Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations of Samples
2.3. Synthesis of the Different Sizes of Graphite Sheets
2.4. Preparation of EGx/C1P0.2EP Composite Epoxy Coatings
3. Results
3.1. Characterization of Samples and Coatings
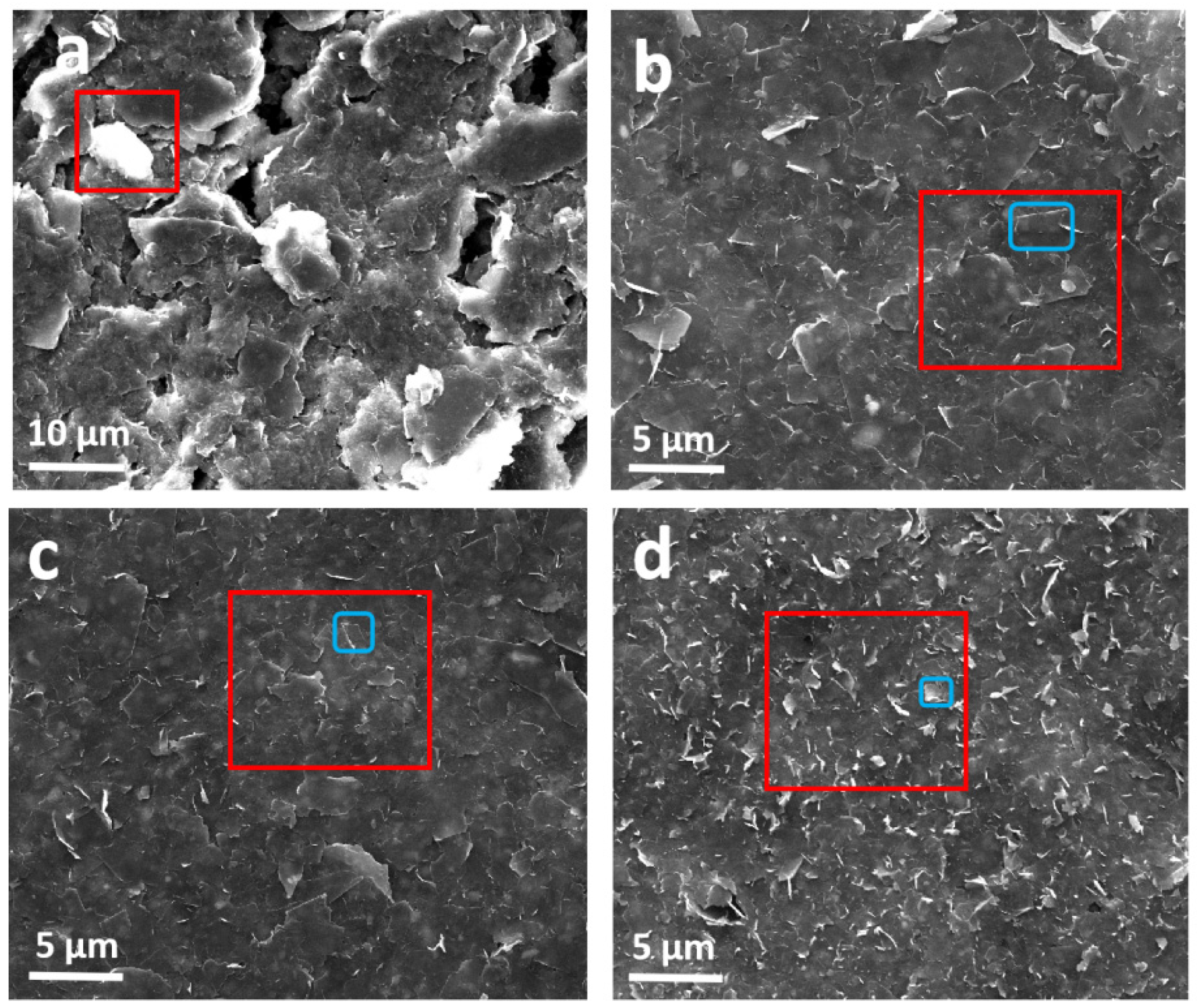
3.1.1. Morphology Characterization of Samples and Coatings
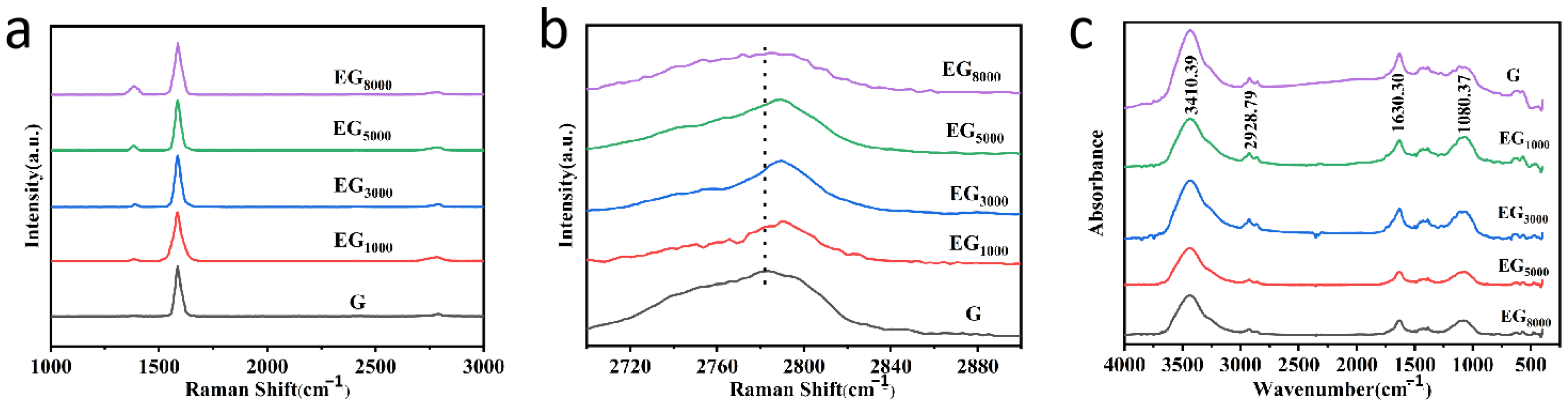
3.1.2. Structural Characterization of Samples and Coatings
3.1.3. Wettability Characterization of Samples and Coatings
3.2. Anti-Corrosion Properties of EGx/C1P0.2EP Coatings
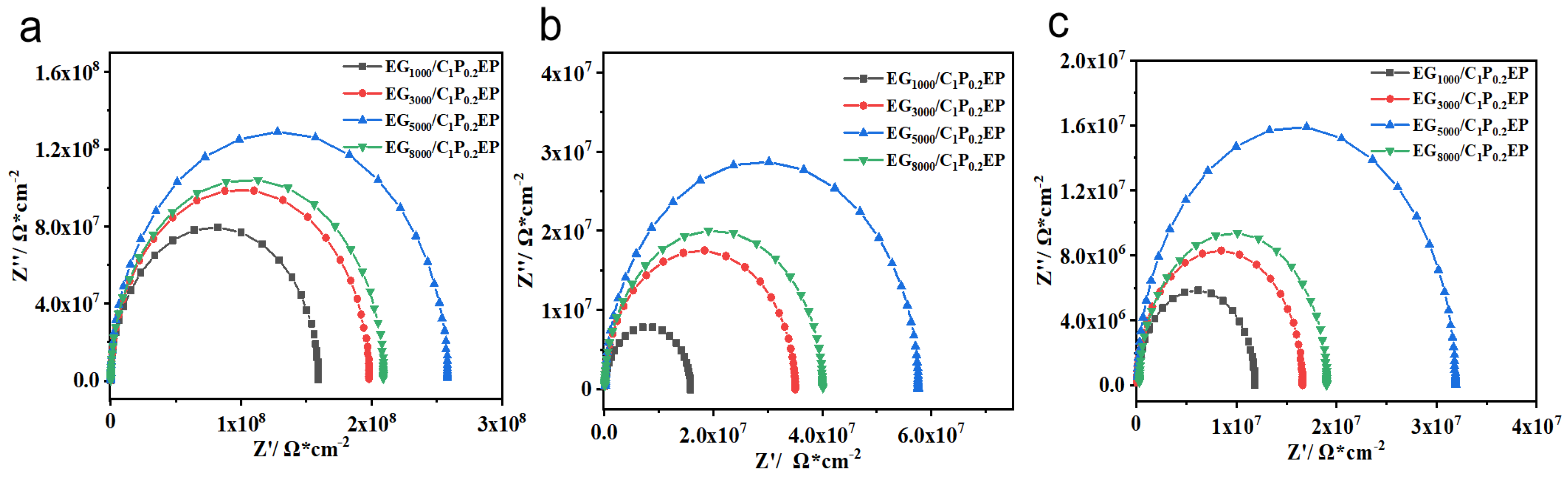
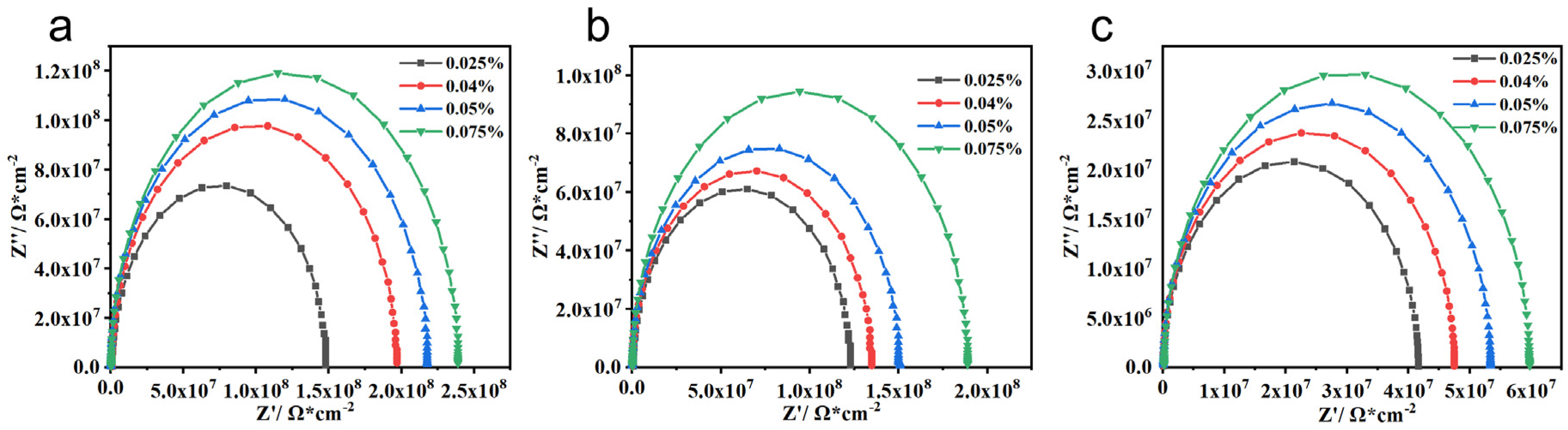
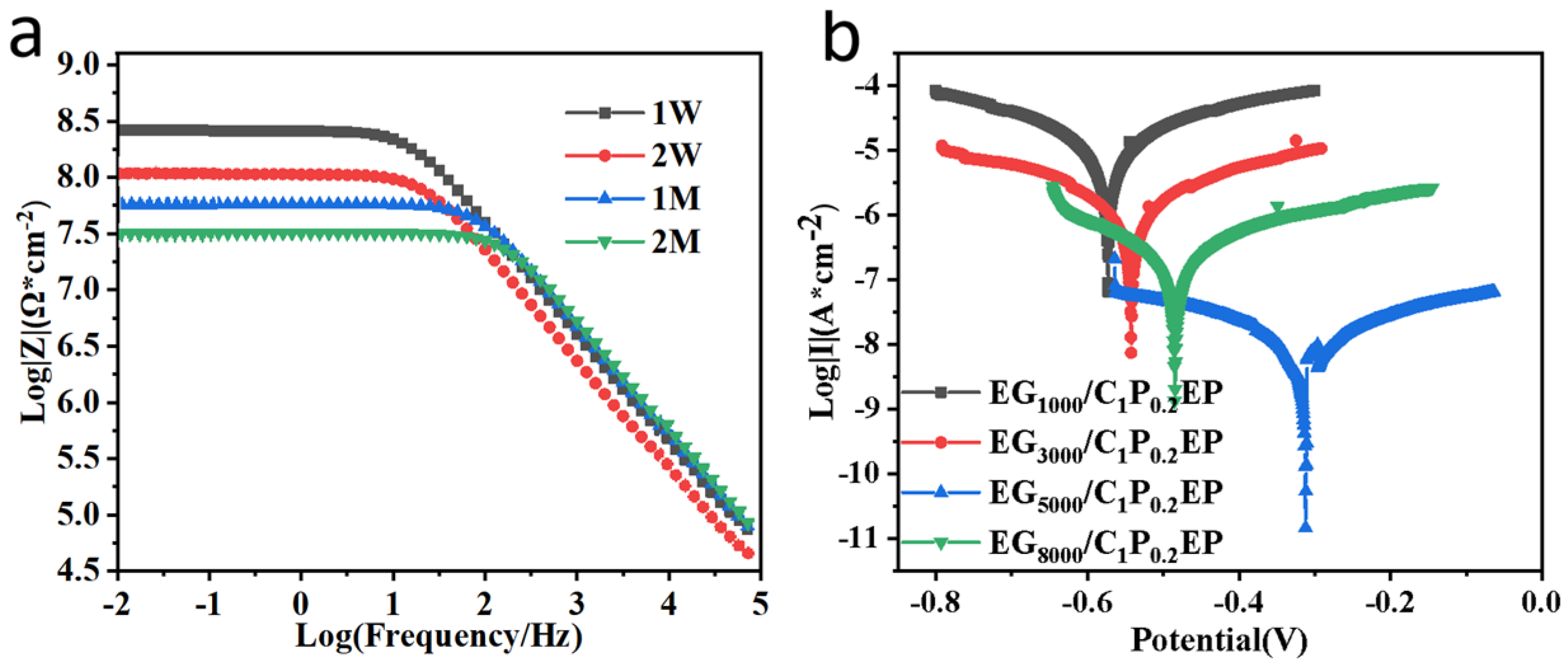
3.2.1. Electrochemical Characteristics
3.2.2. Barrier Properties
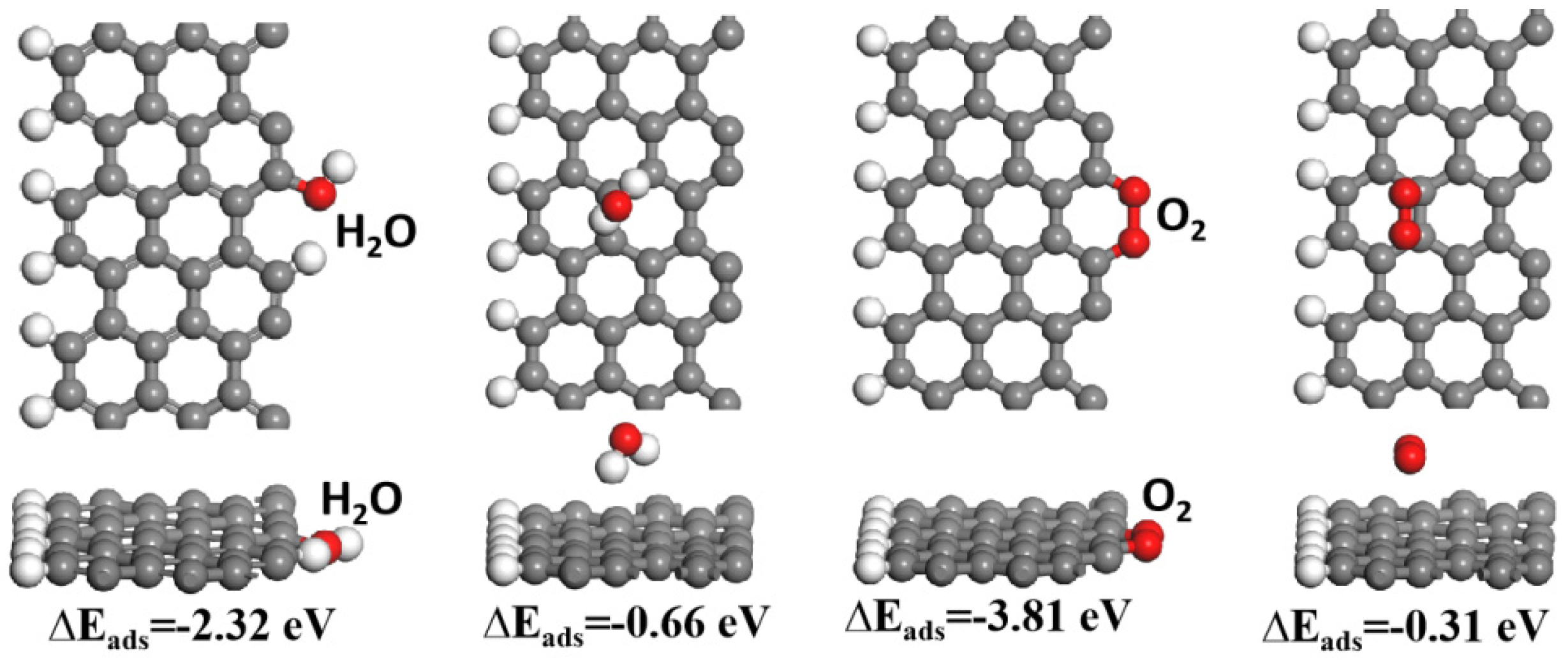
3.3. Theoretical Calculation and Anti-Corrosion Mechanism of EGx/C1P0.2EP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bejinariu, C.; Burduhos-Nergis, D.-P.; Cimpoesu, N. Immersion behavior of carbon steel, phosphate carbon steel and phosphate and painted carbon steel in saltwater. Materials 2021, 14, 188. [Google Scholar] [CrossRef]
- Vovchenko, L.; Lazarenko, O.; Matzui, L.; Perets, Y.; Zhuravkov, A.; Fedorets, V.; Le Normand, F. Mechanical and electrical properties of the epoxy composites with graphite nanoplatelets and carbon nanotubes. Phys. Status Solidi 2014, 211, 336–441. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Zheng, Y.; Zhao, X.; Yu, H. A long-term anticorrsive coating through graphene passivation. Carbon 2018, 138, 197–206. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Li, L.; Zhao, T.; Chen, R. Improvement of rate and cycle performence by rapid polyaniline coating of a MWCNT/Sulfur cathode. J. Phys. Chem. C 2011, 115, 24411–24417. [Google Scholar] [CrossRef]
- Perrin, F.; Phan, T.; Nguyen, D. Synthesis and characterization of polyaniline nanoparticles in phosphonic acid amphiphile aqueous micellar solutions for waterborne corrosion protection coatings. J. Polym. Sci. Polym. Chem. Ed. 2015, 53, 1606–1616. [Google Scholar] [CrossRef]
- Chen, F.; Liu, P. Conducting polyaniline nanoparticles and their dispersion for waterborne corrosion protection coatings. ACS Appl. Mater. Interfaces 2011, 3, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhao, X.; Tong, X.; Ma, J.; Chen, B.; Liu, S.; Zhao, H.; Yu, H.; Chen, J. Facile preparation of polyaniline nanoparticles and their dispersion for waterborne anticorrosion coatings. Int. J. Electrochem. Sci. 2016, 11, 1621–1631. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Berber, H.; Sarac, A.; Yıldırım, H. A comparative study on water-based coatings prepared in the presence of oligomeric and conventional protective colloids. Prog. Org. Coat. 2011, 71, 225–233. [Google Scholar] [CrossRef]
- Wang, N.; Fu, W.; Sun, M.; Zhang, J.; Fang, Q. Effect of different structured TiO2 particle on anticorrosion properties of waterborne epoxy coatings. Corros. Eng. Sci. Technol. 2016, 51, 365–372. [Google Scholar] [CrossRef]
- Wang, N.; Diao, X.; Zhang, J.; Kang, P. Corrosion resistance of waterborne epoxy coatings by incorporation of dopamine treated Mesoporous-TiO2 particles. Coatings 2018, 8, 209. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of water permeation dynamics and anti-corrosion mechanism of Graphene/Zinc coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Sheng, X.; Mo, R.; Ma, Y.; Zhang, X.; Zhang, L.; Wu, H. Waterborne epoxy resin/polydopamine modified zirconium phosphate nanocomposite for anticorrosive coating. Ind. Eng. Chem. Res. 2019, 58, 16571–16580. [Google Scholar] [CrossRef]
- Huang, T.; Su, Y.; Yeh, T.; Huang, H.; Wu, C.; Huang, K.; Chou, Y.; Yeh, J.; Wei, Y. Advanced anticorrosive coatings prepared from electroactive Epoxy-SiO2 hybrid nanocomposite materials. Electrochim. Acta 2011, 56, 6142–6149. [Google Scholar] [CrossRef]
- Martín-Fabiani, I.; Lesage, D.; Schulz, M.; Liu, Y.; Lee, M.; Duffy, B.; Agosto, F.; Lansalot, M.; Keddie, J. Enhanced water barrier properties of surfactant-free polymer films obtained by MacroRAFT-Mediated emulsion polymerization. ACS Appl. Mater. Interfaces 2018, 10, 11221–11232. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Rahman, O.; Peng, W.; Dou, H.; Yu, H. A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne Graphene/Epoxy coatings. Appl. Surf. Sci. 2018, 427, 981–991. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile Preparation of Water Dispersible Graphene Sheets Stabilized by Carboxylated Oligoanilines and Their Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17648. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, J.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Dispersion and parallel assembly of sulfonated graphene in waterborne epoxy anticorrosion coatings. J. Mater. Chem. A 2019, 7, 17937–17946. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxidealumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. Considering the effect of graphene loading in water-based epoxy coatings. J. Coat. Technol. Res. 2018, 15, 923–931. [Google Scholar] [CrossRef]
- Kopsidas, S.; Olowojoba, G.; Kinloch, A.; Taylor, A. Examining the effect of graphene nanoplatelets on the corrosion resistance of epoxy coatings. Int. J. Adhes. Adhes. 2021, 104, 102723. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Tian, Y.; Xie, Y.; Sheng, X.; Jiang, X.; Zhang, X. Exfoliation and functionalization of α-zirconium phosphate in one pot for waterborne epoxy coatings with enhanced anticorrosion performance. Prog. Org. Coat. 2020, 138, 105390. [Google Scholar] [CrossRef]
- Lv, K.; Pan, R.; Zhang, L.; Tian, Y.; Yan, S.; Wan, D. Synergistically assembled graphene/ZnO composite to enhance anticorrosion performance of waterborne epoxy coatings. RSC Adv. 2022, 15, 9069–9076. [Google Scholar] [CrossRef]
- Teng, C.; Xie, D.; Wang, J.; Yang, Z.; Ren, G.; Zhu, Y. Ultrahigh Conductive Graphene Paper Based on Ball-Milling Exfoliated Graphene. Adv. Funct. Mater. 2017, 27, 1700240. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Zhao, H.; Pu, J.; Yu, C. Novel nitrogen doped carbon dots enhancing the anticorrosive performance of waterborne epoxy coatings. Nanoscale Adv. 2019, 1, 3443–3451. [Google Scholar] [CrossRef]
- Irfan, M.; Iqbal, S.; Ahmad, S. Waterborne reduced graphene oxide dispersed sebacic acid modified soy epoxy nanocomposite: A green and sustainable approach for high performance mechanically robust anticorrosive coatings. Prog. Org. Coat. 2022, 170, 106984. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion Resistance of Graphene-Reinforced Waterborne Epoxy Coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]
- Huan, Y.; Dan, L.; Xu, H. Graphene-induced enhanced anticorrosion performance of waterborne epoxy resin coating. Front. Mater. Sci. 2020, 14, 211–220. [Google Scholar]
- Lv, K.; Wan, D.; Pan, R. Curvature of NCNTs induced selectivity of CO2 electroreduction into CO. Carbon Neutralization 2022, 1, 189–197. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krief, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, K.; Bao, Y.; Ma, H.; Liu, X.; Zhu, Y.; Wan, D. Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings. Coatings 2024, 14, 769. https://doi.org/10.3390/coatings14060769
Lv K, Bao Y, Ma H, Liu X, Zhu Y, Wan D. Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings. Coatings. 2024; 14(6):769. https://doi.org/10.3390/coatings14060769
Chicago/Turabian StyleLv, Kuilin, Yiwang Bao, Huachao Ma, Xiaogen Liu, Ying Zhu, and Detian Wan. 2024. "Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings" Coatings 14, no. 6: 769. https://doi.org/10.3390/coatings14060769
APA StyleLv, K., Bao, Y., Ma, H., Liu, X., Zhu, Y., & Wan, D. (2024). Size Effect of Graphite Nanosheet-Induced Anti-Corrosion of Hydrophobic Epoxy Coatings. Coatings, 14(6), 769. https://doi.org/10.3390/coatings14060769




