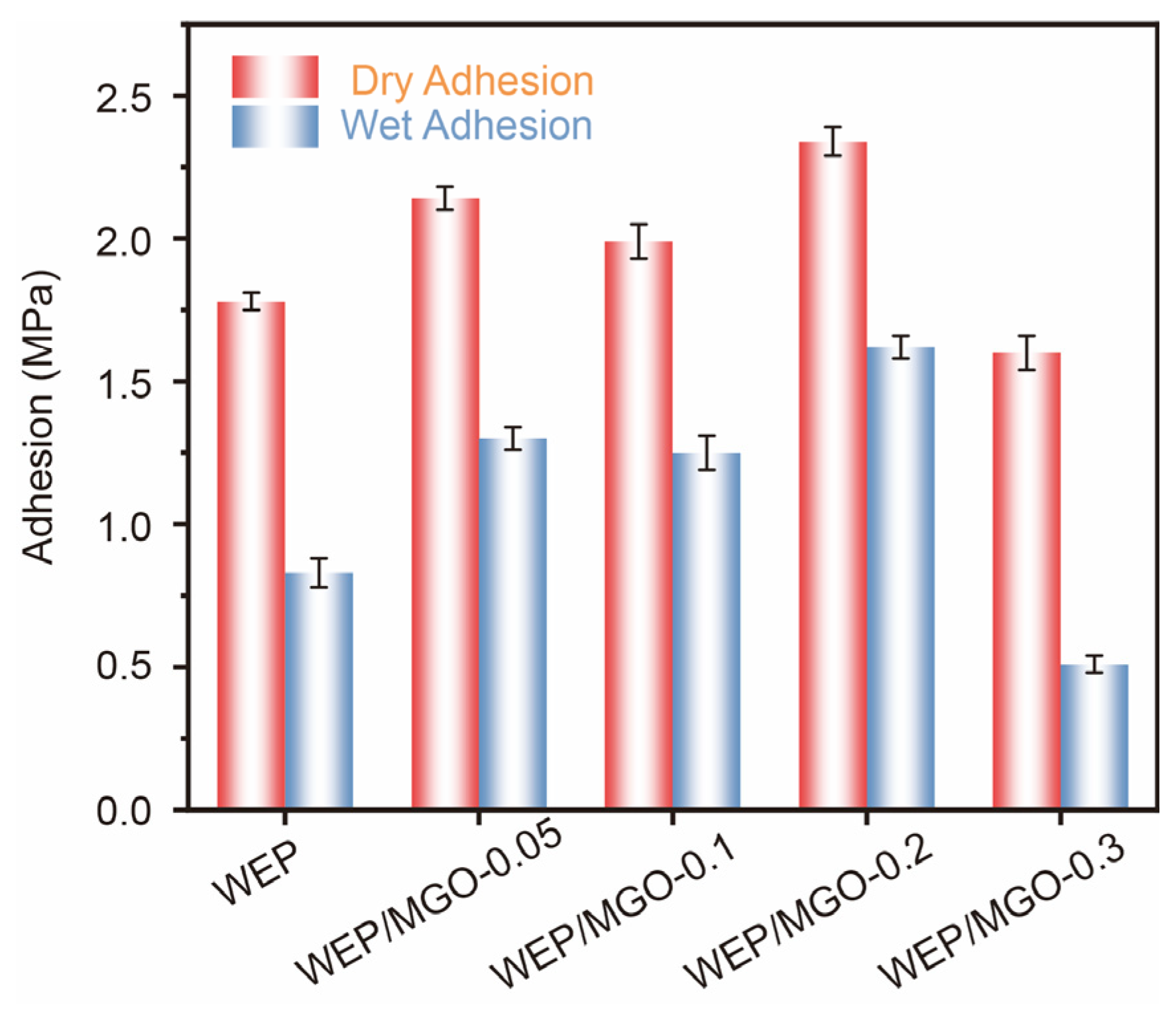

| WEP | WEP/MGO-0.05 | WEP/MGO-0.1 | WEP/MGO-0.2 | WEP/MGO-0.3 | |
|---|---|---|---|---|---|
| Rs (ohm·cm2) | 9.52 × 10−4 | 1.53 × 102 | 9.99 × 10−3 | 1.00 × 10−4 | 6.14 × 10−4 |
| Qc-Y0 (Ω−1·cm−2·sn) | 4.10 × 10−9 | 4.27 × 10−10 | 2.14 × 10−10 | 5.15 × 10−10 | 3.71 × 10−9 |
| Qc-n | 7.61 × 10−1 | 9.22 × 10−1 | 9.33 × 10−1 | 8.56 × 10−1 | 7.77 × 10−1 |
| Rcp (ohm·cm2) | 4.61 × 108 | 8.41 × 108 | 7.20 × 104 | 9.34 × 108 | 1.50 × 108 |
| Qdl-Y0 (Ω−1·cm−2·sn) | 3.92 × 10−11 | 5.96 × 10−10 | 2.03 × 10−9 | 2.16 × 10−7 | 3.06 × 10−7 |
| Qdl-n | 5.92 × 10−1 | 5.23 × 10−1 | 5.57 × 10−1 | 8.72 × 10−1 | 3.83 × 10−1 |
| Rct (ohm·cm2) | 5.33 | 2.69 × 109 | 7.26 × 108 | 3.76 | 1.30 × 102 |
| Samples | Icorr (A/cm2) | CR (mm/Year) |
|---|---|---|
| WEP | 3.76 × 10−11 | 1.73 × 10−5 |
| WEP/MGO-0.05 | 1.55 × 10−11 | 7.13 × 10−6 |
| WEP/MGO-0.1 | 4.59 × 10−12 | 2.11 × 10−6 |
| WEP/MGO-0.2 | 1.89 × 10−11 | 8.57 × 10−6 |
| WEP/MGO-0.3 | 7.42 × 10−11 | 3.41 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, D.; Chen, J.; Huo, D.; Gao, X.; Dong, J.; Yin, Y.; Liu, J.; Nan, D. Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings. Coatings 2024, 14, 488. https://doi.org/10.3390/coatings14040488
Li X, Li D, Chen J, Huo D, Gao X, Dong J, Yin Y, Liu J, Nan D. Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings. Coatings. 2024; 14(4):488. https://doi.org/10.3390/coatings14040488
Chicago/Turabian StyleLi, Xin, Dongsheng Li, Jie Chen, Dongxia Huo, Xin Gao, Junhui Dong, Yue Yin, Jun Liu, and Ding Nan. 2024. "Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings" Coatings 14, no. 4: 488. https://doi.org/10.3390/coatings14040488






