Preparation of Silicone Coating and Its Anti-Ice and Anti-Corrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
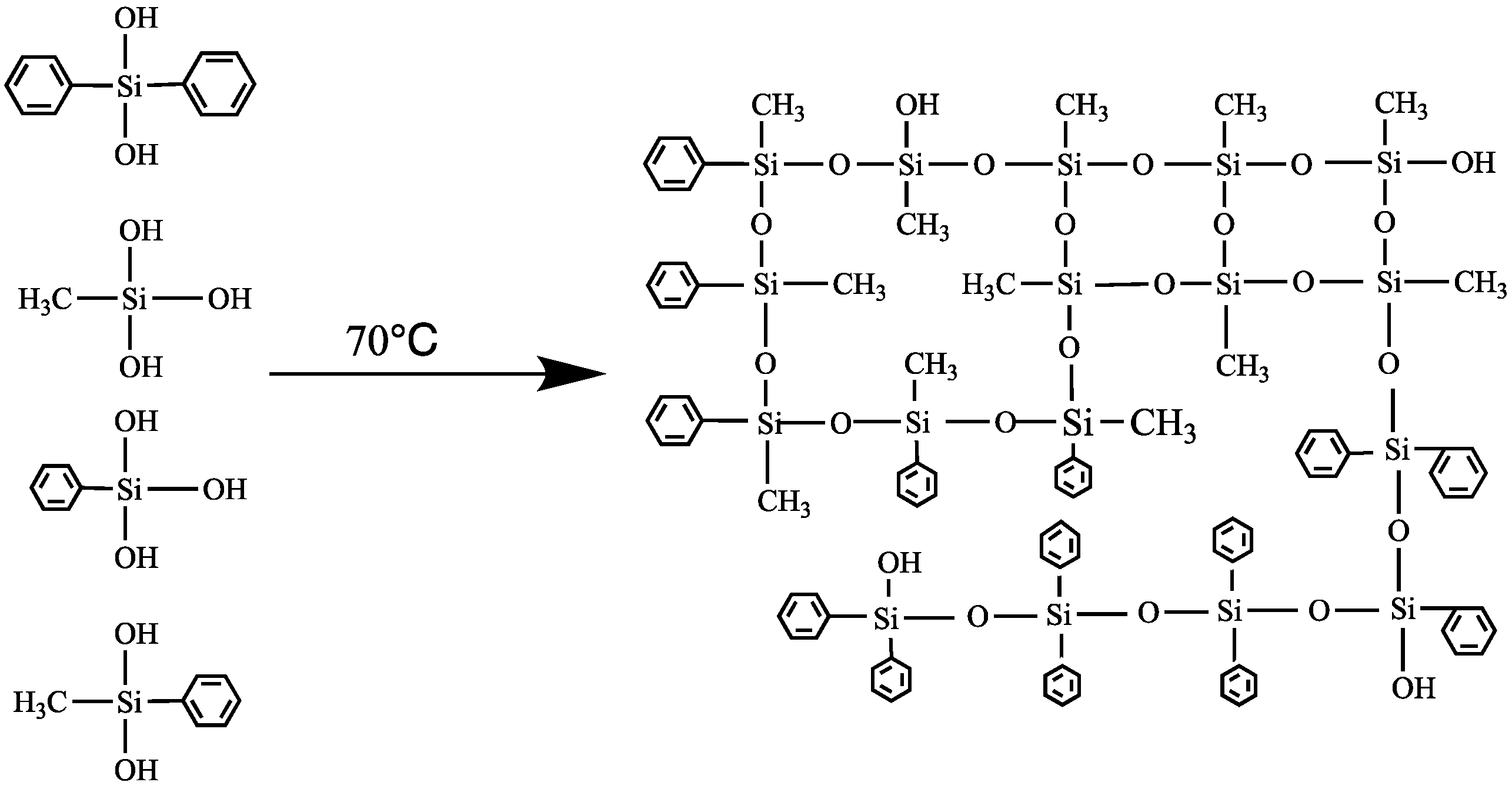
2.2. Preparation of Silicone Resin
2.3. Preparation of Silicone Coating
2.4. Characterization
3. Results
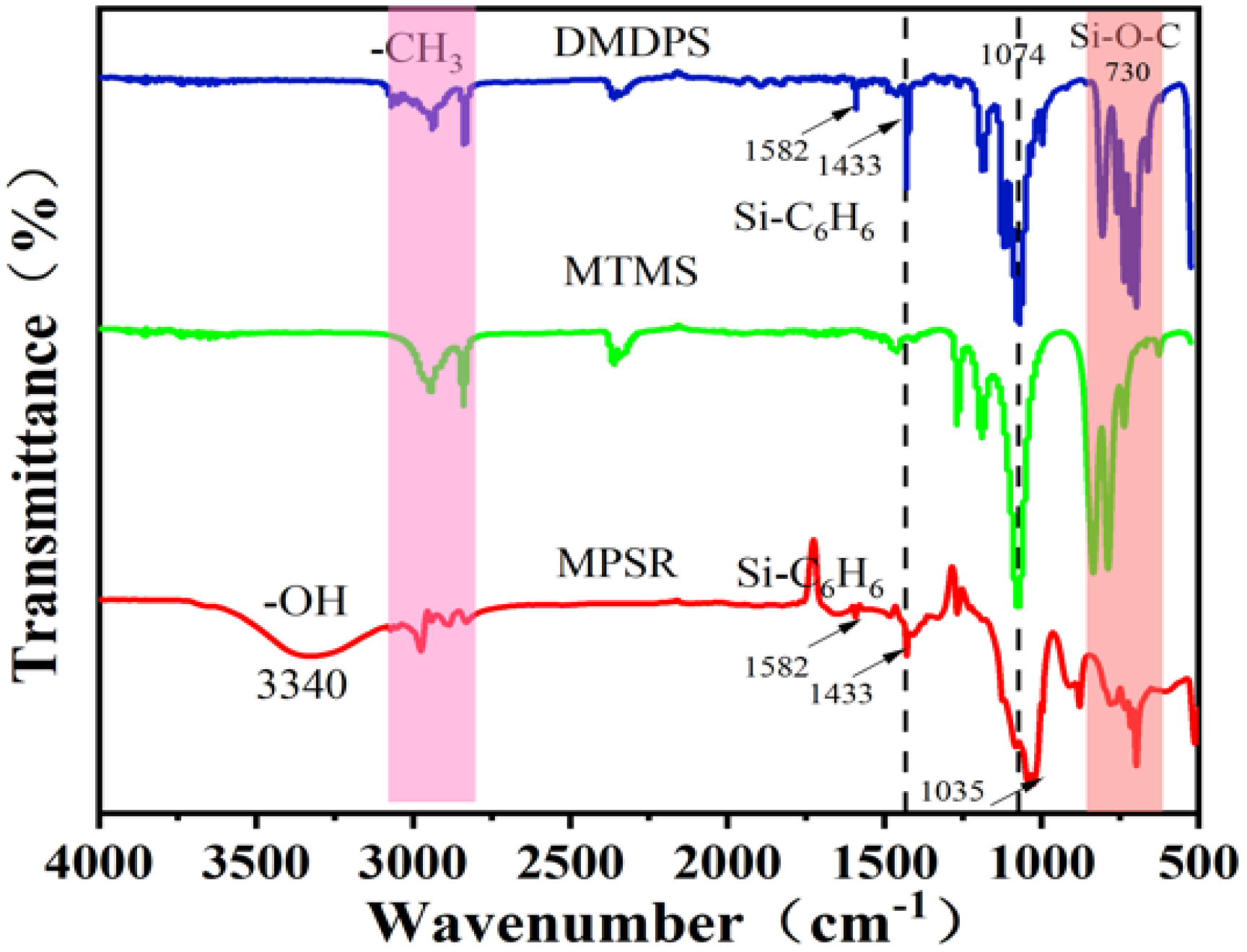
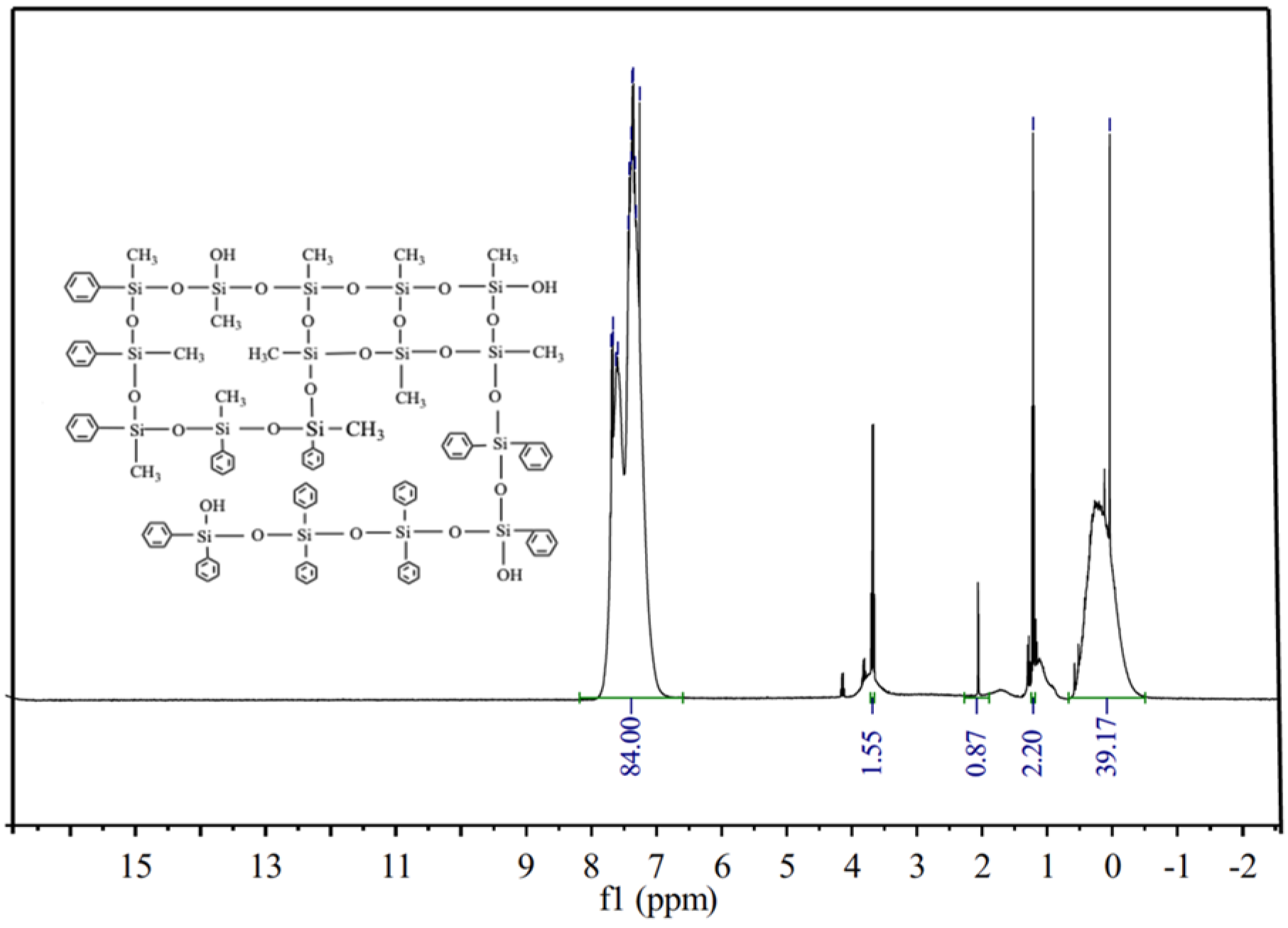
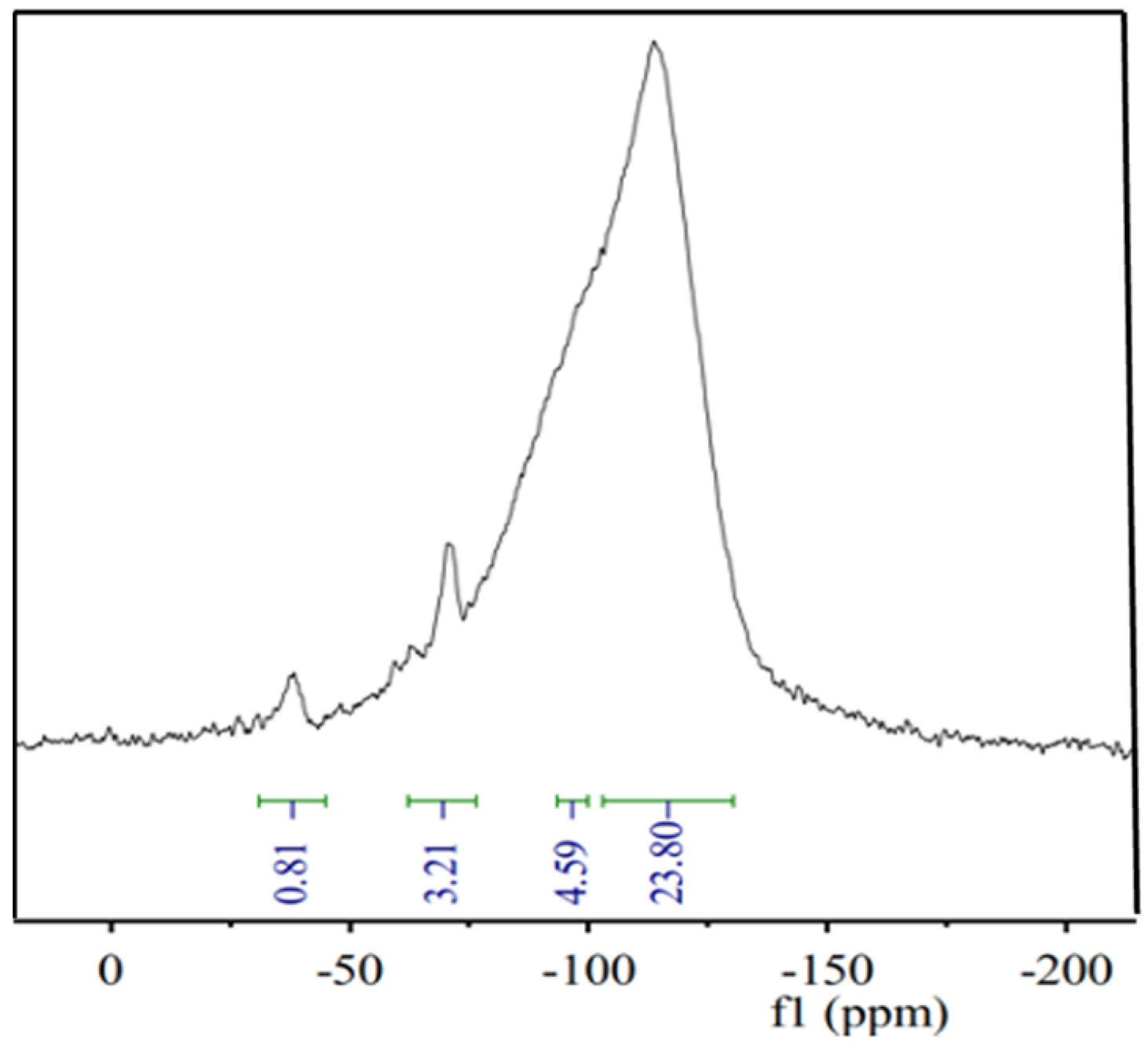
3.1. Structure Characterization of Silicone Resin
3.2. Silicone Resin Coating Properties
3.3. Anti-Icing Performance of Silicone Resin
3.3.1. Icing Time
3.3.2. Desorption Time
3.3.3. Theoretical Calculation of Icing Time
- (a)
- The law of conservation of energy governs heat transfer and exchange, with no heat loss considered.
- (b)
- The contact area between the liquid and coating is assumed to be roughly circular, and the influence of gravity is disregarded.
- (c)
- Heat transfer beyond the liquid and substrate is not considered.
- (1)
- The amount of heat that needs to be given off/absorbed when water freezes/ice melts:
- (2)
- The heat transfer rate equation is:
- (3)
- Icing time
3.4. Corrosion Resistance
3.4.1. Saltwater Resistance and Salt Spray Resistance
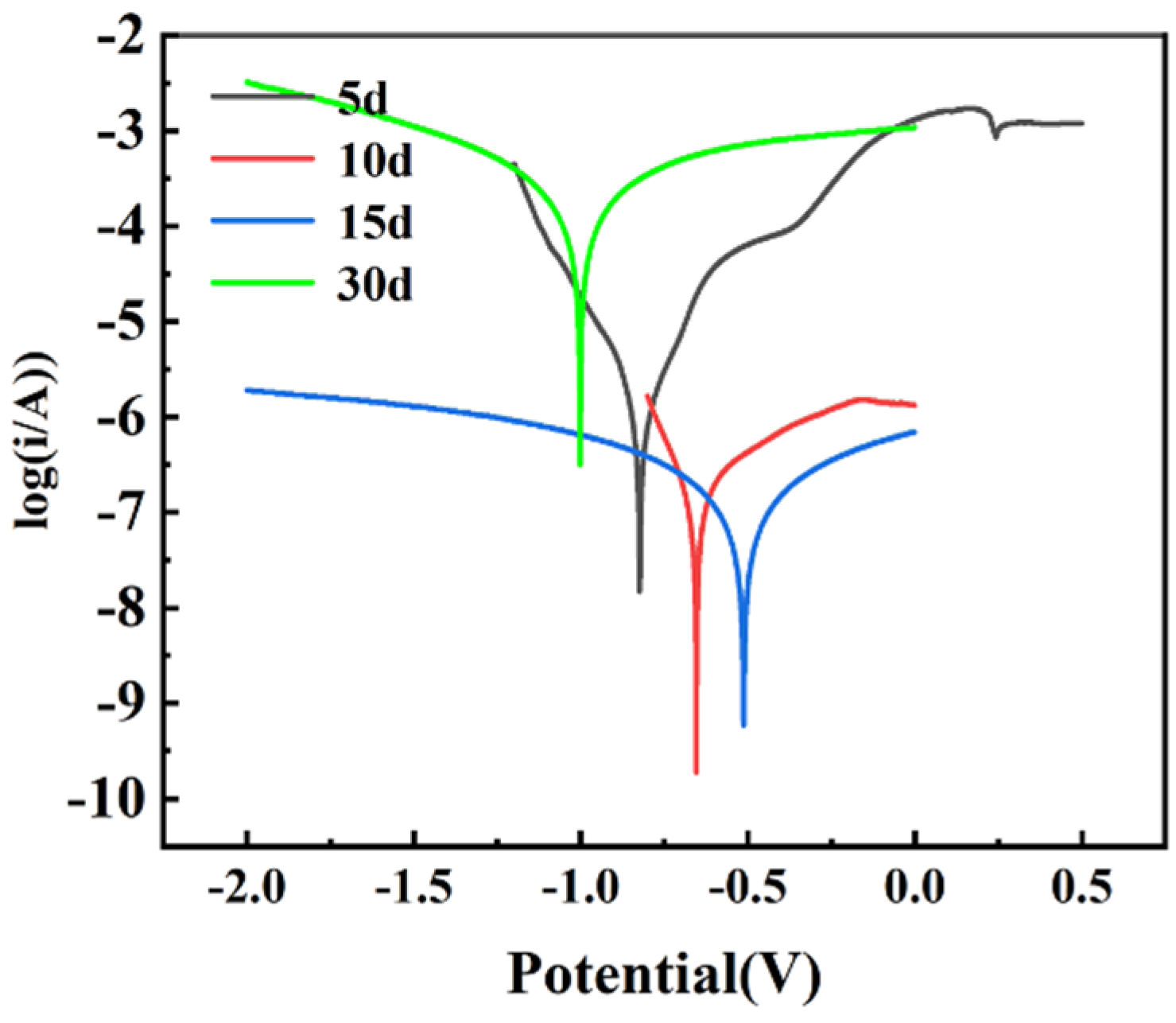
3.4.2. Tafel Polarization Curves
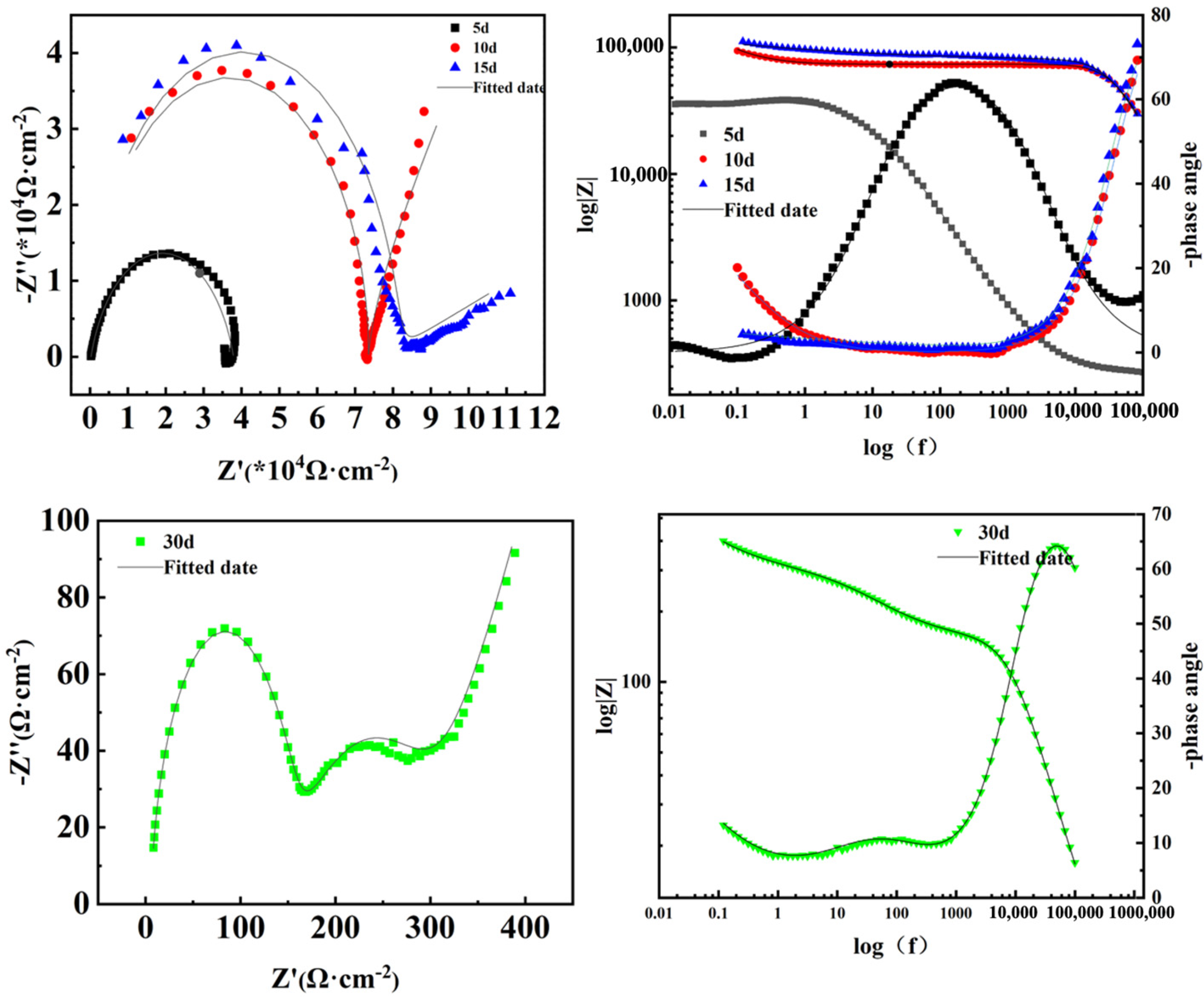
3.4.3. Electrochemical Impedance Spectra
3.5. Anti-Corrosion Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajagopal, S.; Zhang, P. How widespread is the usage of the Northern Sea Route as a commercially viable shipping route? A statistical analysis of ship transits from 2011 to 2018 based on empirical data. Mar. Policy 2021, 125, 104300. [Google Scholar] [CrossRef]
- Biresselioglu, M.E.; Demir, M.H.; Solak, B.; Kayacan, A.; Altinci, S. Investigating the trends in arctic research: The increasing role of social sciences and humanities. Sci. Total Environ. 2020, 729, 139027. [Google Scholar] [CrossRef]
- Kondratenko, A.A.; Kujala, P.; Hirdaris, S.E. Holistic and sustainable design optimization of Arctic ships. Ocean. Eng. 2023, 275, 114095. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Han, S.; Meng, Q.; Xing, K.; Araby, S.; Yu, Y.; Mouritz, A.; Ma, J. Epoxy/graphene film for lifecycle self-sensing and multifunctional applications. Compos. Sci. Technol. 2020, 198, 108312. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Benedetti, A.V. Self-healing ability based on hydrogen bonds in organic coatings for corrosion protection of AA1200. Corros. Sci. 2020, 177, 108984. [Google Scholar] [CrossRef]
- Xie, C.; Jia, Y.; Xue, M.; Yin, Z.; Luo, Y.; Hong, Z.; Liu, W. Anti-corrosion and self-healing behaviors of waterborne polyurethane composite coatings enhanced via chitosan-modified graphene oxide and phosphate intercalated hydrotalcite. Prog. Org. Coat. 2022, 168, 106881. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. 2-Mercaptobenzimidazole-inbuilt metal-organic-frameworks modified graphene oxide towards intelligent and excellent anti-corrosion coating. Corros. Sci. 2021, 191, 109715. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M.; Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, S.; Lu, Y.F.; Liu, Z.X.; Lu, F. Experimental Study on Corrosion Prevention of a Multilayer Coating System. Adv. Mater. Res. 2014, 881–883, 1307–1311. [Google Scholar] [CrossRef]
- Krishnan, A.; Krishnan, A.V.; Ajith, A.; Shibli, S. Influence of materials and fabrication strategies in tailoring the anticorrosive property of superhydrophobic coatings. Surf. Interfaces 2021, 25, 26. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Caldona, E.B.; Leng, W.; Street, J.; Wang, G.; Zhang, Z. Anticorrosive epoxy coatings containing ultrafine bamboo char and zinc particles. J. Environ. Chem. Eng. 2021, 9, 105707. [Google Scholar] [CrossRef]
- Yue, Q.X.; Wu, L.F.; Lv, J.; Wang, A.M.; Ding, R.; Wang, Y.Y.; Yue, L.; Gao, W.; Li, X.L.; Li, X.Y.; et al. Study on anti-corrosion performance and mechanism of epoxy coatings based on basalt flake loaded aniline trimer. Colloid Interface Sci. Commun. 2021, 45, 100505. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Park, S.; Lim, H. Effects of morphology parameters on anti-icing performance in superhydrophobic surfaces. Appl. Surf. Sci. 2018, 435, 585–591. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust Anti-Icing Performance of a Flexible Superhydrophobic Surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- Heydari, G.; Thormann, E.; Järn, M.; Tyrode, E.; Claesson, P.M. Hydrophobic Surfaces: Topography Effects on Wetting by Supercooled Water and Freezing Delay. J. Phys. Chem. C 2013, 117, 21752–21762. [Google Scholar] [CrossRef]
- Xie, H.; Wei, J.; Duan, S.; Zhu, Q.; Yang, Y.; Chen, K.; Zhang, J.; Li, L.; Zhang, J. Non-fluorinated and durable photothermal superhydrophobic coatings based on attapulgite nanorods for efficient anti-icing and deicing. Chem. Eng. J. 2022, 428, 132585. [Google Scholar] [CrossRef]
- Kuo, C.F.J.; Chen, J.B. Study on the synthesis and application of silicone resin containing phenyl group. J. Sol-Gel Sci. Technol. 2015, 76, 66–73. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Sarmento, V.; Schiavetto, M.; Hammer, P.; Benedetti, A.; Fugivara, C.; Suegama, P.; Pulcinelli, S.; Santilli, C. Corrosion protection of stainless steel by polysiloxane hybrid coatings prepared using the sol–gel process. Surf. Coat. Technol. 2010, 204, 2689–2701. [Google Scholar] [CrossRef]
- Dvornic, P.R.; Lenz, R.W. Exactly alternating silarylene–siloxane polymers. II. The condensation polymerization of arylenedisilanols and bisureidosilanes. J. Polym.Sci. Polym. Chem. Ed. 1982, 20, 951–966. [Google Scholar]
- Huang, W.; Huang, Y.; Yunzhao, Y.U. Synthesis of MQ silicone resins through hydrolytic condensation of ethyl polysilicate and hexamethyldisiloxane. J. Appl. Polym. Sci. 1998, 70, 1753–1767. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, K.S.; Eo, Y.J.; Yoon, K.B.; Bae, B.S. Synthesis of fluorinated hybrid material for UV embossing of a large core optical waveguide structure. J. Mater. Chem. 2005, 15, 465–479. [Google Scholar] [CrossRef]
- Gokulakrishnan, N.; Karbowiak, T.; Bellat, J.P.; Vonna, L.; Saada, M.-A.; Paillaud, J.L.; Soulard, M.; Patarin, J.; Parmentier, J. Improved hydrophobicity of inorganic–organic hybrid mesoporous silica with cage-like pores. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 34–43. [Google Scholar] [CrossRef]
- Chu, H.H.; Chiang, W.L.; Chuang, K.S. Viscoelastic and adhesive properties of PMMA-b-PtBA with tackifier. Int. J. Adhes. Adhes. 2012, 38, 89–94. [Google Scholar] [CrossRef]
- Menaa, B.; Takahashi, M.; Tokuda, Y.; Yoko, T. High dispersion and fluorescence of anthracene doped in polyphenylsiloxane films. J. Sol-Gel Sci. Technol. 2006, 39, 185–194. [Google Scholar] [CrossRef]
- Ono, S.; Tsuge, H.; Nishi, Y.; Hirano, S.-I. Improvement of Corrosion Resistance of Metals by an Environmentally Friendly Silica Coating Method. J. Sol-Gel Sci. Technol. 2004, 29, 147–153. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Morgan, A.B.; Antonucci, J.M.; VanLandingham, M.R.; Harris, R.H., Jr.; Awad, W.H.; Shields, J.R. Thermal and flammability properties of a silica–poly(methylmethacrylate) nanocomposite. J. Appl. Polym. Sci. 2003, 89, 2072–2078. [Google Scholar] [CrossRef]
- Chang, T.C.; Wang, Y.T.; Hong, Y.S.; Chiu, Y.S. Organic–inorganic hybrid materials. V. Dynamics and degradation of poly(methyl methacrylate) silica hybrids. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1972–1980. [Google Scholar] [CrossRef]
- Kuo, C.; Chen, J.B.; Shih, C.Y.; Huang, C.Y. Silicone resin synthesized by tetraethoxysilane and chlorotrimethylsilane through hydrolysis–condensation reaction. J. Appl. Polym. Sci. 2014, 131, 169–172. [Google Scholar] [CrossRef]
- Mehdizade, M.; Eivani, A.R.; Soltanieh, M. Effects of reduced surface grain structure and improved particle distribution on pitting corrosion of AA6063 aluminum alloy. J. Alloys Compd. 2020, 838, 155464. [Google Scholar] [CrossRef]
- Silva, C.L.; Soares, R.B.; Pereira, P.H.R.; Figueiredo, R.B.; Lins, V.F.; Langdon, T.G. The Effect of High-Pressure Torsion on Microstructure, Hardness and Corrosion Behavior for Pure Magnesium and Different Magnesium Alloys. Adv. Eng. Mater. 2019, 21, 1801081. [Google Scholar] [CrossRef]
- Zheng, S.X.; Li, J.H. Inorganic-organic sol gel hybrid coatings for corrosion protection of metals. J. Sol-Gel Sci. Technol. 2010, 54, 174–187. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, C.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic press: Cambridge, MA, USA, 1990. [Google Scholar]
- de Damborenea, J.; Pellegri, N.; de Sanctis, O.; Durán, A. Electrochemical behaviour of SiO2 sol-gel coatings on stainless steel. J. Sol-Gel Sci. Technol. 1995, 4, 239–244. [Google Scholar] [CrossRef]
- Park, S.S.; Mackenzie, J.D. Microstructure Effects in Multidipped Tin Oxide Films. J. Am. Ceram. Soc. 1995, 78, 2669–2672. [Google Scholar] [CrossRef]
- Menaa, B.; Takahashi, M.; Tokuda, Y.; Yoko, T. Preparation and properties of polyphenylsiloxane-based hybrid glass films obtained from a non-aqueous coating sol via a single-step dip-coating. Opt. Mater. 2007, 29, 806–813. [Google Scholar] [CrossRef]

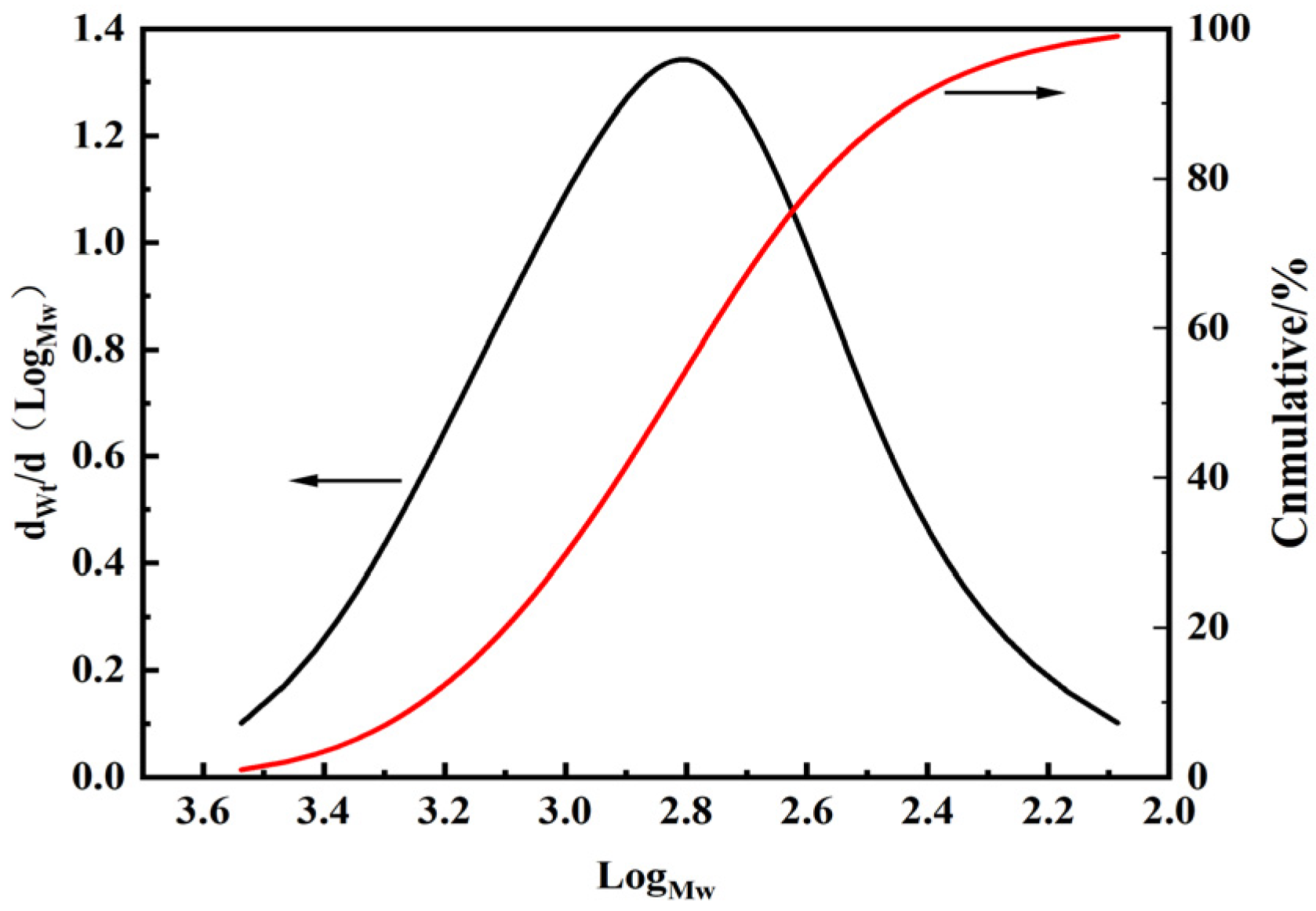
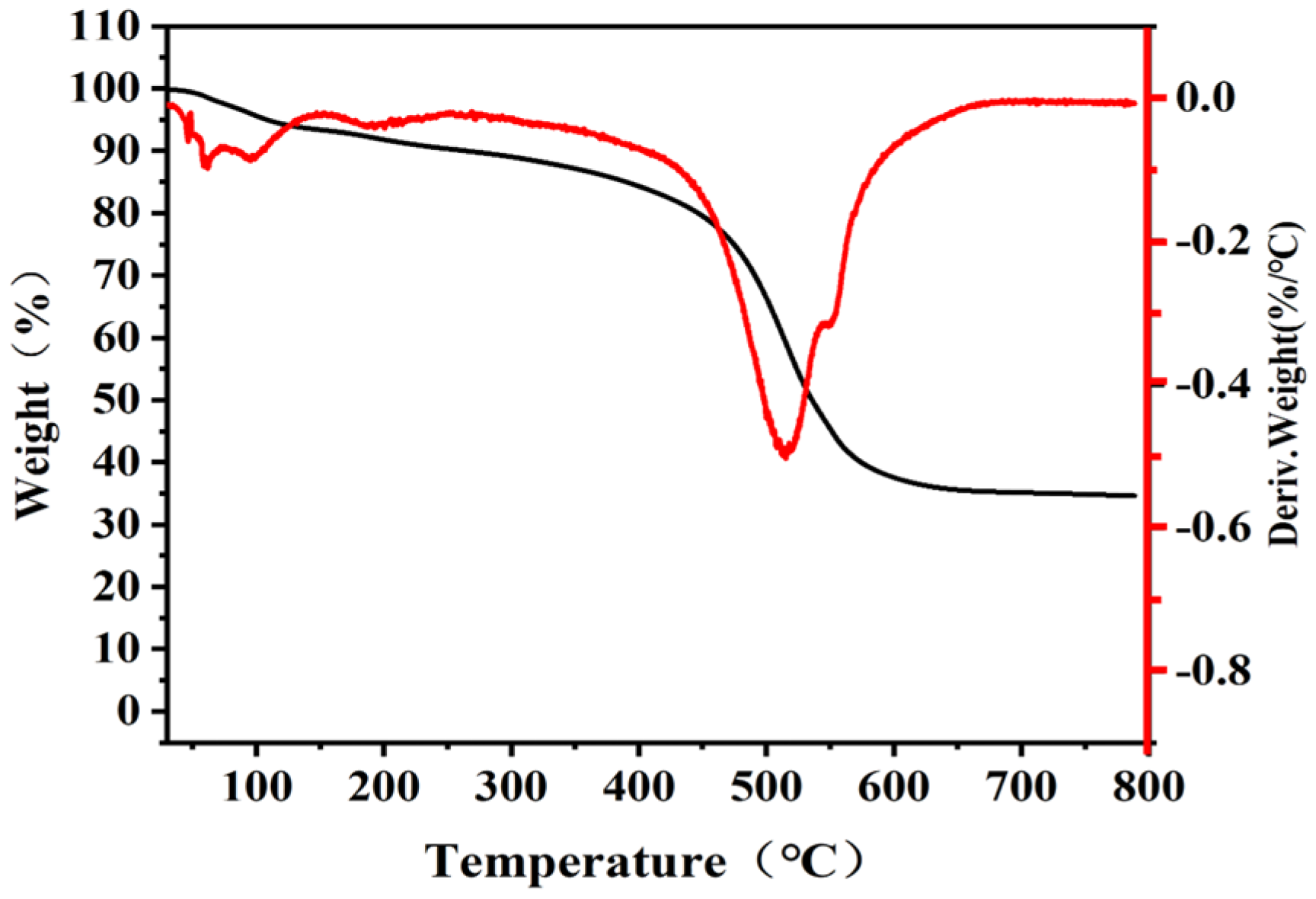




| Name | Mn | Mw | Mp | Mz | Mz+1 | Mw/Mn | Mz/Mw | Mz+1/Mw |
|---|---|---|---|---|---|---|---|---|
| Numeric value | 528 | 867 | 625 | 1385 | 2050 | 1.64 | 1.60 | 2.36 |
| Samples | Performance |
|---|---|
| Coating thickness (μm) | 50 ± 10 |
| Pendulum hardness (s) | 130 ± 5 |
| Pencil hardness | 3H |
| Gloss degree (GU) | 130 ± 5 |
| Grid adhesion | 0 |
| Pull-out adhesion (MPa) | 0.67 ± 0.1 |
| Impact strength (Kg·cm) | 50 |
| High and low temperature alternating test (−30 °C, 3 h~50 °C, 3 h, 50% humidity/times) | ≥40 |
| Samples | Ecorr (V) | Icorr (μA/cm2) | Rp (Ω) | ba | −bc | |
|---|---|---|---|---|---|---|
| Days | 5 | −0.824 | 1.1140 | 27,951.6 | 7.625 | 6.333 |
| 10 | −0.654 | 0.1503 | 246,075.4 | 3.736 | 8.022 | |
| 15 | −0.512 | 0.6050 | 73,141.5 | 5.619 | 4.206 | |
| 30 | −1.002 | 83.6400 | 527.7 | 4.831 | 5.019 | |
| Samples | Rs (Ω·cm2) | CPEf (Ω−1 s−ncm−2) | Rf (Ω·cm2) | CPEdl (Ω−1 s−ncm−2) | Rct (Ω·cm2) | Zw (Ω−1 s5 cm−2) | |
|---|---|---|---|---|---|---|---|
| Time | 5 d | 268.8 | 1.121 × 10−6 | 37,540 | 5.556 × 10−2 | 5.038 × 105 | 1.18 × 10−10 |
| 10 d | 0.01 | 5.906 × 10−11 | 73,260 | 3.89 × 10−5 | 8.538 × 108 | 1.148 × 10−14 | |
| 15 d | 0.18 | 6.256 × 10−11 | 77,530 | 3.634 × 10−5 | 1.11 × 107 | 2.66 × 10−7 | |
| 30 d | 5.779 | 2.048 × 10−6 | 150 | 3.005 × 10−4 | 156.1 | 9.011 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, J.; Wen, S.; Jiang, S.; Song, J.; Ding, S.; Wu, H. Preparation of Silicone Coating and Its Anti-Ice and Anti-Corrosion Properties. Coatings 2024, 14, 699. https://doi.org/10.3390/coatings14060699
Wang H, Wang J, Wen S, Jiang S, Song J, Ding S, Wu H. Preparation of Silicone Coating and Its Anti-Ice and Anti-Corrosion Properties. Coatings. 2024; 14(6):699. https://doi.org/10.3390/coatings14060699
Chicago/Turabian StyleWang, Haopeng, Jihu Wang, Shaoguo Wen, Sihong Jiang, Jia Song, Shengnan Ding, and Hui Wu. 2024. "Preparation of Silicone Coating and Its Anti-Ice and Anti-Corrosion Properties" Coatings 14, no. 6: 699. https://doi.org/10.3390/coatings14060699




