Design of Debondable PU Coating for Degradation on Demand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bio-Based Trigger Degradable PU Dispersion
2.3. Fabric Coating
2.4. Characterisation
3. Results
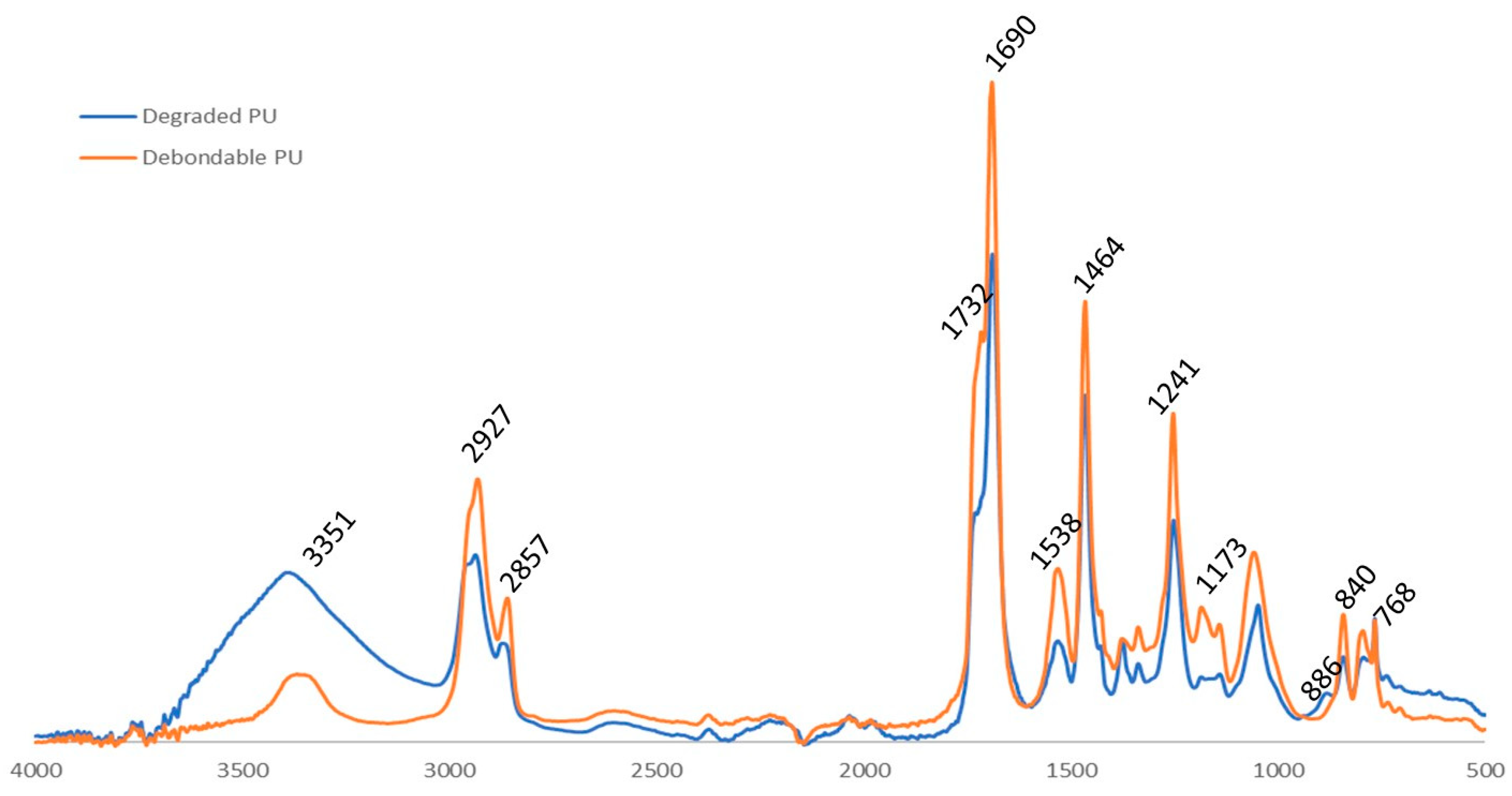
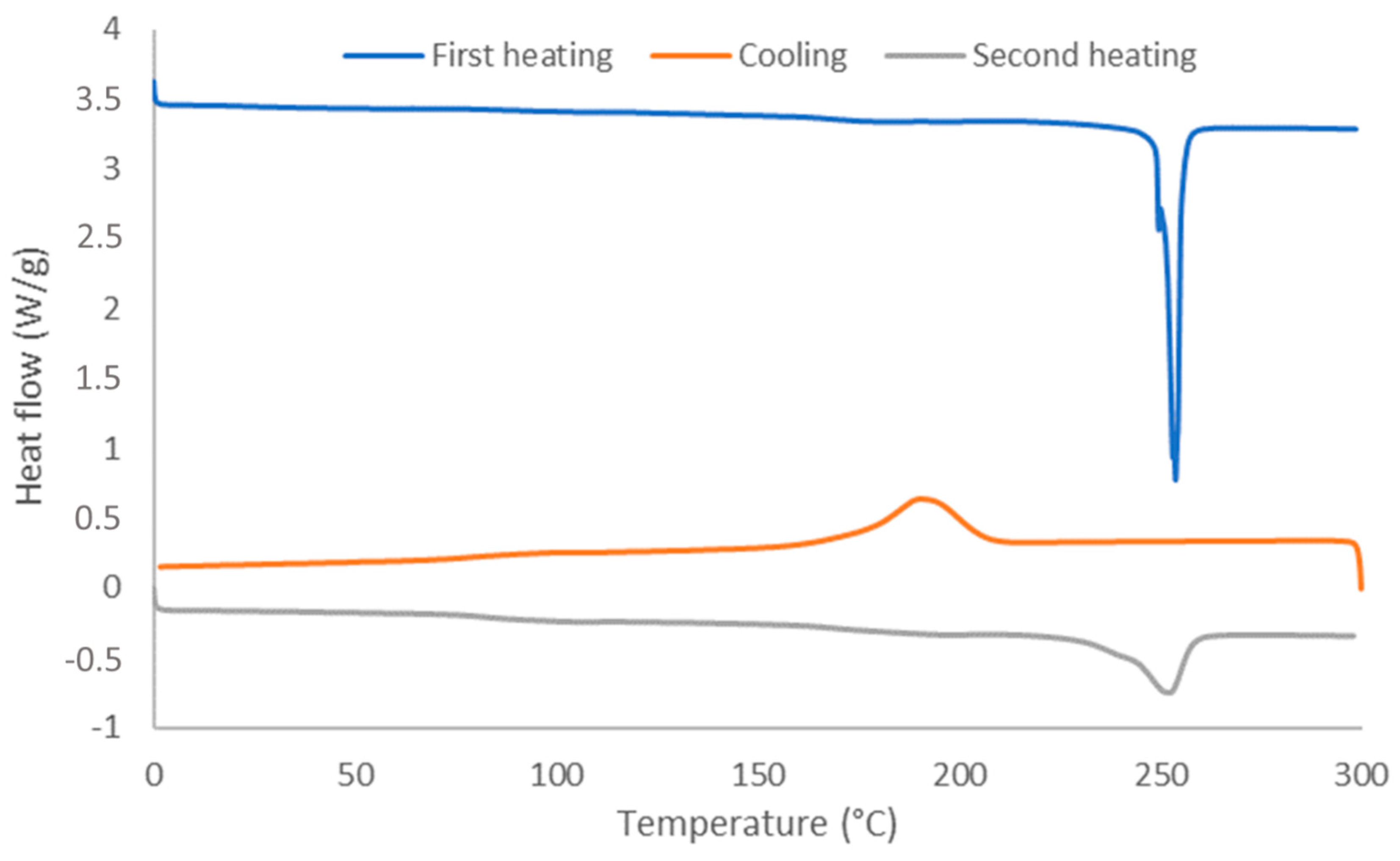
3.1. Structural Characterisation of PU Coating
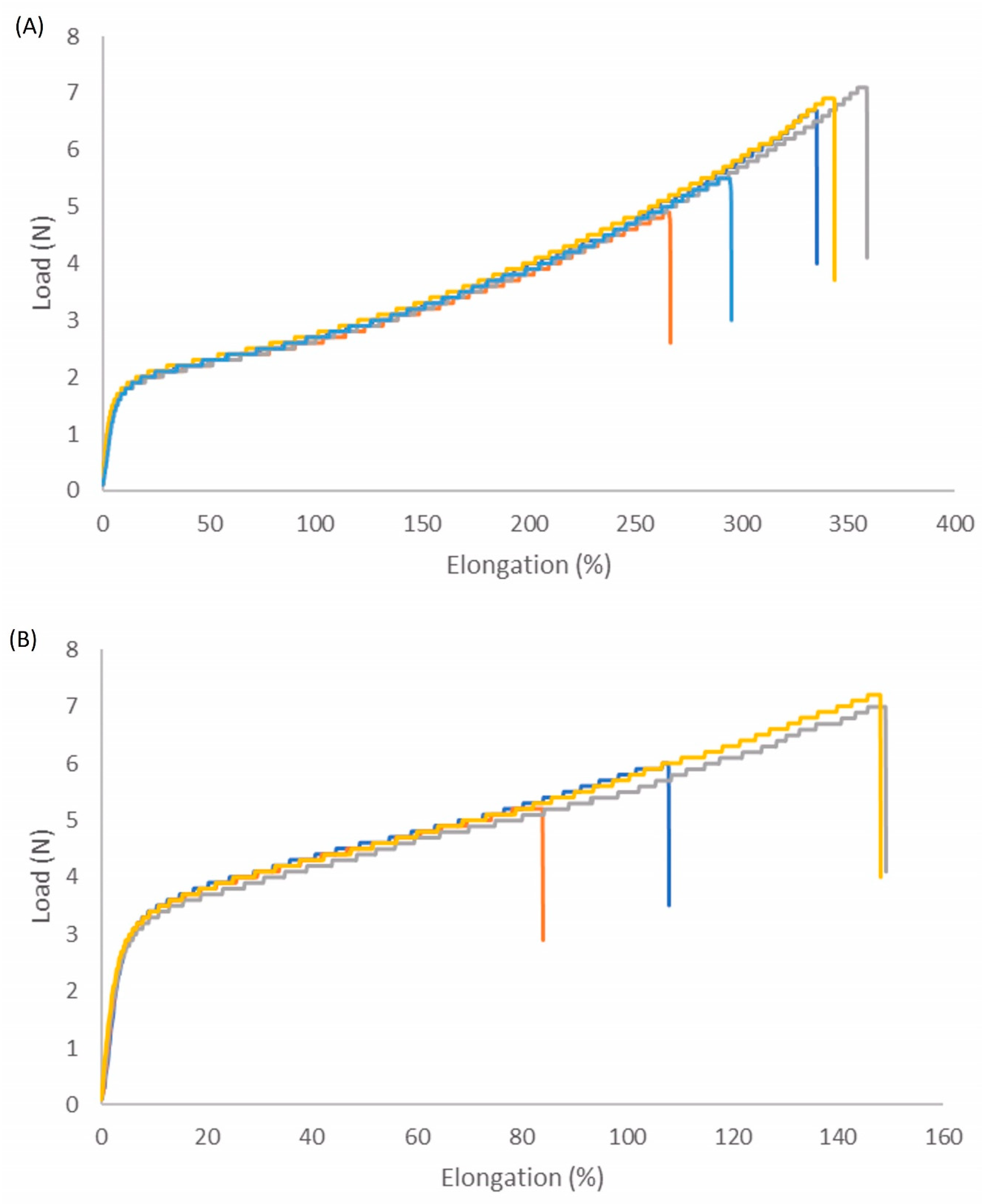
3.2. Properties of the Coating
3.3. Degradation Study
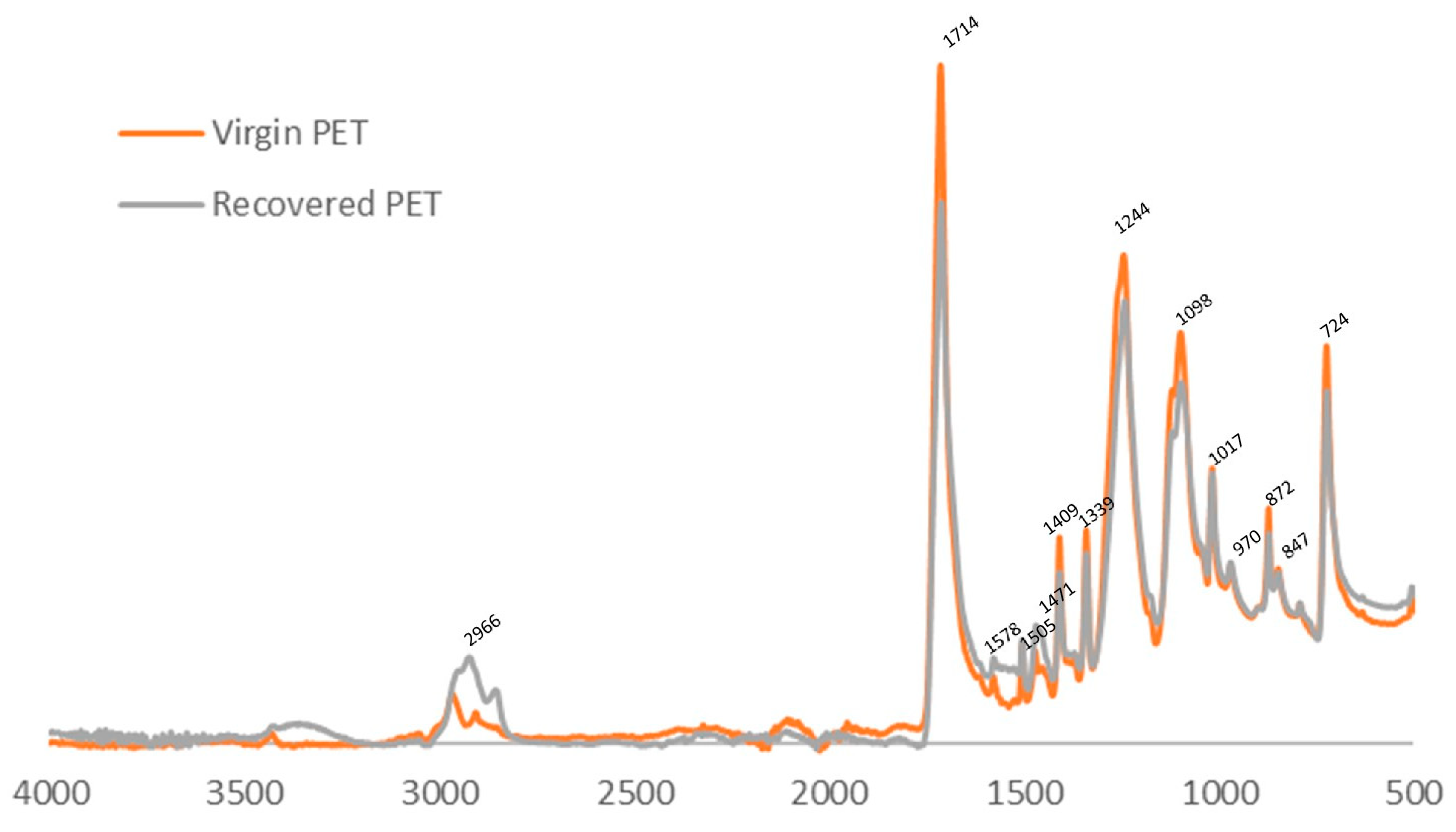
3.4. Characterisation of Recovered PET Fabric
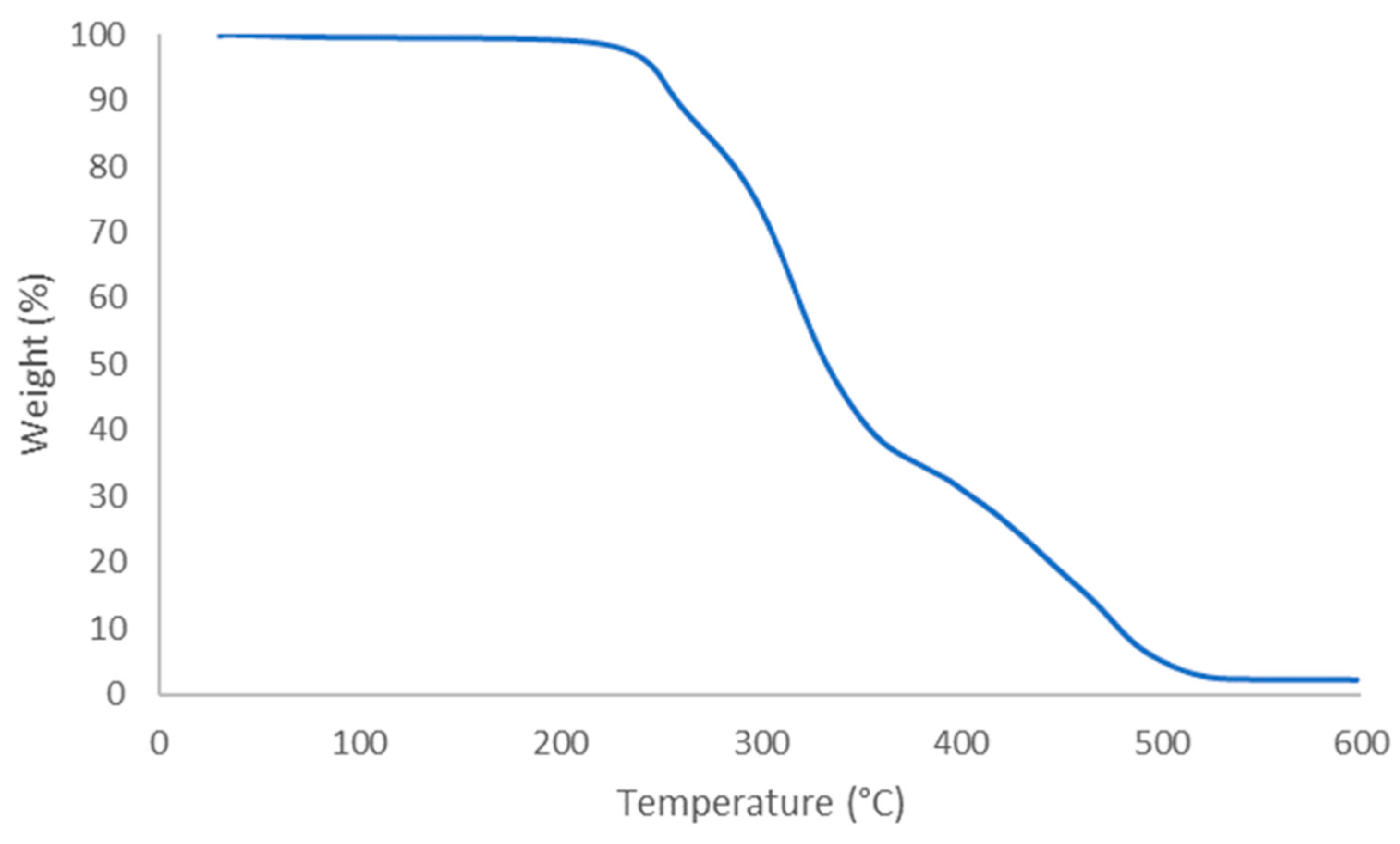
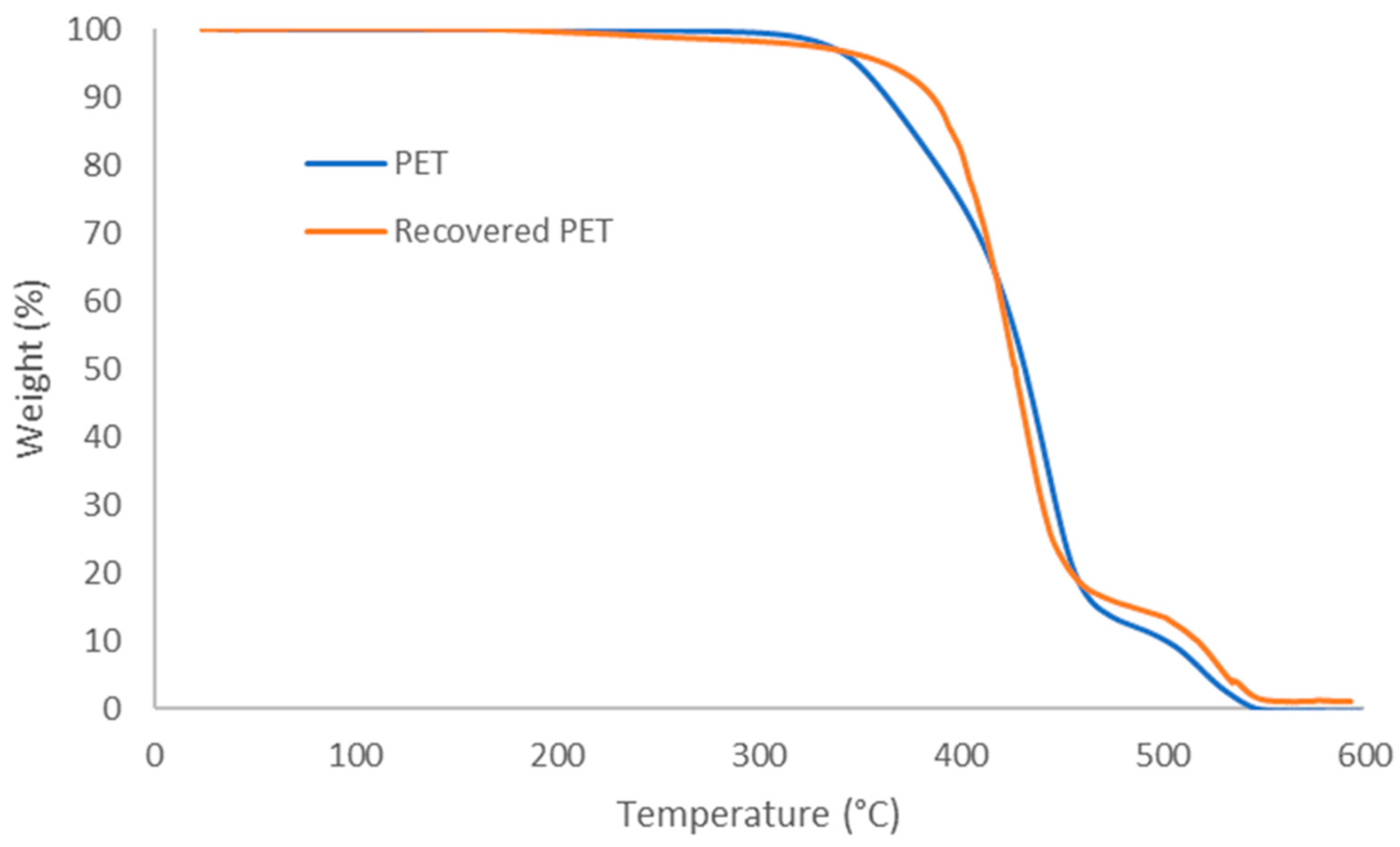
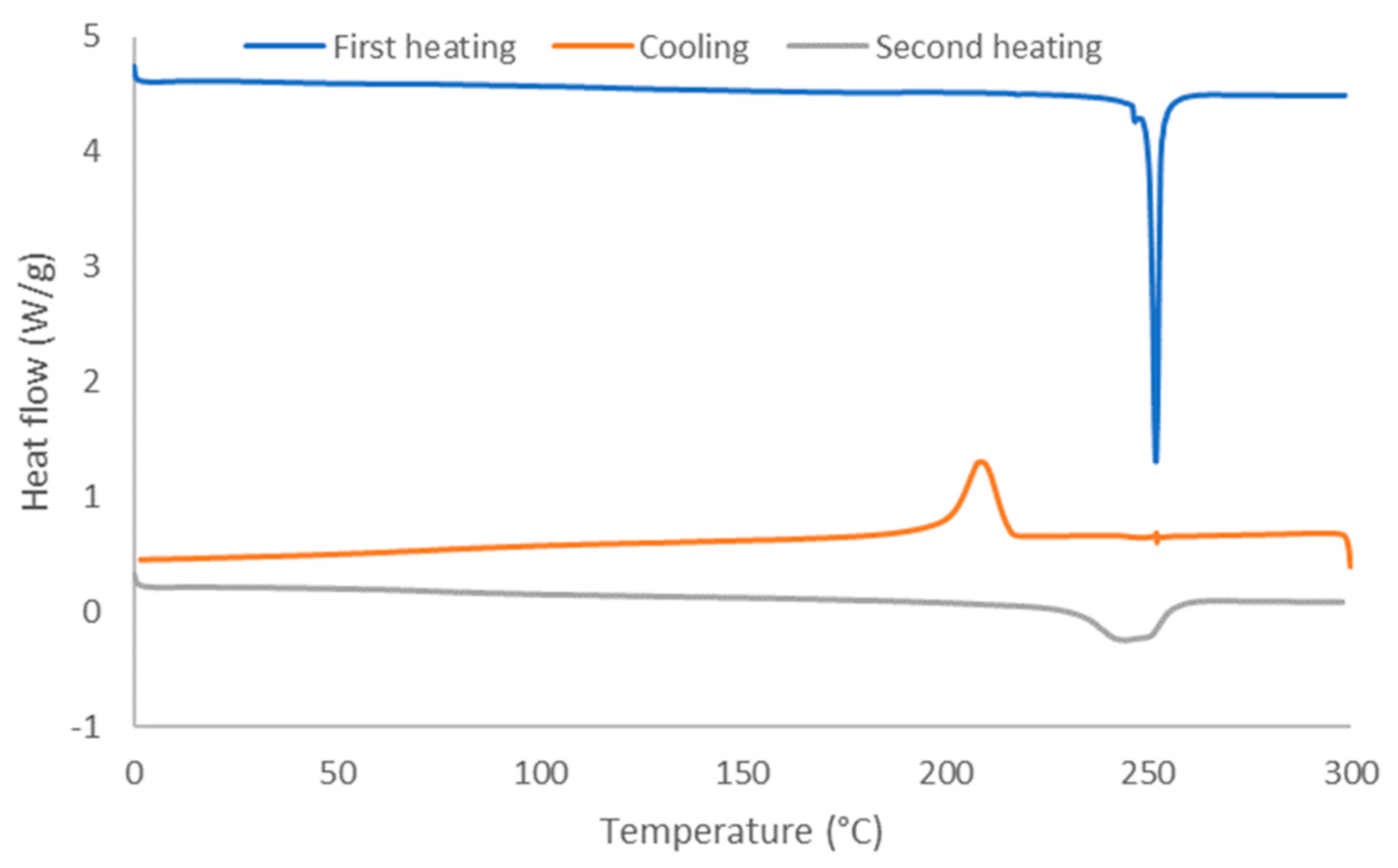
3.5. Thermal Properties of Recovered PET Fabric
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Song, H.; Wang, J.; Wang, Y.; Jia, S.; Deng, T.; Hou, X. Controllable degradation of polyurethane elastomer via selective cleavage of C-O and C-N bonds. J. Clean. Prod. 2018, 176, 873–879. [Google Scholar] [CrossRef]
- De Smet, D.; Verjans, J.; Vanneste, M. Selective Solvolysis of Bio-Based PU-Coated Fabric. Polymers 2022, 14, 5452. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J. Thermo-Chemical Decomposition Study of Polyurethane Elastomer through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef]
- Jutrzenka Trzebiatowska, P.; Beneš, H.; Datta, J. Evaluation of the glycerolysis process and valorisation of recovered polyol in polyurethane synthesis. React. Funct. Polym. 2019, 139, 25–33. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Drinčić, A.; Oreški, A.; Onder, O.; Utroša, P.; Pahovnik, D.; Žagar, E. Insight into Chemical Recycling of Flexible Polyurethane Foams by Acidolysis. ACS Sustain. Chem. Eng. 2022, 10, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cadena, L.; Tersac, G.; Coqueret, X.; Gamiño-Arroyo, Z. Solvolysis of acrylate-urethane coatings cured by electron-beam and UV radiation. Prog. Org. Coat. 2021, 136, 105268–105633. [Google Scholar] [CrossRef]
- Zahedifar, P.; Pazdur, L.; Vande Velde, C.; Billen, P. Multistage chemical recycling of polyurethanes and dicarbamates: A glycolysis–hydrolysis demonstration. Sustainability 2021, 13, 3583. [Google Scholar] [CrossRef]
- Dispinar, T.; Colard, C.; Du Prez, F. Polyurea microcapsules with a photocleavable shell: UV-triggered release. Polym. Chem. 2013, 4, 763. [Google Scholar] [CrossRef]
- Paula, C.; Pereira, P.; Coelho, J.; Fonseca, A.; Serra, A. Development of light-degradable poly(urethane-urea) hydrogel films. Mater. Sci. Eng. C 2021, 131, 112520. [Google Scholar] [CrossRef]
- Xu, Y.; Morado, E.; Zimmerman, S. Construction from destruction using a photo-triggered self-propagating degradable polyurethane as a one-pot epoxy. Polym. Chem. 2020, 11, 6215. [Google Scholar] [CrossRef]
- Wang, B.; Ma, S.; Xu, X.; Li, Q.; Yu, T.; Wang, S.; Yan, S.; Liu, Y.; Zhu, J. High-Performance, Biobased, Degradable Polyurethane Thermoset and Its Application in Readily Recyclable Carbon Fiber Composites. ACS Sustain. Chem. Eng. 2020, 8, 11162–11170. [Google Scholar] [CrossRef]
- Bachelder, E.; Beaudette, T.; Broaders, K.; Paramonov, S.; Dashe, J.; Fréchet, J. Acid-degradable polyurethane particles for protein-based vaccines: Biological evaluation and in vitro analysis of particle degradation products. Mol. Pharm. 2008, 5, 876. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhang, X.; Yu, P.; Li, Q.; Qin, Y.; Xin, Z. Facile fabrication of degradable polyurethane thermosets with high mechanical strength and toughness via the cross-linking of triple boron–urethane bonds. J. Mater. Chem. A 2021, 9, 22410. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, X.; Wang, J.; Du, J.; Zhu, Y. Vanillin-Based Degradable Polyurethane Thermosets Demonstrating High Bio-Content and Mechanical Properties. ACS Appl. Polym. Mater. 2023, 5, 4536. [Google Scholar] [CrossRef]
- Xu, Y.; Sen, S.; Wu, O.; Zhong, X.; Ewoldt, R.; Zimmerman, S. Base-triggered self-amplifying degradable polyurethanes with the ability to translate local stimulation to continuous long-range degradation. Chem. Sci. 2020, 11, 3326. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jin, B.; Wang, H.; Wu, W.; Cao, Z.; Wu, J.; Huang, G. A Degradable and Self-Healable Vitrimer Based on Non-isocyanate Polyurethane. Front. Chem. 2020, 8, 585569. [Google Scholar] [CrossRef] [PubMed]
- Bols, M.; Pedersen, C. Silyl-protective groups influencing the reactivity and selectivity in glycosylations. Beilstein J. Org. Chem. 2017, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Blasco, M.; Moyano, M.; Hernández-Fernández, C.; Sierra-Molero, F.; Pastor, I.; Alonso, D.; Arán-Aís, F.; Orgilés-Calpena, E. Polyurethane Adhesives with Chemically Debondable Properties via Diels–Alder Bonds. Polymers 2024, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Babra, T.; Trivedi, A.; Warriner, C.; Bazin, N.; Castiglione, D.; Sivour, C.; Hayes, W.; Greenland, B. Fluoride degradable and thermally debondable polyurethane based adhesive. Polym. Chem. 2017, 8, 7207. [Google Scholar] [CrossRef]
- Babra, T.; Warriner, C.; Bazin, N.; Hayes, W.; Greenland, W. A fluoride degradable crosslinker for debond-on-demand polyurethane based crosslinked adhesives. Mater. Today Commun. 2021, 26, 101777. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; Patil, M.; John, J. Addressing the Sustainability Conundrums and Challenges within the Polymer Value Chain. Sustainability 2023, 15, 15758. [Google Scholar] [CrossRef]
- ISO 13934-1:2013; Textiles—Tensile Properties of Fabrics. Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. ISO: Geneva, Switzerland, 2013.
- ISO 811:2018; Textiles—Determination of Resistance to Water Penetration—Hydrostatic Pressure Test. ISO: Geneva, Switzerland, 2018.
- ISO 6330:2021; Textiles—Domestic Washing and Drying Procedures for Textile Testing. ISO: Geneva, Switzerland, 2021.
- Wilhelm, C.; Gardette, J.-L. Infrared analysis of the photochemical behaviour of segmented polyurethanes: 1. Aliphatic poly(ester-urethane). Polymer 1997, 38, 4019. [Google Scholar] [CrossRef]
- Irusta, L.; Fernanadez-Berridi, J. Aromatic poly(ester-urethanes): Effect of the polyol molecular weight on the photochemical behavior. Polymer 2000, 41, 3297. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra: A Practical Approach in Encyclopedia of Analytical Chemistry; Mayers, R., Ed.; Wiley: Hoboken, NJ, USA, 2000; p. 10815. [Google Scholar]
- Kaushiva, B.; McCartney, S.; Rossmy, G.; Wilkes, G. Surfactant level influences on structure and properties of flexible slabstock polyurethane foams. Polymer 2000, 41, 285. [Google Scholar] [CrossRef]
- Ahmad, S.; Zafar, F.; Sharmin, E.; Garg, N.; Kashif, M. Synthesis and characterization of corrosion protective polyurethanefattyamide/silica hybrid coating material. Prog. Org. Coat. 2012, 73, 112. [Google Scholar] [CrossRef]
- Anand, A.; Kulkarni, R.; Gite, V. Preparation and properties of eco-friendly two pack PU coatings based on renewable source (sorbitol) and its property improvement by nano ZnO. Prog. Org. Coat. 2012, 74, 764. [Google Scholar] [CrossRef]
- Marathe, R.; Tatiya, P.; Chaudhari, A.; Lee, J.; Mahulikar, P.; Sohn, D.; Gite, V. Neem acetylated polyester polyol—Renewable source based smart PU coatings containing quinoline (corrosion inhibitor) encapsulated polyurea microcapsules for enhance anticorrosive property. Ind. Crop. Prod. 2015, 77, 239–250. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, C.-C. Synthesis of 1,3-Bis(4-Hydroxybutyl)tetramethyldisiloxane and α,ω-Bis(4-Hydroxybutyl)-polydimethylsiloxane. Iran. Polym. J. 2007, 16, 153. [Google Scholar]
- Holland, B.; Hay, J. The thermal degradation of PET and analogous polyesters measured by thermal analysis—Fourier Transform infrared spectroscopy. Polymer 2002, 43, 1835–1847. [Google Scholar] [CrossRef]
- dos Santos Pereira, A.; da Silva, M.; Lima Júnior, E.; dos Santos Paula, A.; Tommasini, F. Processing and characterization of PET composites reinforced with geopolymer concrete waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.; Aburiazaiza, A.; Khan, H.; Ismail, I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic pyrolysis of plastic waste: Moving toward pyrolysis based biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Sustaita-Rodríguez, J.; Medellín-Rodríguez, F.; Olvera-Mendez, D.; Gimenez, A.; Luna-Barcenas, G. Thermal Stability and Early Degradation Mechanisms of High-Density Polyethylene, Polyamide 6 (Nylon 6), and Polyethylene Terephthalate. Polym. Eng. Sci. 2019, 59, 2016–2023. [Google Scholar] [CrossRef]
- Mulcahy, K.; Kilpatrick AHarper, G.; Walton, A.; Abbott, A. Debondable adhesives and their use in recycling. Green Chem. 2022, 24, 36–61. [Google Scholar] [CrossRef]
- Thomas, J.; Patil, R.S.; John, J.; Patil, M. A Comprehensive Outlook of Scope within Exterior Automotive Plastic Substrates and Its Coatings. Coatings 2023, 13, 1569. [Google Scholar] [CrossRef]





| Priplast 3172 (g) | IPDI (g) | DMPA (g) | HBTMS (g) | Propanediol (g) | |
|---|---|---|---|---|---|
| Control PUD | 20 | 24.34 | 2.36 | - | 2 |
| Trigger degradable PUD | 20 | 12.26 | 2.36 | 2 | - |
| Tensile Strength (MPa) | Elongation (%) | |
|---|---|---|
| Trigger degradable PU | 15 | 319 |
| Crosslinked trigger degradable PU | 16 | 122 |
| Tensile Strength (MPa) | Elongation (%) | |
|---|---|---|
| Virgin PET | 120 | 37 |
| Recovered PET | 110 | 37 |
| Tg (°C) | Tm (°C) | Xc (%) | |
|---|---|---|---|
| Virgin PET | 83 | 252 | 39 |
| Recycled PET | 73 | 244 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Smet, D.; Vanneste, M. Design of Debondable PU Coating for Degradation on Demand. Coatings 2024, 14, 731. https://doi.org/10.3390/coatings14060731
De Smet D, Vanneste M. Design of Debondable PU Coating for Degradation on Demand. Coatings. 2024; 14(6):731. https://doi.org/10.3390/coatings14060731
Chicago/Turabian StyleDe Smet, David, and Myriam Vanneste. 2024. "Design of Debondable PU Coating for Degradation on Demand" Coatings 14, no. 6: 731. https://doi.org/10.3390/coatings14060731






