Dispersed CeO2 Nanorods with Low-Speed Mixing for Mechanical Properties Promotion of PTA Steel Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Alloy Steel Coatings
2.3. Physical and Chemical Characterization
2.4. Mechanical Properties
3. Results and Discussion
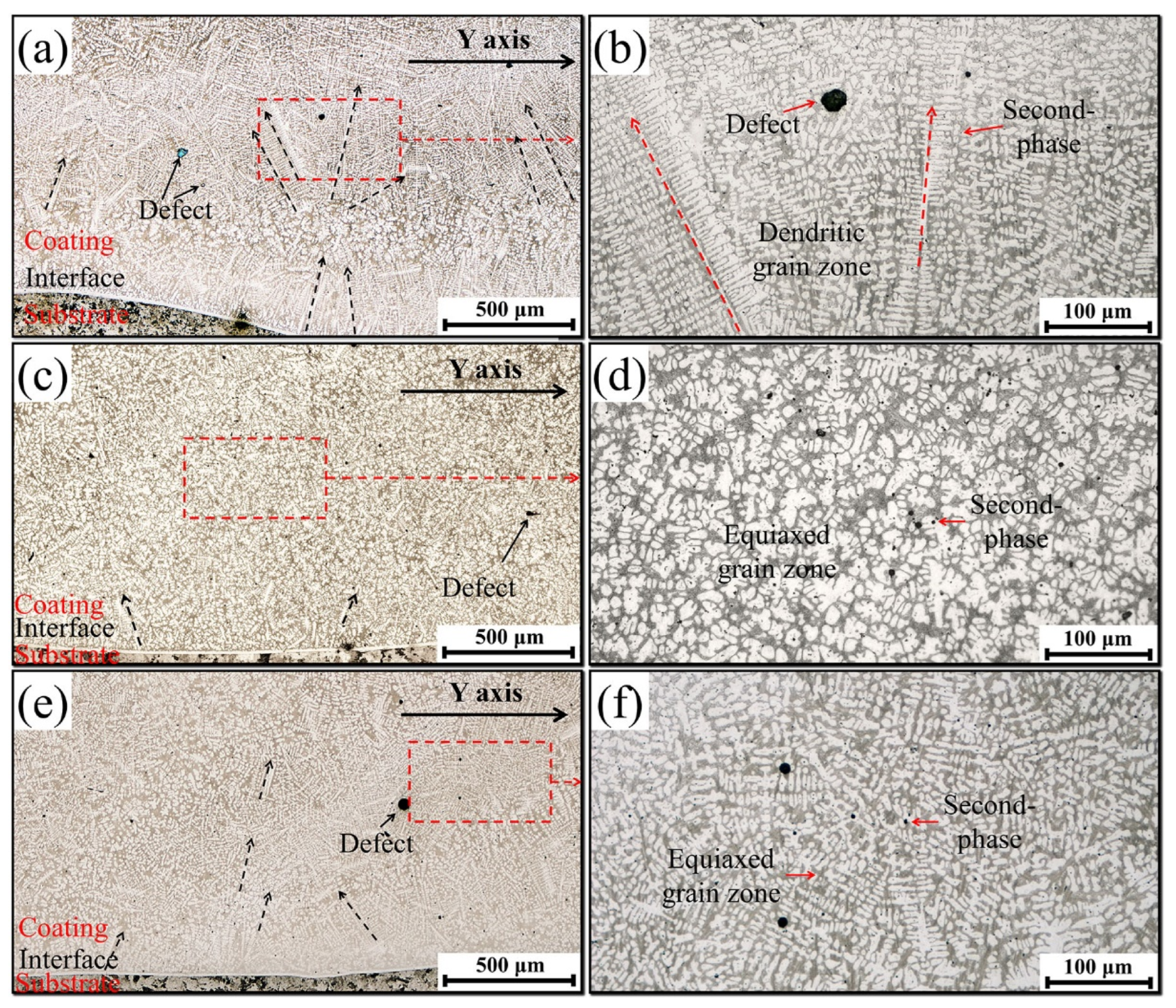
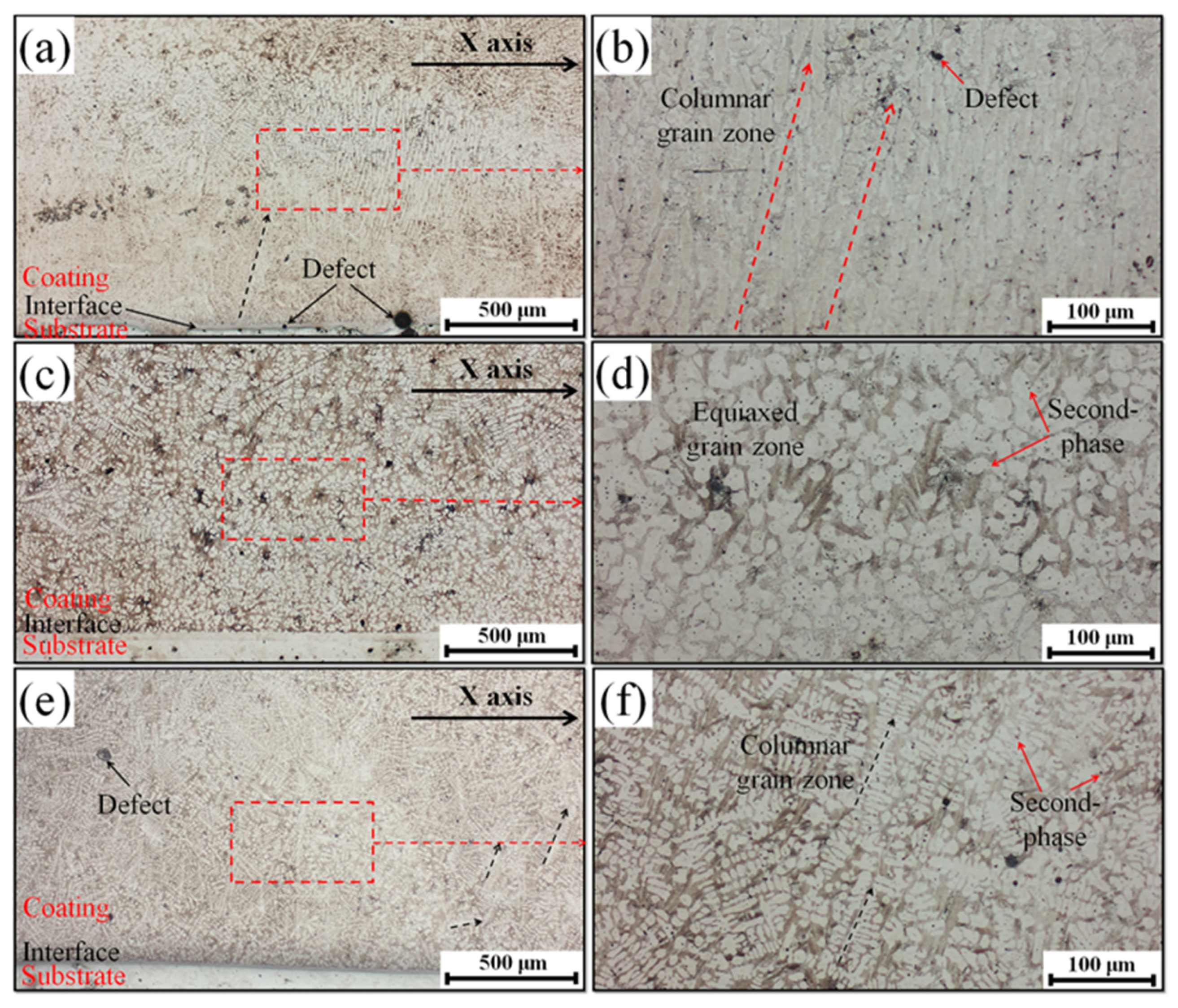
3.1. Microstructural Characterization
3.2. XRD Analysis
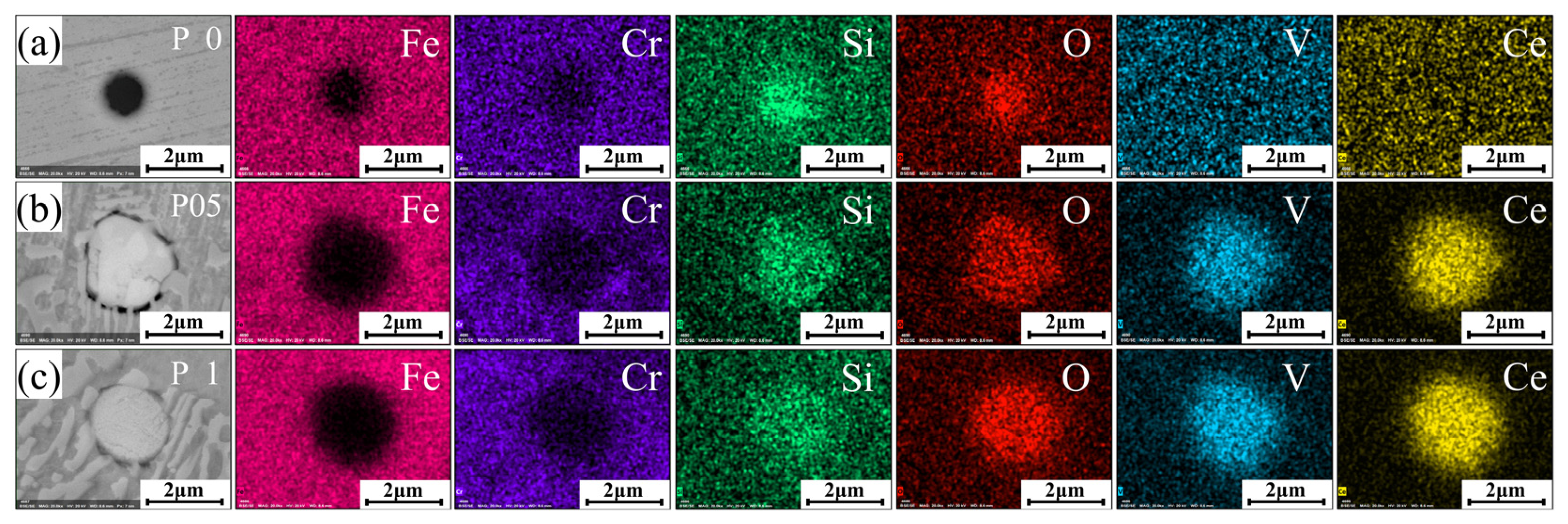
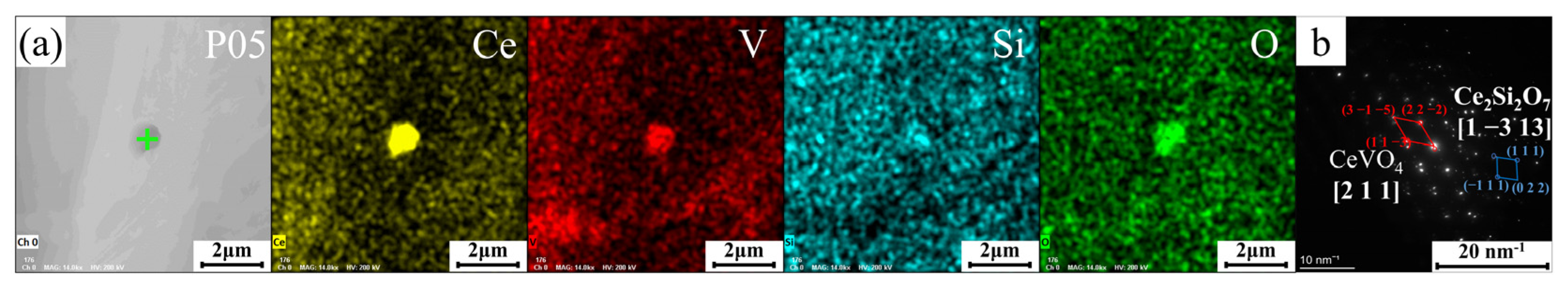
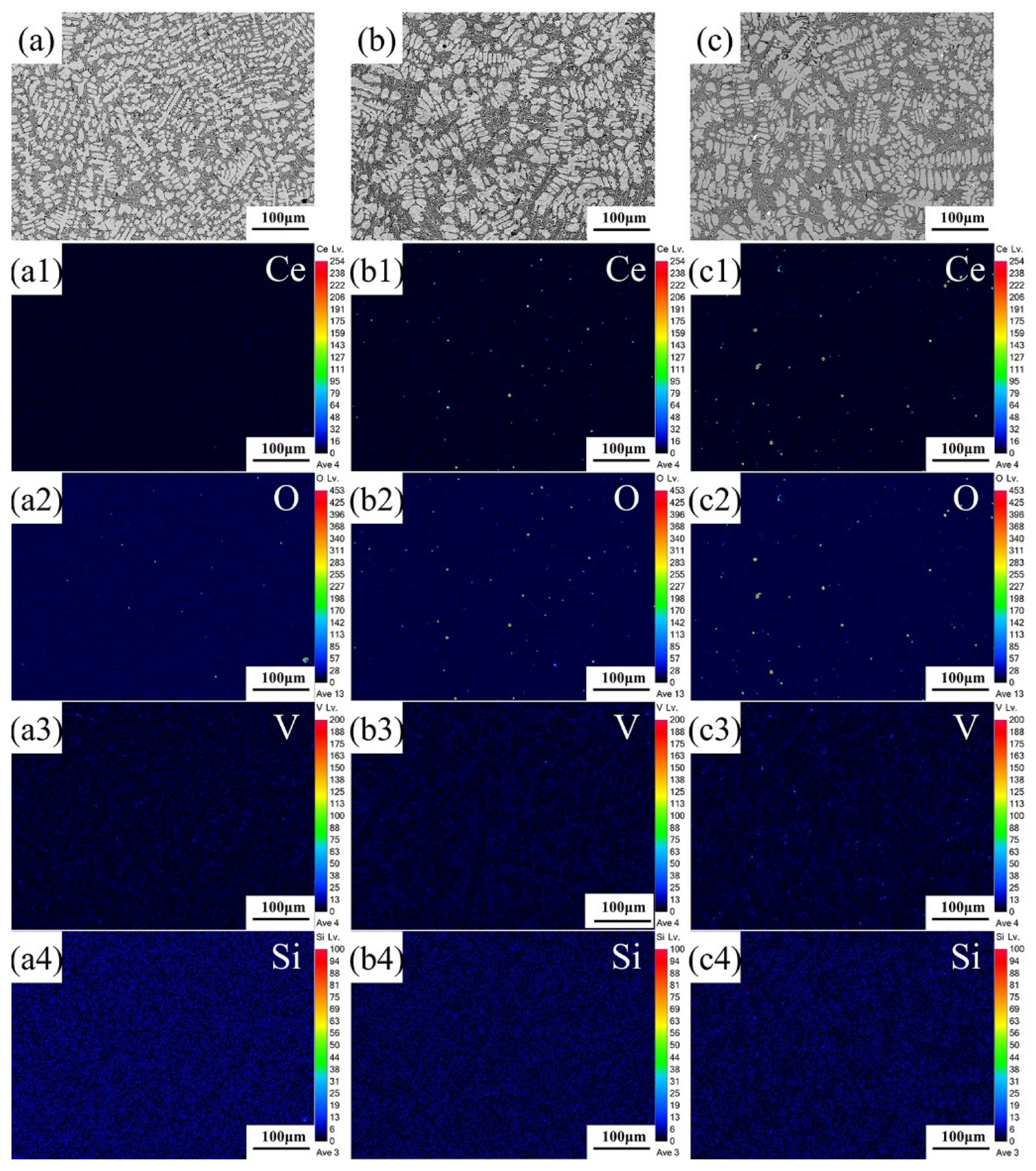
3.3. Analysis of Elemental Distribution
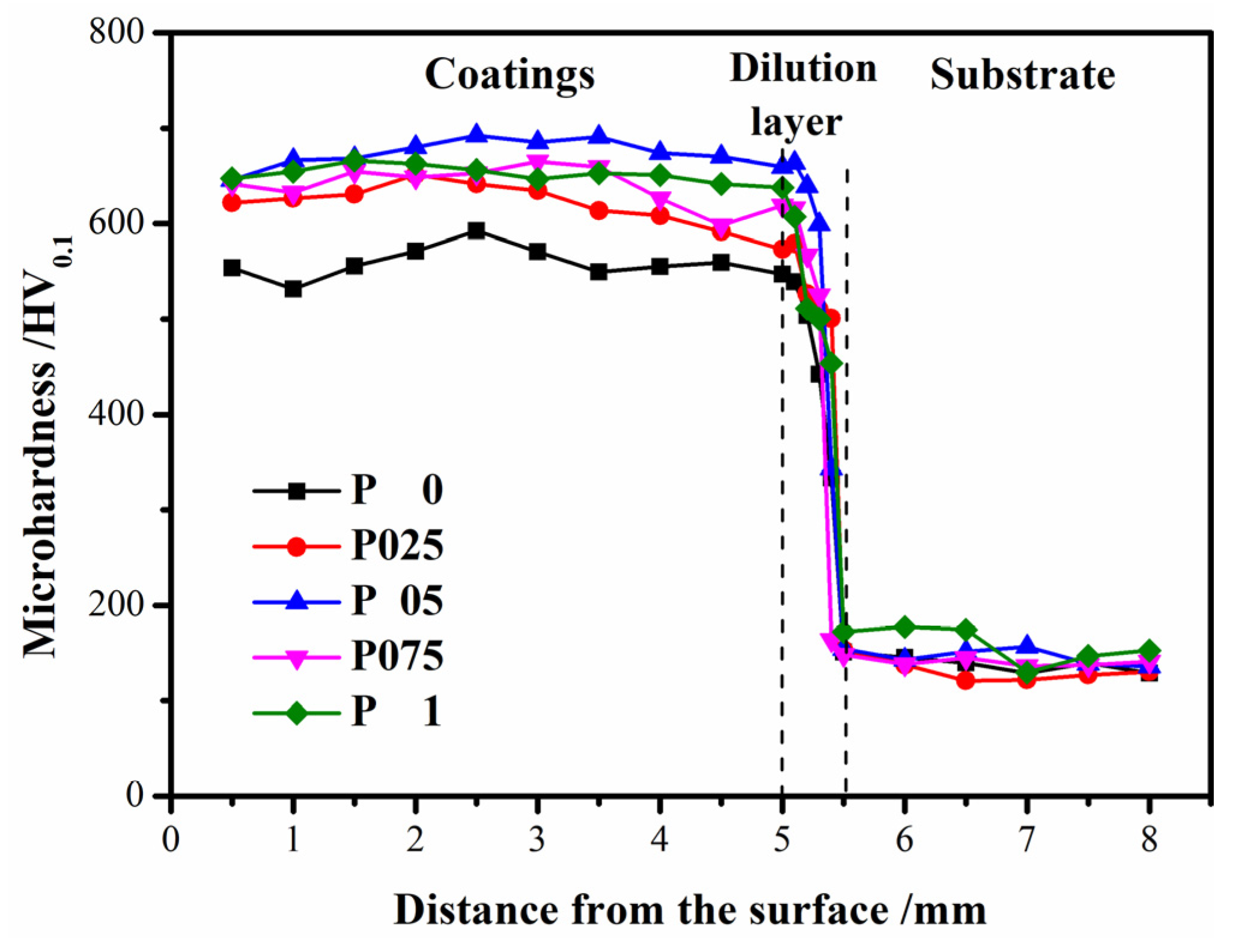
3.4. Mechanical Properties
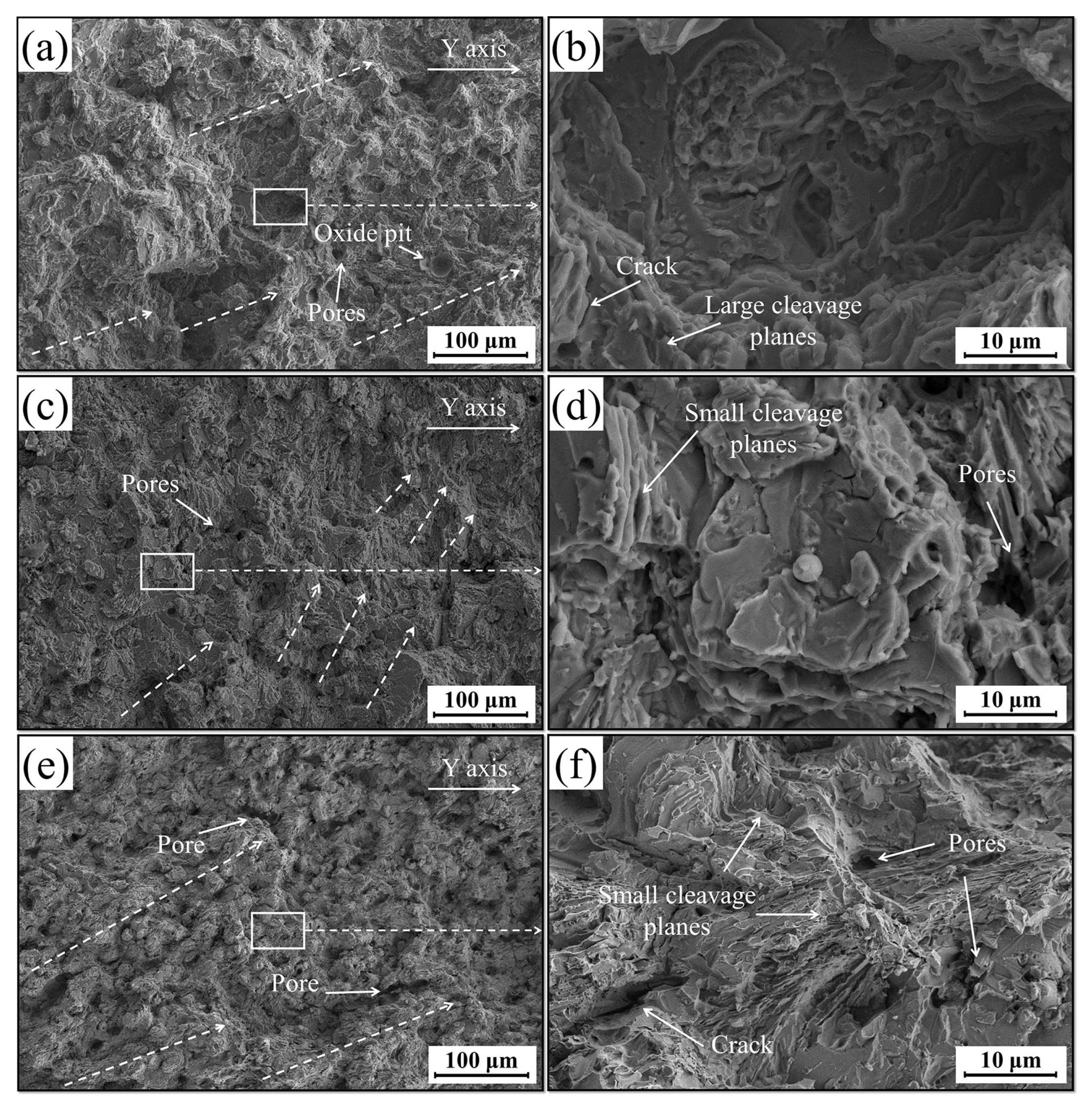
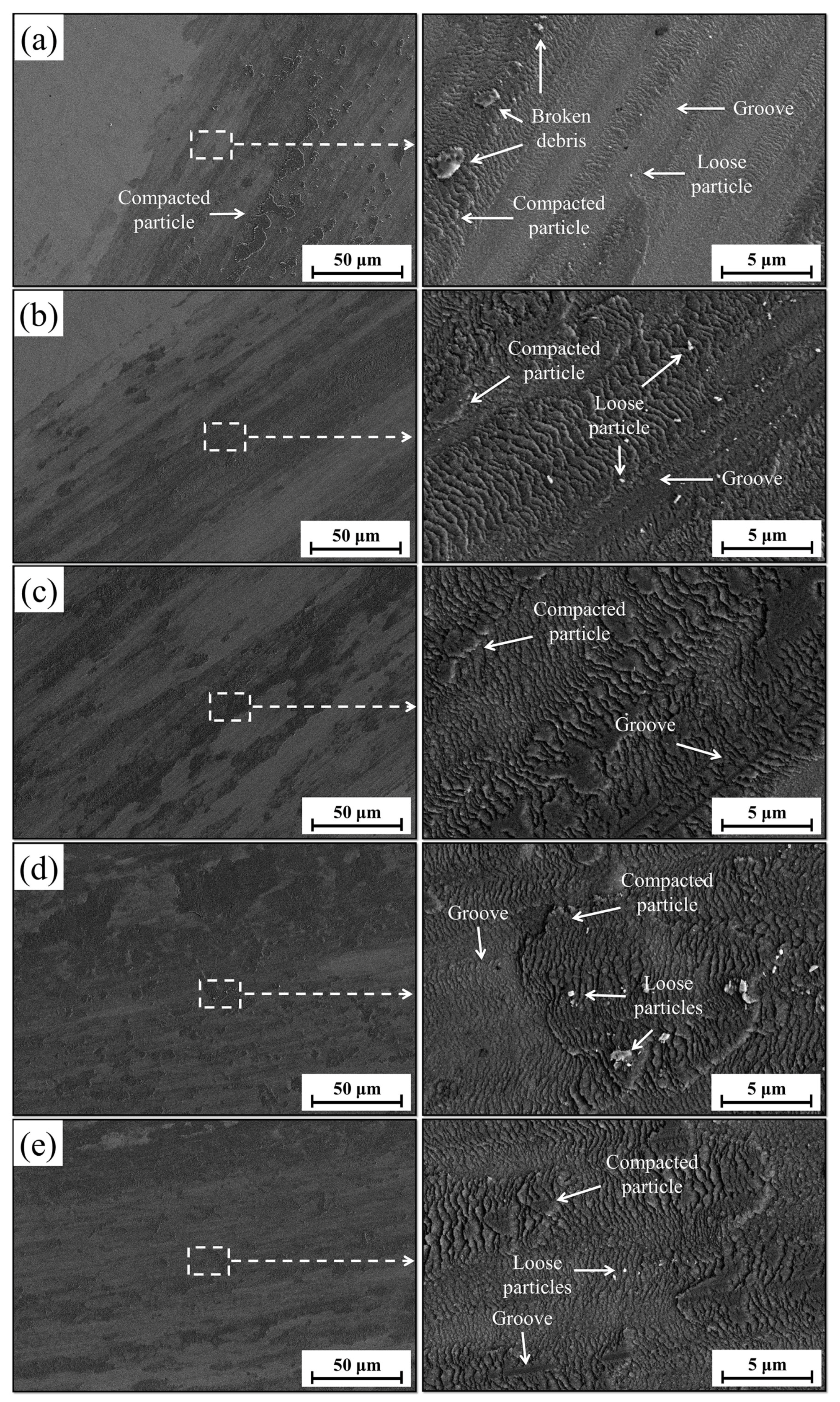
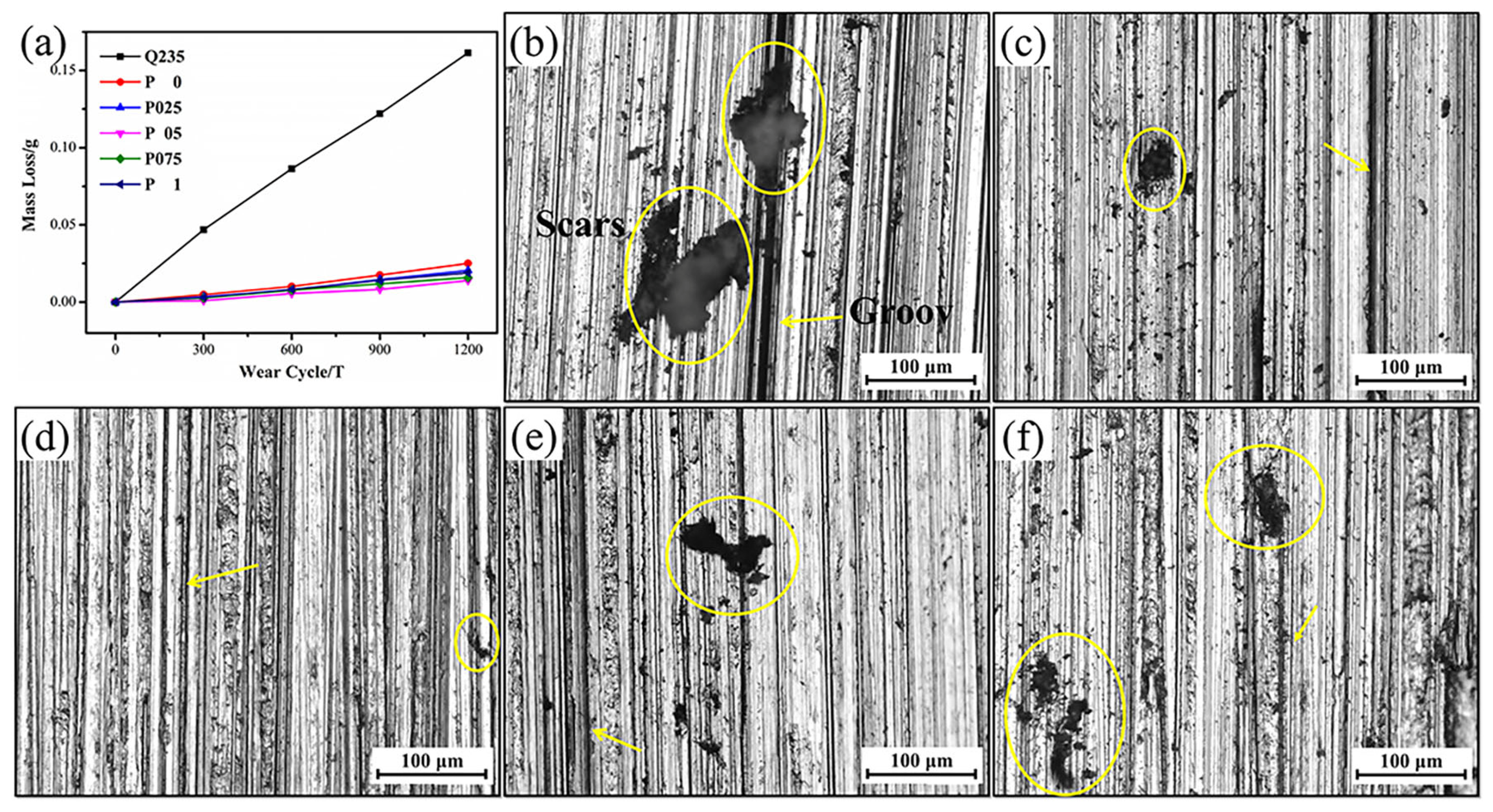
3.5. Wear Performance
4. Conclusions
- These Ce ions hindered the directional growth of the long-range dendritic solidification structure in the P coatings. Meanwhile, during the rapid solidification process, Ce combined with trace amounts of V and Si elements to form dispersed submicron Ce-rich particles as nucleation sites, promoting equiaxed crystal growth;
- With the optimal addition of 0.5 wt.% CNRs, the mechanical properties of the P coating improved significantly. In the deposition direction, the microhardness and tensile strength exhibited respective increases of 20.4% and 97.6%, while the elongation demonstrated an improvement of 59%. There was an 88.1% enhancement in the tensile strength in the perpendicular deposition direction, while the elongation was augmented by 33.9%. Meanwhile, the wear weight loss decreased by 44.2%, accompanied by an augmentation in the relative wear resistance by 79.3%;
- Excessive CNRs beyond 0.5 wt.% increased the O element content within the P coatings, leading to the formation of inclusions and subsequently causing a decline in their mechanical properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Wu, Y.P. Microstructure and wear behavior of the (AlCoCrFeNi)x/(WC–10Co)1−x composite coatings produced via high velocity oxy-fuel thermal spraying. Ceram. Int. 2023, 49, 28560–28570. [Google Scholar] [CrossRef]
- Omar, A.; Rehan, A. Influence of Feedstock and Thermal Spray Process on the Phase Composition of Alumina Coatings and Their Sliding Wear. J. Therm. Spray Technol. 2023, 32, 2028–2053. [Google Scholar]
- MohammadBagher, S.; Hossein, T. Utilization of Ni-Cu/Al2O3 co-deposition composite coatings on mild steel surface via electroplating method and evaluation of its tribological, electrochemical properties. Surf. Coat. Technol. 2023, 475, 130118. [Google Scholar]
- Ullah, Z.A.; Yasir, M.K. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process 2021, 68, 225–273. [Google Scholar]
- Liu, Y.A.; Ding, Y. Research and progress of laser cladding on engineering alloys: A review. J. Manuf. Process 2021, 66, 341–363. [Google Scholar] [CrossRef]
- Li, Y.; Cui, X.F. Interfacial bonding properties between cobalt-based plasma cladding layer and substrate under tensile conditions. Mater. Des. 2017, 123, 54–63. [Google Scholar] [CrossRef]
- Liu, T.Z.; Chang, M. Characteristics of WC reinforced Ni-based alloy coatings prepared by PTA + PMI method. Surf. Coat. Technol. 2020, 383, 125232. [Google Scholar] [CrossRef]
- Lai, Y.B.; Wang, D.Y. Influence of Process Parameter on the Residual Stress of the Co-based Alloy Plasma Cladding. Surf. Technol. 2019, 48, 314–321. [Google Scholar]
- Zhou, Y.X.; Zhang, J. Microstructure and properties of NiCrBSi coating by plasma cladding on gray cast iron. Surf. Coat. Technol. 2019, 361, 270–279. [Google Scholar] [CrossRef]
- Huang, H.H.; Han, G. Characterizing the magnetic memory signals on the surface of plasma transferred arc cladding coating under fatigue loads. J. Magn. Magn. Mater. 2017, 443, 281–286. [Google Scholar] [CrossRef]
- Gnyusov, S.F.; Degterev, A.S. The effect of plasma torch weaving on microstructural evolution in multiple-pass plasma-transferred arc Fe-Cr-V-Mo-C coating. Surf. Coat. Technol. 2018, 344, 75–84. [Google Scholar] [CrossRef]
- Deng, X.K.; Zhang, G.J. Investigations on microstructure and wear resistance of Fe-Mo alloy coating fabricated by plasma transferred arc cladding. Surf. Coat. Technol. 2018, 350, 480–487. [Google Scholar] [CrossRef]
- Li, X.; Lai, Y.B. Process optimization and properties of Fe-Cr-C alloy coating prepared by plasma cladding. Surf. Technol. 2020, 49, 177–184. [Google Scholar]
- Zhang, D.K.; Xue, H.B. Effect of Y2O3 on Microstructure and Properties of Fe-Al-Si-B Cladding by Plasma Transferred Arc. Rare Met. Mater. Eng. 2018, 47, 469–473. [Google Scholar]
- Sigolo, E.; Soyama, J. Wear resistant coatings of boron-modified stainless steels deposited by Plasma Transferred Arc. Surf. Coat. Technol. 2016, 302, 255–264. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Wang, Q.Y. Effect of Cr3C2 content on microstructure and properties of 310 stainless steel coating by PTA welding. Surf. Eng. 2020, 14, 464–471. [Google Scholar] [CrossRef]
- Deng, L.; Luo, J.R. Achieving excellent mechanical properties of ODS steel by Y2O3 addition. Mater. Sci. Eng. A 2023, 872, 145008. [Google Scholar] [CrossRef]
- Yue, J.Y.; Liu, X.Y. Combined effect of Y2O3 nanoparticles and Si second-phase oxide on microstructure and wear resistance of plasma-clad steel coating. Surf. Coat. Technol. 2020, 403, 126348. [Google Scholar] [CrossRef]
- Yue, J.Y.; Sui, Y. Influence of Y Nano-Oxide and Its Secondary Phase on Microstructure, Mechanical Properties, and Wear Behavior of the Stainless Steel Coatings Fabricated by Plasma Transfer Arc. Metals 2022, 12, 942. [Google Scholar] [CrossRef]
- Singla, Y.K.; Arora, N. Dry sliding adhesive wear characteristics of Fe-based hard facing alloys with different CeO2 additives—A statistical analysis. Tribol. Int. 2017, 105, 229–240. [Google Scholar] [CrossRef]
- Wang, W.J.; Fu, Z.K. The role of lanthanum oxide on wear and contact fatigue damage resistance of laser cladding Fe-based alloy coating under oil lubrication condition. Tribol. Int. 2016, 94, 470–478. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, M. Microstructure and properties of laser clad TiC+NiCrBSi+rare earth composite coatings. Surf. Coat. Technol. 2002, 161, 195–199. [Google Scholar] [CrossRef]
- Quazi, M.M.; Fazal, M.A. Effect of rare earth elements and their oxides on the tribomechanical performance of laser claddings: A review. J. Rare Earths 2016, 34, 549–564. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y. Laser cladding in-situ NbC particle reinforced Fe-based composite coatings with rare earth oxide addition. Surf. Coat. Technol. 2014, 239, 102–107. [Google Scholar] [CrossRef]
- Ding, L.; Hu, S.S. Effect of nano-CeO2 on microstructure and wear resistance of Co-based coatings. Surf. Coat. Technol. 2015, 276, 565–572. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostructured ferritic alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S.; Kaito, T.; Sakasegawa, H.; Chikata, N.; Hayashi, S.; Ohnuki, S. High-temperature strength characterization of advanced 9Cr-ODS ferritic steels. Mater. Sci. Eng. A 2009, 510, 115–120. [Google Scholar]
- London, A.J.; Santra, S. Effect of Ti and Cr on dispersion, structure and composition of oxide nano-particles in model ODS alloys. Acta Mater. 2015, 97, 223–233. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sui, Y. Laser metal deposited steel alloys with uniform microstructures and improved properties prepared by addition of small amounts of dispersed Y2O3 nanoparticles. Mater. Sci. Eng. A 2021, 806, 140827. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sui, Y. Dimension effect of Y2O3 nanomaterial on microstructure and tensile properties of laser metal deposited stainless steel coatings. Surf. Coat. Technol. 2021, 419, 127259. [Google Scholar] [CrossRef]
- GB/T 228.1-2021; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. Chinese Standard: Beijing, China, 2021.
- Zhang, L.S.; Zhang, M. Effects of nano-CeO2 on microstructure and properties of Ni625 alloy prepared by laser cladding. J. Alloys Compd. 2022, 918, 165571. [Google Scholar] [CrossRef]
- Yang, C.; Jing, C.N. Effect of CeO2 on the microstructure and properties of AlCoCrFeNi2.1 laser cladding coatings. J. Alloys Compd. 2024, 976, 172948. [Google Scholar] [CrossRef]
- Han, G.Y.; Zhang, Y. Effect of the Addition of CeO2 on the Microstructure and Corrosion of in-situ TiB/Ti Composite Coatings Prepared by Laser Cladding Technology. Int. J. Electrochem. Sc. 2021, 2, 16. [Google Scholar] [CrossRef]
- Gao, Z.T.; Ren, H.B. Effect of CeO2 on the microstructure and microhardensss of laser-cladded Ni60 on 35CrMoV alloys. Micron 2021, 150, 103146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Sun, R.L. Effects of CeO2 on Microstructure and Properties of Tic/ti2ni Reinforced Ti-based Laser Cladding Composite Coatings. Opt. Laser Eng. 2019, 120, 84–94. [Google Scholar]
- Wei, C.; Wei, Y.S. Improving Resistance to Crack and Corrosion of Laser-Cladded TiC/Co-based Composite Coatings by Doping Minor CeO2. Int. J. Electrochem. Sci. 2022, 17, 220622. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Huang, G.K. Crack-free Fe-based amorphous coating synthesized by laser cladding. Mater. Lett. 2017, 210, 46–50. [Google Scholar] [CrossRef]
- Fatih, D.G.; Erhan, D.R. Tribology Study of Pulse Electrodeposited Ni-B-SWCNT Composite Coating. TMS 2022, 74, 574–583. [Google Scholar]
- Sheng, L.Y.; Xiao, Y.N. Influence of layer number on microstructure, mechanical properties and wear behavior of the TiN/Ti multilayer coatings fabricated by high-power magnetron sputtering deposition. J. Manuf. Process 2021, 70, 529–542. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lu, X.C. Rare earth effect on microstructure, mechanical and tribological properties of CoCrW coatings. Mater. Sci. Eng. C 2007, A444, 92–98. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lu, X.C. Rare earth effect on the microstructure and wear resistance of Ni-based coatings. Mater. Sci. Eng. A 2007, 454–455, 194–202. [Google Scholar] [CrossRef]
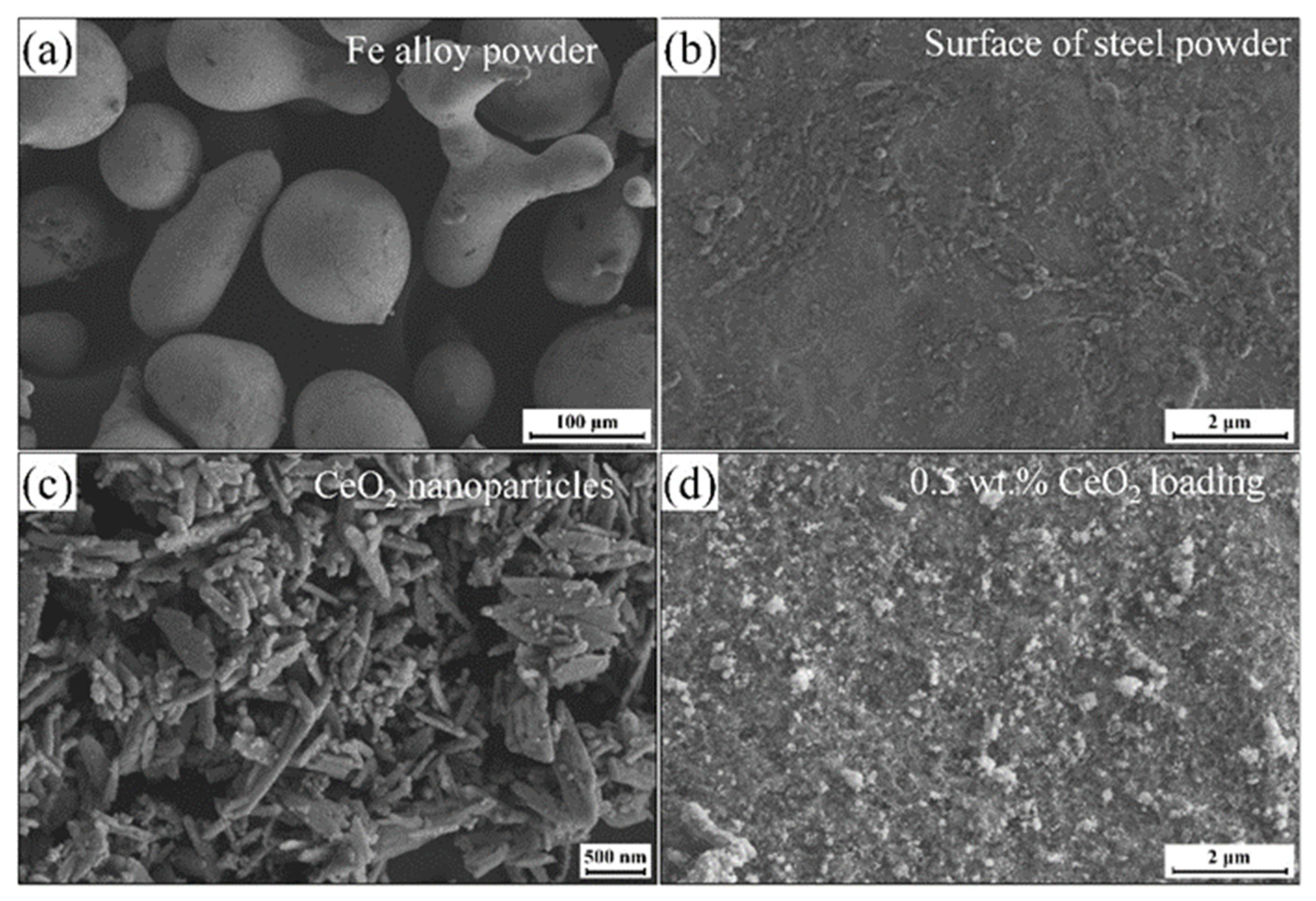



| EL | Parameter |
|---|---|
| Arc length (mm) | 10 |
| Current (A) | 175 |
| Plasma gas flow (L/s) | 0.1 |
| Protective gas flow (L/s) | 0.12 |
| Feeding gas flow (L/s) | 0.1 |
| Powder feeding rate (mg/s) | 400 |
| Scanning velocity (mm/s) | 1 |
| Overlap (%) | 40 |
| P0 | P025 | P05 | P075 | P1 | |
|---|---|---|---|---|---|
| Ce (wt.%) | 0 | 0.038 | 0.040 | 0.044 | 0.046 |
| O (wt.%) | 0.011 | 0.021 | 0.023 | 0.026 | 0.027 |
| Coating Condition | Average Hardness Values (HV0.1) | Tensile Strength in X-Axis (MPa) | Elongation in X-Axis (%) | Elastic Modulus in X-Axis (Gpa) | Tensile Strength in Y-Axis (MPa) | Elongation in Y-Axis (%) | Elastic Modulus in Y-Axis (Gpa) |
|---|---|---|---|---|---|---|---|
| confidence intervals of 95% | (565.28, 691.92) | (627.87 989.73) | (2.551, 4.225) | (135.77, 162.39) | (573.34, 932.26) | (3.09, 4.09) | (73.06, 87.464) |
| P0 | 559 | 681 | 2.89 | 118.2 | 504 | 3.06 | 68.03 |
| P025 | 619 | 1021 | 3.15 | 147.5 | 606 | 3.17 | 78.57 |
| P05 | 673 | 1346 | 4.59 | 161.1 | 948 | 4.28 | 88.87 |
| P075 | 640 | 1076 | 3.34 | 166.3 | 899 | 4.11 | 86.67 |
| P1 | 652 | 920 | 2.97 | 153.3 | 707 | 3.33 | 79.17 |
| Bare Surface (Q235) | P0 | P025 | P05 | P075 | P1 | Confidence Intervals of 95% | |
|---|---|---|---|---|---|---|---|
| Weight loss (g) | 0.1614 | 0.0251 | 0.0206 | 0.014 | 0.016 | 0.0189 | (0.0103, 0.0259) |
| Relative wear resistance | 1 | 6.430 | 7.835 | 11.529 | 10.151 | 8.540 | (7.146, 10.248) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.-Y.; Jiao, P.-C.; Sui, Y.; Lu, F.; Zhang, R.-Y.; Chen, W.-D.; Zhao, L.-S. Dispersed CeO2 Nanorods with Low-Speed Mixing for Mechanical Properties Promotion of PTA Steel Coatings. Coatings 2024, 14, 713. https://doi.org/10.3390/coatings14060713
Yue J-Y, Jiao P-C, Sui Y, Lu F, Zhang R-Y, Chen W-D, Zhao L-S. Dispersed CeO2 Nanorods with Low-Speed Mixing for Mechanical Properties Promotion of PTA Steel Coatings. Coatings. 2024; 14(6):713. https://doi.org/10.3390/coatings14060713
Chicago/Turabian StyleYue, Jun-Yu, Peng-Cheng Jiao, Yi Sui, Fei Lu, Rui-Ying Zhang, Wei-Dong Chen, and Li-Sha Zhao. 2024. "Dispersed CeO2 Nanorods with Low-Speed Mixing for Mechanical Properties Promotion of PTA Steel Coatings" Coatings 14, no. 6: 713. https://doi.org/10.3390/coatings14060713
APA StyleYue, J.-Y., Jiao, P.-C., Sui, Y., Lu, F., Zhang, R.-Y., Chen, W.-D., & Zhao, L.-S. (2024). Dispersed CeO2 Nanorods with Low-Speed Mixing for Mechanical Properties Promotion of PTA Steel Coatings. Coatings, 14(6), 713. https://doi.org/10.3390/coatings14060713






