Effects of Annealing Temperature on Bias Temperature Stress Stabilities of Bottom-Gate Coplanar In-Ga-Zn-O Thin-Film Transistors
Abstract
:1. Introduction
2. Method
3. Results
3.1. XPS Characterization
3.1.1. IGZO Layer
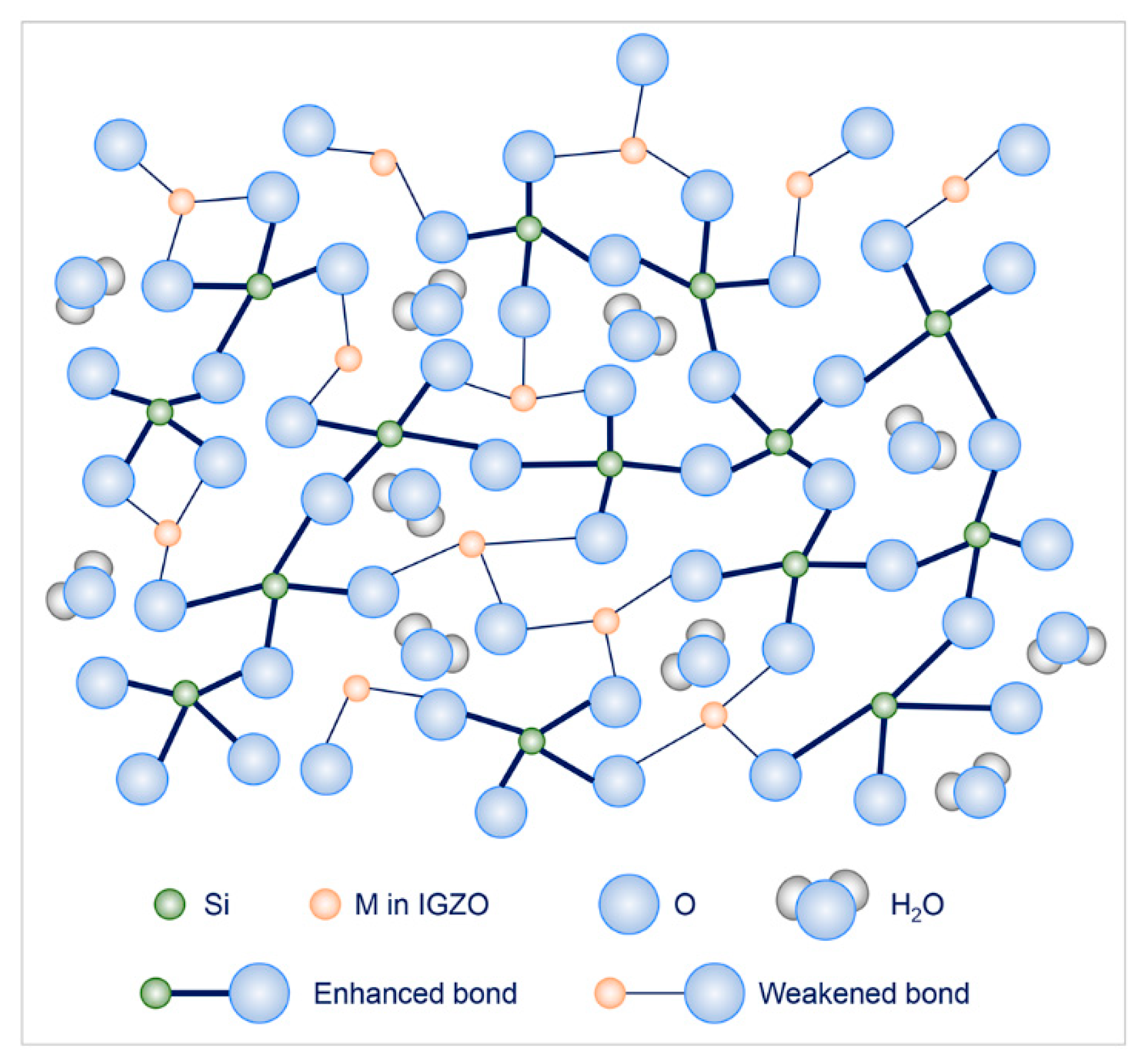
3.1.2. IGZO-SiO2 Interfacial Mixing Layer
3.1.3. SiO2 Layer
3.2. SEM Characterization
3.3. Bias Stress Stabilities
3.3.1. Transfer Characteristics
3.3.2. Positive Bias Temperature Stress (PBTS) Stabilities
3.3.3. Negative Bias Temperature Stress (NBTS) Stabilities
| Configuration | Active Layer | VGS (V) | Temp (°C) | Time (s) | ΔVth (V) | Reference |
|---|---|---|---|---|---|---|
| Coplanar_BG | IGZO | +20 | 60 | 3000 | 2.5 | [47] |
| Staggered_BG | IGZO | +20 | 60 | 3600 | 0.5 | [48] |
| Staggered_BG | IGZTO | +20 | 60 | 7200 | 1.8 | [49] |
| Staggered_BG | IZO | +20 | 60 | 7200 | 3.0 | [50] |
| Staggered_BG | IZO:Pr | +20 | 60 | 7200 | 2.0 | [50] |
| Coplanar_BG | IGZO | +20 | 60 | 10,800 | 1.3 | This work |
| Staggered_BG | SZTO | −20 | 60 | 3600 | −2 | [51] |
| Staggered_BG | SZTO | −20 | 60 | 3600 | −2.73 | [52] |
| Staggered_BG | IGZO | −20 | 60 | 3600 | −0.5 | [48] |
| Staggered_BG | IGZTO | −20 | 60 | 7200 | 0.5 | [49] |
| Staggered_BG | IZO | −20 | 60 | 7200 | −0.8 | [50] |
| Staggered_BG | IZO:Pr | −20 | 60 | 7200 | −0.71 | [50] |
| Staggered_BG | IZO | −20 | 60 | 10,800 | −3.5 | [53] |
| Staggered_BG | HIZO | −20 | 60 | 10,800 | −3~−1 | [54] |
| Coplanar_BG | IGZO | −20 | 60 | 10,800 | −2 | This work |
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamiya, T.; Hosono, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef]
- Jeong, J.K. The status and perspectives of metal oxide thin-film transistors for actrix matrix flexible displays. Semicond. Sci. Technol. 2011, 26, 034008. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.-J.; Kim, H.-S.; Park, J.-S. Reivew of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Conley, J.F. Instabilities in amorphous oxide semiconductor thin-film transistors. IEEE Trans. Device Mater. Reliab. 2010, 10, 460–475. [Google Scholar] [CrossRef]
- Jeong, J.K. Photo-bias instability of metal oxide thin film transistors for advanced active matrix displays. J. Mater. Res. 2013, 28, 2071–2084. [Google Scholar] [CrossRef]
- Tang, H.; Ide, K.; Hiramatsu, H.; Ueda, S.; Ohashi, N.; Kumomi, H.; Hosono, H.; Kamiya, T. Effects of thermal annealing on elimination of deep defects in amorhpous In-Ga-Zn-O thin-film transistors. Thin Solid Films 2016, 614, 73–78. [Google Scholar] [CrossRef]
- Ide, K.; Nomura, K.; Hiramatsu, H.; Kamiya, T.; Hosono, H. Structural relaxation in amorphous oxide semiconductor, a-In-Ga-Zn-O. J. Appl. Phys. 2012, 111, 073513. [Google Scholar] [CrossRef]
- Takenaka, K.; Nunomura, S.; Hayashi, Y.; Komatsu, H.; Toko, S.; Tampo, H.; Setsuhara, Y. Stability and gap states of amorphous In-Ga-Zn-Ox thin film transistors: Impact of sputtering configuration and post-annealing on device performance. Thin Solid Films 2024, 790, 140203. [Google Scholar] [CrossRef]
- Chiang, H.Q.; McFarlane, B.R.; Hong, D.; Presley, R.E.; Wager, J.F. Processing effects on the stability of amorphous indium gallium zinc oxide thin-film transistors. J. Non-Cryst. Solids 2008, 354, 2826–2830. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, T.-C.; Li, H.-W.; Chen, S.-C.; Chung, W.-F.; Chen, Y.-H.; Tai, Y.-H.; Tseng, T.-Y.; Huang, F.-S.Y. Surface states related the bias stability of amorphous In-Ga-Zn-O thin film transistors under different ambient gases. Thin Solid Films 2011, 520, 1432–1436. [Google Scholar] [CrossRef]
- Fuh, C.-S.; Sze, S.M.; Liu, P.-T.; Teng, L.-F.; Chou, Y.-T. Role of environmental and annealing conditions on the passivation-free In-Ga-Zn-O TFT. Thin Solid Films 2011, 520, 1489–1494. [Google Scholar] [CrossRef]
- Shin, H.S.; Ahn, B.D.; Rim, Y.S.; Kim, H.J. Annealing temperature dependence on the positive bias stability of IGZO thin-film transistors. J. Inf. Disp. 2011, 12, 209–212. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, J.H.; Woo, C.H.; Lee, J.Y.; Cho, H.K. Effect of annealing temperature on the electrical performances of solution-processed InGaZnO thin film transistors. Thin Solid Films 2011, 519, 5146–5149. [Google Scholar] [CrossRef]
- Jeon, S.-J.; Chang, J.-W.; Choi, K.-S.; Kar, J.P.; Lee, T.-I.; Myoung, J.-M. Enhancement in electrical performance of indium gallium zinc oxide-based thin film transistors by low temperature thermal annealing. Mater. Sci. Semicond. Process. 2010, 13, 320–324. [Google Scholar] [CrossRef]
- Chen, X.F.; He, G.; Gao, J.; Zhang, J.W.; Xiao, D.Q.; Jin, P.; Deng, B. Substrate temperature dependent structural, optical and electrical properties of amorphous InGaZnO thin films. J. Alloys Compd. 2015, 632, 533–539. [Google Scholar] [CrossRef]
- Watanabe, K.; Lee, D.-H.; Sakaguchi, I.; Nomura, K.; Kamiya, T.; Haneda, H.; Hosono, H.; Ohashi, N. Surface reactivity and oxygen migration in amorphous indium-gallium-zinc oxide films annealed in humid atmosphere. Appl. Phys. Lett. 2013, 103, 201904. [Google Scholar] [CrossRef]
- Cho, E.N.; Kim, C.E.; Yun, I. Analysis of bias stress instability in amorphous InGaZnO thin-film transistors. IEEE Trans. Device Mater. Reliab. 2011, 11, 112–117. [Google Scholar] [CrossRef]
- Fung, T.-C.; Abe, K.; Kumomi, H.; Kanicki, J. Electrical instability of RF sputter amorphous In-Ga-Zn-O thin-film transistors. J. Disp. Technol. 2009, 5, 452–461. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Hirano, M.; Hosono, H. Origins of threshold voltage shifts in room-temperature deposited and annealed a-In-Ga-Zn-O thin-film transistors. Appl. Phys. Lett. 2009, 95, 013502. [Google Scholar] [CrossRef]
- van Berkel, C.; Powell, M.J. Resolution of amorphous silicon thinfilm transistor instability mechanism using ambipolar transistors. Appl. Phys. Lett. 1987, 51, 1094–1096. [Google Scholar] [CrossRef]
- Brotherton, S.D. Introduction to thin Film Transistors: Physics and Technology of TFTs, 1st ed.; Springer: Cham, Switzerland, 2013. [Google Scholar]
- Vidor, F.F.; Wirth, G.I.; Hilleringmann, U. Fundamentals. In ZnO Thin-Film Transistors for Cost-Efficient Flexible Electronics; Springer: Cham, Switzerland, 2018; pp. 26–30. [Google Scholar]
- Schön, G. Auger and direct electron spectra in X-ray photoelectron studies of zinc, zinc oxide, gallium, and gallium oxide. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 75–86. [Google Scholar] [CrossRef]
- Gross, T.; Ramm, M.; Sonntag, H.; Unger, W.; Weijers, H.M.; Adem, E.H. An XPS analysis of different SiO2 modifications employing a C 1s as well as an Au 4f7/2 static charge reference. Surf. Interface Anal. 1992, 18, 59–64. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Liu, W.K.; Yuen, W.T.; Stradling, R.A. Preparation of InSb substrates for molecular beam epitaxy. J. Vac. Sci. Technol. 1995, 13, 1539–1545. [Google Scholar] [CrossRef]
- Abliz, A. Effects of hydrogen plasma treatment on the electrical performances and reliability of InGaZnO thin-film transistors. J. Alloys Compd. 2020, 831, 154694. [Google Scholar] [CrossRef]
- Rivas-Aguilar, M.E.; Hernandez-Como, N.; Gutierrez-Heredia, G.; Sánchez-Martínez, A.; Mireles Ramirez, M.; Mejia, I.; Quevedo-López, M.A. Specific contact resistance of IGZO thin film transistors with metallic and transparent conductive oxides electrodes and XPS study of the contact/semiconductor interfaces. Curr. Appl. Phys. 2018, 18, 834–842. [Google Scholar] [CrossRef]
- Yang, S.-H.; Kim, J.Y.; Park, M.J.; Choi, K.-H.; Kwak, J.S.; Kim, H.-K.; Lee, J.-M. Low resistance ohmic contacts to amorphous IGZO thin films by hydrogen plasma treatment. Surf. Coat. Technol. 2012, 206, 5067–5071. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of XPS O 1s signal at 531-532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Ikenaga, E.; Yanagi, H.; Kobayashi, K.; Hosono, H. Depth analysis of subgap electronic states in amorphous oxide semiconductor, a-In-Ga-Zn-O, studied by hard x-ray photoelectron spectroscopy. J. Appl. Phys. 2011, 109, 073726. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1995. [Google Scholar]
- Magari, Y.; Makino, H.; Furuta, M. Carrier generation mechanism and origin of subgap states in Ar- and He-plasma-treated In-Ga-Zn-O thin films. J. Solid State Sci. Technol. 2017, 6, Q101–Q107. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier Science & Technology: Oxford, UK, 2004. [Google Scholar]
- Mitchell, B.S. An Introduction to Materials Engineering and Science, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chowdhury, M.D.H.; Um, J.G.; Jang, J. Remarkable changes in interface O vacancy and metal-oxide bonds in amorphous indium-gallium-zinc-oxide thin-film transistors by long time annealing at 250 °C. Appl. Phys. Lett. 2014, 105, 233504. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, D.; Xu, L.; Wu, Q.; Xie, H.; Dong, C. Thermal stability of amorphous InGaZnO thin film transistors passivated by AlOx layers. Solid-State Electron. 2015, 104, 39–43. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Q.; Xu, L.; XIe, H.; Liu, G.; Zhang, L.; Dong, C. Ambient effect on thermal stability of amorphous InGaZnO thin film transistors. Solid-State Electron. 2016, 126, 170–174. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, F.; Ge, F.; Xu, G.; Huang, F. Ga-doped ZnO films magnetron sputtered at ultralow discharge voltages: Significance of controlling defect generation. Thin Solid Films 2018, 660, 840–845. [Google Scholar] [CrossRef]
- Ide, K.; Nomura, K.; Hosono, H.; Kamiya, T. Electronic defects in amorphous oxide semiconductors: A review. Phys. Status Solid A 2015, 109, 37–41. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Lu, H.; Yu, G.; Ren, F.; Chen, D.; Zhang, R.; Zheng, Y. Study on interface characteristics in amorphous indium-gallium-zinc oxide thin-film transistros by using low-frequency noise and temperature dependent mobility measurement. Solid-State Electron. 2015, 109, 37–41. [Google Scholar] [CrossRef]
- Kagan, C.R. Thin-Film Transistors, 1st ed.; MARCEL DEKKER, INC.: New York, NY, USA, 2003. [Google Scholar]
- Jeong, J.K.; Jeong, J.H.; Yang, H.W.; Park, J.-S.; Mo, Y.-G.; Kim, H.D. High performance thin film transistors with cosputtered amorphous indium gallium zinc oxide channel. Appl. Phys. Lett. 2007, 91, 113505. [Google Scholar] [CrossRef]
- Cho, S.-I.; Ko, J.B.; Lee, S.H.; Kim, J.; Park, S.-H.K. Remarkably stable high mobility of self-aligned oxide TFT by investigating the effect of oxygen plasma time during PEALD of SiO2 gate insulator. J. Alloys Compd. 2022, 893, 162308. [Google Scholar] [CrossRef]
- Han, Y.; Cui, C.; Yang, J.; Tsai, M.-Y.; Chang, T.-C.; Zhang, Q. H2O induced hump phenomenon in capacitance-voltage measurements of a-IGZO thin-film transistors. IEEE Trans. Device Mater. Reliab. 2016, 16, 20–24. [Google Scholar] [CrossRef]
- Shiah, Y.-S.; Sim, K.; Shi, Y.; Abe, K.; Ueda, S.; Sasase, M.; Kim, J.; Hosono, H. Mobility-stability trade-off in oxide thin-film transistors. Nat. Electron. 2021, 4, 800–807. [Google Scholar] [CrossRef]
- Ryu, M.-K.; Park, S.-H.K.; Hwang, C.-S.; Yoon, S.-M. Comparative studies on electrical bias temperature instabilities of In-Ga-Zn-O thin film transistors with different device configurations. Solid-State Electron. 2013, 89, 171–176. [Google Scholar] [CrossRef]
- Kim, J.-L.; Lee, C.K.; Kim, M.J.; Lee, S.H.; Jeong, J.K. Role of MoTi diffusion barrier in amorphous indium-gallium-zinc-oxide thin-film transistors with a copper source/drain electrode. Thin Solid Films 2021, 731, 138759. [Google Scholar] [CrossRef]
- Ochi, M.; Morita, S.; Takanashi, Y.; Tao, H.; Goto, H.; Kugimiya, T.; Kanamura, M. Electrical characterization of BCE-TFTs with a-IGZTO oxide semiconductor compatible with Cu and Al interconnections. Soc. Inf. Disp. 2015, 46, 853–856. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Chen, W.; Li, M.; Wu, W.; Xu, H.; Zou, J.; Tao, H.; Wang, L.; XU, M.; et al. Improving thermal stability of solution process indium zinc oxide thin film transistors by praseodymium oxide doping. Appl. Mater. Interfaces 2018, 10, 28764–28771. [Google Scholar] [CrossRef]
- Byum, J.M.; Lee, S.Y. Effect of channel thickness on the electrical performance and the stability of amorphous SiZnSnO thin film transistor. Mater. Sci. Semicond. Process. 2020, 117, 105183. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Lee, S.Y. Investigation on the change of the performance of Si-Zn-Sn-O thin film transistors under negative bias temperature stress depending on the channel thickness. Solid-State Electron. 2018, 153, 93–98. [Google Scholar] [CrossRef]
- Son, K.-S.; Park, J.S.; Kim, T.S.; Kim, H.-S.; Seo, S.-J.; Kim, S.-J.; Seon, J.B.; Ji, K.H.; Jeong, J.K.; Ryu, M.K.; et al. Improvement of photo-induced negative bias stability of oxide thin film transistors by reducing the density of sub-gap states related to oxygen vacancies. Appl. Phys. Lett. 2013, 102, 122108. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Jung, J.S.; Son, K.S.; Lee, K.-H.; Park, J.S.; Kim, T.S.; Park, J.-S.; Choi, R.; Jeong, J.K.; Koo, B.; et al. Investigation of light-induced bias instability in Hf-In-Zn-O thin film transistors: A cation combinatorial approach. J. Electrochem. Soc. 2011, 158, H433–H437. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.-H.; Yoon, S.Y.; Jang, J.N.; Hong, M.P. Low-temperature fabrication (<150 °C) of amorphous IGZO TFTs via high density CVD and superimposed rf/dc magnetron sputtering. Curr. Appl. Phys. 2012, 12, S48–S51. [Google Scholar] [CrossRef]
- Jia, J.; Torigoshi, Y.; Shigesato, Y. In situ analyses on negative ions in the indium-gallium-zinc oxide sputtering process. Appl. Phys. Lett. 2013, 103, 013501. [Google Scholar] [CrossRef]
- Hanyu, Y.; Abe, K.; Domen, K.; Nomura, K.; Hiramatsu, H.; Kumomi, H.; Hosono, H.; Kamiya, T. Effects of high-temperature annealing on operation characteristics of a-In-Ga-Zn-O TFTs. J. Disp. Technol. 2014, 10, 979–983. [Google Scholar] [CrossRef]

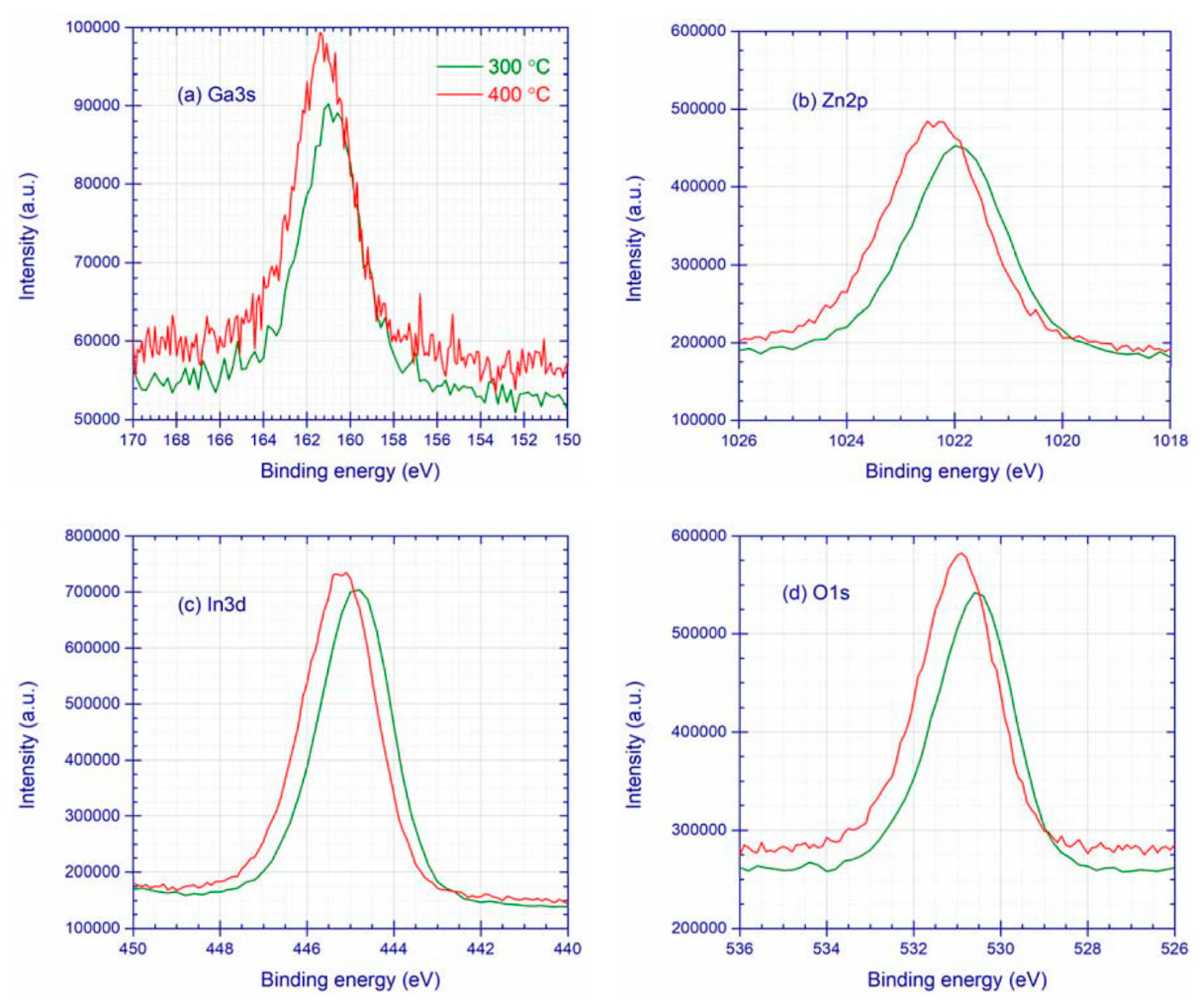
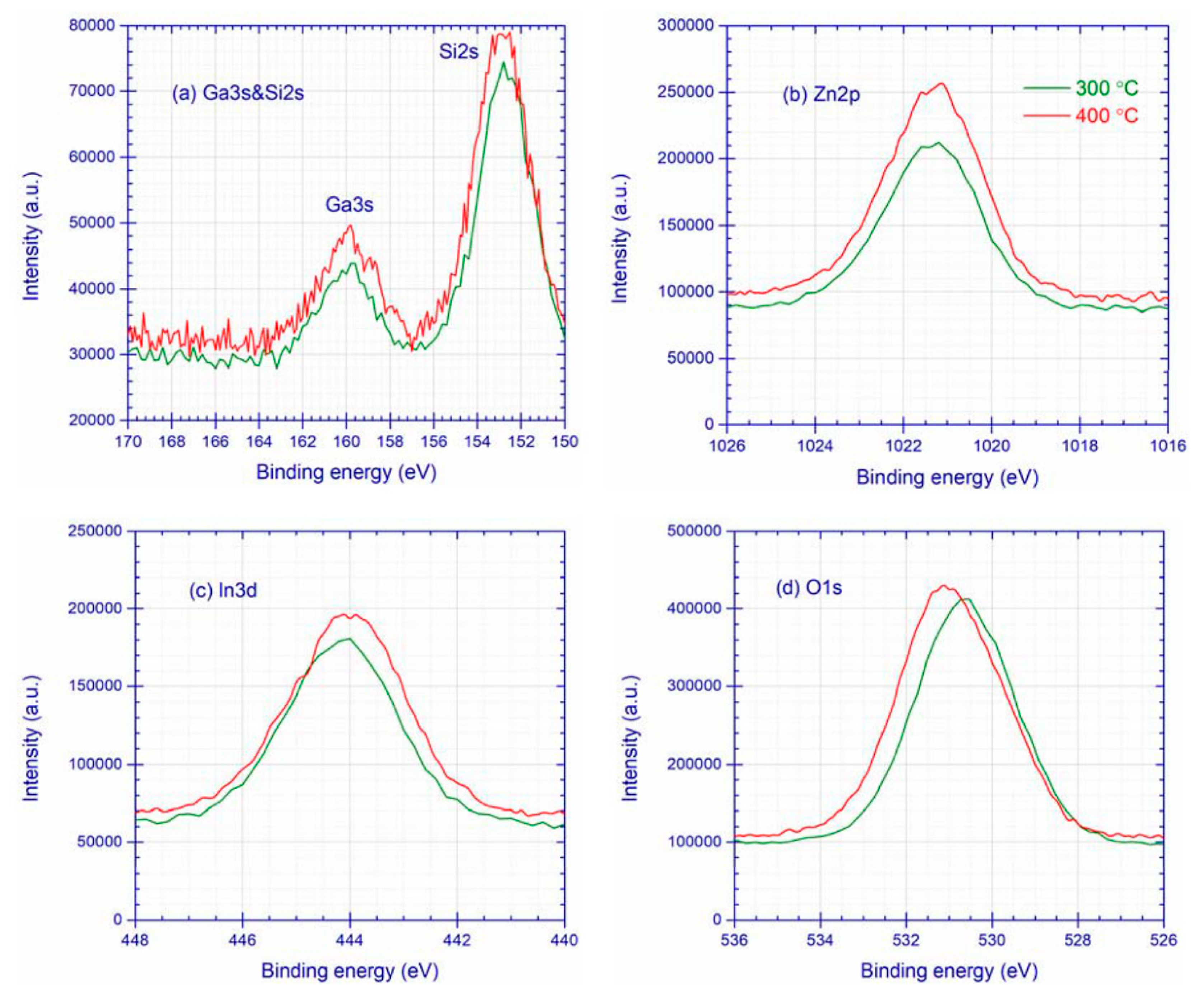



| Ta (°C) | Ga3s | Zn2p | In3d | O1s | |||
|---|---|---|---|---|---|---|---|
| Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | AOII (%) | |
| 300 | 160.9 | 3.3 | 1022.0 | 2.2 | 444.9 | 2.0 | 21 |
| 400 | 161.2 | 3.3 | 1022.3 | 2.2 | 445.2 | 2.0 | 21 |
| Ta (°C) | Ga3s | Zn2p | In3d | Si2s | O1s | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | AOII%(%) | |
| 300 | 159.91 | 2.8 | 1021.38 | 2.5 | 444.13 | 2.4 | 152.5 | 3.1 | 20 |
| 400 | 159.89 | 3.0 | 1021.35 | 2.5 | 444.02 | 2.5 | 152.8 | 3.1 | 24 |
| Ta (°C) | Si2s | O1s | ||
|---|---|---|---|---|
| Position (eV) | FWHM (eV) | Position (eV) | FWHM (eV) | |
| 300 | 153.2 | 3.2 | 531.5 | 2.3 |
| 400 | 153.3 | 3.1 | 531.6 | 2.4 |
| Ta (°C) | State | μ (cm2/Vs) | SS (V/dec) | Ion/Ioff | Vth (V) | ΔVth (V) |
|---|---|---|---|---|---|---|
| 300 | As-fabricated | 95 | 0.77 | 7.6 × 106 | 1.7 | +1.5 |
| 3h PBTS | 103 | 0.72 | 2.5 × 106 | 3.2 | ||
| 400 | As-fabricated | 93 | 0.44 | 3.7 × 107 | −3.2 | 0.0 |
| 3h PBTS | 105 | 0.44 | 4.2 × 107 | −3.2 | ||
| 300 | As-fabricated | 103 | 0.57 | 8.4 × 106 | 1.3 | −1.9 |
| 3h NBTS | 124 | 0.63 | 5.6 × 107 | −0.6 | ||
| 400 | As-fabricated | 95 | 0.40 | 6.0 × 107 | −2.5 | −4.5 |
| 3h NBTS | 102 | 0.54 | 3.0 × 107 | −7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shen, Y.; Chen, Y.; Xu, G.; Liu, Y.; Huang, R. Effects of Annealing Temperature on Bias Temperature Stress Stabilities of Bottom-Gate Coplanar In-Ga-Zn-O Thin-Film Transistors. Coatings 2024, 14, 555. https://doi.org/10.3390/coatings14050555
Chen Y, Shen Y, Chen Y, Xu G, Liu Y, Huang R. Effects of Annealing Temperature on Bias Temperature Stress Stabilities of Bottom-Gate Coplanar In-Ga-Zn-O Thin-Film Transistors. Coatings. 2024; 14(5):555. https://doi.org/10.3390/coatings14050555
Chicago/Turabian StyleChen, Yuyun, Yi Shen, Yuanming Chen, Guodong Xu, Yudong Liu, and Rui Huang. 2024. "Effects of Annealing Temperature on Bias Temperature Stress Stabilities of Bottom-Gate Coplanar In-Ga-Zn-O Thin-Film Transistors" Coatings 14, no. 5: 555. https://doi.org/10.3390/coatings14050555
APA StyleChen, Y., Shen, Y., Chen, Y., Xu, G., Liu, Y., & Huang, R. (2024). Effects of Annealing Temperature on Bias Temperature Stress Stabilities of Bottom-Gate Coplanar In-Ga-Zn-O Thin-Film Transistors. Coatings, 14(5), 555. https://doi.org/10.3390/coatings14050555







