Enhancing Tribological Performance of Micro-Arc Oxidation Coatings on 6061 Aluminum Alloy with h-BN Incorporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Micro-Arc Oxidation Coatings
2.2. Preparation Process
2.3. Structural and Morphology Characterization
2.4. Hardness Tests
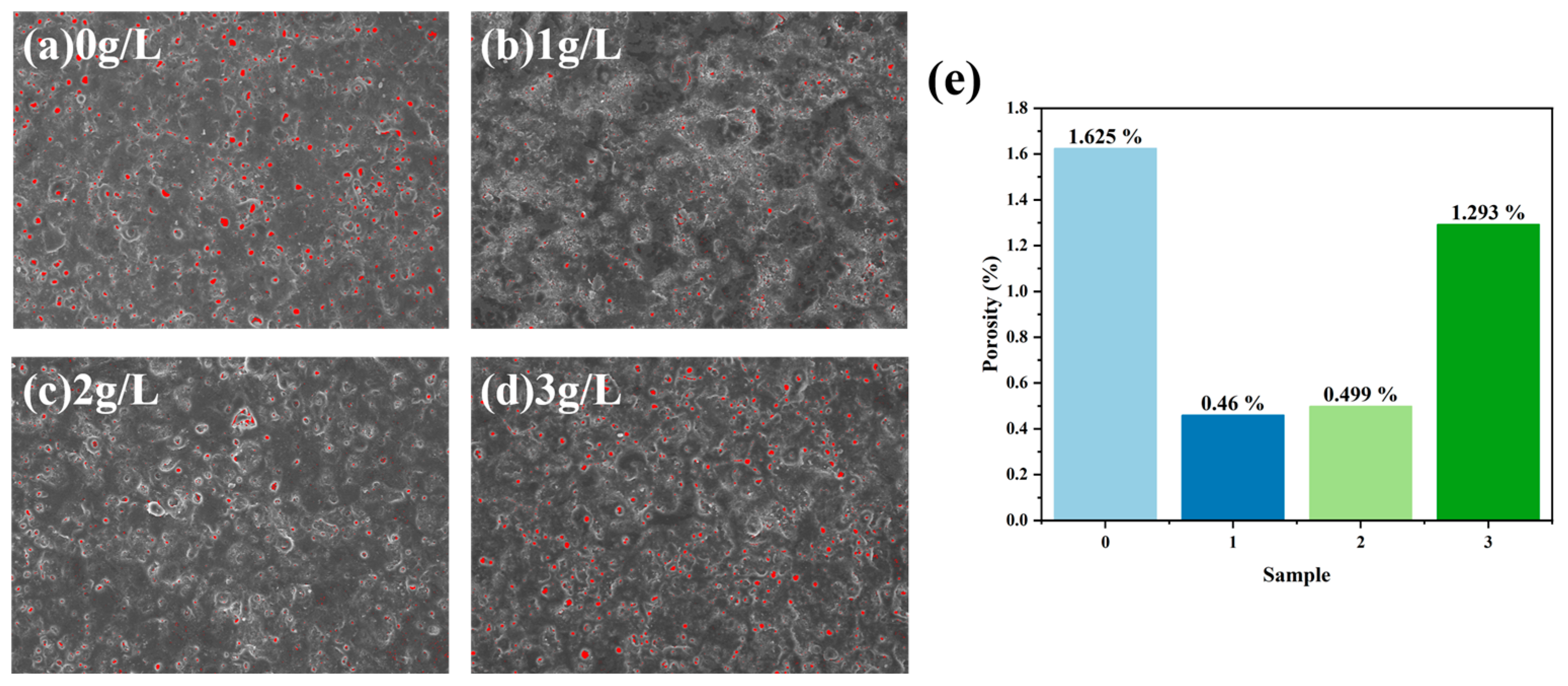
2.5. Porosity and Roughness Measurements
2.6. Friction Tests
2.7. Corrosion Tests
3. Results
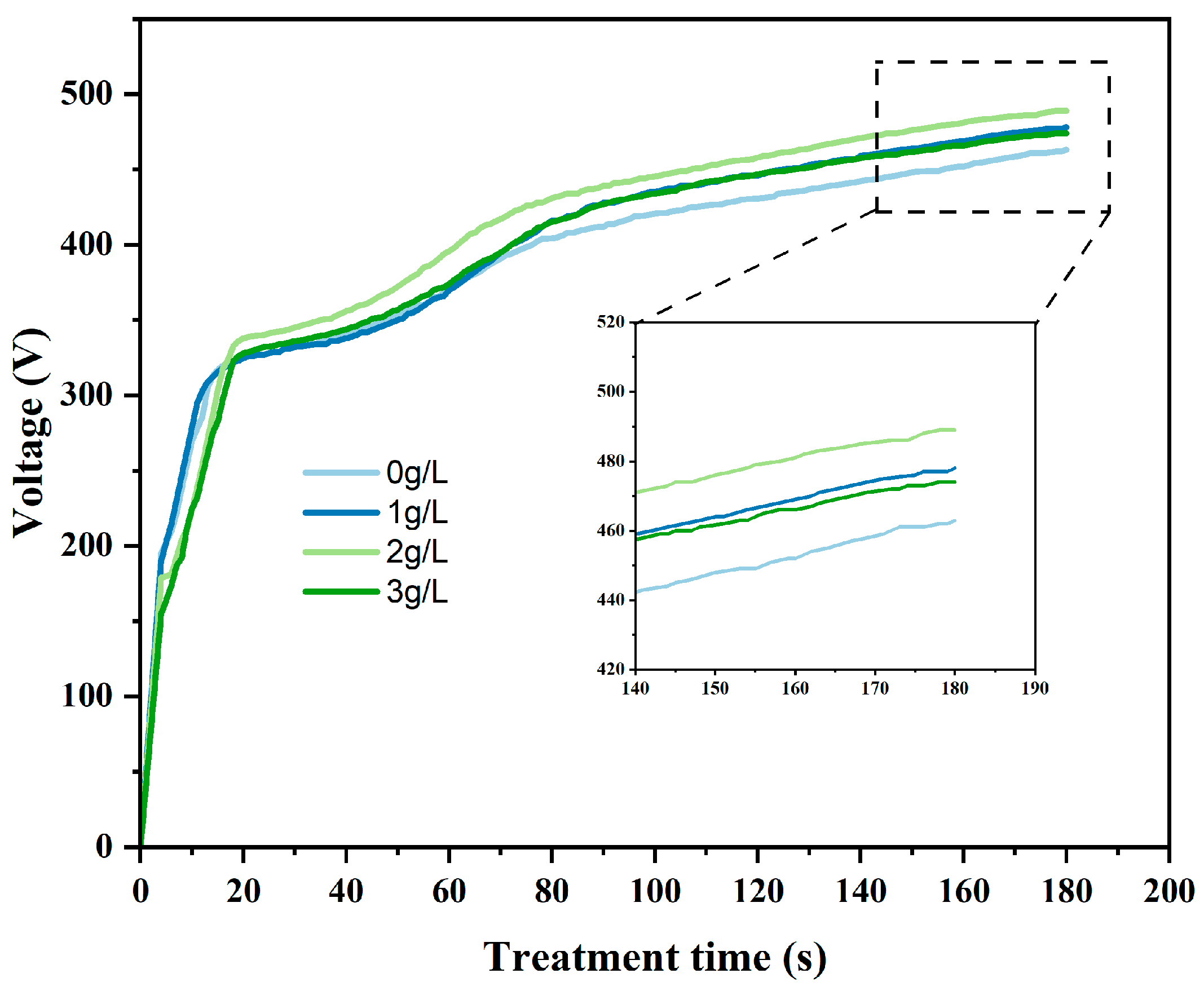
3.1. Voltage–Time Response
3.2. Analysis of Electrochemical Corrosion Test Results
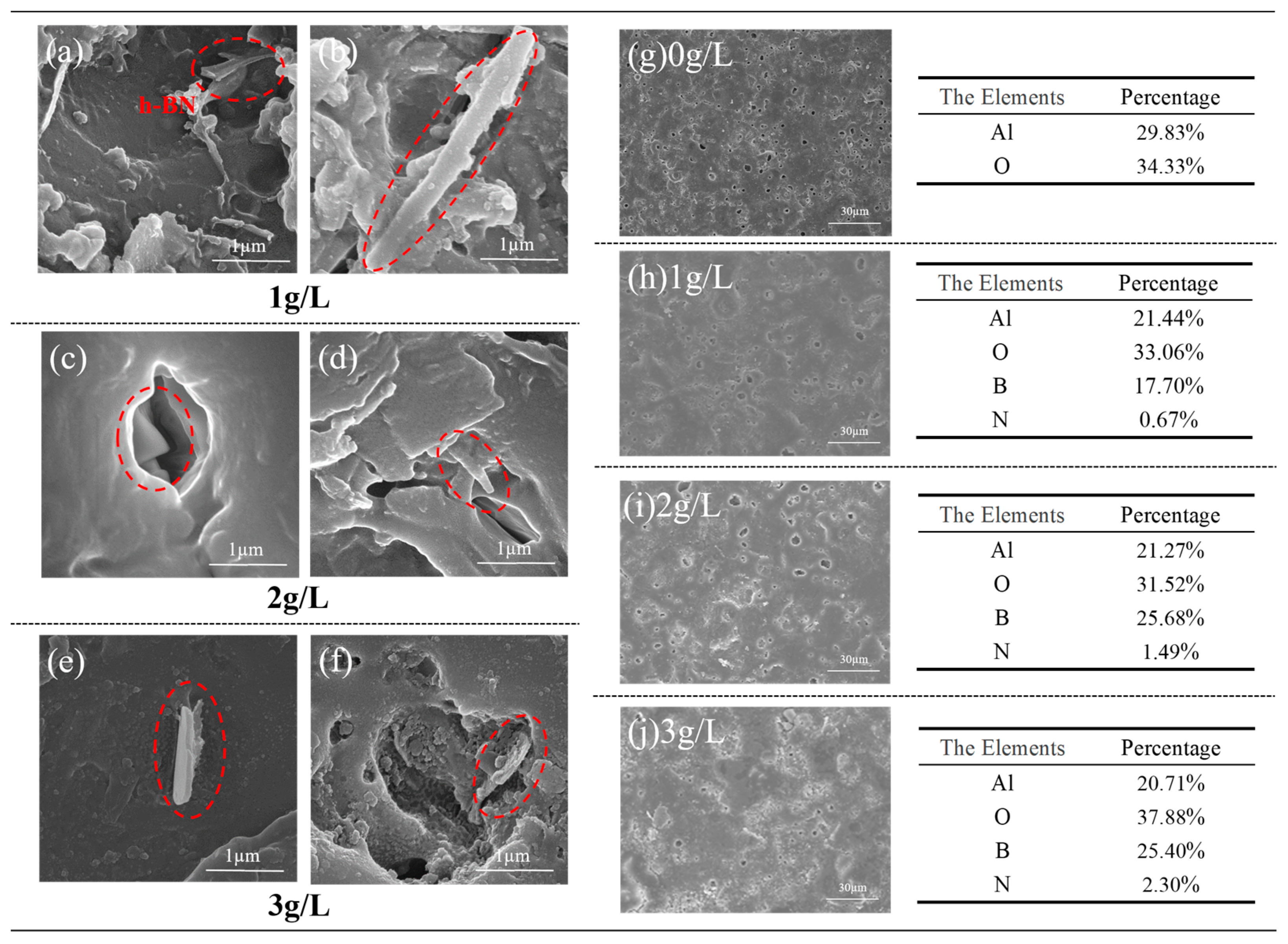
3.3. Surface Morphology and Composition Analysis of Micro-Arc Oxidation Coating
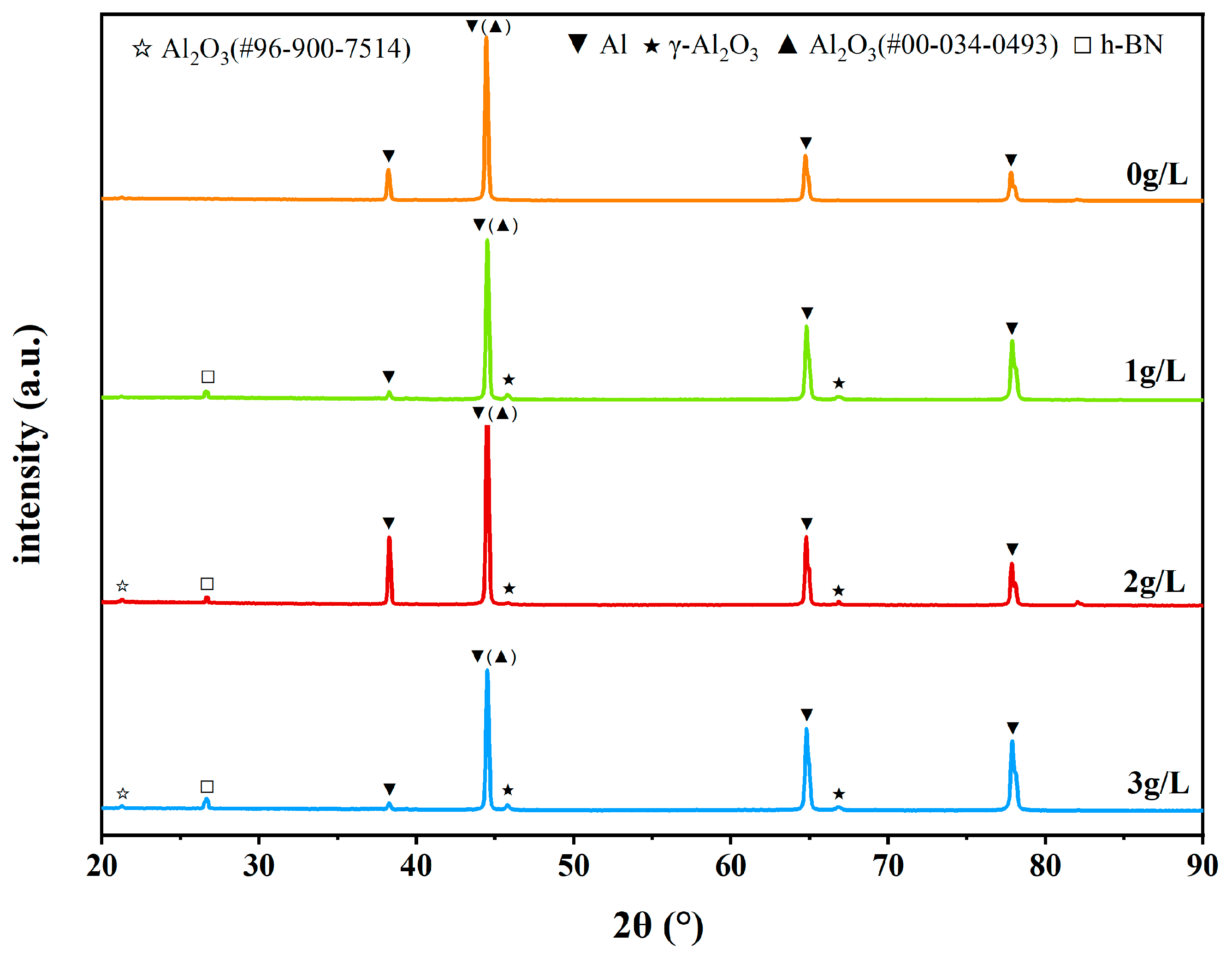
3.4. Analysis of the Phase Structure of Micro-Arc Oxidation Coating
3.5. Wear Morphology Analysis of Micro-Arc Oxidation Coating
4. Discussion
- (1)
- Porosity and surface roughness are directly proportional to the concentration of h-BN nanoparticles. Compared to MAO coatings without h-BN, the porosity of coatings with 1 g/L decreased by 30%.
- (2)
- The incorporation of h-BN significantly increased the hardness and thickness of the coatings. Due to the high hardness of h-BN nanoparticles, the MAO coating at a concentration of 2 g/L had the highest hardness, which was 2.5 times greater than that of the coating at 0 g/L. The best corrosion resistance of the coating was also obtained for the 2 g/L sample.
- (3)
- The addition of h-BN nanoparticles effectively reduced the COF of the MAO coatings. h-BN provided a certain level of lubrication; the coating at 2 g/L showed the most stable friction coefficient curve with the lowest COF. With the lubricating effect of h-BN particles, the wear resistance of the 2 g/L h-BN/Al2O3 coating had a friction coefficient of about 25% compared to the coating without h-BN.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kammer, C. Aluminum and Aluminum Alloys. In Springer Handbook of Materials Data; Springer: Berlin/Heidelberg, Germany, 2018; pp. 161–197. [Google Scholar]
- Li, K.; Gong, H.H.; Zhang, C.C.; Sha, H.J. Influence of Shot Peening Treatment on the Corrosion Resistance of 6061 Aluminum Alloy. J. Hunan Univ. Arts Sci. (Nat. Sci. Ed.) 2024, 36, 27–31. [Google Scholar]
- Zhang, G.H.; Huang, G.Q.; Xv, P.; Yu, M.Z. Research progress in surface treatment of aluminum alloys. J. China Inst. Metrol. 2010, 21, 174–178. [Google Scholar]
- Yuting, D.; Zhiyang, L.; Guofeng, M. The research progress on micro-arc oxidation of aluminum alloy. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012055. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Z.; Tang, S.; Dong, W.; Tong, Q.; Li, W. Influence of graphene particles on the micro-arc oxidation behaviors of 6063 aluminum alloy and the coating properties. Appl. Surf. Sci. 2017, 423, 939–950. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, Z.; Wu, H.; Ji, S.; Ma, Z.; Wu, Z.; Chen, P.; Zhu, J.; Fu, R.K.Y.; Lin, H.; et al. Corrosion behavior of ZnO-reinforced coating on aluminum alloy prepared by plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 374, 1015–1023. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Huang, Y.; Ovri, H.; Zheludkevich, M.L.; Kainer, K.U. Plasma electrolytic oxidation coatings on Mg alloy with addition of SiO2 particles. Electrochim. Acta 2016, 187, 20–33. [Google Scholar] [CrossRef]
- Xu, R.; Li, Y.; Fan, B.; Weng, Y.; Zhou, Y.; Yan, F. One-step preparation of molybdate-stannate-tungstate composite conversion coating on magnesium alloy AZ91D and its microstructure and corrosion resistance. Int. J. Electrochem. Sci. 2023, 18, 100398. [Google Scholar] [CrossRef]
- Yürektürk, Y.; Muhaffel, F.; Baydoğan, M. Characterization of micro arc oxidized 6082 aluminum alloy in an electrolyte containing carbon nanotubes. Surf. Coat. Technol. 2015, 269, 83–90. [Google Scholar] [CrossRef]
- Nimura, K.; Sugawara, T.; Jibiki, T.; Ito, S.; Shima, M. Surface modification of aluminum alloy to improve fretting wear properties. Tribol. Int. 2016, 93, 702–708. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3/TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
- Samanipour, F.; Bayati, M.R.; Golestani-Fard, F.; Zargar, H.R.; Mirhabibi, A.R.; Shoaei-Rad, V.; Abbasi, S. Innovative fabrication of ZrO2–HAp–TiO2 nano/micro-structured composites through MAO/EPD combined method. Mater. Lett. 2011, 65, 926–928. [Google Scholar] [CrossRef]
- Roostaei, M.; Aghajani, H.; Abbasi, M.; Abasht, B. Formation of Al2O3/MoS2 nanocomposite coatings by the use of electro spark deposition and oxidation. Ceram. Int. 2021, 47, 11644–11653. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Lin, Y.; Zhang, Z.; Feng, S.; Talha, M.; Shi, Y.; Shi, T. Effects of graphene on structure and corrosion resistance of plasma electrolytic oxidation coatings formed on D16T Al alloy. Appl. Surf. Sci. 2019, 475, 645–659. [Google Scholar] [CrossRef]
- Li, X.; Ding, Q.; Zhao, G.; Hu, Q. Effects of Graphene Oxide on the Mechanical and Tribological Properties of Micro-Arc Oxidation Coatings on Aluminum Alloy. Mater. Prot. 2023, 56, 1–9. [Google Scholar]
- Wang, Y.W.; Xiao, W.Q.; Zhou, H.J.; Zhou, X.H. Preparation, Modification, and Application of Hexagonal Boron Nitride. Chem. New Mater. 2022, 50, 32–37. [Google Scholar]
- Cui, S.; Kan, H.; Zhang, N.; Ru, H.Q. Preparation, application and research progress of hexagonal boron nitride. J. Funct. Mater. 2020, 51, 8072–8077. [Google Scholar]
- Yang, M.Y.; Zhang, B.; Zhang, X.; Zhou, T.G. Research on the Geometric Structure, Electronic Structure, Magnetism, and Edge Characteristics of Graphene and Hexagonal Boron Nitride Nanosheets. J. At. Mol. Phys. 2018, 35, 673–680. [Google Scholar]
- Chen, T.; Wang, X.; Liu, Z.; Deng, C.J.; Yu, C.; Ding, J.; Zhu, H.X. Effect of h-BN on Microstructure and Properties of Al2O3-C Refractories at Different Nitriding Temperatures. J. Chin. Ceram. Soc. 2022, 50, 3305–3313. [Google Scholar]
- Wu, C.; Wang, Y.; Li, J.F.; Zhu, Z.J.; You, Z.Y.; Tu, Z.Y. Advances in the Preparation and Thermal Conductivity Properties of Hexagonal and Cubic Boron Nitride. Foundry Technol. 2023, 44, 1–8. [Google Scholar]
- Tang, K.; Ni, Z.Y.; Liu, Q.H.; Quhe, R.G.; Zheng, Q.Y.; Zheng, J.X.; Fei, R.X.; Gao, Z.X.; Lu, J. Electronic and transport properties of a biased multilayer hexagonal boron nitride. Eur. Phys. J. B 2012, 85, 301. [Google Scholar] [CrossRef]
- GB/T 3191-2010; Aluminium and Aluminium Alloys Extruded Bars, Rods, Wire, Profiles, and Tubes. Standardization Administration of China: Beijing, China, 2011.
- Li, Z.; Di, S. The Microstructure and Wear Resistance of Microarc Oxidation Composite Coatings Containing Nano-Hexagonal Boron Nitride (HBN) Particles. J. Mater. Eng. Perform. 2017, 26, 1551–1561. [Google Scholar] [CrossRef]
- Wang, G.; Guo, L.; Ruan, Y.; Zhao, G.; Zhang, X.; Liu, Y.; Kim, D.-E. Improved wear and corrosion resistance of alumina alloy by MAO and PECVD. Surf. Coat. Technol. 2024, 479, 130556. [Google Scholar] [CrossRef]
- Wu, H.; Shi, Z.; Zhang, X.; Qasim, A.M.; Xiao, S.; Zhang, F.; Wu, Z.; Wu, G.; Ding, K.; Chu, P.K. Achieving an acid resistant surface on magnesium alloy via bio-inspired design. Appl. Surf. Sci. 2019, 478, 150–161. [Google Scholar] [CrossRef]
- Tran, Q.-P.; Chin, T.-S.; Kuo, Y.-C.; Jin, C.-X.; Trung, T.; Tuan, C.V.; Dang, D.Q. Diamond powder incorporated oxide layers formed on 6061 Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Li, X. Theoretical Study on the Conductivity Differences between Graphene and Hexagonal Boron Nitride. Univ. Chem. 2024, 39, 364–368. [Google Scholar] [CrossRef]
- Lü, C.; Wang, C.; Gu, J. First-principles study of structural, elastic, thermodynamic, electronic and optical properties of cubic boron nitride and hexagonal boron nitride at high temperature and high pressure. Acta Phys. Sin. 2019, 68, 077102. [Google Scholar] [CrossRef]
- Kim, S.M.; Hsu, A.; Park, M.H.; Chae, S.H.; Yun, S.J.; Lee, J.S.; Cho, D.-H.; Fang, W.; Lee, C.; Palacios, T.; et al. Synthesis of large-area multilayer hexagonal boron nitride for high material performance. Nat. Commun. 2015, 6, 8662. [Google Scholar] [CrossRef]
- Xu, B.; Sun, J.; Han, J.; Yang, Z.; Zhou, H.; Xiao, L.; Xu, S.; Han, Y.; Ma, A.; Wu, G. Effect of hierarchical precipitates on corrosion behavior of fine-grain magnesium-gadolinium-silver alloy. Corros. Sci. 2022, 194, 109924. [Google Scholar] [CrossRef]
- Xi, K.; Wu, H.; Zhou, C.; Qi, Z.; Yang, K.; Fu, R.K.Y.; Xiao, S.; Wu, G.; Ding, K.; Chen, G.; et al. Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates. Appl. Surf. Sci. 2022, 585, 152693. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, S.; Wu, H.; Wu, C.; Chen, G.; Yin, Y.; Chu, P.K. Effect of h-BN nanoparticles incorporation on the anti-corrosion and anti-wear properties of micro-arc oxidation coatings on 2024 aluminum alloy. Ceram. Int. 2023, 49, 37475–37485. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5 N NaCl aqueous solution: Part II. EIS interpretation of corrosion behaviour. Corros. Sci. 2023, 45, 1257–1273. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials 2021, 14, 4037. [Google Scholar] [CrossRef]
- Letyagin, N.V.; Akopyan, T.K.; Sokorev, A.A.; Sviridova, T.A.; Cherkasov, S.O.; Mansurov, Y.N. The Characterization of Coatings Formed on As-Cast Al, Al–Si, and Al–Ca Aluminum Substrates by Plasma Electrolytic Oxidation. Metals 2023, 13, 1509. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Zhou, Y.; Guo, L.; Ouyang, J.-H. Microstructure and corrosion resistance of modified 2024 Al alloy using surface mechanical attrition treatment combined with microarc oxidation process. Corros. Sci. 2011, 53, 473–480. [Google Scholar] [CrossRef]
- Zhu, X.; Fu, J.; Ma, D.; Ma, C.; Fu, Y.; Zhang, Z. Effect of nano h-BN particles on growth regularity and tribological behavior of PEO composite ceramic coating of ZL109 alloy. Sci. Rep. 2022, 12, 995. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Wu, X.; Lai, Y. Tribological Behavior of Microarc Oxidation Coatings on Aluminum Alloy. ISIJ Int. 2016, 46, 287–291. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.; Wang, S.; Zhao, Q.; Zhang, X.; Zhang, M. Microstructure and Tribological Behavior of a TiO2/hBN Composite Ceramic Coating Formed via Micro-arc Oxidation of Ti–6Al–4V Alloy. J. Mater. Sci. Technol. 2016, 32, 1071–1076. [Google Scholar] [CrossRef]




| Element | Cu | Mn | Mg | Zn | Cr | Ti | Si | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.15~0.4 | 0.15 | 0.8~1.2 | 0.25 | 0.04~0.35 | 0.15 | 0.4~0.8 | 0.7 | Base |
| Name of the Drug | Fineness | Manufacturer |
|---|---|---|
| NaOH | Analytical purity | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Na2SiO3 | ||
| (NaPO3)6 | ||
| h-BN |
| Sample | 0 g/L | 1 g/L | 2 g/L | 3 g/L |
|---|---|---|---|---|
| Equivalent Circuit | R(Q(R(QR)(QR))) | |||
| RS (Ohm⋅cm2) | 4.44 | 3.61 | 3.66 | 4.40 |
| Yf (Ohm−2·cm−2·s−n) | 9.56 × 10−4 | 2.52 × 10−6 | 4.01 × 10−5 | 3.47 × 10−5 |
| nf | 0.64 | 0.88 | 0.69 | 0.88 |
| Rpore (Ohm⋅cm2) | 1.26 | 1.61 × 10−7 | 1.15 × 10−7 | 1.10 |
| Ydl (Ohm−2·cm−2·s−n) | 4.98 × 10−4 | 3.40 × 10−4 | 7.21 × 10−4 | 4.72 × 10−4 |
| ndl | 0.98 | 1.00 | 0.93 | 0.93 |
| Rct (Ohm⋅cm2) | 586.80 | 472.70 | 1218 | 359.90 |
| Ydiff (Ohm−2·cm−2·s−n) | 1.28 × 10−2 | 2.31 × 10−2 | 7.43 × 10−3 | 1.31 × 10−3 |
| ndiff | 0.43 | 0.36 | 0.33 | 0.72 |
| Rdiff (Ohm⋅cm2) | 493.80 | 12.37 | 15.35 | 7.59 |
| Sample | 0 g/L | 1 g/L | 2 g/L | 3 g/L |
|---|---|---|---|---|
| Roughness (µm) | 2.505 | 1.138 | 1.654 | 1.704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Song, J.; Lin, J.; Wang, G.; Zhao, G. Enhancing Tribological Performance of Micro-Arc Oxidation Coatings on 6061 Aluminum Alloy with h-BN Incorporation. Coatings 2024, 14, 771. https://doi.org/10.3390/coatings14060771
Zhao X, Song J, Lin J, Wang G, Zhao G. Enhancing Tribological Performance of Micro-Arc Oxidation Coatings on 6061 Aluminum Alloy with h-BN Incorporation. Coatings. 2024; 14(6):771. https://doi.org/10.3390/coatings14060771
Chicago/Turabian StyleZhao, Xia, Jingfu Song, Jingyi Lin, Guoqing Wang, and Gai Zhao. 2024. "Enhancing Tribological Performance of Micro-Arc Oxidation Coatings on 6061 Aluminum Alloy with h-BN Incorporation" Coatings 14, no. 6: 771. https://doi.org/10.3390/coatings14060771
APA StyleZhao, X., Song, J., Lin, J., Wang, G., & Zhao, G. (2024). Enhancing Tribological Performance of Micro-Arc Oxidation Coatings on 6061 Aluminum Alloy with h-BN Incorporation. Coatings, 14(6), 771. https://doi.org/10.3390/coatings14060771








