Abstract
This paper deals with the evaluation of the surface of structural steel whose samples were deliberately contaminated with transparent spray primer, adhesive label glue, and welding sprays prior to hot-dip galvanizing. The galvanized samples were studied by optical microscopy, GDOES, adhesion tests, and condensation humidity tests. The effect of surface contamination on the quality of the zinc coating was found to be significant. In some cases, the zinc coating is damaged (after contamination with welding sprays), in others, it is completely absent (after contamination with spray primer or adhesive label glue).
1. Introduction
Hot-dip galvanizing is one of the most effective methods of protecting steel against corrosion in various industries [1]. It makes it possible to obtain high-quality coatings and thus ensure long-term corrosion protection at relatively low operating costs for coatings [2]. Despite the fact that the galvanization process has been known for more than 150 years, this technology is still evolving [3]. Standards EN ISO 1461 [4] and EN ISO 14713-2 [5] provide the technical and qualitative requirements for hot dip galvanizing of steel parts.
Corrosion [6,7] is the disintegration of a metal due to its electrochemical or chemical reaction with a corrosive environment. The consequences are integrity damage, material destruction, fatigue, embrittlement, fractures, leaks, perforation; as well as changes in shape and surface quality, deterioration of appearance, changes in technical parameters, changes in functional properties, etc. Zinc coatings are widely used to protect steel parts against corrosion [8]. These coatings act as a barrier on the steel surface and also protect it as a sacrificial anode [9,10].
The technology of hot-dip galvanizing is based on the formation of an alloy coating of hot-dip zinc on the surface of a steel component. In hinge hot dip galvanizing [11], steel products (cleaned of grease, scale, rust, and other impurities and fixed on hinges) are immersed in baths containing molten zinc. Galvanizing is a complex process of diffusion processes, elementary metallurgical reactions, and thermodynamic changes [12].
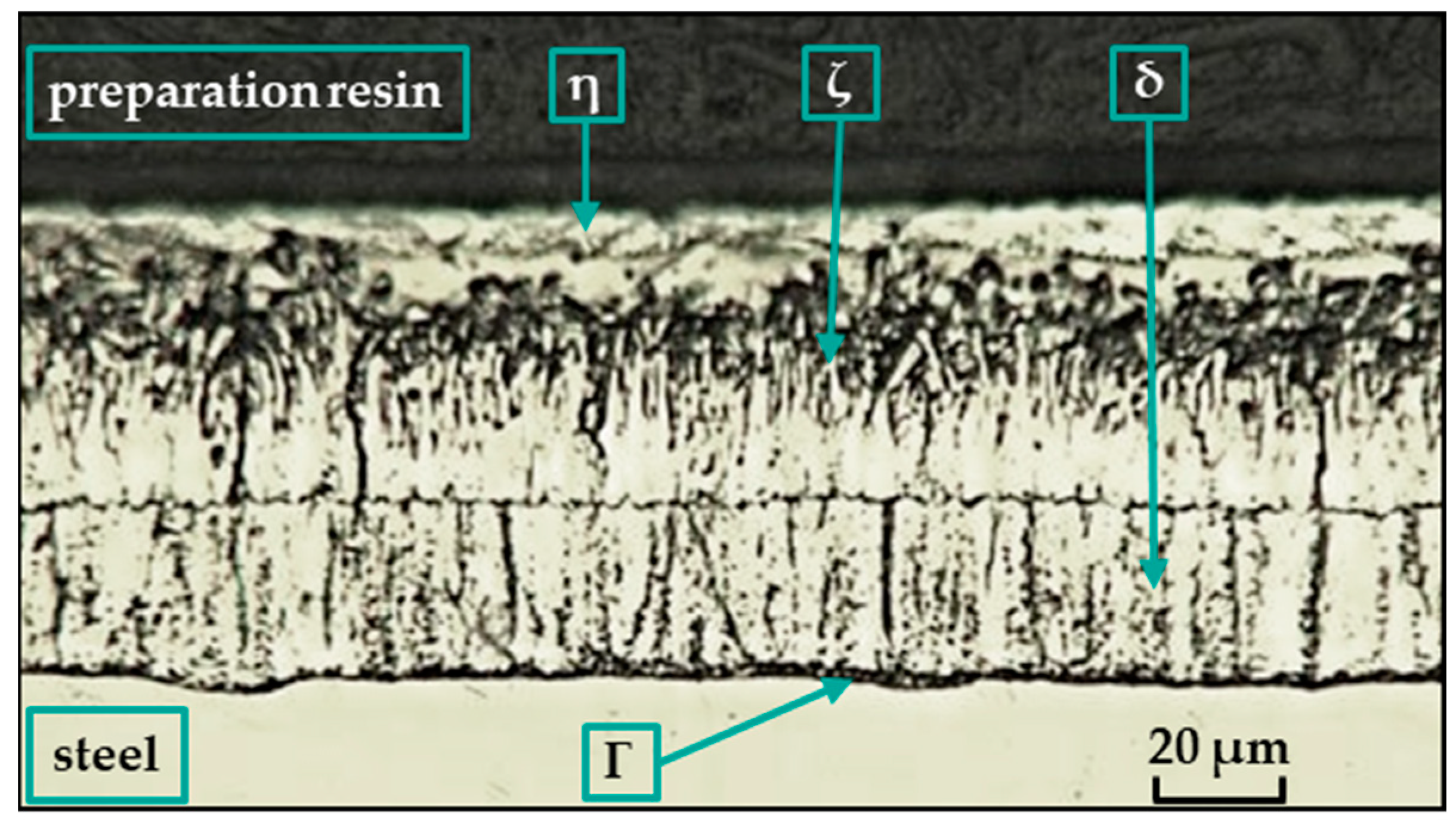
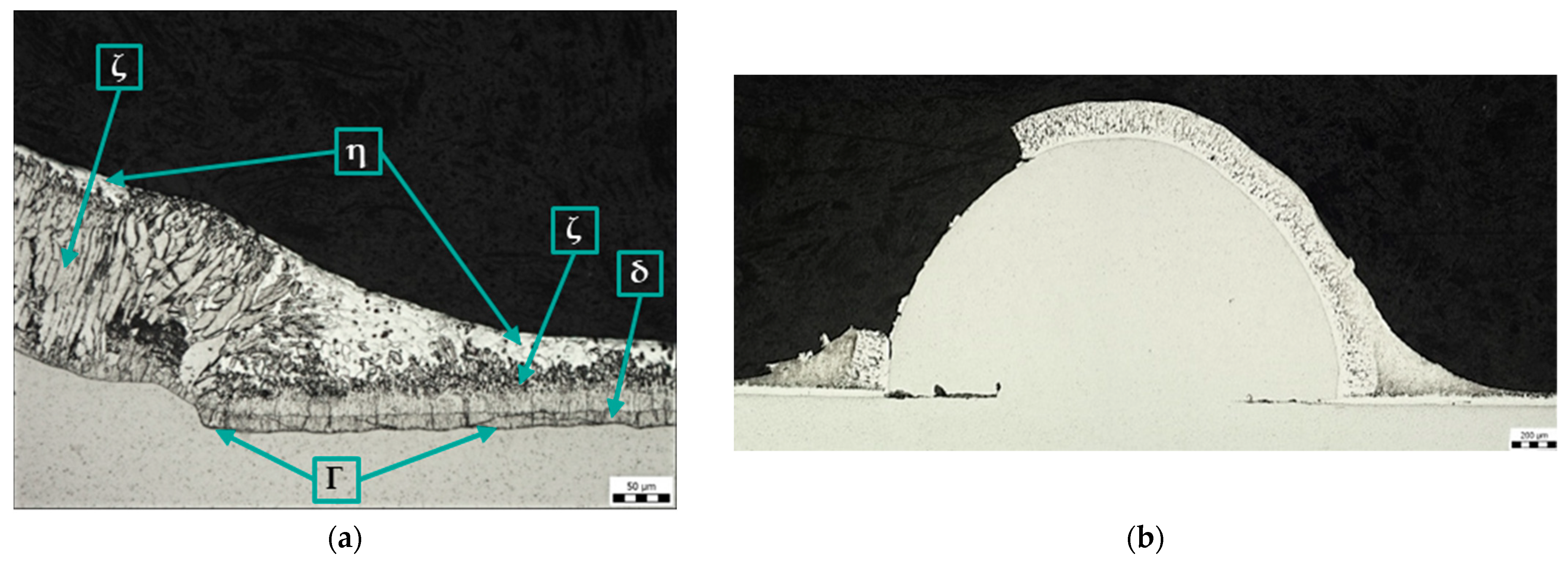
Depending on the composition of the steel, the temperature and composition of the bath, the thickness of the galvanized part, the immersion time in the bath, the surface conditions, and the method and rate of cooling, a coating composed of different Fe-Zn intermetallic compounds is formed [13] (see Figure 1). The layers composed of these compounds have different grain compositions morphologies, thicknesses, and mechanical properties. They are usually marked gamma (Γ), delta (δ), zeta (ζ), or eta (η).
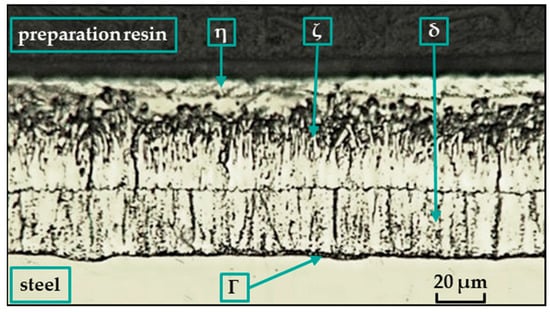
Figure 1.
Structure of hot-dip zinc coatings [25].
In the hot-dip galvanizing process, the zinc partially diffuses into the ferrite (Feα), where it forms a substitution solution in the surface layer. The iron–zinc alloy phases (see Figure 1) then grow on this substrate according to the instantaneous equilibrium conditions, and their relative presence in the coating changes with immersion time. The ferrite and zinc form a substitutional solid solution α with a zinc content of up to 4% at 450 °C. The metallurgical reaction during hot-dip galvanizing produces intermediate iron–zinc phases with different crystal structures [14]. Each of these alloy phases is characterized by a certain ratio in which zinc binds with iron. A thin layer of the Γ-phase [15,16,17] is covered by a consistent layer of closely arranged hexagonal crystals of the δ-phase [18,19,20,21], on which grow monoclinic crystals of the ζ-phase [19,22,23] penetrating into the zinc melt. Finally, the η-phase is formed [13], which adheres to the alloy coating when it emerges from the zinc bath [24].
The thickness and structure of the resulting coating and the individual intermetallic phases depend not only on the chemical composition of the material to be coated [26] and the bath, but also on other external factors influencing the course of the metallurgical reaction: temperature of the galvanizing bath, surface structure of the galvanized steel, type of mechanical or heat treatment of the galvanized steel, thickness of the galvanized steel, presence of foreign substances in the surface layer of the steel, immersion time in the bath, method, and time of cooling of the product after galvanizing.
The combination of these factors leads to the fact that the coating created during the hot-dip galvanizing process in commercial galvanizing plants is characterized by a whole range of different morphological deviations [11]: scale and algae, lumps, hard zinc staining around the drainage hole, hard zinc crystals growing out of the substrate, mixed coating structures, blisters, crusts, primary coating delamination, embedded zinc ash, etc.
The aim of this work was to assess the quality of the zinc coating in the case that the steel surface was contaminated before galvanizing and was not sufficiently cleaned or modified. In practice, the production of incorrectly plated components cannot be avoided. Very often, the customer’s failure to inform the galvanizer of the need for surface treatment beyond normal cleaning and pickling is to blame. Therefore, the prepared samples were contaminated and/or welded, and the quality of the formed zinc coating was assessed visually, metallographically, by an adhesion test, and a corrosion resistance test in a condensation chamber. This research was conducted because this issue is insufficiently described in the literature.
2. Materials and Methods
For the presented experiment, structural steel marked Kosmalt E300T (U. S. Steel Košice, s.r.o., Košice, Slovak Republic) was chosen. Its chemical composition was determined using a Spectruma Analytik GMBH (Hof, Bayern, Germany) glow discharge optical emission spectrometer (model GDA 750) under excitation conditions of 700 V and 35 mA [27,28,29] and elemental analysis (Eltra CS 2000) (Haan, Germany) [30,31] (elements marked *) and is shown in Table 1.

Table 1.
Chemical composition of Kosmalt E300T steel.
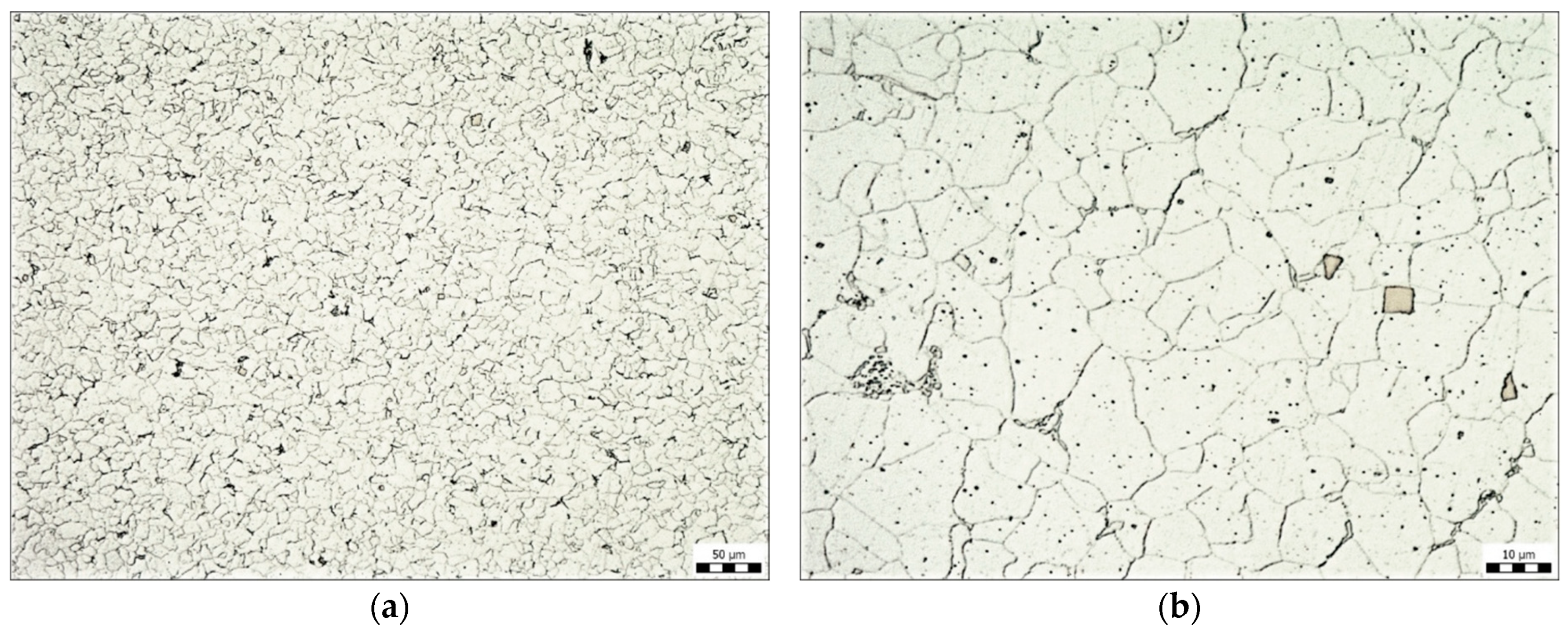
The pictures (Figure 2) show the microstructure of this steel. The microstructure consists of ferrite, rarely disintegrated pearlite (at the grain boundaries), and titanium carbonitrides (Figure 2b). An Olympus GX51 (Nagano, Japan) optical microscope was used to determine the structure of the base material as well as the thickness of the zinc coatings. The samples were analyzed in cross sections, embedded in resin, and ground and polished in a standard manner. Etching was performed with a Nital 4% etching agent.
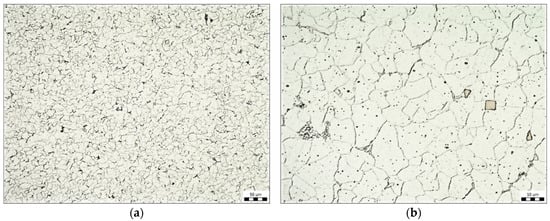
Figure 2.
Microstructure of Kosmalt E300T steel, magnification 200× (a), magnification 1000× (b).
Prior to the experiment, the surfaces of the samples were contaminated with transparent spay primer (sample B) and glue that remained on the sample after removing the label, which is used for marking metal sheets (sample C).
Other samples were welded by GMAW (MAG) (using anti-spatter spray (applied to the weldment to prevent spatter-type defects on the weldment) (sample F) and torch sprays (in practice, these should be applied to the torch, not to the weldment) (samples E and G). Welding was carried out in M21 shielding gas (designated according to EN ISO 14175 [32]) containing 82% Ar and 18% CO2. The consumable was G3Si1 wire (designated according to EN ISO 14341 [33]) with a chemical composition of 0.09 wt.% C, 0.9 wt.% Si, and 1.5 wt.% Mn. The welding was carried out under the following parameters: welding current 200 to 210 A, welding voltage 23 to 24 V and welding speed 7.0 to 7.4 mm/s. The list of samples is given in Table 2.

Table 2.
List of samples for hot-dip galvanizing.
The prepared samples were treated in a standard hot-dip galvanizing process in an industrial galvanizing plant (Figure 3), where they underwent a standard process (degreasing, rinsing, pickling with dilute hydrochloric acid, rinsing, immersion in flux, drying, and immersion in molten zinc).

Figure 3.
Hot-dip galvanizing process of the studied samples in an industrial galvanizing plant; (a) after degreasing, (b) after galvanizing; (photo by the author).
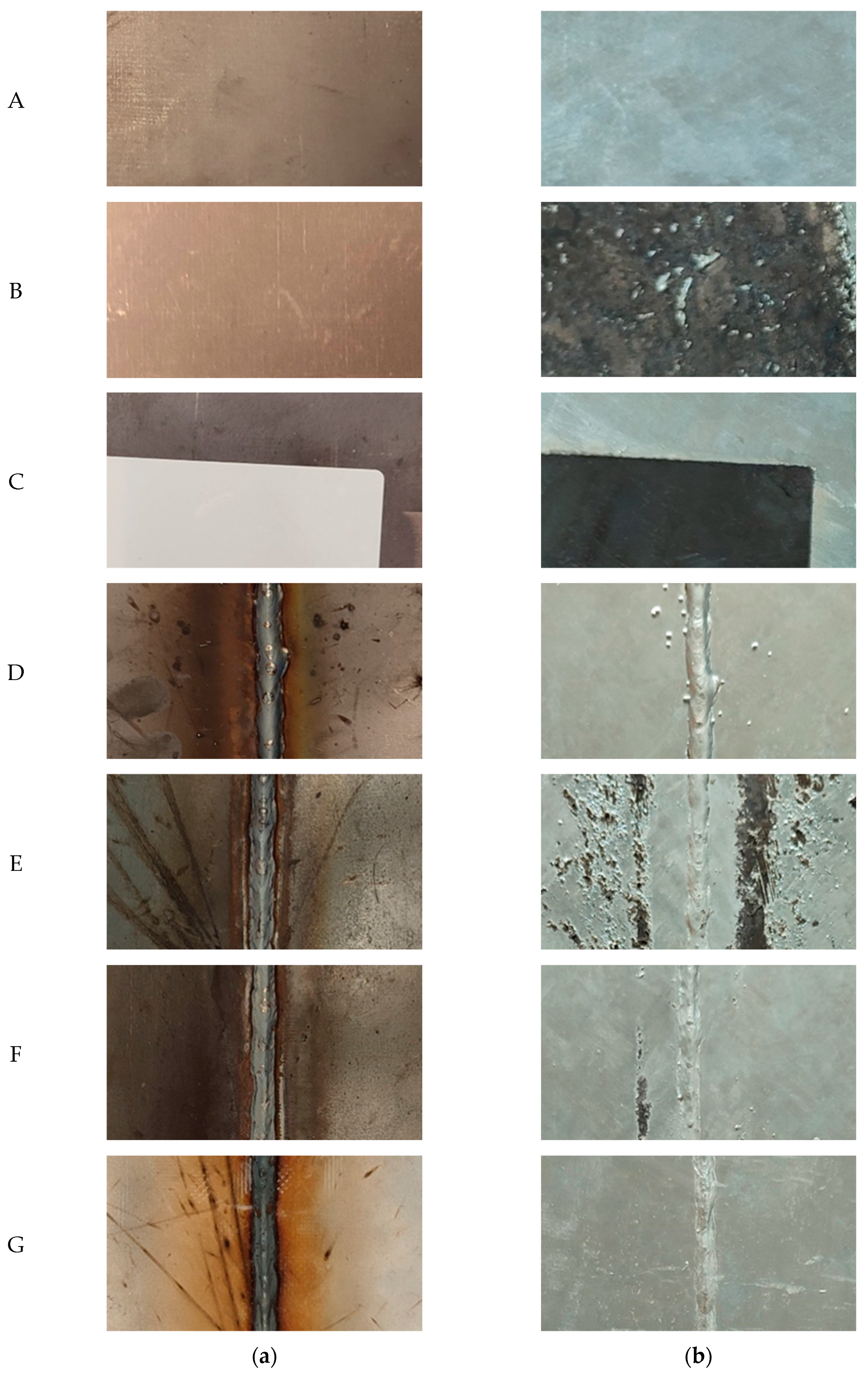
Photographs of the samples before and after hot-dip galvanizing are shown in Figure 4. By visual evaluation, it can be concluded that contamination of the surface before welding and the welding itself have a major influence on the quality of the zinc coating. On the samples with both transparent spay primer and glue (which remain after removing the adhesive label), either an almost completely missing or discontinuous zinc coating layer can be seen.
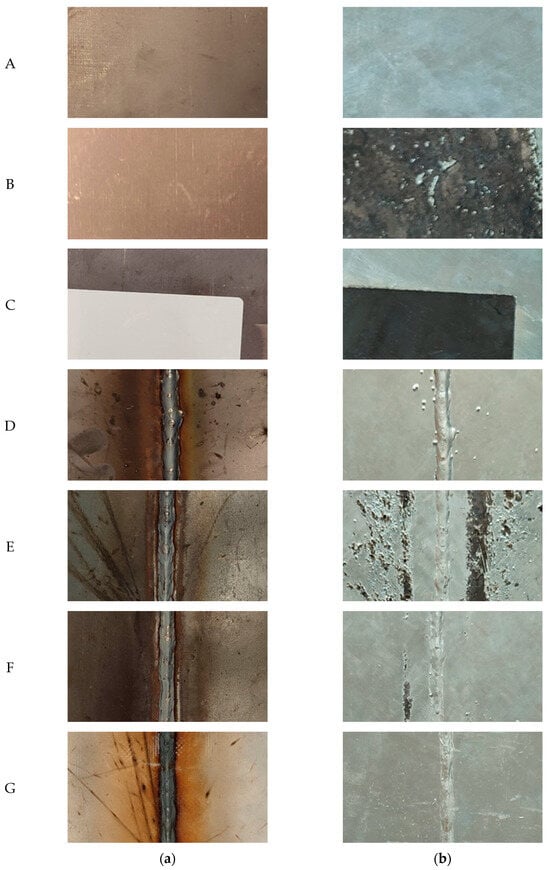
Figure 4.
Photographs of samples before hot-dip galvanizing (a) and after hot-dip galvanizing (b).
On samples E and F, an insufficiently formed zinc coating layer can be seen. When welding without the use of spray (sample D), spatter occurred, resulting in these defects being accentuated by the zinc coating. This leads to a reduction in aesthetic quality but also to the peeling of the zinc on the spatter, which could result in reduced corrosion protection of the zinc coating. There was no visible damage to the zinc surface on sample G.
3. Results
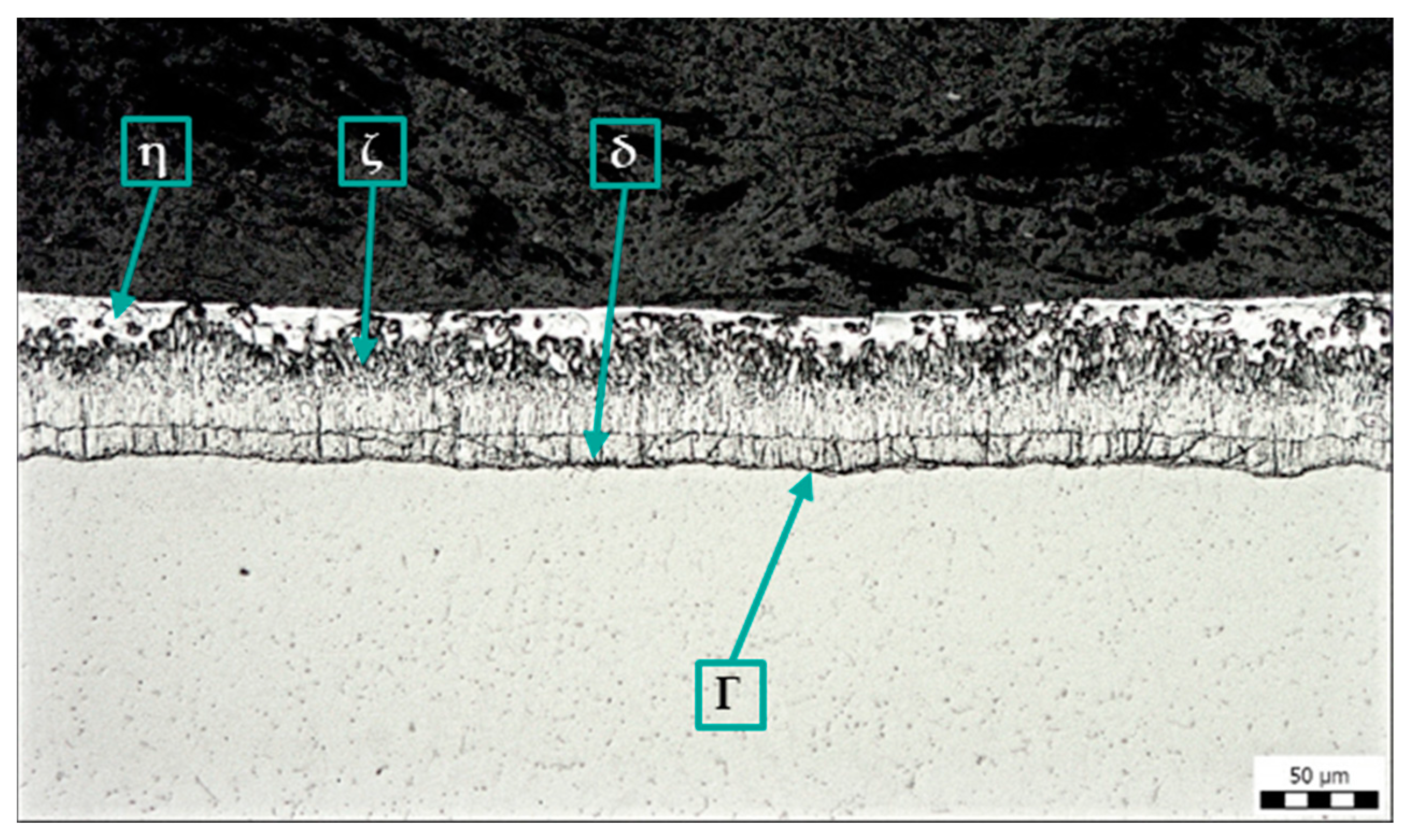
A standard metallographic cross-section was prepared on all samples, and the integrity and quality of the zinc coating were assessed. Figure 5 shows the zinc coating on a sample without surface contamination. The structure of the coating corresponds to the quality requirements of the hot-dip galvanizing coating.
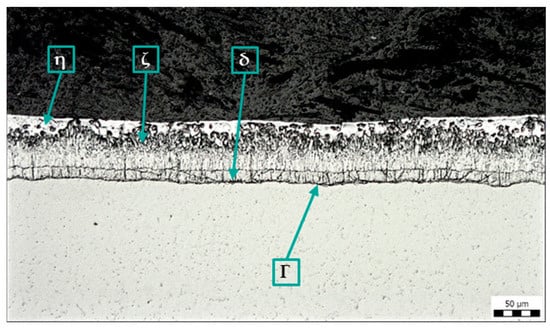
Figure 5.
Microstructure of sample A: Zn coating without contamination, magnification 200×.
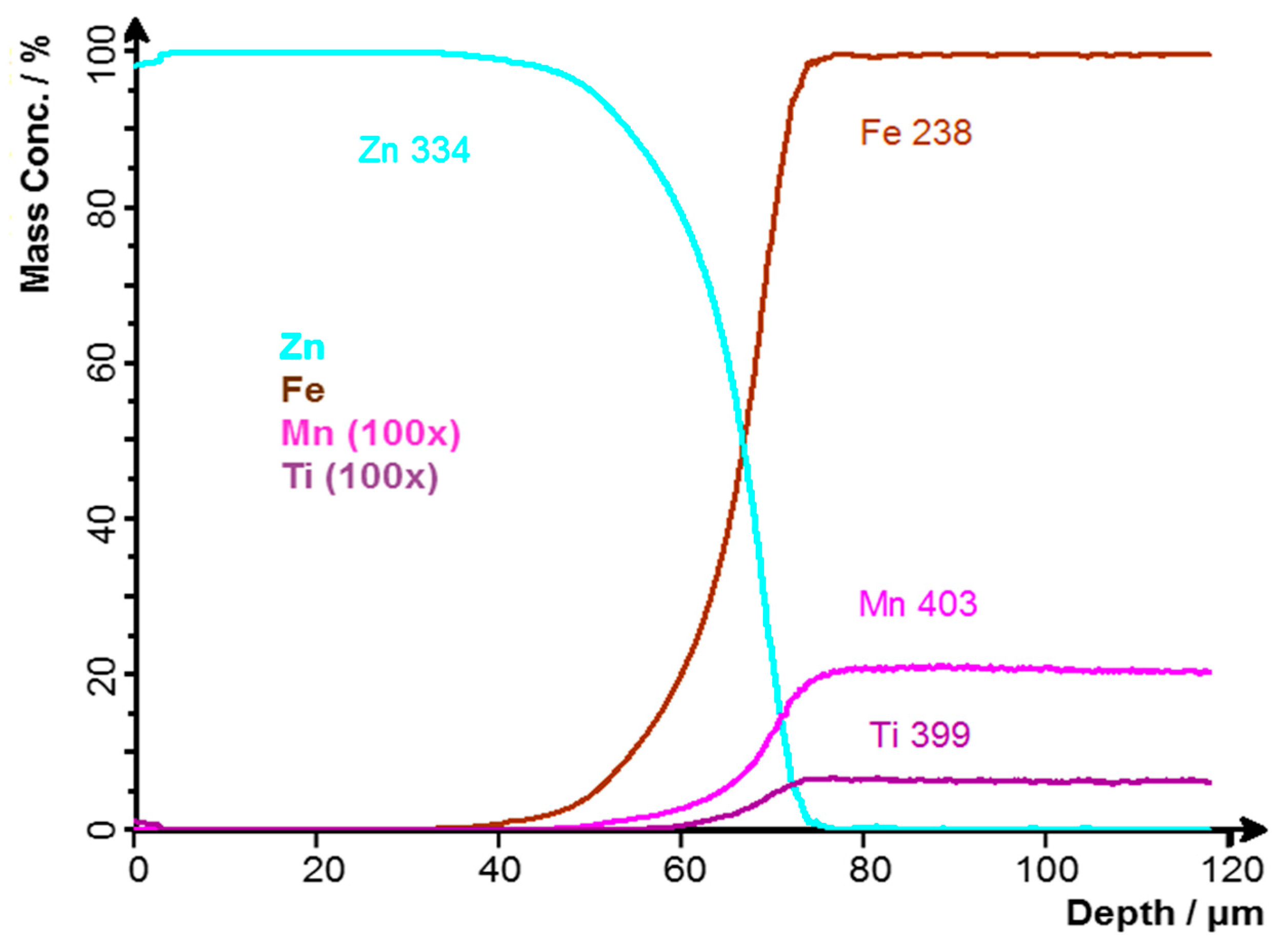
Figure 6 shows a record of the GDOES profile analysis. The GDOES profile analysis was performed under excitation conditions of 1000 V and 15 mA [25] from the surface, approximately 20 mm from the edge of the sample. The curves correspond to the results of optical microscopy and also to the GDOES BULK analysis, the results of which are shown in Table 1. The values of Mn and Ti contents have to be divided by 100, as indicated in the figure legend. The scales of these elements have been changed due to their low concentrations. The subtracted Zn coating thickness was approximately 67 μm.
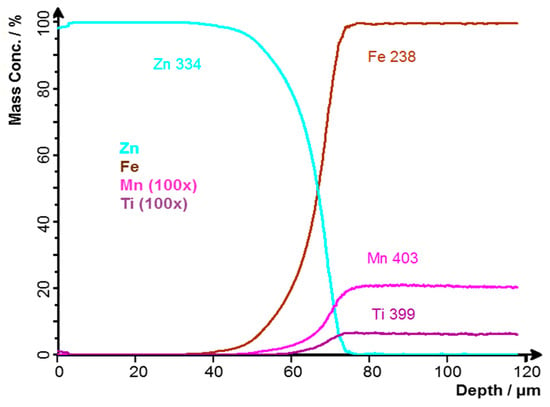
Figure 6.
GDOES profile analysis of sample A: Zn coating without contamination.
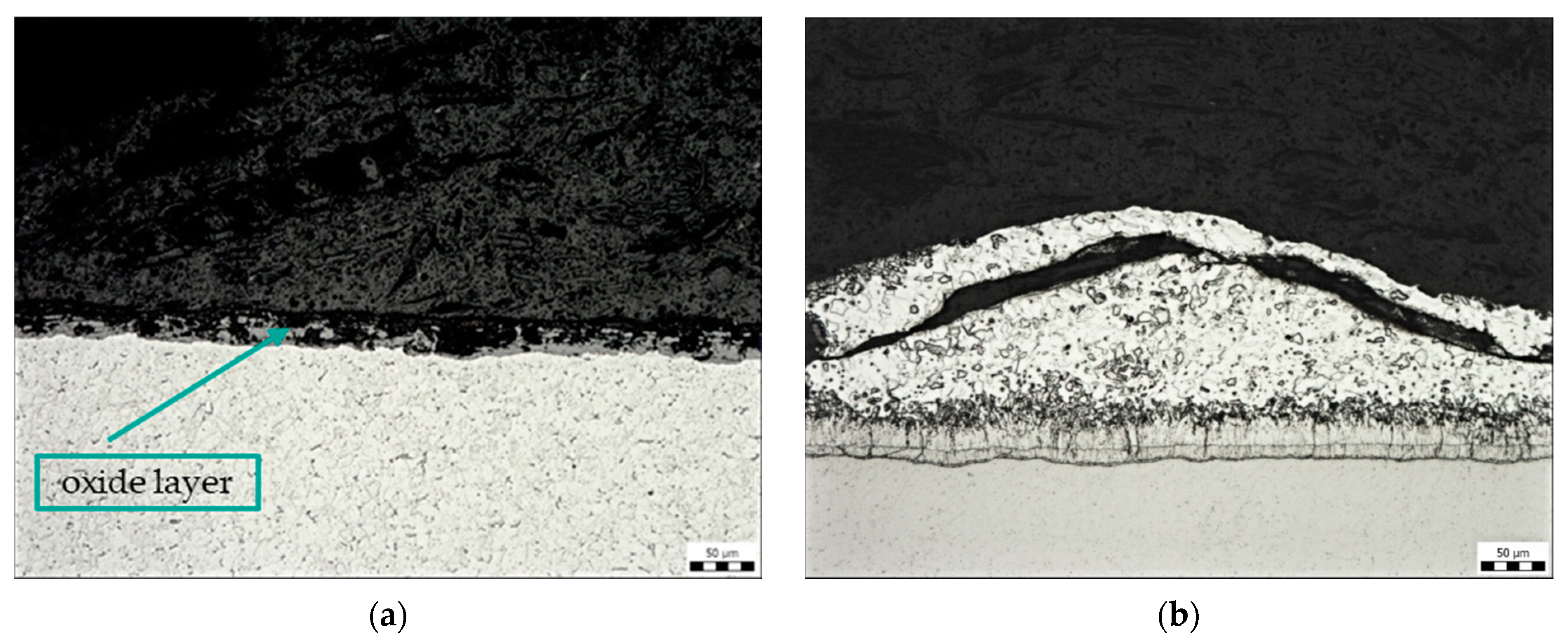
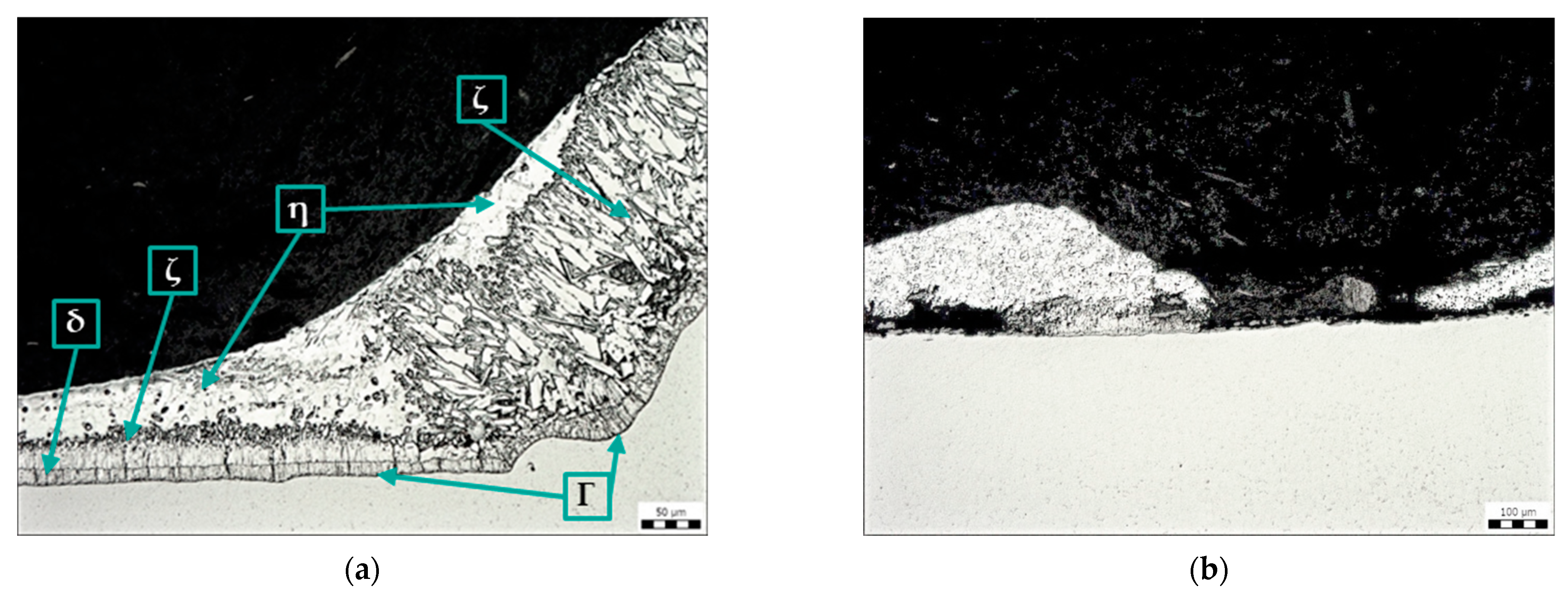
Figure 7a shows the oxide layer on the steel surface, which was formed on a sample contaminated with transparent spay primer (sample B). Residues of the Zn phase were observed on the oxide layer. Figure 7b shows a fragment of zinc coating on the contaminated surface. At this location, there was an increase in the η phase of the zinc coating, i.e., the layer that is formed when the component is removed from the molten zinc bath.
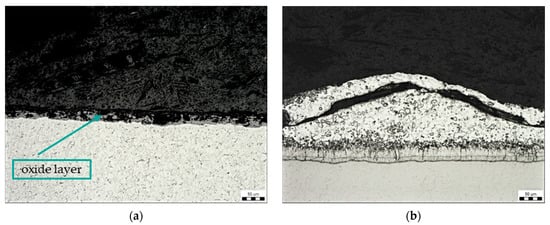
Figure 7.
Microstructure of sample B: contamination with transparent spray primer, magnification 200×: (a) oxide layer, (b) Zn coating fragment.
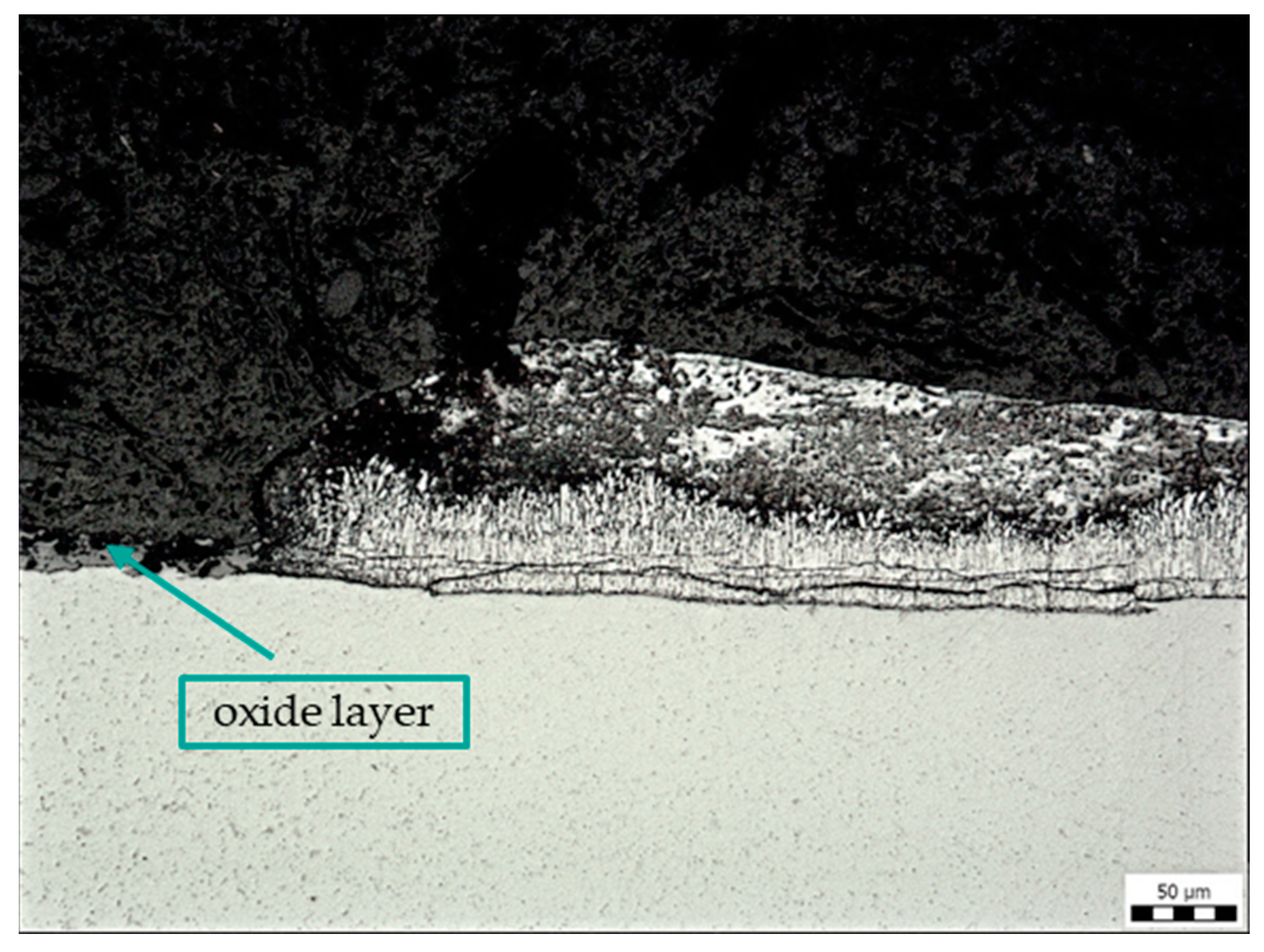
Figure 8 shows the edge of the removed label (sample C) without Zn coating (left side of the picture). The Zn coating is uneven around the label, and there are cracks inside, especially in the lower δ and Γ phases, which are formed by diffusion after immersion of the steel in the molten zinc bath. In the area of the removed label, there is no zinc coating on the base material but a layer of oxides.
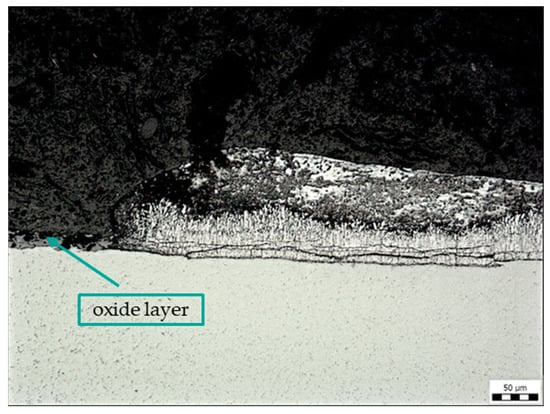
Figure 8.
Microstructure of sample C: contamination with adhesive label glue, magnification 200×.
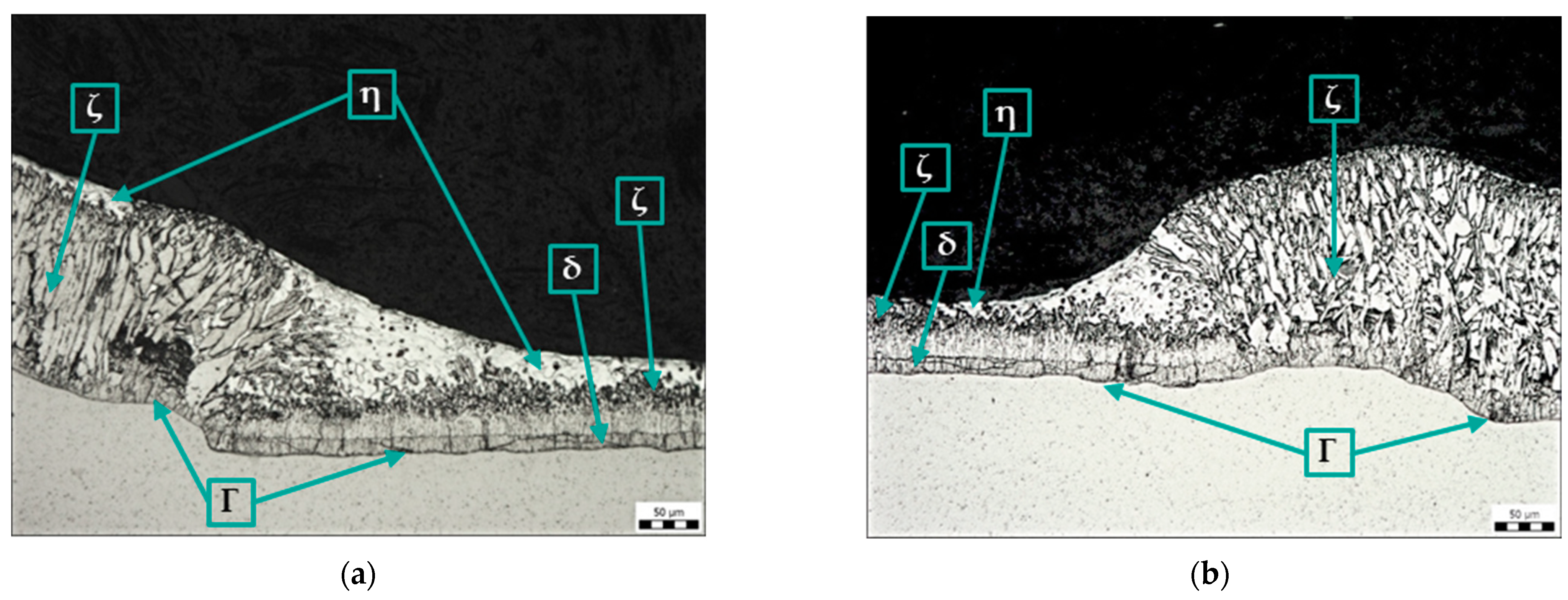
The following figure (Figure 9a) shows the microstructure of the zinc coating on the base material and the weld metal without the use of any anti-spatter sprays (sample D). The zinc coating on the weld metal is continuous, but its structure and thickness are different from the coating on the base metal (BM), which is due to the different chemical composition of the weld metal (G3Si1) and the base metal (Kosmalt 300T). In the structure of the zinc coating, the η phase (pure zinc) is absent, and the ζ phase reaches the surface of the coating.
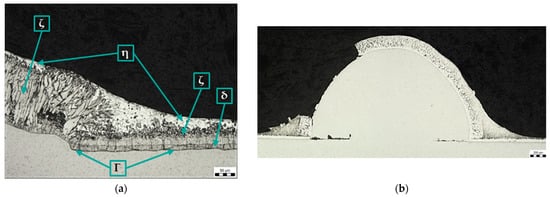
Figure 9.
Microstructure of sample D: (a) Zn coating in the area of transition from base metal to weld metal, magnification 200×; (b) Zn coating on the spatter, magnification 50×.
The picture below (Figure 9b) shows a panoramic image of a spatter with a broken zinc coating. Again, the different structure and thickness of the Zn coating on the base metal and on the weld metal can be seen. In addition, the lack of bonding between the spatter and the base material can be seen.
Figure 10a shows the microstructure of the zinc coating at the transition between the base metal and the weld metal using torch spray (sample E). The Zn coating on the weld metal is continuous, but its structure and thickness are different from those on the base metal. As in sample D, the structure of the zinc coating on the weld metal lacks the η phase (pure zinc), and the ζ phase reaches the surface of the coating. Figure 10b shows a discontinuous Zn coating (area outside the weld) that is damaged due to the use of torch spray.
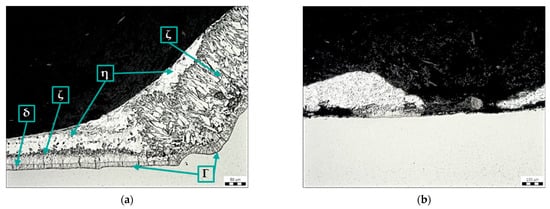
Figure 10.
Microstructure of sample E: (a) Zn coating in the area of transition from base metal to weld metal, magnification 200×; (b) Zn coating on base material, magnification 100×.
Figure 11a shows the transition between the base metal and the weld metal of sample F (surface sprayed with anti-spatter spray before welding) and the Zn coating at this transition. As in samples D and E, the structure of the zinc coating on the weld metal lacks the η phase (pure zinc), and the ζ phase reaches the surface of the coating.
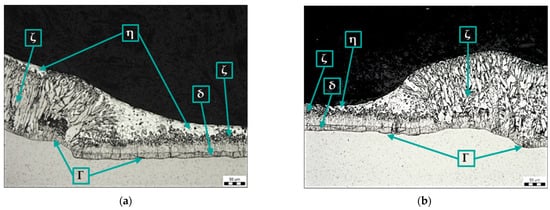
Figure 11.
Microstructure of samples F (a) and G (b): Zn coating in the area of transition from base metal to weld metal, magnification 200×.
Figure 11b shows the microstructure of the zinc coating at the transition between the base metal and the weld metal using a ceramic torch spray (sample G). The structure and thickness of the coating on the weld metal are different from those on the base metal, as well as on samples D, E, and F. No damage to the zinc coating is evident on the surface of sample G.
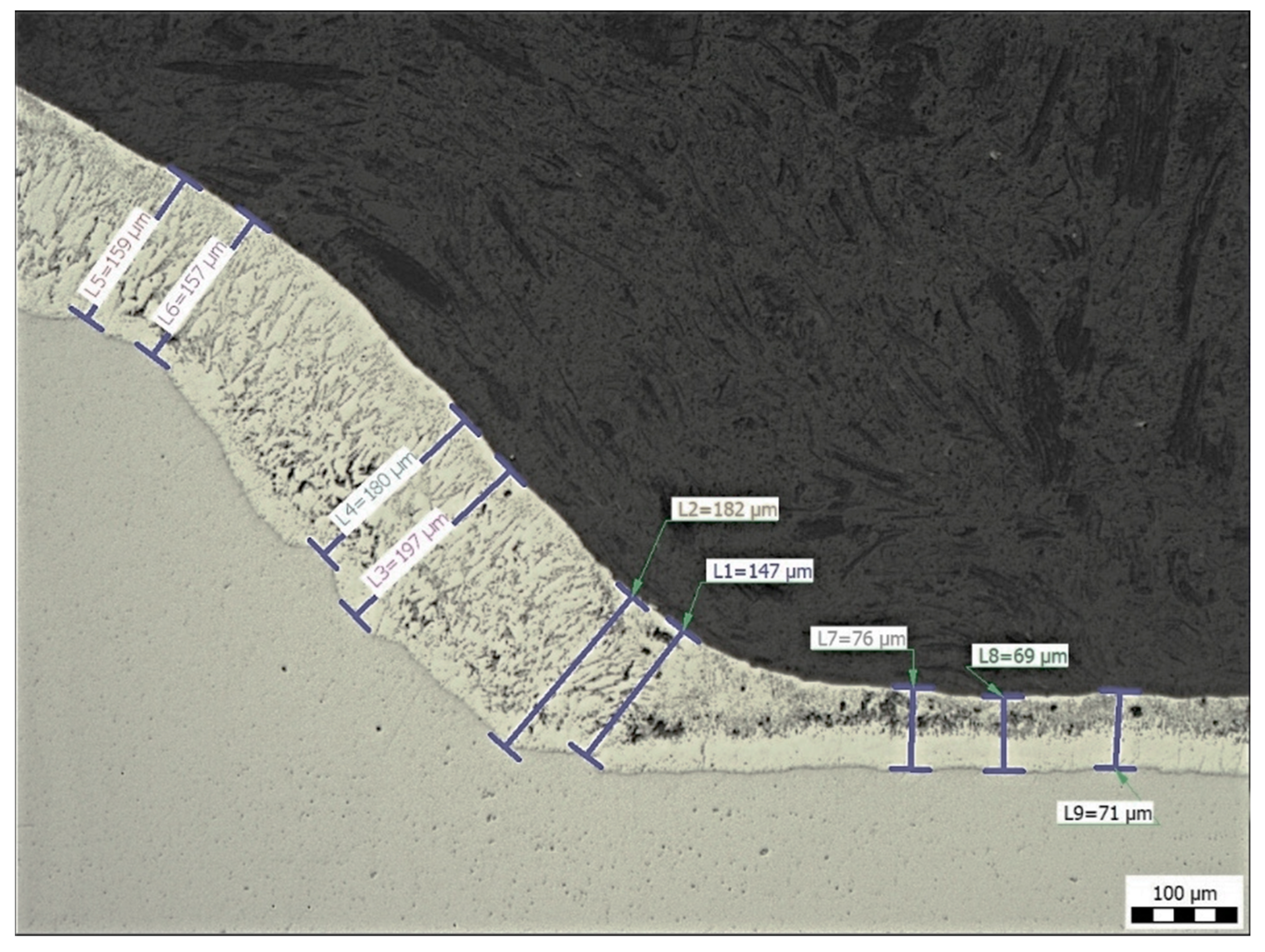
Figure 12 shows the measurement of the coating thickness at the transition zone from the base material to the weld metal (sample E). The results of the thickness measurements for all samples outside the transition zone (n = 15) are summarized in Table 3.
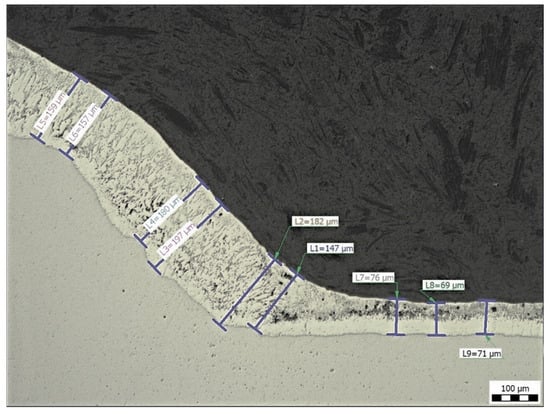
Figure 12.
Sample E: Measurement of Zn coating thickness at the transition from base material to weld metal, magnification 100×.

Table 3.
Thickness measurement results for samples A-G (µm).
The average thicknesses of the zinc coating on the base metal vary from 58 to 71 µm and from 160 to 171 µm on the weld metal (see Table 3). The samples were evaluated only in areas without zinc coating damage. The results are not statistically different. In locations where the Zn coating was not damaged, contamination by welding sprays did not affect the Zn coating thickness.
To assess the quality of the zinc coating, an adhesion test was performed. This test was carried out according to ASTM A123 [34] “Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel Products” and DIN 50978 [35] on hot-dip galvanized base material (sample A, Figure 13), on a sample to which an adhesive label had been applied (sample C), and also on galvanized sheet metal welds contaminated with welding sprays (samples E–G). Sample B was not tested because a continuous zinc coating did not form on its surface, which was contaminated with transparent spay primer.
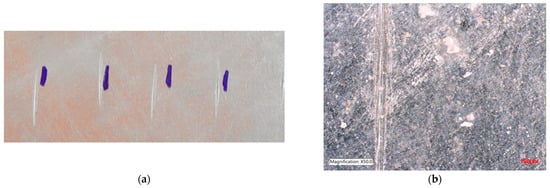
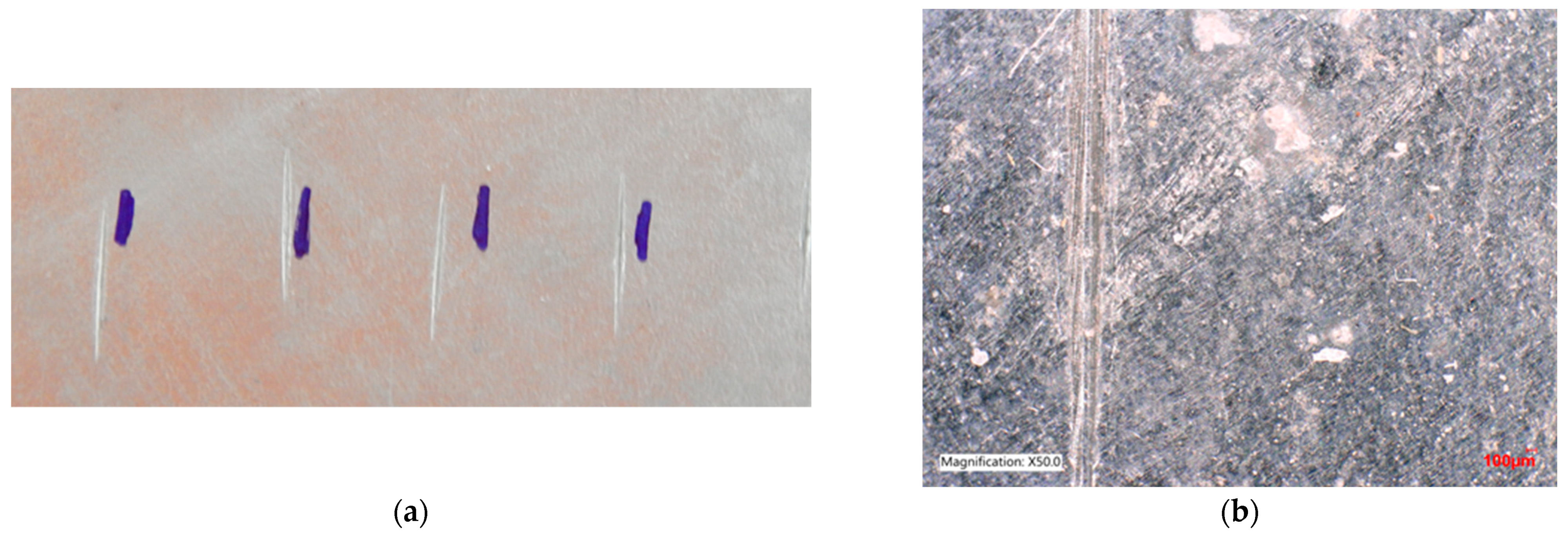
Figure 13.
Sample A: (a) image after adhesion test; (b) surface after the test—3D microscope, magnification 50×.
The adhesion test was carried out using a Hammer LKA SCANGALV AB (Järpen, Sweden), and a Keyence VHX-5000 3D microscope (Osaka, Japan) was used to evaluate this test.
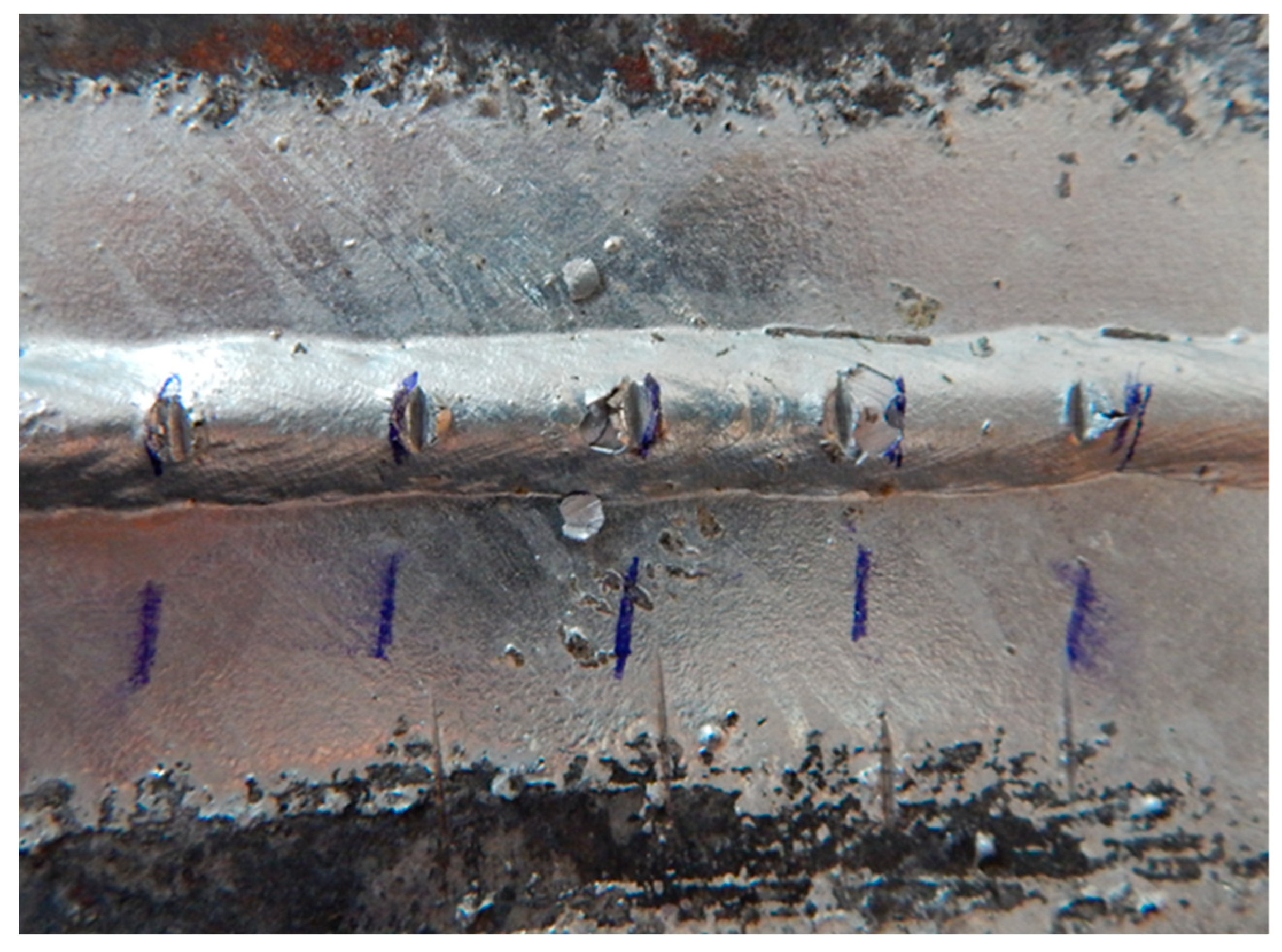
In Figure 13, it can be seen that after the test, there was no cracking or peeling of the Zn coating between the individual scratches. The coating is stable, firmly adhering to the surface of the sheet. Color unevenness is typical for hot zinc and does not reduce the quality of corrosion protection.
The adhesion test at the transition between the contaminated area (on which the zinc coating is not present) and the uncontaminated part of the sample shows that there was no peeling or cracking of the Zn coating between the individual scratches.
The scratches on sample E are visible in Figure 14 and Figure 15. It can be seen in Figure 14 that the spray contamination significantly affected the galvanization of the sheet, but there was no peeling of the zinc coating on the surface with a uniform zinc layer after the test. The scratches in the zinc coating on the weld metal are slightly cracked but still meet the parameters given by the DIN 50978 standard [35] (no loss of adhesion of the zinc coating on 50% of the area between scratches).
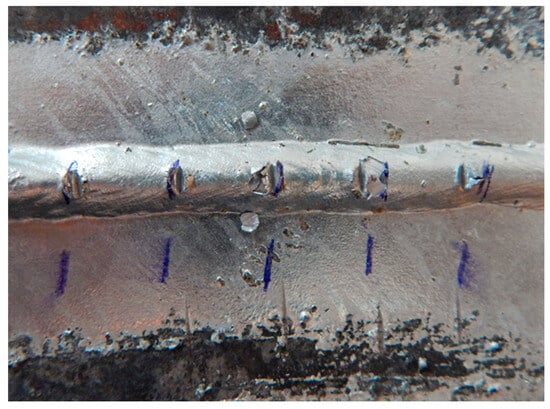
Figure 14.
Sample E: Image after adhesion test.

Figure 15.
Sample E: Image after adhesion test: (a) detail of the scratch on the weld metal, magnification 50×; (b) detail of scratch outside the weld metal, magnification 20×.
Similar results were also achieved by testing samples F and G. There was only a very slight cracking of the zinc coating in the immediate vicinity of the indentation.
All samples were further tested in a condensation chamber (CSN 03 8131, eqv. ASTM D4585) [36,37].
The test was carried out in the CCT chamber for 200 h at 35 °C, 100% relative humidity, and water condensation. The following figures (Figure 16a–g) show photographs of the samples after exposure in the test chamber.

Figure 16.
Samples A–G (a–g): image after condensation humidity test.
Figure 16a is a photograph of sample A after exposure in the test chamber. This is the original hot-dip galvanized material, the surface of which has not been contaminated or mechanically or thermally affected. It can be seen that the sample shows no indications of corrosion due to the corrosive environment. The zinc coating is only slightly covered by a thin layer of white-grey corrosion products of zinc.
Samples B and C (Figure 16b,c) are samples contaminated with transparent spray primer and glue after the removal of the adhesive label. Here, a significant corrosion attack on the underlying steel is evident, but this is consistent with the assumption given that a zinc layer has not formed on such a contaminated substrate.
Samples D–G are made of the same material as samples A–C, but they are welded by MAG technology using welding sprays (E–G). The condensation humidity tests in the CCT chamber (Figure 16d–g) showed that corrosion attack was only evident where there was no continuous zinc coating.
On the weld, the zinc coating has a significantly greater thickness, so the result that welding without the use of sprays did not cause a problem with corrosion resistance corresponds to the assumption.
4. Discussion
Based on the experiments performed, it can be concluded that the effect of surface contamination on the quality of the zinc coating is significant.
The use of a label (marking of the steel grade) has a significant negative effect on the subsequent zinc coating. It is essential that the customer inform the galvanizer that he has removed the label or left it on. Similarly, the intentional or unintentional use of transparent synthetic spray leads to the subsequent absence of zinc coating.
The results presented in this paper also show that the use of welding sprays affects the quality of the subsequently applied hot-dip zinc coating.
In this work, three types of sprays were tested:
- torch spray (sample E)—causes extensive defects in the zinc coating around the weld,
- anti-spatter spray (sample F)—causes defects in the zinc coating around the weld,
- ceramic torch spray (sample G)—does not cause defects in the zinc coating around the weld.
It is important to note that sprays intended for torches (E, G) are not intended for spraying weldments. The samples tested were contaminated with them deliberately to demonstrate the effect of the mishandling of these sprays during welding. It is surprising that the anti-spatter spray (sample F), despite being intended for spraying the surroundings of the weld, caused defects in the subsequent zinc coating. The only one of the tested sprays that did not cause defects during subsequent galvanizing of the steel was the ceramic protective spray (sample G).
The achieved results can be applied to Kosmalt E300T steel and steels of similar chemical composition, i.e., low-carbon, unalloyed steels with a low content of silicon and phosphorus.
5. Conclusions
The steel surface contamination before galvanizing cannot be easily identified, but it has a significant negative effect on the final zinc coating. The tests performed show changes in phase structure—missing some phases and their thickness. These changes locally affected the appearance and corrosion of the coating. In some cases, the zinc coating is damaged (after contamination with welding sprays); in others, it is completely absent (after contamination with spray primer or adhesive label glue).
Steel structure producers should pay attention to steel surface quality before galvanizing in order to obtain a hot dip coating for long-term protection.
Author Contributions
Conceptualization, J.V. and P.M.; methodology, J.V., P.M. and K.K.; validation, J.V., P.M. and K.K.; formal analysis, J.V.; investigation, J.V., P.M. and K.K.; resources, J.V., P.M. and K.K.; data curation, J.V., P.M. and K.K.; writing—original draft preparation, J.V.; writing—review and editing, J.V., P.M. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This article was created with the contribution of the specific research project No. SP2024/062 “Influence of production, processing parameters and degradation mechanisms on the resulting material properties and lifetime of structural materials” and the specific research project No. SP2024/049 “Research and Optimisation of Engineering Technologies”, both solved in 2024 at the VSB-Technical University of Ostrava.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Autor Kateřina Kreislová was employed by the company SVÚOM s.r.o. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Jiřina Vontorová and Petr Mohyla have no conflict of interest.
References
- Kania, H. Structure and Corrosion Resistance of Coatings Obtained by the Batch Double Hot Dip Method in Eutectoid ZnAl Bath with the Addition of Mg and Si. Coatings 2022, 12, 1207. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef] [PubMed]
- Marder, A.R. Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- ISO 1461; Hot Dip Galvanized Coatings on Fabricated Iron and Steel Articles Specifications and Test Methods. ISO: Geneva, Switzerland, 2022.
- ISO 14713-2; Zinc Coatings—Guidelines and Recommendations for the Protection against Corrosion of Iron and Steel in Structures—Part 2: Hot Dip Galvanizing. ISO: Geneva, Switzerland, 2019.
- Kreislova, K.; Knotkova, D. The Results of 45 Years of Atmospheric Corrosion Study in the Czech Republic. Materials 2017, 10, 394. [Google Scholar] [CrossRef]
- Halama, M.; Kreislova, K.; Van Lysebettens, J. Prediction of Atmospheric Corrosion of Carbon Steel Using Artificial Neural Network Model in Local Geographical Regions. Corrosion 2011, 67, 065004-1. [Google Scholar] [CrossRef]
- Bhat, R.S.; Balakrishna, M.K.; Parthasarathy, P.; Hegde, A.C. Structural Properties of Zn-Fe Alloy Coatings and Their Corrosion Resistance. Coatings 2023, 13, 772. [Google Scholar] [CrossRef]
- Boshkov, N.S.; Petrov, K.P.; Kovacheva, D.; Vitkova, S.D.; Nemska, S. Influence of the Alloying Component on the Protective Ability of Some Zinc Galvanic Coatings. Electrochim. Acta 2005, 51, 77–84. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Lagaris, D.A.; Vatista, P.C. Adhesion and Corrosion Behaviour of Zn-Co Electrodeposits on Mild Steel. Mater. Chem. Phys. 2011, 126, 398–403. [Google Scholar] [CrossRef]
- Kuklík, V. AČSZ–Drsnost Povlaků Žárového Zinku Nanášených Podle ČSN EN ISO 1461 (Roughness of Hot Dipped Zinc Coatings Applied According to EN ISO 1461). Konstrukce 2012. [Google Scholar]
- Kuklík, V.; Kudláček, J. Hot-Dip Galvanizing of Steel Structures; Elsevier: Amsterdam, The Netherlands; Butterworth-Heinemann: Oxford, UK, 2016; ISBN 9780081007532. [Google Scholar]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 4th ed.; Pearson Education Limited: Harlow, UK, 2012; ISBN 978-0-273-74275-3. [Google Scholar]
- Yeomans, S.R. Galvanized Steel Reinforcement in Concrete; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 978-0080445113. [Google Scholar]
- Hu, X.; Watanabe, T. Relationship between the Crystallographic Structure of Electrodeposited Fe-Zn Alloy Film and Its Thermal Equilibrium Diagram. Mater. Trans. 2001, 42, 1969–1976. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.; Zhou, W.; Zhang, J.; Wu, J. Cross-Sectional TEM Observation of Iron–Zinc Intermetallic Γ and Γ1 Phases in Commercial Galvannealed IF Steel Sheets. Mater. Des. 2007, 28, 249–253. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, X. Effects of Steel Coatings Microstructure on Weldability in Resistance Spot Welding of Galvannealed Steel Sheets. Adv. Mater. Res. 2010, 139–141, 610–613. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Inomoto, M.; Adachi, H.; Takebayashi, H.; Inui, H. Micropillar Compression Deformation of Single Crystals of the Intermetallic Compound ς-FeZn13. Acta Mater. 2014, 65, 229–239. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Kashioka, D.; Inomoto, M.; Inui, H.; Takebayashi, H.; Yamaguchi, S. Compression Deformability of Γ and ζ Fe–Zn Intermetallics to Mitigate Detachment of Brittle Intermetallic Coating of Galvannealed Steels. Scr. Mater. 2013, 69, 307–310. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Tanaka, K.; Yasuhara, A.; Inui, H. Structure Refinement of the Δ1p Phase in the Fe-Zn System by Single-Crystal X-Ray Diffraction Combined with Scanning Transmission Electron Microscopy. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 275–282. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Yasuhara, A.; Inui, H. Order–Disorder Structure of the Δ1k Phase in the Fe–Zn System Determined by Scanning Transmission Electron Microscopy. Acta Mater. 2014, 81, 345–357. [Google Scholar] [CrossRef]
- Gellings, P.J.; Gierman, G.; Koster, D.; Kuit, J. Synthesis and Characterization of Homogeneous Intermetallic Fe-Zn Compounds. Int. J. Mater. Res. 1980, 71, 70–75. [Google Scholar] [CrossRef]
- Belin, R.; Tillard, M.; Monconduit, L. Redetermination of the Iron-Zinc Phase FeZn13. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 267–268. [Google Scholar] [CrossRef]
- Kuklík, V. Povlaky Žárového Zinku Na Pálených Plochách (Hot Dipped Zinc Coatings On Thermal Cut Surfaces). Konstrukce 2011. [Google Scholar]
- Vontorová, J.; Mohyla, P. Use of GDOES Method for Evaluation of the Quality and Thickness of Hot Dip Galvanised Coating. Trans. IMF 2018, 96, 313–318. [Google Scholar] [CrossRef]
- Pokorný, P.; Kolisko, J.; Balík, L.; Novak, P. Effect of Chemical Composition of Steel on the Structure of Hot-Dip Galvanized Coating. Metalurgija 2016, 55, 115–118. [Google Scholar]
- Weiss, Z. Emission Yields and the Standard Model in Glow Discharge Optical Emission Spectroscopy: Links to the Underlying Physics and Analytical Interpretation of the Experimental Data. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 121–133. [Google Scholar] [CrossRef]
- Vontorová, J.; Váňová, P. Determination of Carburized Layer Thickness by GDOES Method. AIMS Mater. Sci. 2018, 5, 34–43. [Google Scholar] [CrossRef]
- Priamushko, T.S.; Mikhaylov, A.A.; Babikhina, M.N.; Kudiiarov, V.N.; Laptev, R.S. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals 2018, 8, 372. [Google Scholar] [CrossRef]
- Veverka, J.; Vilémová, M.; Chlup, Z.; Hadraba, H.; Lukáč, F.; Csáki, Š.; Matějíček, J.; Vontorová, J.; Chráska, T. Evolution of Carbon and Oxygen Concentration in Tungsten Prepared by Field Assisted Sintering and Its Effect on Ductility. Int. J. Refract. Met. Hard Mater. 2021, 97, 105499. [Google Scholar] [CrossRef]
- Čegan, T.; Cagala, M.; Kursa, M.; Kawulok, P.; Rusz, S.; Juřica, J.; Vontorová, J. Effect of Ti2AlC Particles on the Microstructure and Elevated-Temperature-Deformation Properties of γ-TiAl Alloys. Mater. Tehnol. 2014, 48, 831–835. [Google Scholar]
- ISO 14175; Welding Consumables—Gases and Gas Mixtures for Fusion Welding and Allied Processes. ISO: Geneva, Switzerland, 2008.
- ISO 14341; Welding Consumables—Wire Electrodes and Deposits for Gas Shielded Metal Arc Welding of Non Alloy and Fine Grain Steels-Classification. ISO: Geneva, Switzerland, 2020.
- ASTM A123; Standard Specification for Zinc (Hot-Dip Galvanized) Coatings on Iron and Steel Products. ASTM: West Conshohocke, PA, USA, 2017.
- DIN 50978; Prüfung Metallischer Überzüge; Haftvermögen von Durch Feuerverzinken Hergestellten Überzügen. DIN: Berlin, Germany, 1985.
- ČSN 03 8131; Korozní Zkouška v Kondenzační Komoře (Corrosion Test in the Condensing Chamber). ÚNMZ: Praha, Czech Republic, 1973.
- ASTM D4585; Standard Practice for Testing Water Resistance of Coatings Using Controlled Condensation. ASTM: West Conshohocke, PA, USA, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).