PbS Colloidal Quantum Dots: Ligand Exchange in Solution
Abstract
1. Introduction
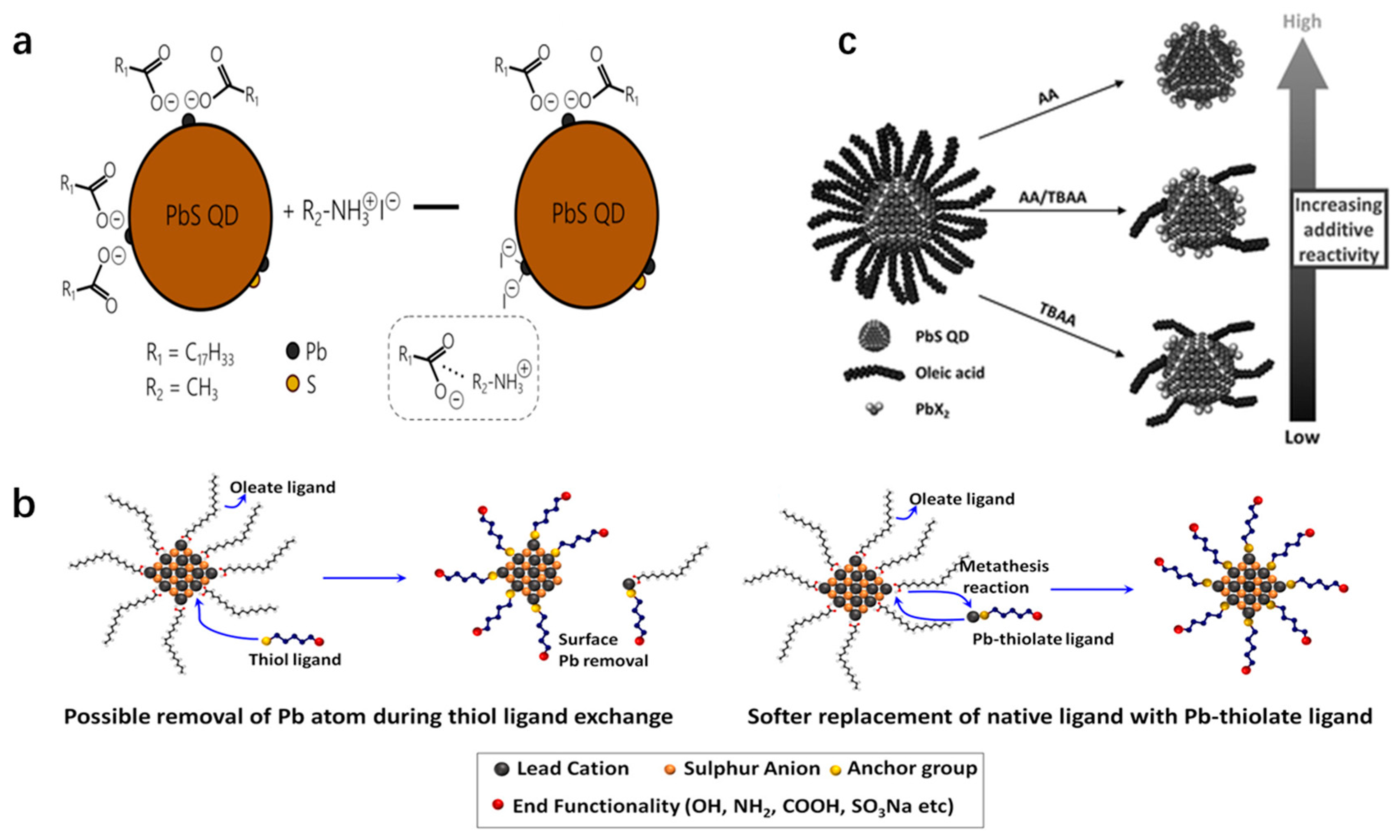
2. Solution Phase Ligand Exchange
3. Characterization of CQDs and Devices
4. Principles of Ligand Exchange in Solution
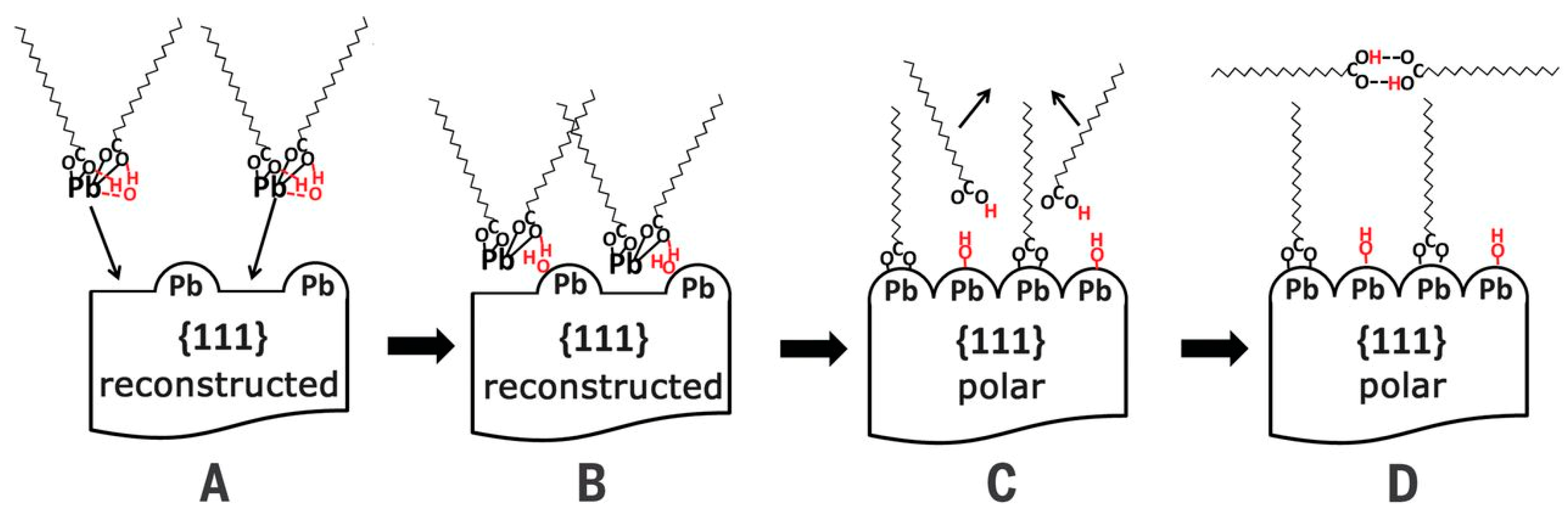
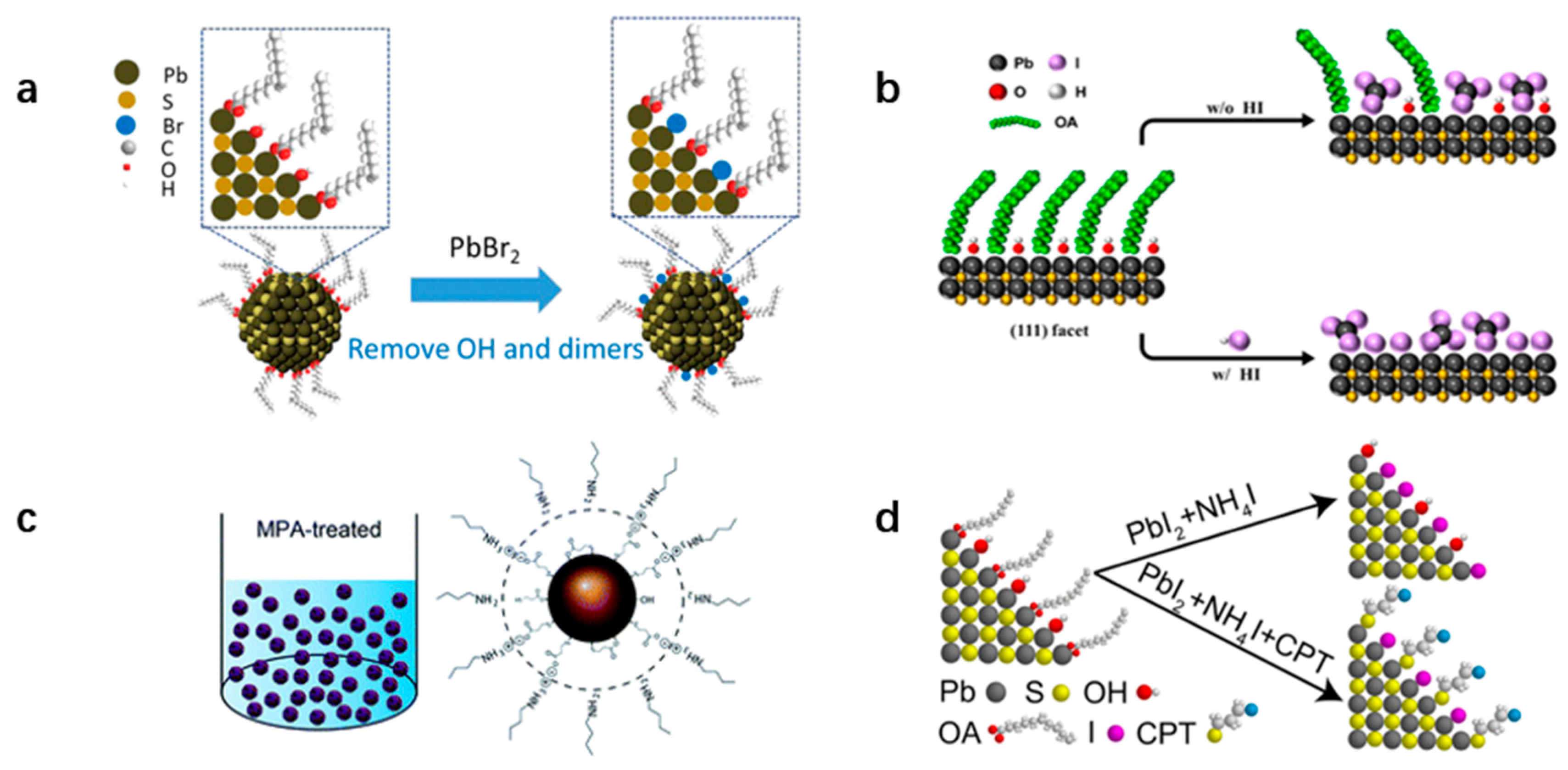
4.1. Ligand Exchange of (111) Facet

4.2. Passivation of (100) Facet
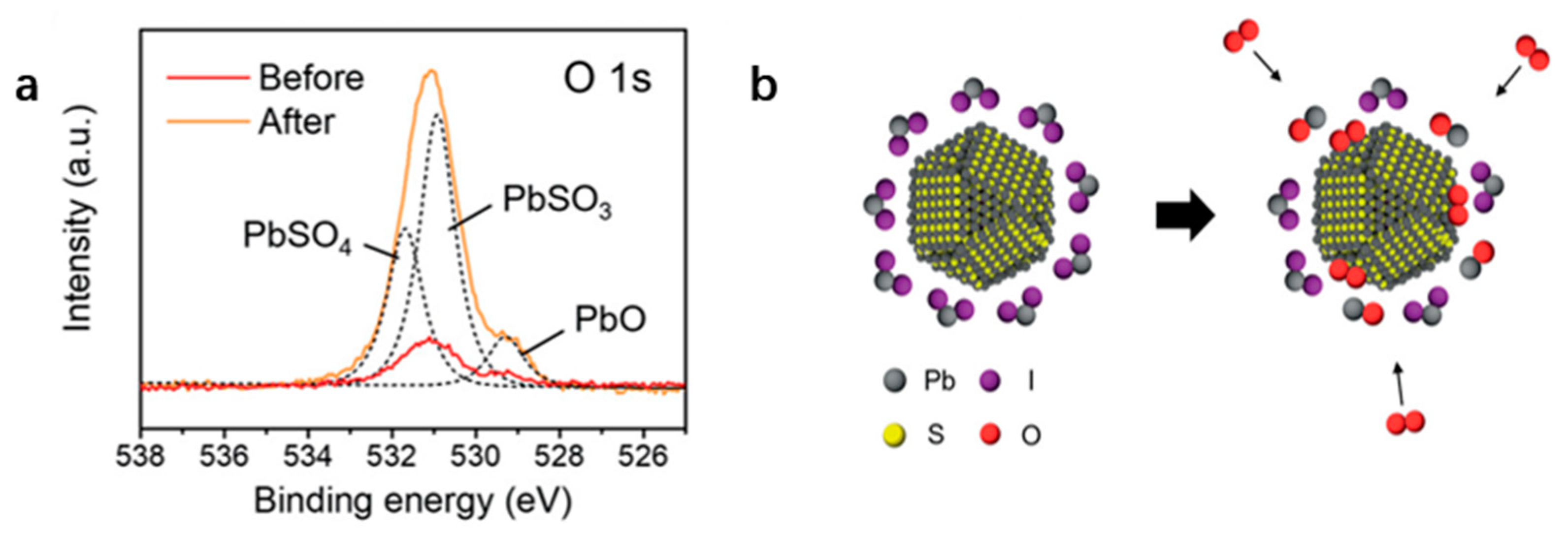
4.3. Removal of Hydroxyl (-OH)
4.4. Energy State Distribution Adjustment
5. Conclusions
Funding
Conflicts of Interest
References
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor Quantum Dots and Quantum Dot Arrays and Applications of Multiple Exciton Generation to Third-Generation Photovoltaic Solar Cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal Quantum Dot Solar Cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef] [PubMed]
- Keuleyan, S.; Lhuillier, E.; Brajuskovic, V.; Guyot-Sionnest, P. Mid-Infrared HgTe Colloidal Quantum Dot Photodetectors. Nat. Photonics 2011, 5, 489–493. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor CQDs: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef] [PubMed]
- Edward, H. Sargent. Infrared Quantum Dots. Adv. Mater. 2005, 17, 515–522. [Google Scholar]
- Choi, H.; Ko, J.H.; Kim, Y.H.; Jeong, S. Steric-Hindrance-Driven Shape Transition in PbS Quantum Dots: Understanding Size-Dependent Stability. J. Am. Chem. Soc. 2013, 135, 5278–5281. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371. [Google Scholar] [CrossRef]
- Beygi, H.; Sajjadi, S.A.; Babakhani, A.; Young, J.F.; van Veggel, F.C. Surface chemistry of as-synthesized and air-oxidized PbS Quantum Dots. Appl. Surf. Sci. 2018, 457, 1–10. [Google Scholar] [CrossRef]
- Shrestha, A.; Batmunkh, M.; Tricoli, A.; Qiao, S.Z.; Dai, S. Near-infrared Active Lead Chalcogenide Quantum Dots: Preparation, Post-Synthesis Ligand Exchange, and Applications in Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 5202–5224. [Google Scholar] [CrossRef]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Cha, D.; et al. Colloidal-quantum-dot photovoltaics using atomic-ligand passivation. Nat. Mater. 2011, 10, 765–771. [Google Scholar] [CrossRef]
- Carey, G.H.; Chou, K.W.; Yan, B.; Kirmani, A.R.; Amassian, A.; Sargent, E.H. Materials processing strategies for colloidal quantum dot solar cells: Advances, present-day limitations, and pathways to improvement. MRS Commun. 2013, 3, 83–90. [Google Scholar] [CrossRef]
- Fischer, A.; Rollny, L.; Pan, J.; Carey, G.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Kim, J.Y.; Bakr, O.M.; et al. Directly Deposited Quantum Dot Solids Using a Colloidally Stable Nanoparticle Ink. Adv. Mater. 2013, 25, 5742–5749. [Google Scholar] [CrossRef]
- Wang, R.; Shang, Y.; Kanjanaboos, P.; Zhou, W.; Ning, Z.; Sargent, E.H. Colloidal quantum dot ligand engineering for high performance solar cells. Energy Environ. Sci. 2016, 9, 1130–1143. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Phuyal, D.; Du, J.; Tian, L.; Öberg, V.A.; Johansson, M.B.; Cappel, U.B.; Karis, O.; Liu, J.; et al. Inorganic CsPbI3 Perovskite Coating on PbS Quantum Dot for Highly Efficient and Stable Infrared Light Converting Solar Cells. Adv. Energy Mater. 2018, 8, 1702049. [Google Scholar] [CrossRef]
- Hu, L.; Lei, Q.; Guan, X.; Patterson, R.; Yuan, J.; Lin, C.; Kim, J.; Geng, X.; Younis, A.; Wu, X.; et al. Optimizing Surface Chemistry of PbS Colloidal Quantum Dot for Highly Efficient and Stable Solar Cells via Chemical Binding. Adv. Sci. 2021, 8, 2003138. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Chen, J.; Zheng, S.; Phuyal, D.; Yu, M.; Tian, L.; Liu, J.; Karis, O.; Rensmo, H.; Johansson, E.M.J.; et al. Highly Stabilized Quantum Dot Ink for Efficient Infrared Light Absorbing Solar Cells. Adv. Energy Mater. 2019, 9, 1902809. [Google Scholar] [CrossRef]
- Liu, M.; Voznyy, O.; Sabatini, R.; de Arquer, F.P.G.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic–inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nat. Mater. 2017, 16, 258–263. [Google Scholar] [CrossRef]
- Mandal, D.; Dambhare, N.V.; Rath, A.K. Reduction of Hydroxyl Traps and Improved Coupling for Efficient and Stable Quantum Dot Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 46549–46557. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, T.; Park, T.; Jeong, S. Suppression of hydroxylation on the surface of colloidal quantum dots to enhance the open-circuit voltage of photovoltaics. J. Mater. Chem. A 2020, 8, 4844–4849. [Google Scholar] [CrossRef]
- Jo, J.W.; Choi, J.; García de Arquer, F.P.; Seifitokaldani, A.; Sun, B.; Kim, Y.; Ahn, H.; Fan, J.; Quintero-Bermudez, R.; Kim, J.; et al. Acid-Assisted Ligand Exchange Enhances Coupling in Colloidal Quantum Dot Solids. Nano Lett. 2018, 18, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- van Veggel, F.C.J.M. Near-Infrared CQDs and Their Delicate Synthesis, Challenging Characterization, and Exciting Potential Applications. Chem. Mater. 2014, 26, 111–122. [Google Scholar] [CrossRef]
- Ning, Z.; Gong, X.; Comin, R.; Walters, G.; Fan, F.; Voznyy, O.; Yassitepe, E.; Buin, A.; Hoogland, S.; Sargent, E.H. Quantum-Dot-in-Perovskite Solids. Nature 2015, 523, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Goswami, P.N.; Rath, A.K. Thiol and Halometallate, Mutually Passivated Quantum Dot Ink for Photovoltaic Application. ACS Appl. Mater. Interfaces 2019, 11, 26100–26108. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Ouellette, O.; de Arquer, F.P.G.; Voznyy, O.; Kim, Y.; Wei, M.; Proppe, A.H.; Saidaminov, M.I.; Xu, J.; Liu, M.; et al. Multibandgap quantum dot ensembles for solar-matched infrared energy harvesting. Nat. Commun. 2018, 9, 4003. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Z.; Andersen, N.T.; Biondi, M.; Todorović, P.; Sun, B.; Ouellette, O.; Abed, J.; Sagar, L.K.; Choi, M.-J.; Hoogland, S.; et al. Mixed Lead Halide Passivation of Quantum Dots. Adv. Mater. 2019, 31, 1904304. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Che, F.; Sun, B.; Voznyy, O.; Proppe, A.; Munir, R.; Wei, M.; Quintero-Bermudez, R.; Hu, L.; Hoogland, S.; et al. Controlled Steric Hindrance Enables Efficient Ligand Exchange for Stable, Infrared-Bandgap Quantum Dot Inks. ACS Energy Lett. 2019, 4, 1225–1230. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; de Arquer, F.P.G.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% Certified Colloidal Quantum Dot Solar Cells via Solvent-Polarity-Engineered Halide Passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Zherebetskyy, D.; Scheele, M.; Zhang, Y.; Bronstein, N.; Thompson, C.; Britt, D.; Salmeron, M.; Alivisatos, P.; Wang, L.W. Hydroxylation of the surface of PbS nanocrystals passivated with oleic acid. Science 2014, 344, 1380–1384. [Google Scholar] [CrossRef]
- Choi, J.; Choi, M.J.; Kim, J.; Dinic, F.; Todorovic, P.; Sun, B.; Wei, M.; Baek, S.W.; Hoogland, S.; Garcia de Arquer, F.P.; et al. Stabilizing Surface Passivation Enables Stable Operation of Colloidal Quantum Dot Photovoltaic Devices at Maximum Power Point in an Air Ambient. Adv. Mater. 2020, 32, 1906497. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Che, F.; Jo, J.W.; Choi, J.; de Arquer, F.P.G.; Voznyy, O.; Sun, B.; Kim, J.; Choi, M.; Quintero-Bermudez, R.; et al. A Facet-Specific Quantum Dot Passivation Strategy for Colloid Management and Efficient Infrared Photovoltaics. Adv. Mater. 2019, 31, 1805580. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Wang, H.; Zhang, Y.; Cheng, C.; Zhai, T.; Chen, B.; Liu, X.; Jono, R.; Mao, X.; Liu, Y.; et al. The effect of water on colloidal quantum dot solar cells. Nat. Commun. 2021, 12, 4381. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Vafaie, M.; Levina, L.; Wei, M.; Dong, Y.; Gao, Y.; Kung, H.T.; Biondi, M.; Proppe, A.H.; Chen, B.; et al. Ligand-Assisted Reconstruction of Colloidal Quantum Dots Decreases Trap State Density. Nano Lett. 2020, 20, 3694–3702. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wang, Y.; Yang, F.; Lu, K.; Xue, Y.; Wu, T.; Fang, H.; Zhou, S.; Zhang, Y.; Ling, X.; et al. Stable PbS Quantum Dot Ink for Efficient Solar Cells by Solution-Phase Ligand Engineering. J. Mater. Chem. A 2019, 7, 15951–15959. [Google Scholar] [CrossRef]
- Jo, J.W.; Kim, Y.; Choi, J.; de Arquer, F.P.G.; Walters, G.; Sun, B.; Ouellette, O.; Kim, J.; Proppe, A.H.; Quintero-Bermudez, R.; et al. Enhanced Open-Circuit Voltage in Colloidal Quantum Dot Photovoltaics via Reactivity-Controlled Solution-Phase Ligand Exchange. Adv. Mater. 2017, 29, 1703627. [Google Scholar] [CrossRef]
- Shestha, A.; Yin, Y.; Andersson, G.G.; Spooner, N.A.; Qiao, S.; Dai, S. Versatile PbS Quantum Dot Ligand Exchange Systems in the Presence of Pb-Thiolates. Small 2017, 13, 1602956. [Google Scholar] [CrossRef]



| Additives | Chemical Equations |
|---|---|
| NH4Ac and PbX2 (Br, I) | PbS-PbX2 (Br, I) CQD |
| NH4Ac, PbI2 and CsI | PbS-CsPbI3-P CQD |
| Carboxylates (formate, acetate and propionate) | PbS-OA CQD + PbI2 + RCOO− PbS-PbI2 + RCOO− CQD |
| CPT, NH4I, and PbX2 (Br, I) | PbS-OA CQD + CPT + NH4I + PbX2 (Br, I) PbS-CPT + PbX2 (Br, I) CQD |
| NH4I | PbS-OA CQD + AI PbS-AI CQD |
| NaAc and PbX2 (Cl, Br, I) | PbS-OA CQD + NaAc + PbX2 (Cl, Br, I) PbS-NaAc + PbX2 (Cl, Br, I) CQD |
| Additives | Chemical Equations |
|---|---|
| KI | PbS-KI + PbX2 (Br, I) CQD |
| KI3 | PbS-KI3 + PbX2 (Br, I) CQD |
| NaAc | PbS-OA CQD + PbX2 (Br, I) + NaAc PbS-NaAc + PbX2 (Br, I) CQD |
| Additives | Chemical Equations |
|---|---|
| PbBr2 was added to the synthesis process | PbS-OH− CQD + PbBr2 PbS-PbBr2 CQD PbS-PbX2 (Br, I) CQD |
| HI | PbS-OA + OH− CQD + HI + PbX2 (Br, I) + NaAc PbS-PbX2 (Br, I) + HI CQD |
| CPT and NH4I | PbS-OA + OH− CQD + CPT + PbX2 (Br, I) + NH4I PbS-CPT + PbX2 (Br, I) CQD |
| MPA was added to butylamine | PbS-OA + OH− PbS-MPA + PbI2 CQD |
| Additives | Chemical Equations |
|---|---|
| MAI | PbS-OA CQD + MAI + TBAI PbS-MAI + TBAI CQD |
| Pb-thiolate | PbS-OA CQD + Pb-MESNA(GSH) PbS-MESNA(GSH) CQD |
| NH4Ac and TBAA | PbS-OA + PbX2 (Br, I) CQD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Han, D.; Zhang, X. PbS Colloidal Quantum Dots: Ligand Exchange in Solution. Coatings 2024, 14, 761. https://doi.org/10.3390/coatings14060761
Zhang C, Han D, Zhang X. PbS Colloidal Quantum Dots: Ligand Exchange in Solution. Coatings. 2024; 14(6):761. https://doi.org/10.3390/coatings14060761
Chicago/Turabian StyleZhang, Chuanxi, Dong Han, and Xiaoyu Zhang. 2024. "PbS Colloidal Quantum Dots: Ligand Exchange in Solution" Coatings 14, no. 6: 761. https://doi.org/10.3390/coatings14060761
APA StyleZhang, C., Han, D., & Zhang, X. (2024). PbS Colloidal Quantum Dots: Ligand Exchange in Solution. Coatings, 14(6), 761. https://doi.org/10.3390/coatings14060761






