A Multifunctional Magnetic Fluorescent Nanoprobe for Copper(II) Using ZnS-DL-Mercaptosuccinic Acid-Modified Fe3O4 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Characterization
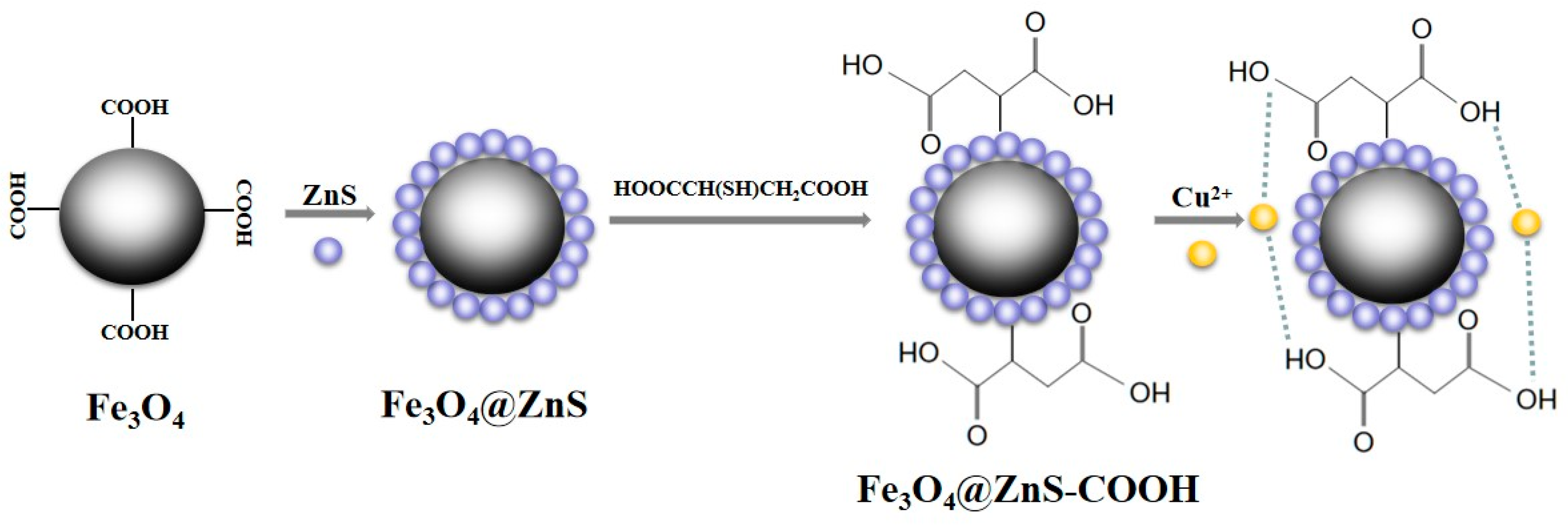
2.3. Synthesis of Fe3O4 Nanoparticles
2.4. Synthesis of Fe3O4@ZnS Nanoparticles
2.5. Synthesis of Fe3O4@ZnS-COOH Nanoparticles
2.6. The Selective and Competitive Experiments
2.7. Adsorption Experiments
3. Results and Discussion
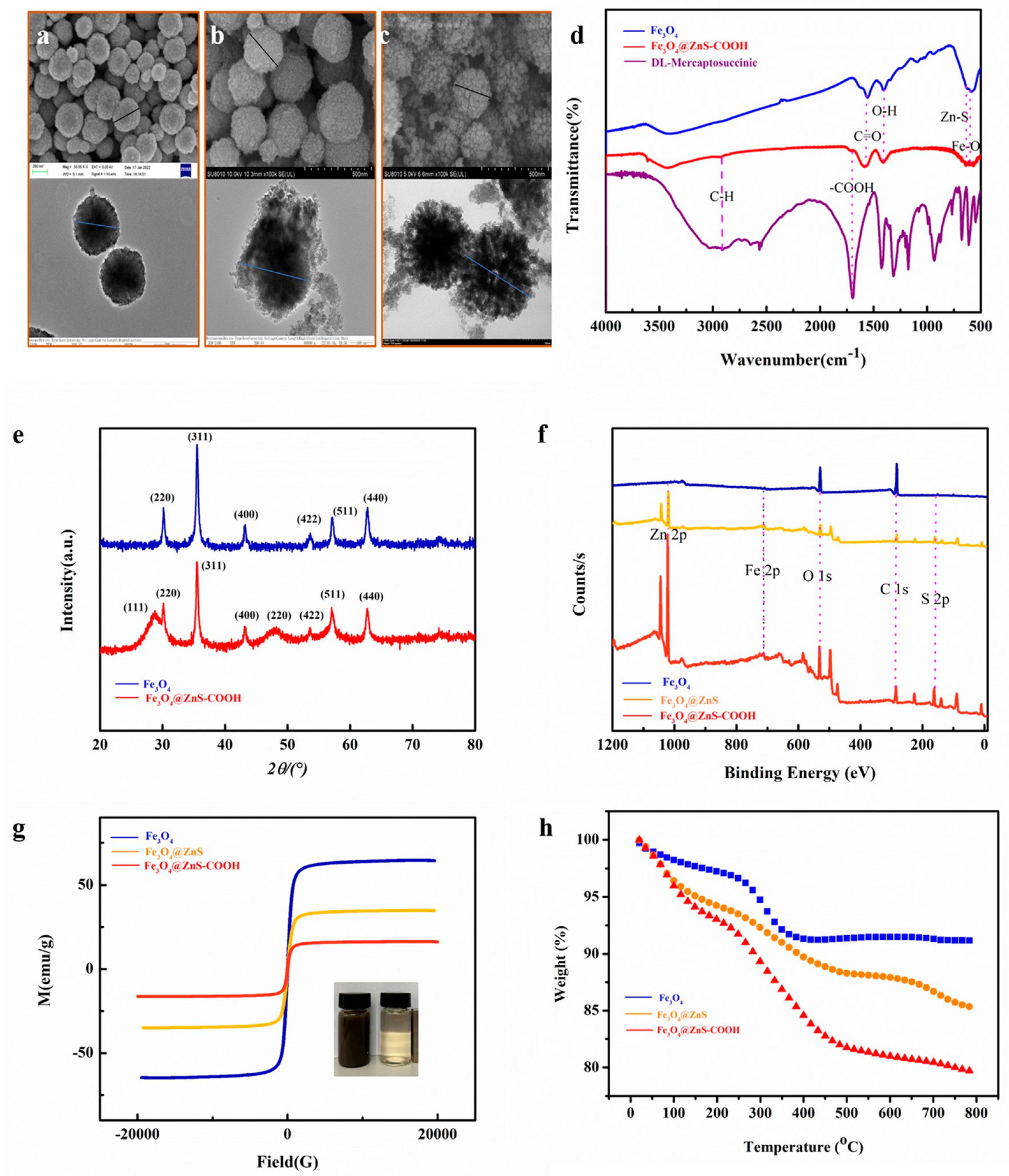
3.1. Characterization of Fe3O4, Fe3O4@ZnS, and Fe3O4@ZnS@ DL-Mercaptosuccinic
3.2. Spectroscopic Characteristics of the Magnetic Fluorescence Nanoprobe
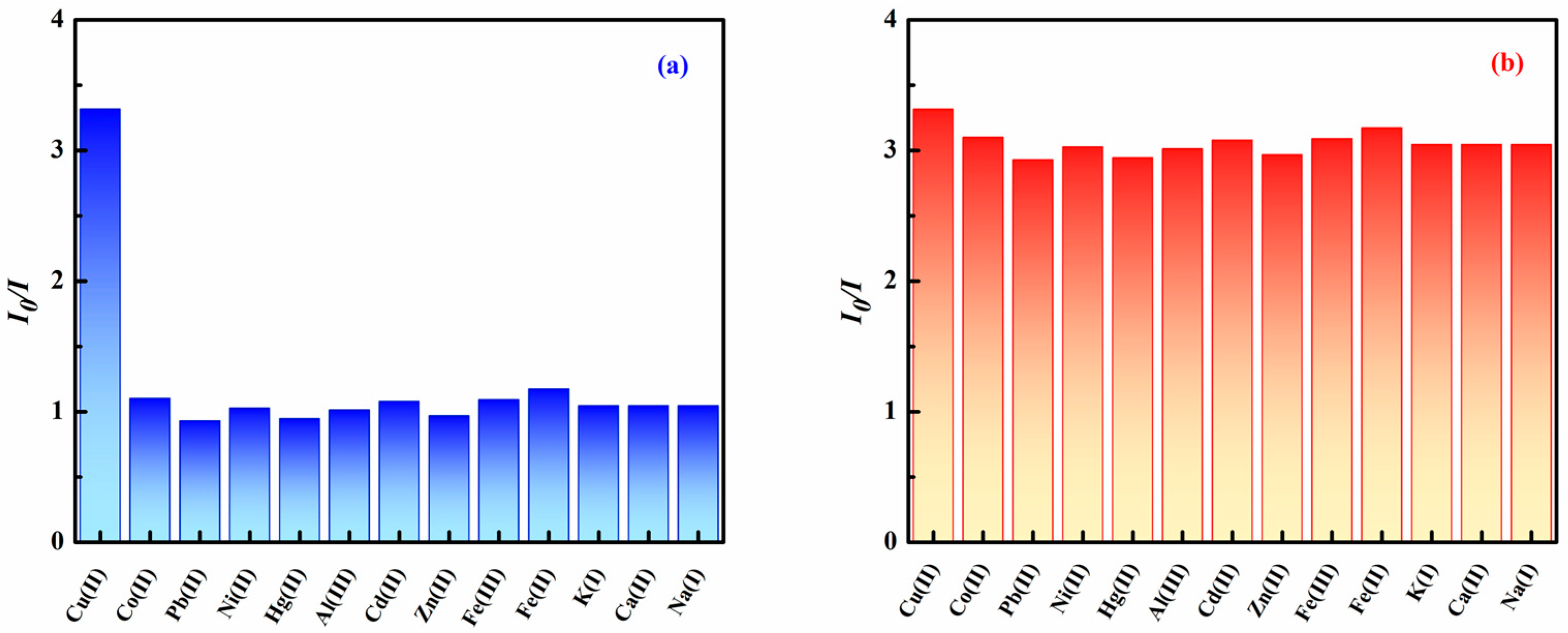
3.3. Specificity and Anti-Interference Capability of the Magnetic Fluorescent Nanoprobe
3.4. Adsorption Capacity and Removal Rate Study
3.5. The Practicability of Magnetic Fluorescent Nanoprobe on the Detection of Cu2+
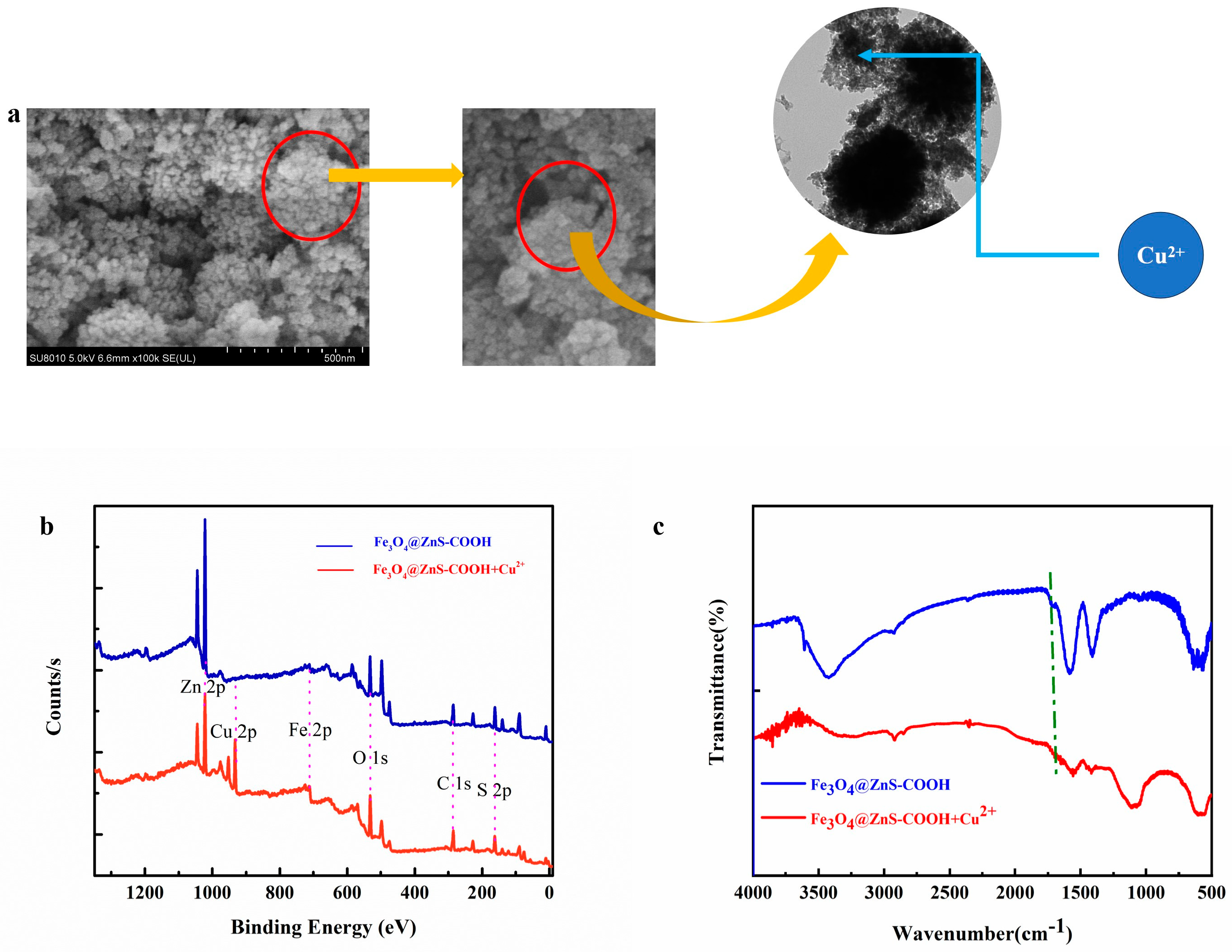
3.6. Detection Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amoah, C.; Obuah, C.; Ainooson, M.K.; Hamenu, L.; Oppong, A. A new sulfonamide-based chemosensor for potential fluorescent detection of Cu2+ and Zn2+ ions. Tetrahedron 2023, 133, 133276. [Google Scholar] [CrossRef]
- Li, S.X.; Zhao, B.; Kan, W.; Song, T.S.; Zheng, W.; Qi, X.; Wang, L.Y.; Song, B. A fluorescent covalent organic framework with a brick-wall topology for the detection of Cu2+ promoted by the associated mechanism. Dye. Pigment. 2023, 214, 111178. [Google Scholar] [CrossRef]
- Choudhury, N.; Saha, B.; De, P. Recent progress in polymer-based optical chemosensors for Cu2+ and Hg2+ Ions: A comprehensive review. Eur. Polym. J. 2021, 145, 110233. [Google Scholar] [CrossRef]
- Segura, C.; Yañez, O.; Galdámez, A.; Tapia, V.; Núñez, M.T.; Osorio-Román, I.; García, C.; García-Beltrán, O.J. Synthesis and characterization of a novel colorimetric and fluorometric probe “Turn-on” for the detection of Cu2+ of derivatives rhodamine. Photoch. Photobio. A 2023, 434, 114278. [Google Scholar] [CrossRef]
- Liu, L.; Cui, Y.Y.; Yang, Y.X.; Zhu, W.J.; Li, C.; Fang, M. A novel lipid droplets/lysosomes-targeting colorimetric and ratiometric fluorescent probe for Cu2+ and its application. Spectrochim. Acta A 2023, 291, 122333. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, T.; Jiang, Y.; Sun, W.; Zhao, Y.; Xin, J.; Hou, Y.; Yang, W. Green, fast, and large-scale synthesis of highly fluorescent Au nanoclusters for Cu2+ detection and temperature sensing. Analyst 2018, 143, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Kumamaru, T.; Okamoto, Y.; Hara, S.; Matsuo, H.; Kiboku, M. Simultaneous determination of copper and lead by graphite-furnace atomic absorption spectrometry after liquid-liquid extraction of the ion pair with zephiramine. Anal. Chim. Acta 1989, 218, 173–178. [Google Scholar] [CrossRef]
- Ebrahimi-Najafabadi, H.; Pasdaran, A.; Bezenjani, R.R.; Bozorgzadeh, E. Determination of toxic heavy metals in rice samples using ultrasound assisted emulsification microextraction combined with inductively coupled plasma optical emission spectroscopy. Food Chem. 2019, 289, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Es’haghi, Z.; Azmoodeh, R. Hollow fiber supported liquid membrane microextraction of Cu2+ followed by flame atomic absorption spectroscopy determination. Arab J. Chem. 2010, 3, 21–26. [Google Scholar] [CrossRef]
- Zhao, L.X.; Chen, K.Y.; Xie, K.B.; Hu, J.J.; Deng, M.Y.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. A benzothiazole-based “on-off” fluorescence probe for the specific detection of Cu2+ and its application in solution and living cells. Dye. Pigment. 2023, 210, 110943. [Google Scholar] [CrossRef]
- Loya, M.; Ghosh, S.; Atta, A.K. A review on dual detection of Cu2+ and Ni2+ ions by using single fluorometric and colorimetric organic molecular probes. J. Mol. Struct. 2023, 1278, 134949. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Yao, G.X.; La, Y.T.; Dong, W.K. Structural insights into three novel Cu(II), Ni(II) and Zn(II) complexes derived of a pyridine-containing N4-cavity bisoxime ligand. Inorg. Chim. Acta 2022, 533, 120775. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Wang, Z.L.; Liang, Y.Y.; Zhou, G.C.; Li, X.Y.; Xu, X.; Yang, Y.Q.; Wang, S.F. A naphthalimide functionalized chitosan-based fluorescent probe for specific detection and efficient adsorption of Cu2. Int. J. Biol. Macromol. 2023, 239, 124261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhu, T.; Xu, Y.; Yang, Y.; Sheng, D.L.; Ma, Q.L. Quaternized salicylaldehyde Schiff base side-chain polymer-grafted magnetic Fe3O4 nanoparticles for the removal and detection of Cu2+ ions in water. Appl. Surf. Sci. 2023, 611, 155632. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Seidi, F.; Deng, C.; Li, R.Y.; Xu, T.T.; Xiao, H.N. Porphyrin derived dual-emissive carbon quantum dots: Customizable synthesis and application for intracellular Cu2+ quantification. Sensor Actuat. B 2021, 343, 130072. [Google Scholar] [CrossRef]
- Hubetska, T.S.; Kobylinska, N.G.; Menendez, J.R.G. Application of Hydrophobic Magnetic Nanoparticles as Cleanup Adsorbents for Pesticide Residue Analysis in Fruit, Vegetable, and Various Soil Samples. J. Agric. Food Chem. 2020, 68, 13550–13561. [Google Scholar] [CrossRef]
- Naini, N.; Kalal, H.S.; Almasian, M.R.; Niknafs, D.; Taghiof, M.; Hoveidi, H. Phosphine-functionalized Fe3O4/SiO2/composites as efficient magnetic nanoadsorbents for the removal of palladium ions from aqueous solution: Kinetic, thermodynamic and isotherm studies. Mater. Chem. Phys. 2022, 287, 126242. [Google Scholar] [CrossRef]
- Liu, Z.G.; Lei, M.; Zeng, W.; Li, Y.; Li, B.L.; Liu, D.X.; Liu, C. Synthesis of magnetic Fe3O4@SiO2-(-NH2/-COOH) nanoparticles and their application for the removal of heavy metals from wastewater. Ceram. Int. 2023, 49, 20470–20479. [Google Scholar] [CrossRef]
- Goswami, Y.C.; Bisauriya, R.; Hlaing, A.A.; Moe, T.T.; Aryanto, D.; Yudianti, R. Highly fluorescent ZnS encapsulated flexible nanocellulose grown by two-step hydrothermal route and their optical, structural and morphological characterization. Mat. Sci. Eng. B 2023, 296, 116708. [Google Scholar]
- Mili, K.; Hsine, Z.; Chevalier, Y.; Ledou, G.; Mlika, R. Application of thiol capped ZnS quantum dots as a fluorescence probe for determination of tetracycline residues. Solid. State Commun. 2023, 360, 115040. [Google Scholar] [CrossRef]
- Zhang, J.R.; Wang, Y.Z.; Xu, Z.Y.; Shi, C.; Yang, X.T. A sensitive fluorescence-visualized sensor based on an InP/ZnS quantum dots-sodium rhodizonate system for monitoring fish freshness. Food Chem. 2022, 384, 132521. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Lin, J.W.; Zhuang, Y.F.; Huang, S.Q.; Chen, J.H.; Han, Z.Z. Facile one-step fabrication of Cu-doped carbon dots as a dual-selective biosensor for detection of pyrophosphate ions and measurement of pH. Spectrochim. Acta A 2022, 270, 120851. [Google Scholar] [CrossRef] [PubMed]
- Mahata, T.; Das, G.M.; Dantham, V.R. Study of surface enhanced fluorescence of CdSeS/ZnS alloyed quantum dots using cytop layer as a dielectric spacer. Spectrochim. Acta A 2022, 283, 121739. [Google Scholar] [CrossRef]
- Yang, C.K.; Pang, Y.; Han, Y.; Zhan, X.H.; Wang, H.; Liu, J.Y.; Gao, R.; Liu, H.R.; Shi, H.X. Removal of trace concentration Sb(V) in textile wastewater by Mn-doped Fe3O4: The mechanisms of Mn affect adsorption performance. Micropor. Mesopor. Mat. 2022, 343, 112150. [Google Scholar] [CrossRef]
- Beketov, I.V.; Safronov, A.P.; Medvedev, A.I.; Alonso, J.; Kurlyandskaya, G.V.; Bhagat, S.M. Iron oxide nanoparticles fabricated by electric explosion of wire: Focus on magnetic nanofluids. AIP Adv. 2012, 2, 022154. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.J.; He, X.; Zhao, Y.; Zada, A.; Peng, A.Z.; Qi, K.Z. Fe3O4@MIL-100(Fe) modified ZnS nanoparticles with enhanced sonocatalytic degradation of tetracycline antibiotic in water. Ultrason. Sonochem. 2023, 95, 106409. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, X.; Dong, W.; Ma, J.; Chao, J.; Li, C.; Wang, L.; Dong, C. A novel fluorescein-based colorimetric probe for Cu2+ detection. RSC Adv. 2016, 6, 59677–59683. [Google Scholar] [CrossRef]
- Huang, A.; Yang, X.; Xia, T.; He, D.; Zhang, R.; Li, Z.; Yang, S.; Liu, Y.; Wen, X. A fluorescence probe of sulfur quantum dots for sensitive detection of copper ions in Paris polyphylla var. yunnanensis. Microchem. J. 2022, 179, 107639. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, B.; Zhao, L.; Zhao, L.; Wang, Q.; Wang, C.; Xu, B. A dansyl-based fluorescent probe for sensing Cu2+ in aqueous solution. Spectrochim. Acta A 2021, 261, 120009. [Google Scholar] [CrossRef]
- Lian, J.J.; Liu, P.; Liu, Q.Y. Nano-scale minerals in-situ supporting CeO2 nanoparticles for off-on colorimetric detection of L–penicillamine and Cu2+ ion. J. Hazard Mater. 2022, 433, 128766. [Google Scholar] [CrossRef]
- Trung, L.G.; Subedi, S.; Dahal, B.; Truong, P.L.; Gwag, J.S.; Tran, N.T.; Nguyen, M.K. Highly efficient fluorescent probes from chitosan-based amino-functional carbon dots for the selective detection of Cu2+ traces. Mater. Chem. Phys. 2022, 291, 126772. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.W. Adsorption of Pb2+ and Cu2+ in wastewater by lignosulfonate adsorbent prepared from corn straw. Int. J. Biol. Macromol. 2023, 247, 125820. [Google Scholar] [CrossRef] [PubMed]
- Rahmatpour, A.; Alijani, N. An all-biopolymer self-assembling hydrogel film consisting of chitosan and carboxymethyl guar gum: A novel bio-based composite adsorbent for Cu2+ adsorption from aqueous solution. Int. J. Biol. Macromol. 2023, 242, 124878. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.P.; Zhang, H.Z.; Wang, P.G.; Lan, Y.L.; Ma, W.W.; Shi, X.Q. NiCo bimetallic and the corresponding monometallic organic frameworks loaded CMC aerogels for adsorbing Cu2+: Adsorption behavior and mechanism. Int. J. Biol. Macromol. 2023, 244, 125169. [Google Scholar] [CrossRef]
- Xie, L.Q.; Peng, S.; Xin, Y.N.; Liu, B.; Jiang, X.Y.; Yu, J.G. Preparation of three-dimensional 2-mercaptothiazoline modified GO aerogel for selective adsorption of Cu2+ in aqueous solution. J. Environ. Chem. Eng. 2023, 11, 110332. [Google Scholar] [CrossRef]
- Lv, X.Y.; Hu, H.Y.; Yao, L.F.; Deng, L.L.; Liu, X.H.; Yu, L.D.; He, H.F. Surface-Enhanced Raman scattering (SERS) filter paper substrates decorated with silver nanoparticles for the detection of molecular vibrations of Acyclovir drug. Spectrochim. Acta A 2023, 298, 122723. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Cai, Y.; Xie, H.; Han, S.; Hao, C.J. Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB. Hazard. Mater. 2022, 423, 127191. [Google Scholar] [CrossRef]


| Probe | Liner Range | LOD | References |
|---|---|---|---|
| CSFH | 0–14 μM | 0.25 μM | [27] |
| SQDs | 20–200 μM | 6.78 μM | [28] |
| D-GT | 0–20 μM | 0.69 μM | [29] |
| CeO2 nanoparticles | 20–80 μM | 9.8 μM | [30] |
| Carbon dots | 0–100 μM | 0.106 μM | [31] |
| Fe3O4@ZnS-COOH | 0–400 μM | 0.489 μM | this work |
| Nanomaterials | Q (mg/g) | References |
|---|---|---|
| Lignosulfonate adsorbent | 450.3 | [32] |
| CS/CMGG composite hydrogel | 151.51 | [33] |
| Ni-MOF-CMC | 216.95 | [34] |
| 3D GO-MT aerogels | 33.91 | [35] |
| Cu2+ ion-imprinted polymers (RH-CIIP) | 87.8 | [36] |
| Fe3O4@ZnS-COOH | 310.239 | This work |
| Sample | Added (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 1 | 0.93 | 93.00 | 2.00 |
| 5.0 | 4.92 | 98.40 | 2.52 | |
| 10.0 | 10.50 | 105.00 | 3.21 | |
| Bottled water | 1 | 1.08 | 108.00 | 2.93 |
| 5.0 | 5.13 | 102.60 | 3.26 | |
| 10.0 | 10.45 | 104.50 | 2.85 | |
| Electrolysis wastewater | 1 | 0.83 | 83.00 | 4.53 |
| 5.0 | 4.20 | 84.00 | 3.93 | |
| 10.0 | 8.75 | 87.50 | 4.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Chen, X.; Chen, J.; Yu, S.; Zeng, X.; Liu, Z. A Multifunctional Magnetic Fluorescent Nanoprobe for Copper(II) Using ZnS-DL-Mercaptosuccinic Acid-Modified Fe3O4 Nanocomposites. Coatings 2024, 14, 685. https://doi.org/10.3390/coatings14060685
Xu P, Chen X, Chen J, Yu S, Zeng X, Liu Z. A Multifunctional Magnetic Fluorescent Nanoprobe for Copper(II) Using ZnS-DL-Mercaptosuccinic Acid-Modified Fe3O4 Nanocomposites. Coatings. 2024; 14(6):685. https://doi.org/10.3390/coatings14060685
Chicago/Turabian StyleXu, Ping, Xin Chen, Jie Chen, Shihua Yu, Xiaodan Zeng, and Zhigang Liu. 2024. "A Multifunctional Magnetic Fluorescent Nanoprobe for Copper(II) Using ZnS-DL-Mercaptosuccinic Acid-Modified Fe3O4 Nanocomposites" Coatings 14, no. 6: 685. https://doi.org/10.3390/coatings14060685






