Improving Trace Detection of Methylene Blue by Designing Nanowire Array on Boron-Doped Diamond as Electrochemical Electrode
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Electrodes
2.3. Characterization of Electrodes
2.4. Electrochemical Measurement
3. Results and Discussion
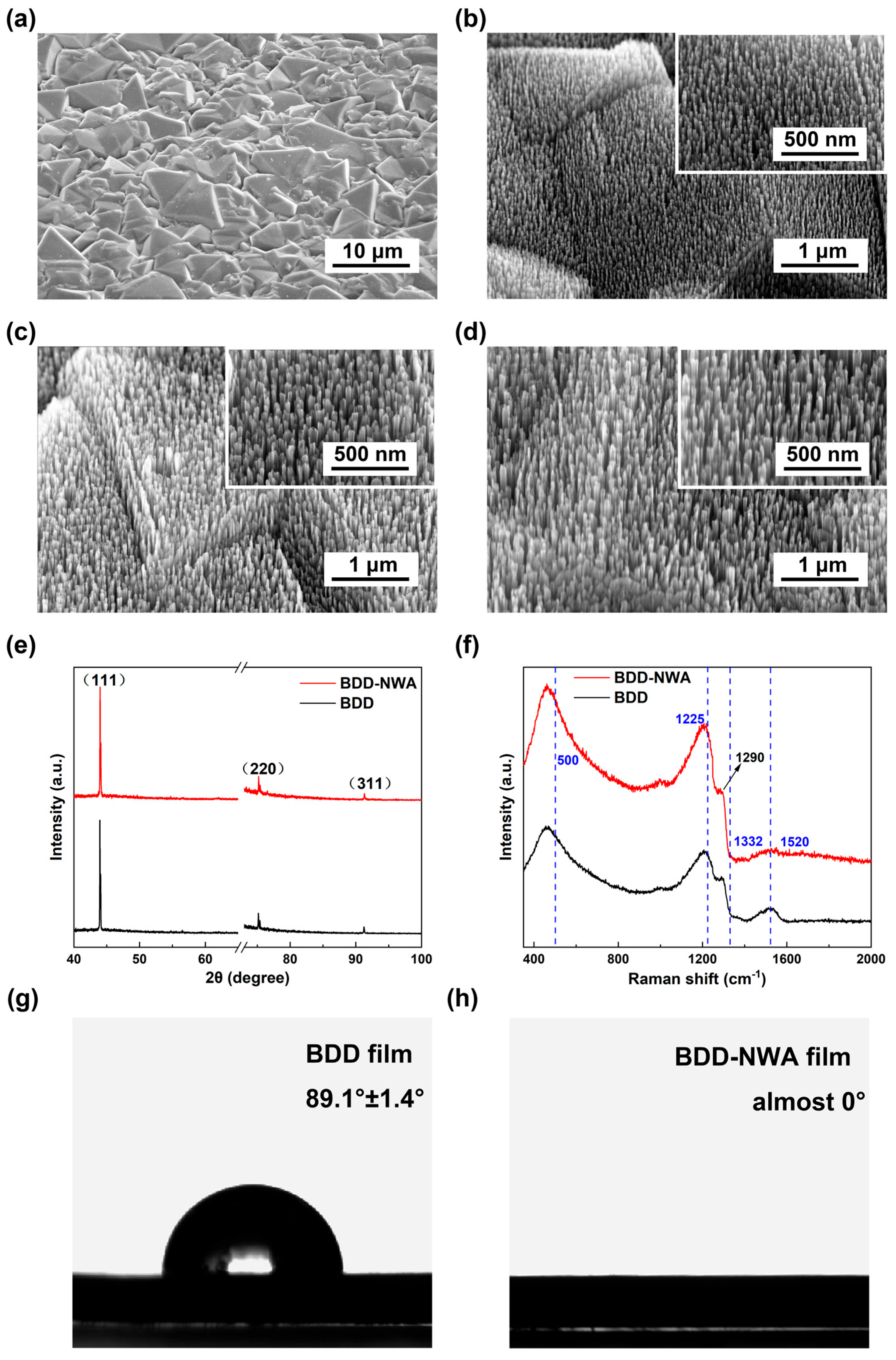
3.1. Characterization of BDD-NWA Electrode
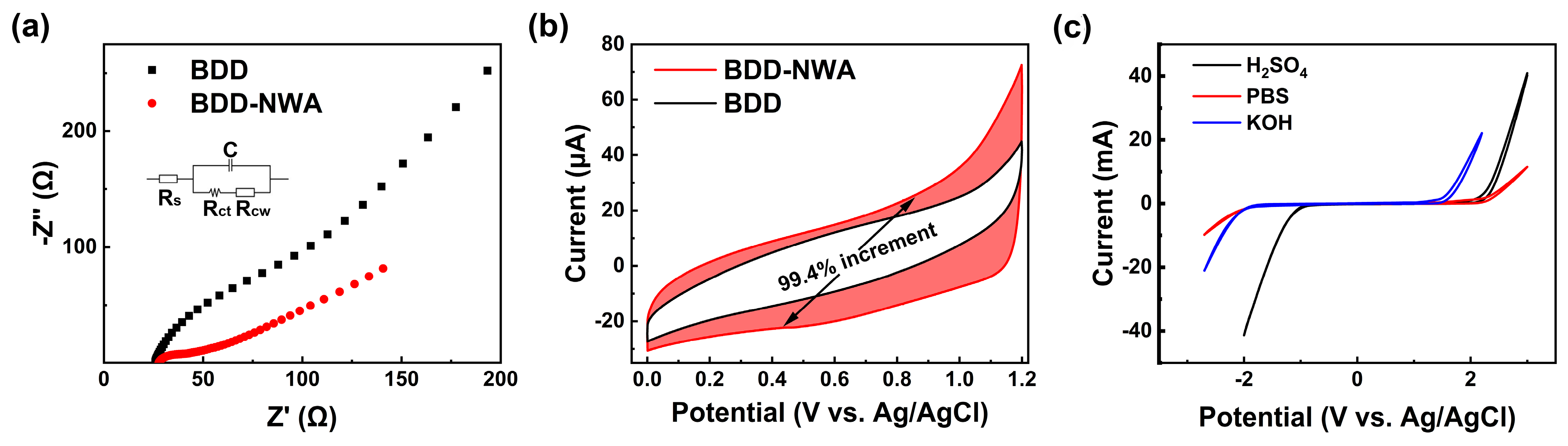
3.2. Electrochemical Characterization of BDD-NWA Electrode
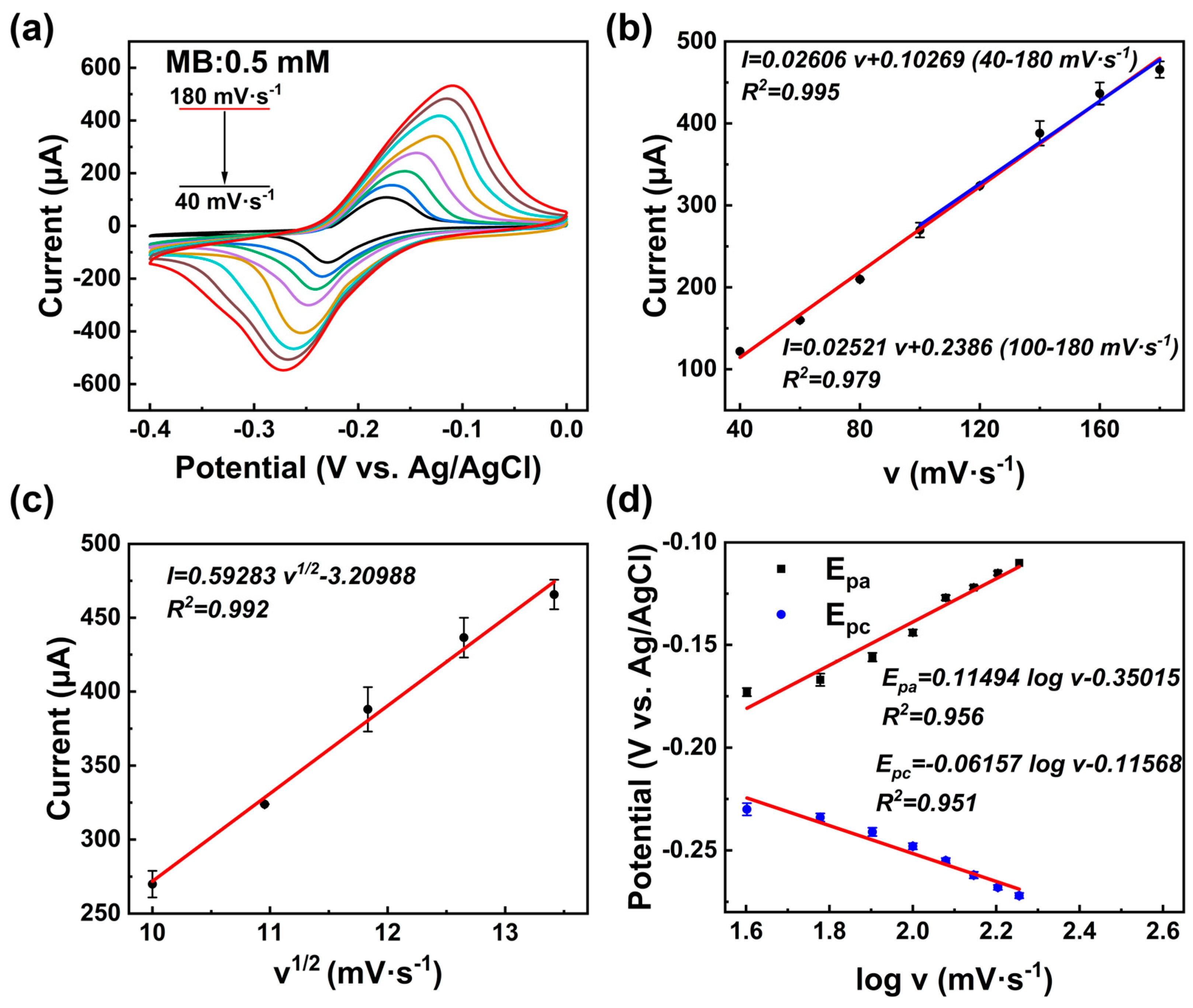
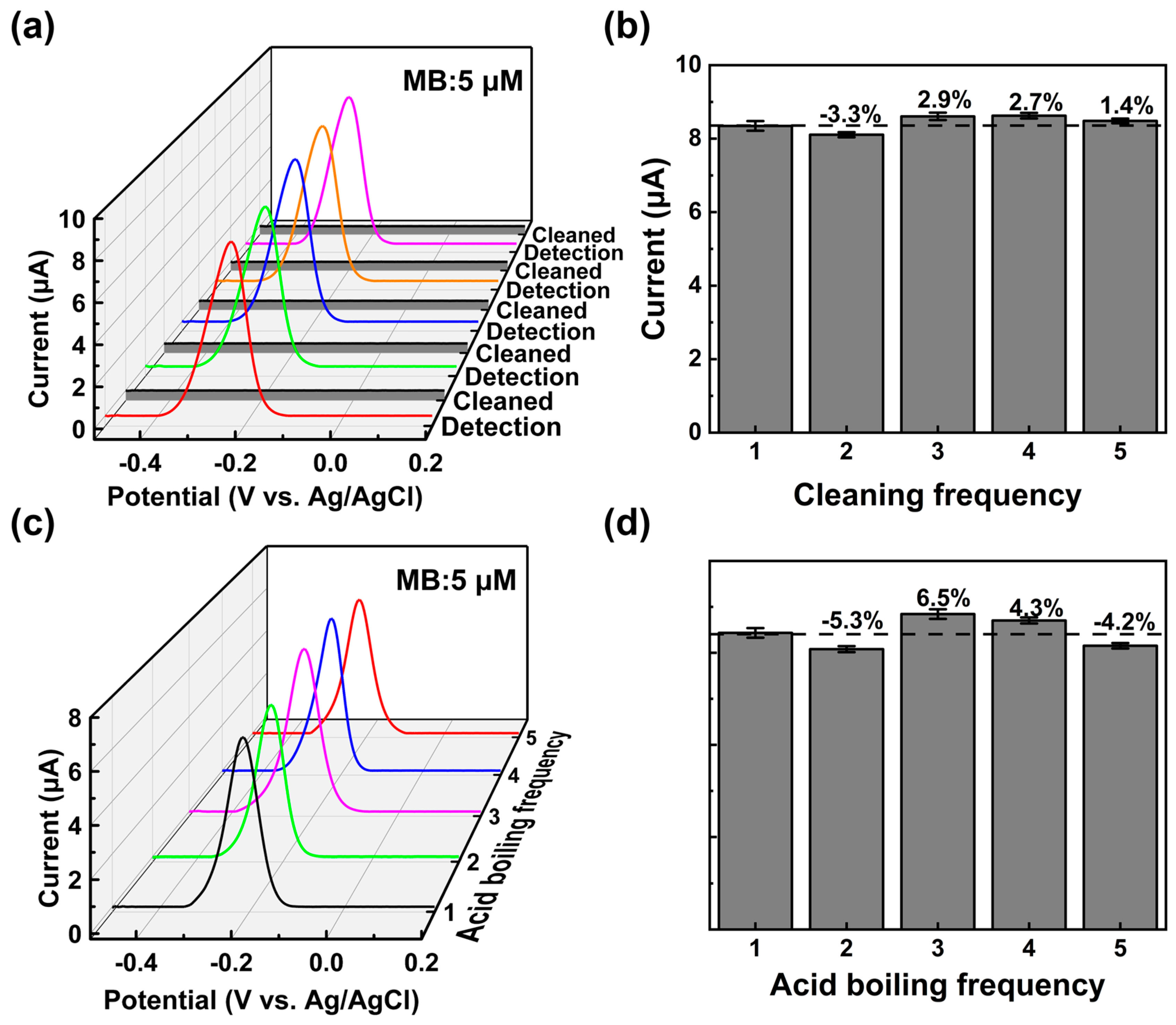
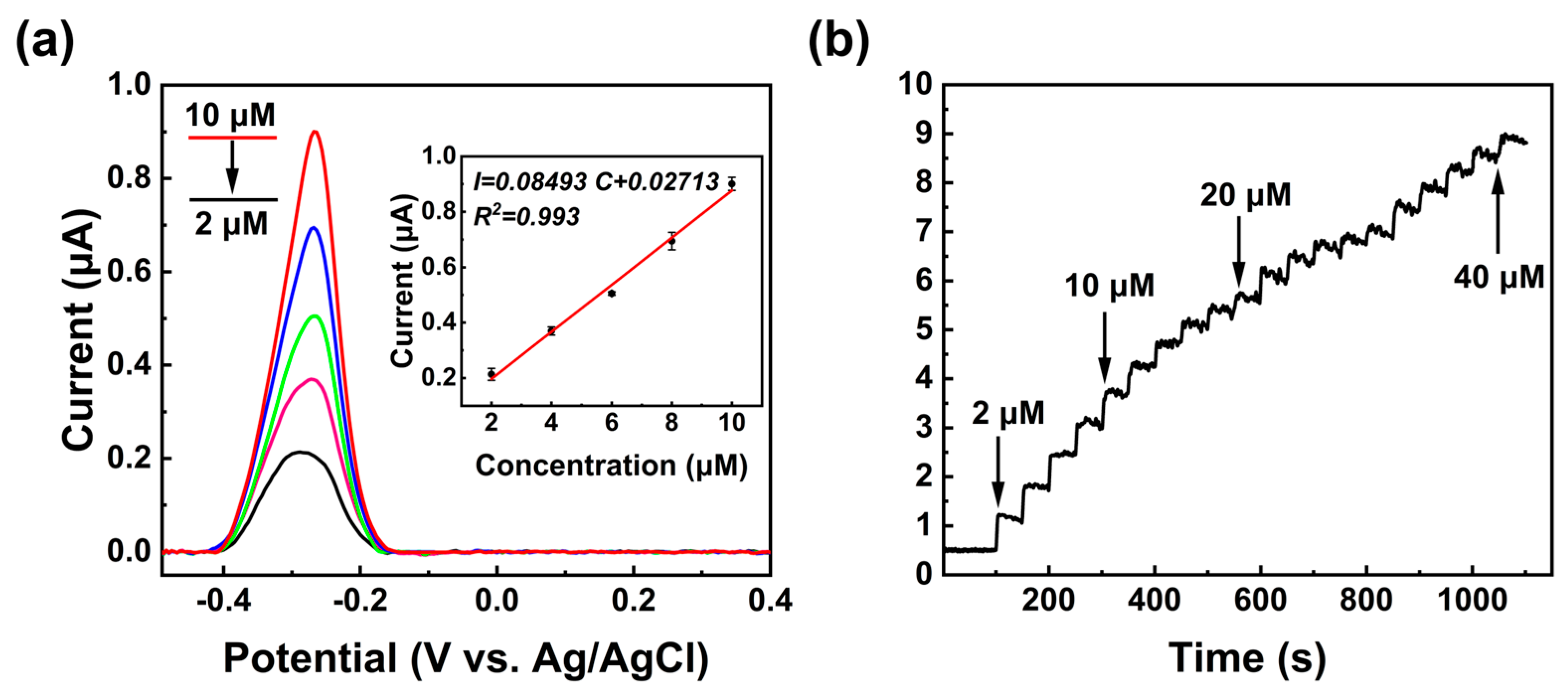
3.3. Detection of MB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, S.S.; Sirajuddin; Solangi, A.R.; Agheem, M.H.; Junejo, Y.; Kalwar, N.H.; Tagar, Z.A. Ultra-fast catalytic reduction of dyes by ionic liquid recoverable and reusable mefenamic acid derived gold nanoparticles. J. Hazard. Mater. 2011, 190, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, S.S.; Adesibikan, A.A. Bio-fabricated green silver nano-architecture for degradation of methylene blue water contaminant: A mini-review. Water Environ. Res. 2021, 93, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Chen, L.; Yang, S.S.; Chen, Z.Q.; Qian, G.N.; Zhang, S.X.; Jing, J.J. A novel technique for localization of small pulmonary nodules. Chest 2007, 131, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J. A Review of Acute Cyanide Poisoning with a Treatment Update. Crit. Care Nurse 2011, 31, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Mathelin, C.; Croce, S.; Brasse, D.; Gairard, B.; Gharbi, M.; Andriamisandratsoa, N.; Bekaert, V.; Francis, Z.; Guyonnet, J.L.; Huss, D.; et al. Methylene Blue Dye, an Accurate Dye for Sentinel Lymph Node Identification in Early Breast Cancer. Anticancer Res. 2009, 29, 4119–4125. [Google Scholar] [PubMed]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Salhab, M.; Al Sarakbi, W.; Mokbel, K. Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. Int. Semin. Surg. Oncol. ISSO 2005, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.W.; Huh, E.H.; Jung, D.J.; Park, J.H.; Rha, K.H.; Kim, S.K.; Park, K.H. Urinary tract injuries during pelvic surgery: Incidence rates and predisposing factors. Int. Urogynecol. J. 2006, 17, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Lipskikh, O.I.; Korotkova, E.I.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for voltammetric determination of food azo dyes—A critical review. Electrochim. Acta. 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Rengelshausen, J.; Burhenne, J.; Fröhlich, M.; Tayrouz, Y.; Singh, S.K.; Riedel, K.D.; Müller, O.; Hoppe-Tichy, T.; Haefeli, W.E.; Mikus, G.; et al. Pharmacokinetic interaction of chloroquine and methylene blue combination against malaria. Eur. J. Clin. Pharmacol. 2004, 60, 709–715. [Google Scholar] [CrossRef]
- Xu, J.Z.; Dai, L.; Wu, B.; Ding, T.; Zhu, J.J.; Lin, H.; Chen, H.L.; Shen, C.Y.; Jiang, Y. Determination of methylene blue residues in aquatic products by liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2009, 32, 4193–4199. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, S.H.D.; Rodrigues, P.R.P.; Agostinho, S.M.L.; Rubim, J.C. Electrochemical and spectroelectrochemical (SERS) studies of the reduction of methylene blue on a silver electrode. J. Electroanal. Chem. 2002, 527, 103–111. [Google Scholar] [CrossRef]
- Razmara, R.S.; Daneshfar, A.; Sahrai, R. Determination of methylene blue and sunset yellow in wastewater and food samples using salting-out assisted liquid-liquid extraction. J. Ind. Eng. Chem. 2011, 17, 533–536. [Google Scholar] [CrossRef]
- Yuan, X.X.; Gao, N.; Gao, X.; Qiu, D.C.; Xu, R.; Sun, Z.L.; Jiang, Z.G.; Liu, J.S.; Li, H.D. Nanopyramid boron-doped diamond electrode realizing nanomolar detection limit of 4-nonylphenol. Sens. Actuator B-Chem. 2019, 281, 830–836. [Google Scholar] [CrossRef]
- Ma, Y.B.; Xu, X.Y.; Yang, T.Y.; Shen, Y.Y.; Jiang, F.; Zhang, Y.W.; Lv, X.T.; Liu, Y.M.; Feng, B.; Che, G.B.; et al. CO2 reduction synergistic tetracycline degradation toward Co-BiOBr ultrathin nanosheets with rich defects and (102) active faces. J. Alloy Compd. 2024, 970, 10. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Wei, Q.P.; Den, Z.J.; Long, H.Y.; Zheng, K.Z.; Li, H.C.; Guo, Y.H.; Yu, Z.M.; Ma, L.; et al. The effect of heat treatment time on the carbon-coated nickel nanoparticles modified boron-doped diamond composite electrode for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 841, 148–157. [Google Scholar] [CrossRef]
- Yence, M.; Cetinkaya, A.; Ozcelikay, G.; Kaya, S.I.; Ozkan, S.A. Boron-Doped Diamond Electrodes: Recent Developments and Advances in View of Electrochemical Drug Sensors. Crit. Rev. Anal. Chem. 2022, 52, 1122–1138. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.B.; Wu, L.Z.; Zhi, J.F. Fabrication of Boron-Doped Diamond Nanorod Forest Electrodes and Their Application in Nonenzymatic Amperometric Glucose Biosensing. ACS Nano. 2009, 3, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Ficek, M.; Szkodo, M.; Stanislawska, A.; Karczewski, J.; Ryl, J.; Dolega, A.; Siuzdak, K.; Bogdanowicz, R. Tailoring Diffusional Fields in Zwitterion/Dopamine Copolymer Electropolymerized at Carbon Nanowalls for Sensitive Recognition of Neurotransmitters. ACS Nano 2022, 16, 13183–13198. [Google Scholar] [CrossRef]
- Olejnik, A.; Ficek, M.; Siuzdak, K.; Bogdanowicz, R. Multi-pathway mechanism of polydopamine film formation at vertically aligned diamondised boron-doped carbon nanowalls. Electrochim. Acta 2022, 409, 12. [Google Scholar] [CrossRef]
- Boonkaew, S.; Dettlaff, A.; Sobaszek, M.; Bogdanowicz, R.; Jönsson-Niedziólka, M. Electrochemical determination of neurotransmitter serotonin using boron/nitrogen co-doped diamond-graphene nanowall-structured particles. J. Electroanal. Chem. 2022, 926, 12. [Google Scholar] [CrossRef]
- Niedzialkowski, P.; Cebula, Z.; Malinowska, N.; Bialobrzeska, W.; Sobaszek, M.; Ficek, M.; Bogdanowicz, R.; Anand, J.S.; Ossowski, T. Comparison of the paracetamol electrochemical determination using boron-doped diamond electrode and boron-doped carbon nanowalls. Biosens. Bioelectron. 2019, 126, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Sain, S.; Ficek, M.; Olejnik, A.; Sawczak, M.; Bogdanowicz, R.; Roy, S.S. Direct determination of paraquat herbicide by square-wave voltammetry by two-step transfer mechanism at heterogeneous boron-doped carbon nanowall electrodes. Diam. Relat. Mat. 2023, 140, 12. [Google Scholar] [CrossRef]
- Hou, X.L.; Shen, G.J.; Meng, L.; Zhu, L.; Guo, M. Multi-walled carbon nanotubes modified glass carbon electrode and its electrocatalytic activity towards oxidation of paracetamol. Russ. J. Electrochem. 2011, 47, 1262–1267. [Google Scholar] [CrossRef]
- Ma, Y.B.; Liu, J.S.; Li, H.D. Diamond-based electrochemical aptasensor realizing a femtomolar detection limit of bisphenol A. Biosens. Bioelectron. 2017, 92, 21–25. [Google Scholar] [CrossRef] [PubMed]
- He, Y.P.; Dong, Y.J.; Huang, W.M.; Tang, X.Q.; Liu, H.H.; Lin, H.B.; Li, H.D. Investigation of boron-doped diamond on porous Ti for electrochemical oxidation of acetaminophen pharmaceutical drug. J. Electroanal. Chem. 2015, 759, 167–173. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N. New directions in structuring and electrochemical applications of boron-doped diamond thin films. Diam. Relat. Mat. 2001, 10, 1799–1803. [Google Scholar] [CrossRef]
- Sousa, C.P.; Ribeiro, F.W.P.; Oliveira, T.; Salazar-Banda, G.R.; de Lima-Neto, P.; Morais, S.; Correia, A.N. Electroanalysis of Pharmaceuticals on Boron-Doped Diamond Electrodes: A Review. ChemElectroChem 2019, 6, 2350–2378. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Gao, N.; Li, J.S.; Li, H.D. Boron-doped diamond electrochemical aptasensors for trace aflatoxin B 1 detection. Anal. Chim. Acta. 2020, 1122, 70–75. [Google Scholar] [CrossRef]
- Ma, Z.C.; Wang, Q.L.; Gao, N.; Li, H.D. Electrochemical detection of clenbuterol with gold-nanoparticles-modified porous boron-doped diamond electrode. Microchem. J. 2020, 157, 7. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, X.X.; Cui, Z.; Xu, C.Y.; Sun, Z.L.; Li, J.H.; Liu, J.S.; Tian, Y.; Li, H.D. A Nanometer-Sized Graphite/Boron-Doped Diamond Electrochemical Sensor for Sensitive Detection of Acetaminophen. ACS Omega 2021, 6, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Liang, Y.; Yang, Y.; Zhang, Q.; Cui, Z.; Liu, J.; Cheng, S.; Gao, N.; Li, H.; Yuan, X. Electrochemical detection of phenacetin with graphite/boron-doped diamond electrode grown at high methane concentration. Diam. Relat. Mat. 2024, 144, 110965. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Yang, Y.M.; Gao, H.X.; Xu, L.P.; Wang, S.T. Bioinspired superwettable electrodes towards electrochemical biosensing. Chem. Sci. 2022, 13, 5069–5084. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Y.; Dong, Q.R.; Sun, D.W.; Pu, H.B. Synthesis of recyclable SERS platform based on MoS2@TiO2@Au heterojunction for photodegradation and identification of fungicides. Spectroc. Acta Pt. A Molec. Biomolec. Spectr. 2023, 285, 11. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-C.; Gao, N.; Cheng, S.-H.; Liu, J.-S.; Yang, M.-C.; Wang, P.; Feng, Z.-Y.; Wang, Q.-L.; Li, H.-D. Wettability and Surface Energy of Hydrogen- and Oxygen-Terminated Diamond Films. Chin. Phys. Lett. 2020, 37, 046801. [Google Scholar] [CrossRef]
- Leem, J.W.; Yu, J.S. Broadband and wide-angle antireflection subwavelength structures of Si by inductively coupled plasma etching using dewetted nanopatterns of Au thin films as masks. Thin Solid Films 2011, 519, 3792–3797. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Ekimov, E.A.; Lyapin, A.G.; Popova, S.V.; Rakhmanina, A.V.; Stishov, S.M.; Lebedev, V.M.; Katayama, Y.; Kato, K. Lattice parameters and thermal expansion of superconducting boron-doped diamonds. Phys. Rev. B 2006, 74, 4. [Google Scholar] [CrossRef]
- Ashcheulov, P.; Sebera, J.; Kovalenko, A.; Petrák, V.; Fendrych, F.; Nesládek, M.; Taylor, A.; Zivcová, Z.V.; Frank, O.; Kavan, L.; et al. Conductivity of boron-doped polycrystalline diamond films: Influence of specific boron defects. Eur. Phys. J. B 2013, 86, 9. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Muret, P. Non-destructive determination of the boron concentration of heavily doped metallic diamond thin films from Raman spectroscopy. Diam. Relat. Mat. 2004, 13, 282–286. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, Y.B.; Chai, X.; Guo, F.X.; Li, Y.L.; Shi, J.; Gai, Z.G.; Jiang, X. Influence of boron doped level on the electrochemical behavior and seawater salinity detection of boron doped diamond film electrodes. J. Electroanal. Chem. 2023, 931, 9. [Google Scholar] [CrossRef]
- May, P.W.; Ludlow, W.J.; Hannaway, M.; Heard, P.J.; Smith, J.A.; Rosser, K.N. Raman and conductivity studies of boron doped microcrystalline diamond, facetted nanocrystalline diamond and cauliflower diamond films. Chem. Phys. Lett. 2007, 446, 103–108. [Google Scholar] [CrossRef]
- Bernard, M.; Baron, C.; Deneuville, A. About the origin of the low wave number structures of the Raman spectra of heavily boron doped diamond films. Diam. Relat. Mat. 2004, 13, 896–899. [Google Scholar] [CrossRef]
- Yu, Z.M.; Wang, J.; Wei, Q.P.; Meng, L.C.; Hao, S.M.; Long, F. Preparation, characterization and electrochemical properties of boron-doped diamond films on Nb substrates. Trans. Nonferr. Met. Soc. China 2013, 23, 1334–1341. [Google Scholar] [CrossRef]
- Shirai, K.; Dekura, H.; Yanase, A. Utilization of high pressure for synthesizing superconducting semiconductors: Analysis of Ekimov’s method. Phys. Status Solidi B Basic Solid State Phys. 2009, 246, 673–678. [Google Scholar] [CrossRef]
- Gao, G.Y.; Fang, D.Y.; Yu, Y.; Wu, L.Z.; Wang, Y.; Zhi, J.F. A double-mediator based whole cell electrochemical biosensor for acute biotoxicity assessment of wastewater. Talanta. 2017, 167, 208–216. [Google Scholar] [CrossRef]
- Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts 2022, 12, 306. [Google Scholar] [CrossRef]
- Morsi, R.M.M.; Mandour, H.S.; Fathi, A.M.; Awad, H.M. Electrical properties, cyclic voltammetry, and anticancer activities of N-(4-(2-hydrazinyl-2-oxoethoxy)phenyl) acetamide complexes. J. Phys. Org. Chem. 2019, 32, 11. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Belter, M.; Sajnóg, A.; Baralkiewicz, D. Over a century of detection and quantification capabilities in analytical chemistry—Historical overview and trends. Talanta 2014, 129, 606–616. [Google Scholar] [CrossRef]
- Prabhu, B.R.; Varier, M.M.; John, N.S. Fabrication of sandwich structures of Ag/analyte/MoO3 sea urchins for SERS detection of methylene blue dye molecules. Nanotechnology 2023, 34, 14. [Google Scholar]
- Patil, M.K.; Gaikwad, S.H.; Mukherjee, S.P. Phase- and Morphology-Controlled Synthesis of Tunable Plasmonic MoO3–x Nanomaterials for Ultrasensitive Surface-Enhanced Raman Spectroscopy Detection. J. Phys. Chem. C. 2020, 124, 21082–21093. [Google Scholar] [CrossRef]
- Li, C.; Wu, C.Q.; Zhang, K.; Chen, M.Q.; Wang, Y.S.; Shi, J.J.; Tang, Z.Y. The charge transfer effect on SERS in a gold-decorated surface defect anatase nanosheet/methylene blue (MB) system. New J. Chem. 2021, 45, 19775–19786. [Google Scholar] [CrossRef]
- Shaikh, I.; Sartale, S. Spin coated Ag NPs SERS substrate: Trace detection study of methylene blue and melamine. Appl. Phys. A—Mater. Sci. Process. 2023, 129, 12. [Google Scholar] [CrossRef]
- Atta, S.; Vo-Dinh, T. Bimetallic Gold Nanostars Having High Aspect Ratio Spikes for Sensitive Surface-Enhanced Raman Scattering Sensing. ACS Appl. Nano Mater. 2022, 9, 12562–12570. [Google Scholar] [CrossRef]
- Bélaz-David, N.; Decosterd, L.A.; Appenzeller, M.; Ruetsch, Y.A.; Chioléro, R.; Buclin, T.; Biollaz, J. Spectrophotometric determination of methylene blue in biological fluids after ion-pair extraction and evidence of its adsorption on plastic polymers. Eur. J. Pharm. Sci. 1997, 5, 335–345. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Zhao, J.; Zeng, B. Adsorptive and stripping behavior of methylene blue at gold electrodes in the presence of cationic gemini surfactants. Electrochim. Acta 2005, 51, 297–303. [Google Scholar] [CrossRef]
- Soto, P.C.; Salamanca-Neto, C.A.R.; Moraes, J.T.; Sartori, E.R.; Bessegato, G.G.; Lopes, F.; Almeida, L.C. A novel sensing platform based on self-doped TiO2 nanotubes for methylene blue dye electrochemical monitoring during its electro-Fenton degradation. J. Solid State Electrochem. 2020, 24, 1951–1959. [Google Scholar] [CrossRef]
- Tonlé, I.K.; Ngameni, E.; Tcheumi, H.L.; Tchiéda, V.; Carteret, C.; Walcarius, A. Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta 2008, 74, 489–497. [Google Scholar] [CrossRef]
- Hassan, S.S.; Nafady, A.; Sirajuddin; Solangi, A.R.; Kalhoro, M.S.; Abro, M.I.; Sherazi, S.T.H. Ultra-trace level electrochemical sensor for methylene blue dye based on nafion stabilized ibuprofen derived gold nanoparticles. Sens. Actuator B-Chem. 2015, 208, 320–326. [Google Scholar] [CrossRef]

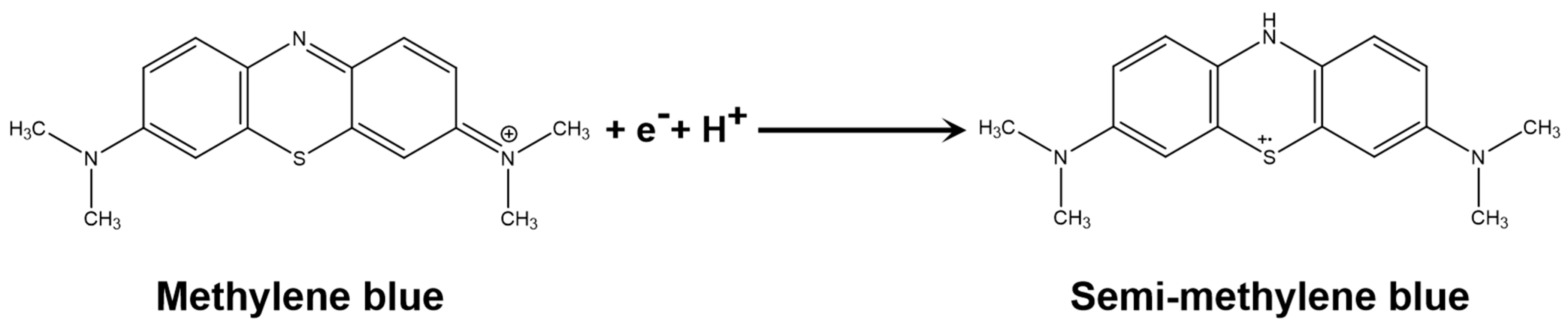

| Electrode Modifiers | Technique | Linear Range (μM) | Detection Limit (nM) | References |
|---|---|---|---|---|
| - | SERS | - | 100 | [51] |
| - | SERS | - | 10 | [52] |
| - | SERS | - | 10 | [53] |
| - | SERS | - | 0.1 | [54] |
| - | SERS | 0.00005–0.03 | 0.042 | [55] |
| - | UV-vis | 0.38–28.5 | - | [56] |
| - | UV-vis | 0.63–21.9 | 190 | [13] |
| Au | CV | 0.2–10 | - | [57] |
| Self-doped TiO2 nanotubes | CV | 1.0–7.94 | 475 | [58] |
| Thiol-functionalized clay | CV | 1.0–14 | 400 | [59] |
| Ibu-AuNPs | DPV | 0.01–1.1 | 3.9 | [60] |
| NH2-fMWCNTs | SWV | 0.01–0.5 | 0.21 | [47] |
| BDD-NWA | SWV | 0.04–10 | 0.72 | This study |
| Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 0 | ND | - | - |
| 2 | 2.15 | 107.5 | 2.1 |
| 4 | 4.03 | 100.8 | 1.5 |
| 6 | 5.63 | 93.8 | 0.6 |
| 8 | 7.86 | 98.3 | 3.1 |
| 10 | 10.28 | 102.8 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Lin, K.; Cheng, S.; Gao, N.; Liu, J.; Li, H. Improving Trace Detection of Methylene Blue by Designing Nanowire Array on Boron-Doped Diamond as Electrochemical Electrode. Coatings 2024, 14, 762. https://doi.org/10.3390/coatings14060762
He S, Lin K, Cheng S, Gao N, Liu J, Li H. Improving Trace Detection of Methylene Blue by Designing Nanowire Array on Boron-Doped Diamond as Electrochemical Electrode. Coatings. 2024; 14(6):762. https://doi.org/10.3390/coatings14060762
Chicago/Turabian StyleHe, Sihan, Kun Lin, Shaoheng Cheng, Nan Gao, Junsong Liu, and Hongdong Li. 2024. "Improving Trace Detection of Methylene Blue by Designing Nanowire Array on Boron-Doped Diamond as Electrochemical Electrode" Coatings 14, no. 6: 762. https://doi.org/10.3390/coatings14060762
APA StyleHe, S., Lin, K., Cheng, S., Gao, N., Liu, J., & Li, H. (2024). Improving Trace Detection of Methylene Blue by Designing Nanowire Array on Boron-Doped Diamond as Electrochemical Electrode. Coatings, 14(6), 762. https://doi.org/10.3390/coatings14060762









