Water-Borne Photo-Thermal Superhydrophobic Coating for Anti-Icing, Self-Cleaning and Oil–Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
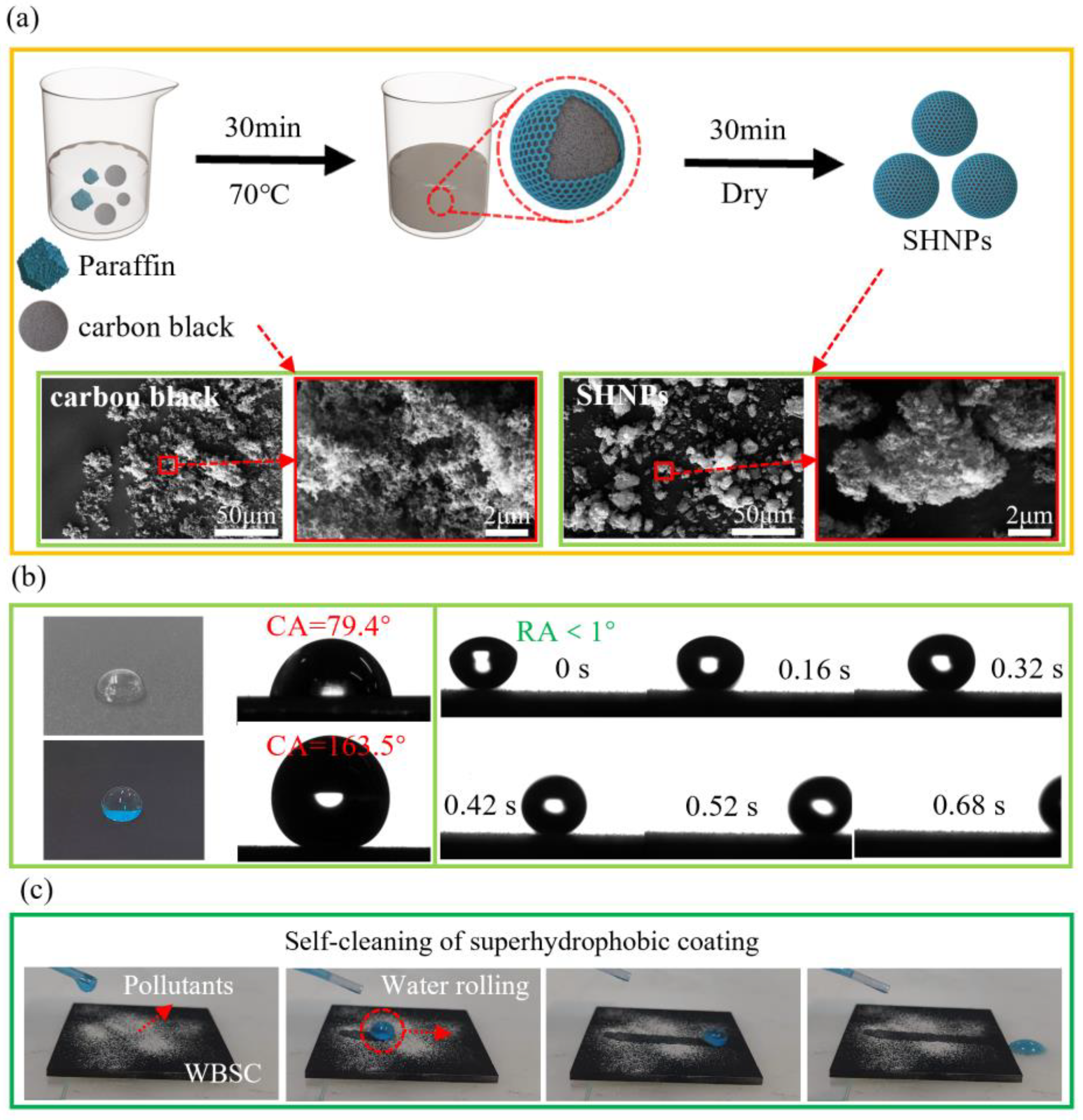
2.2. Preparation of the Superhydrophobic NPs (SHNPs)
2.3. Preparation of the Photo-Thermal Superhydrophobic Coating (WBSC)
2.4. Oil–Water Separation
2.5. Measurements of the Hydrophobicity and Anti-Icing Test
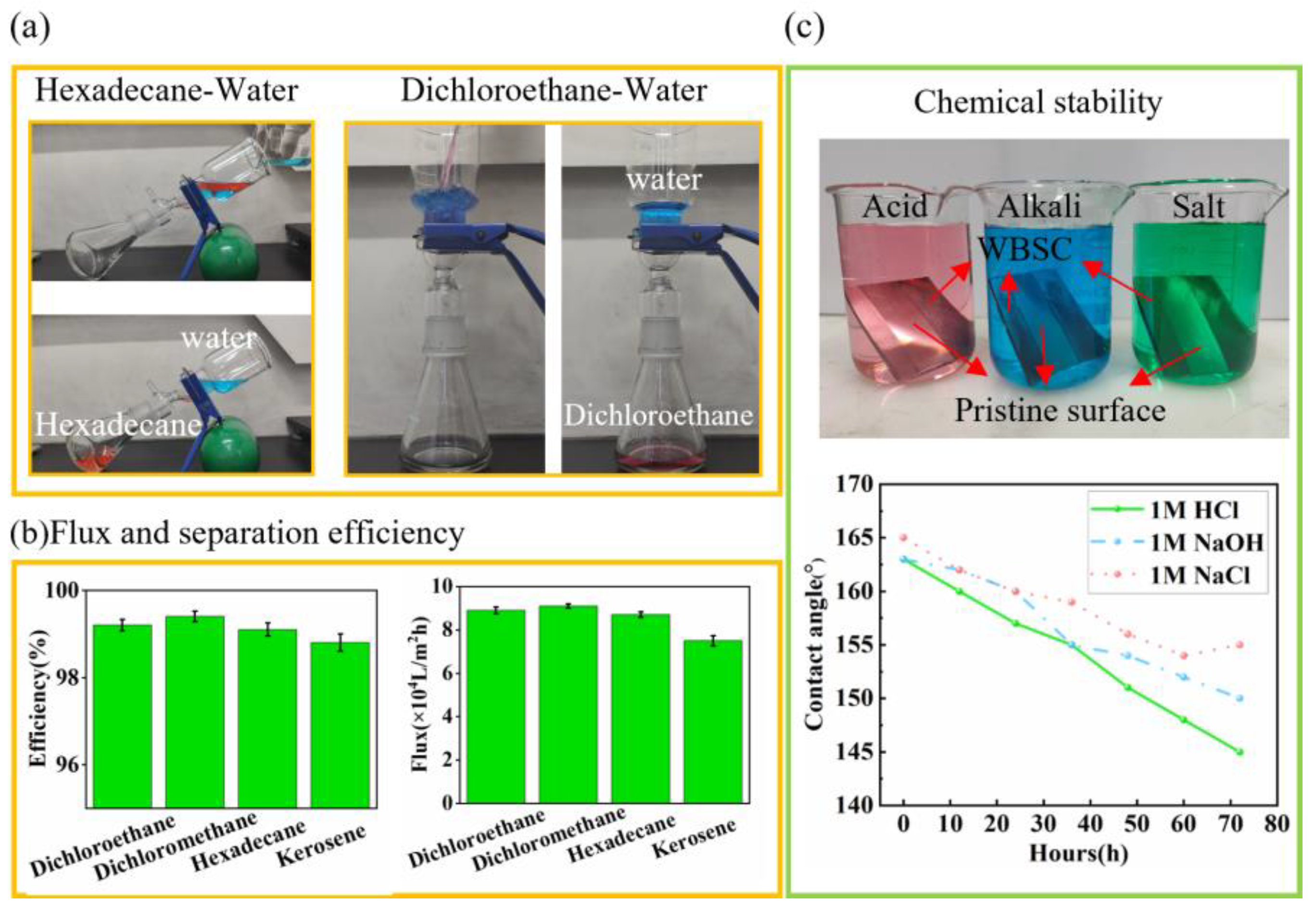
2.6. Chemical Durability Test
2.7. Water Jet Test
2.8. Characterization
3. Results and Discussion
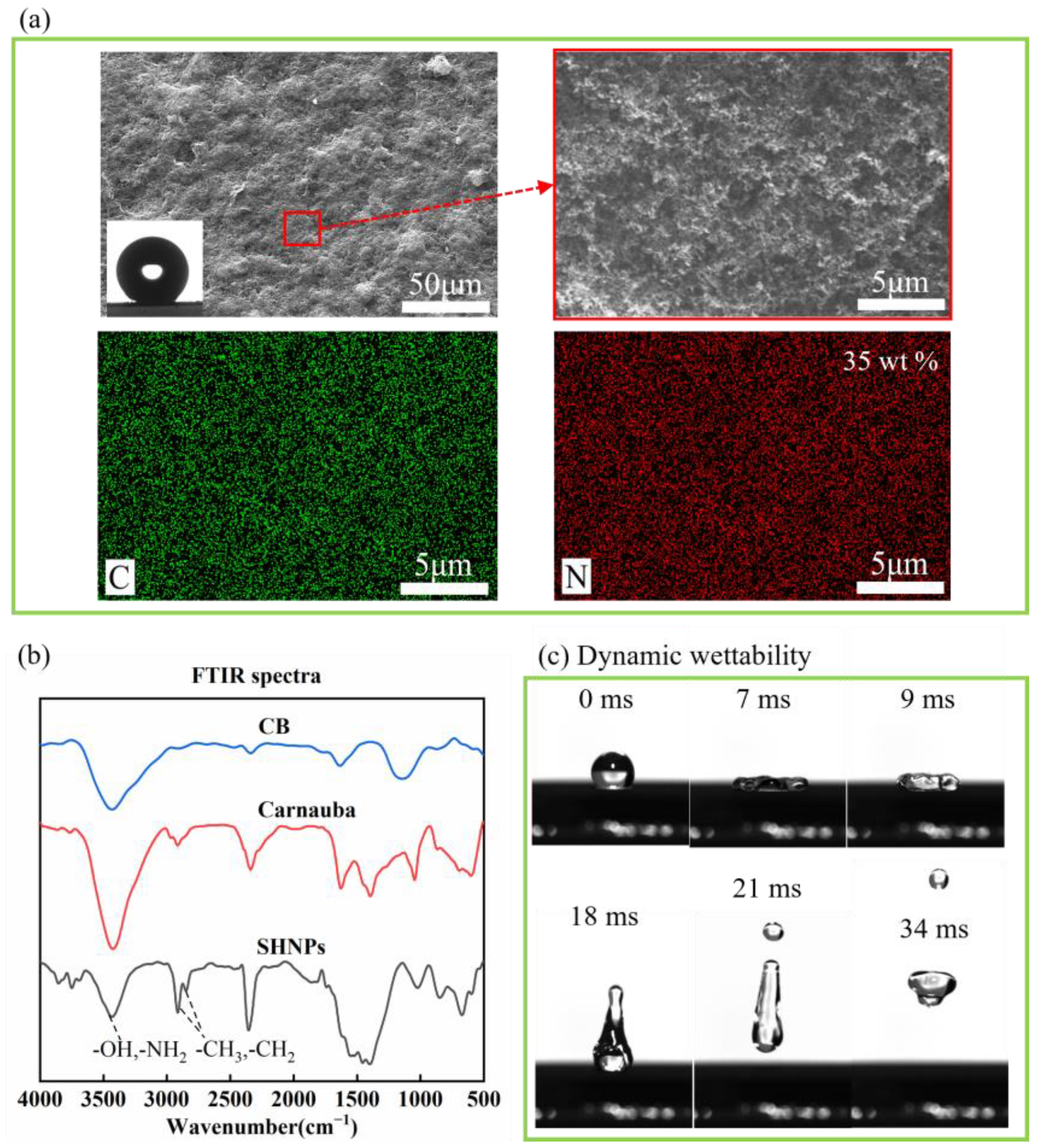
3.1. Preparation and Characterization of the Water-Born Superhydrophobic Coating (WBSC)
3.2. Mechanical Stability of the WBSC Coating
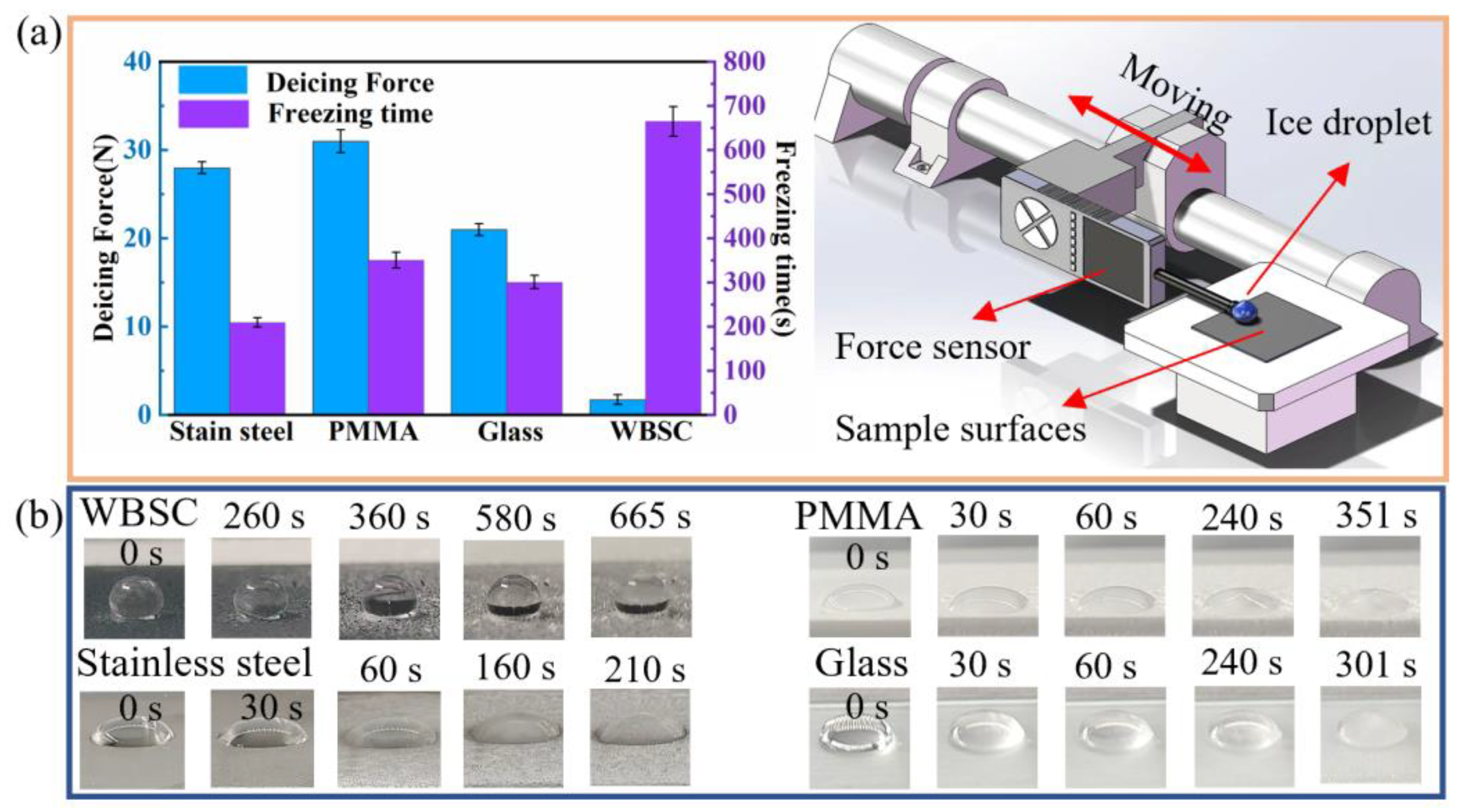
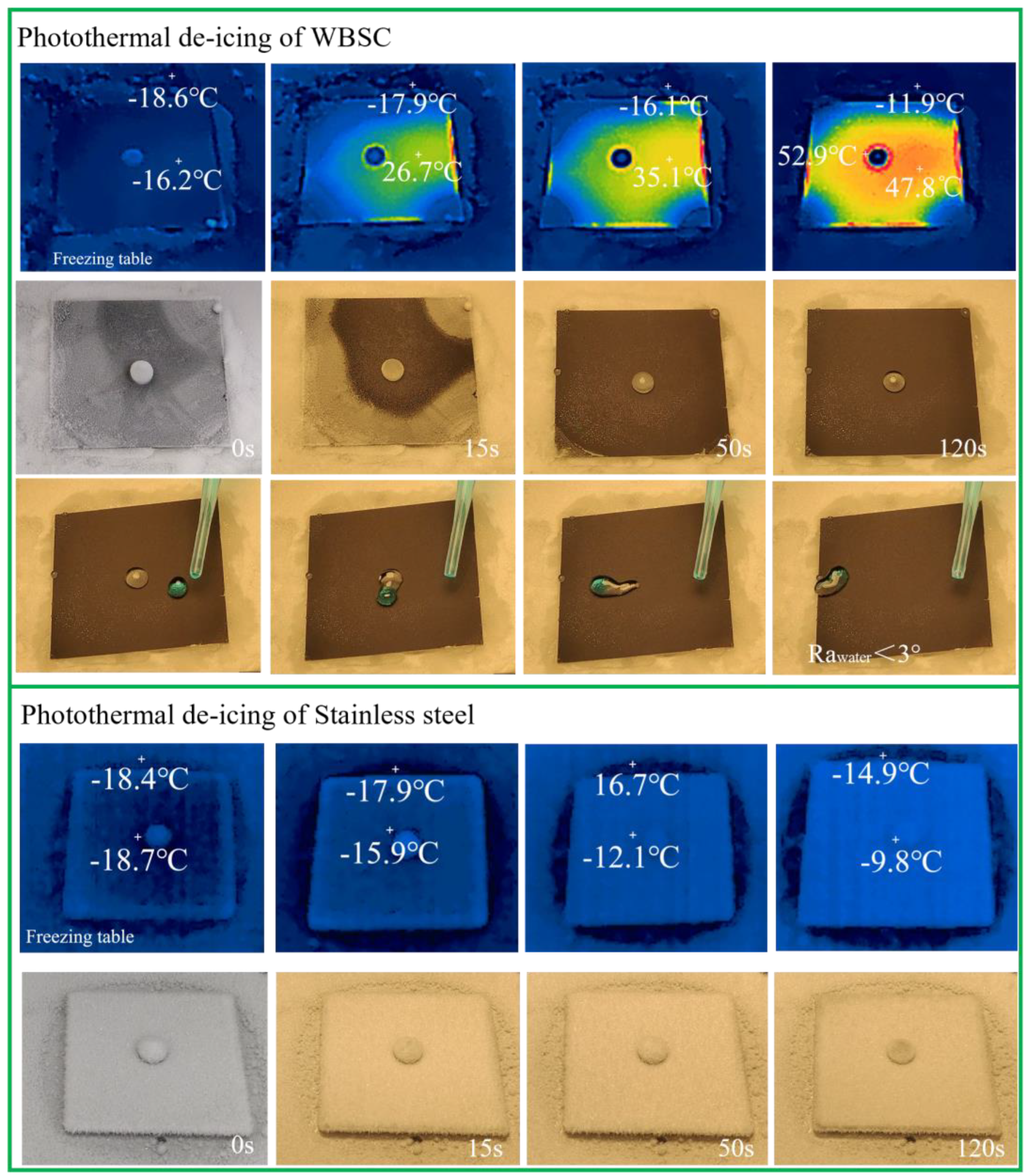
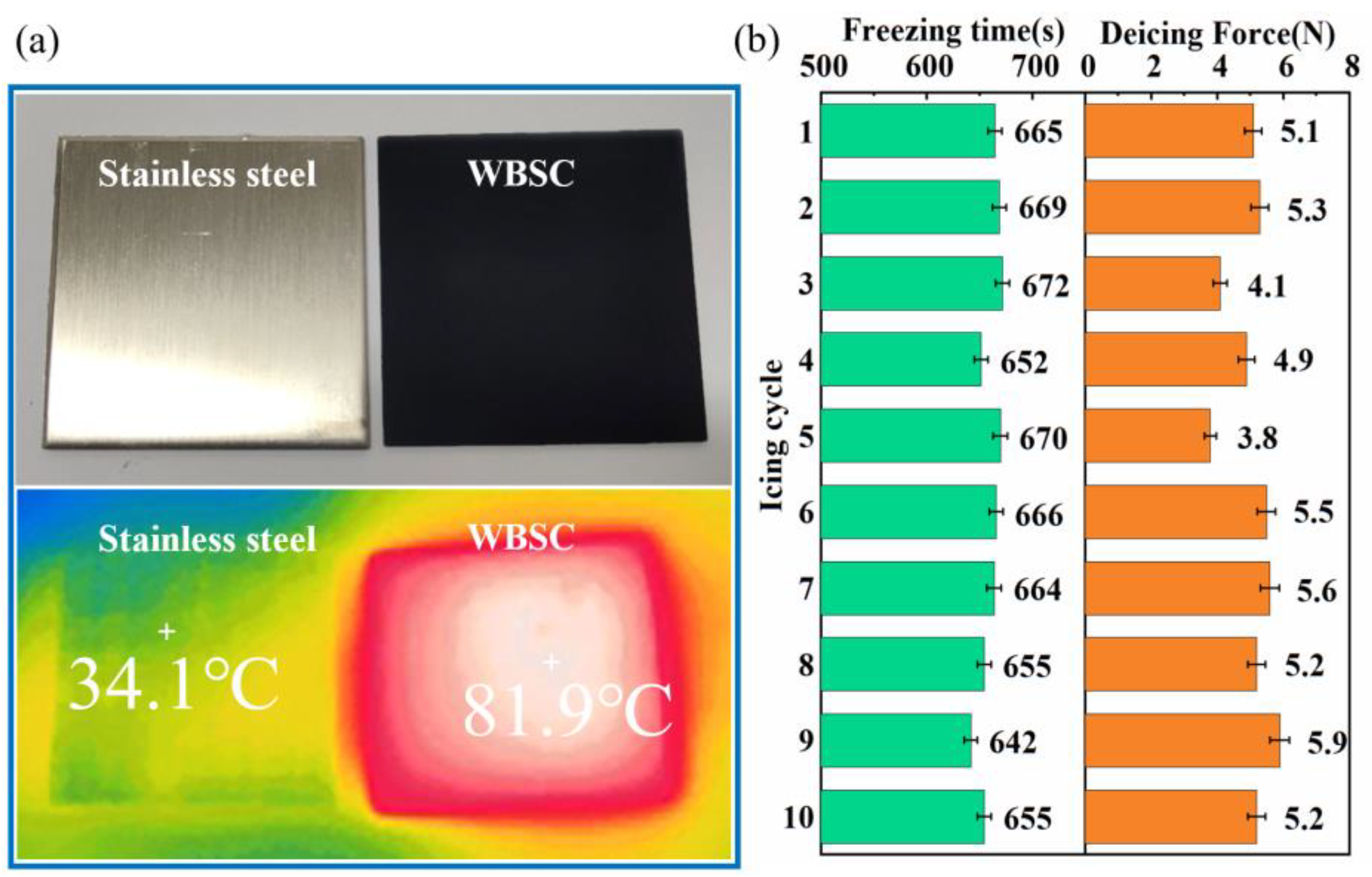
3.3. Anti-/De-Icing Performance and De-Wetting Mechanism
3.4. Oil–Water Separation Based on the WBSC Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Wang, H.; Huang, J.; He, M.; Li, S.; Liu, Y.; Li, F.; You, H. One-step fabrication of robust liquid-repellent mesh induced by femtosecond laser. Chem. Eng. J. 2023, 469, 143853. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Wu, L.; Weng, Z. Construction of ultra-long service life self-cleaning slippery surface on superhydrophobicity functionalized by ATRP treatment. Chem. Eng. J. 2022, 428, 130997. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Wen, G.; Zhu, Y.; Chen, J.; Jing, X.; Sun, S.; Zhang, L.; Liu, X.; Chen, H. Fracture-Promoted Ultraslippery Ice Detachment Interface for Long-Lasting Anti-icing. ACS Nano 2023, 17, 13724–13733. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Y.; Huang, Z.; Wang, F.; Yan, X.; Wu, Z. Electrohydrodynamic behavior of water droplets on a horizontal super hydrophobic surface and its self-cleaning application. Appl. Surf. Sci. 2017, 403, 133–140. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent Progress in Preparation and Anti-Icing Applications of Superhydrophobic Coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, L.; Zhong, L.; Chen, M.; Zhu, L.; Zhang, T.; Han, X.; Hou, Y.; Zheng, Y. Robust Photothermal Icephobic Surface with Mechanical Durability of Multi-Bioinspired Structures. Adv. Mater. 2024, 36, 2305322. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Zhang, B.; Zhou, Y.; Wang, S.; Meng, Y.; Huang, L.; Song, J. Superhydrophobic coating with super-high adhesive strength to substrate. Mater. Lett. 2023, 351, 135084. [Google Scholar] [CrossRef]
- Han, S.; Yao, T.; Yang, X. Preparation and anti-icing properties of a hydrophobic emulsified asphalt coating. Constr. Build. Mater. 2019, 220, 214–227. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, S.; Jia, Y.; Qi, T.; Xiao, L.; Cui, X.; Zhuang, D.; Wei, J. Ultra-black and self-cleaning all carbon nanotube hybrid films for efficient water desalination and purification. Carbon 2020, 169, 134–141. [Google Scholar] [CrossRef]
- Liu, L.; Chen, S.; Hu, Y.; Pan, W.; Dong, T.; Chen, Y.; Lin, L.; Wang, L. Anti-/Deicing Membranes with Damage Detection and Fast Healing. Adv. Funct. Mater. 2024, 2404760. [Google Scholar] [CrossRef]
- Wu, L.; Liu, P.; Hua, X.; Guo, Z.; Liu, W. Photothermal superhydrophobic membrane based on breath figure: Anti-icing and deicing. Chem. Eng. J. 2024, 480, 147553. [Google Scholar] [CrossRef]
- Wu, B.; Cui, X.; Jiang, H.; Wu, N.; Peng, C.; Hu, Z.; Liang, X.; Yan, Y.; Huang, J.; Li, D. A superhydrophobic coating harvesting mechanical robustness, passive anti-icing and active de-icing performances. J. Colloid Interface Sci. 2021, 590, 301–310. [Google Scholar] [CrossRef]
- He, L.; Wang, D.; Ma, T.; Song, J.; Wu, Y.; Li, Y.; Deng, Y.; Zhang, G. Processing and properties of a graphene-reinforced superhydrophobic siloxane. Mater. Des. 2023, 229, 111856. [Google Scholar] [CrossRef]
- Wu, C.; Geng, H.; Tan, S.; Lv, J.; Wang, H.; He, Z.; Wang, J. Highly efficient solar anti-icing/deicing via a hierarchical structured surface. Mater. Horiz. 2020, 7, 2097–2104. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Li, Y.; Chen, T.; Nwokolo, I.K.; Ahmed, S.; Han, E. Modified Graphene Micropillar Array Superhydrophobic Coating with Strong Anti-Icing Properties and Corrosion Resistance. Coatings 2024, 14, 247. [Google Scholar] [CrossRef]
- Li, S.; Zhong, M.; Zou, Y.; Xu, M.; Liu, X.; Xing, X.; Zhang, H.; Jiang, Y.; Qiu, C.; Qin, W.; et al. Fabrication of Micron-Structured Heatable Graphene Hydrophobic Surfaces for Deicing and Anti-Icing by Laser Direct Writing. Coatings 2023, 13, 1559. [Google Scholar] [CrossRef]
- Dai, Z.; Ding, S.; Lei, M.; Li, S.; Xu, Y.; Zhou, Y.; Zhou, B. A superhydrophobic and anti-corrosion strain sensor for robust underwater applications. J. Mater. Chem. A Mater. Energy Sustain. 2021, 9, 15282–15293. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Mu, Z.; Li, Y.; Feng, F. An Experimental Study on Biochar/Polypyrrole Coating for Blade Anti-Icing of Wind Turbines. Coatings 2023, 13, 759. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Zhong, L.; Zhu, L.; Chen, H.; Hou, Y.; Zheng, Y. Robust photothermal superhydrophobic coatings with dual-size micro/nano structure enhance anti-/de-icing and chemical resistance properties. Chem. Eng. J. 2022, 446, 137461. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, S.; Yang, C.; Ji, C.; Long, Y.; Li, G. Femtosecond laser machining of the novel superhydrophobic microstructure for the oil-water separation. Surf. Interfaces 2024, 45, 103873. [Google Scholar] [CrossRef]
- Lin, Z.; Ma, C.; Ma, Z.; Gao, L.; Chen, W.; Chen, G. Laser etching ultra-black coating with novel anti-icing performance. Chem. Eng. J. 2023, 466, 143067. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Hu, Y.; Wang, Z.; Ning, Y.; Bradley, R.; Lou, D.; Zhao, B.; Wu, W. Freestanding Ultrathin Precisely Structured Hierarchical Porous Carbon Blackbody Film for Efficient Solar Interfacial Evaporation. Sol. RRL 2023, 7, 2200803. [Google Scholar] [CrossRef]
- Yu, X.; Ye, J.; Li, C.; Yu, Y.; Yang, H.; Wen, L.; Huang, J.; Xu, W.; Wu, Y.; Zhou, Q.; et al. Superhydrophobic, Highly Conductive, and Trilayered Fabric with Connected Carbon Nanotubes for Energy-Efficient Electrical Heating. ACS Appl. Mater. Interfaces 2024, 16, 26932–26942. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Park, S. Effect of fluorination of carbon nanotubes on superhydrophobic properties of fluoro-based films. J. Colloid Interface Sci. 2010, 342, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, Y.; Hsieh, C.; Chen, Y.; Su, T.; Hsu, J.; Juang, R. Synthesis of magnetic iron oxide nanoparticles onto fluorinated carbon fabrics for contaminant removal and oil-water separation. Sep. Purif. Technol. 2017, 174, 312–319. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Zhang, Z.; Huang, S.; Hao, Z.; Zhao, X. Surfaces with controllable super-wettability and applications for smart oil-water separation. Chem. Eng. J. 2019, 378, 122178. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X. Contact angle hysteresis analysis on superhydrophobic surface based on the design of channel and pillar models. Mater. Des. 2017, 131, 323–333. [Google Scholar] [CrossRef]
- Nadaraia, K.V.; Suchkov, S.N.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Kosianov, D.Y.; Belov, E.A.; Sinebryukhov, S.L.; Gnedenkov, S.V. New superhydrophobic composite coatings on Mg-Mn-Ce magnesium alloy. J. Magnes. Alloys 2023, 11, 1721–1739. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Guo, Z.; Liu, W. Durable mixed edible wax coating with stretching superhydrophobicity. J. Mater. Chem. A Mater. Energy Sustain. 2021, 9, 1495–1499. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Y.; Huang, Z.; Sabin, S.; Xiao, T.; Jiang, L.; Chen, X. Facile Fabrication of a Mechanical, Chemical, Thermal, and Long-Term Outdoor Durable Fluorine-Free Superhydrophobic Coating. Adv. Mater. Interfaces 2021, 8, 2002209. [Google Scholar] [CrossRef]
- Jiang, S.; Diao, Y.; Yang, H. Recent advances of bio-inspired anti-icing surfaces. Adv. Colloid Interface Sci. 2022, 308, 102756. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, Y.; Bhushan, B.; Zhao, X. Mechanochemical robust, magnetic-driven, superhydrophobic 3D porous materials for contaminated oil recovery. J. Colloid Interface Sci. 2019, 538, 25–33. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Lu, S.; Hu, Y.; Liu, L.; You, H. Water-Borne Photo-Thermal Superhydrophobic Coating for Anti-Icing, Self-Cleaning and Oil–Water Separation. Coatings 2024, 14, 758. https://doi.org/10.3390/coatings14060758
Huang J, Lu S, Hu Y, Liu L, You H. Water-Borne Photo-Thermal Superhydrophobic Coating for Anti-Icing, Self-Cleaning and Oil–Water Separation. Coatings. 2024; 14(6):758. https://doi.org/10.3390/coatings14060758
Chicago/Turabian StyleHuang, Jinsong, Shengqi Lu, Yan Hu, Liming Liu, and Hui You. 2024. "Water-Borne Photo-Thermal Superhydrophobic Coating for Anti-Icing, Self-Cleaning and Oil–Water Separation" Coatings 14, no. 6: 758. https://doi.org/10.3390/coatings14060758




