Durable and High-Temperature-Resistant Superhydrophobic Diatomite Coatings for Cooling Applications
Abstract
1. Introduction
2. Materials
3. Coating Synthesis Procedures
3.1. General Aspects
Experimental Procedure with Epoxy Resin as Binder
- Rinsing of the surface of the stainless steel with acetone;
- Ultra-sonication at 100 W of the substrate with nitric acid for 10 min;
- Ultra-sonication at 100 W of the surface of the substrate with hydrogen peroxide for 10 min;
- Drying of the stainless steel substrate with an air jet.
- Mix 8 g of DE, 80 mL of toluene, and 0.1 g of para-toluenesulfonic acid in a glass beaker or vial;
- Gradual addition of 1.7 g (≈1 mL) of PFOTS (perfluoroctyltrichlorosilane) and mixing by vigorous magnetic stirring at 50 °C for 12 h;
- Rinse twice with 20 mL of hexane and discard the supernatants.
- Addition of 30 mL of toluene;
- Gradual addition of 0.75 g (≈0.8 mL) of APTES (amino-propyltriethoxysilane) and mixing by vigorous magnetic stirring at 60 °C for 12 h and discarding of the supernatants;
- Double rinsing of the mixture once with 20 mL of hexane and once with 20 mL of ethanol.
- By stoichiometric analysis (amine equivalent weight) mixing of 17 parts of TEPA for 100 parts of DGEBA;
- Addition into a glass beaker of 25 mL (29 g) of DGEBA and 5 mL (5 g) of TEPA;
- Addition of 23 mL of THF and mixing by vigorous magnetic stirring at room temperature (minimum ratio of 0.3 g to 1 mL of THF according to findings; considered ratio of 1.5 g–1 mL of THF: 23 mL THF for 34 g DGEBA/TEPA).
- Mixing the DE–PFOTS–APTES solution with the EPOXY solution in a glass beaker at room temperature.
- Immersion of the clean substrate into the EPOXY–PFOTS–APTES–DE solution for 5 min before withdrawal for the first dip-coating and for 30 s before withdrawal for the four subsequent immersions;
- Air drying of the substrate after each immersion at room temperature for 1 min.
- Air drying of the coating/substrate set at room temperature for 24 h;
- Heat treatment of the coating/substrate set in an oven at 130 °C for 2 h.
4. Results and Discussion
4.1. Characterization of the Coatings
4.1.1. Fourier Transform Infrared Spectroscopy
- (i)
- Diatomite in the as-received state;
- (ii)
- Diatomite grafted with APTES and then fluorinated with PFOTS;
- (iii)
- Diatomite fluorinated with PFTOS and then grafted with APTES.
- (i)
- CF2 functional group vibration at 696 cm−1;
- (ii)
- Si-O vibration at 754 cm−1;
- (iii)
- Si-O symmetric stretching vibration at 793 cm−1;
- (iv)
- Si-OH vibration at 908 cm−1;
- (v)
- Si-O-Si vibration at 1032 cm−1;
- (vi)
- Si-O in-plane stretching vibration at 1130 cm−1;
- (vii)
- C-C symmetric stretching vibration at 1159 cm−1;
- (viii)
- CF2 functional group symmetric stretching vibration at 1186 cm−1;
- (ix)
- CF2 functional group asymmetric vibration at 1227 cm−1.
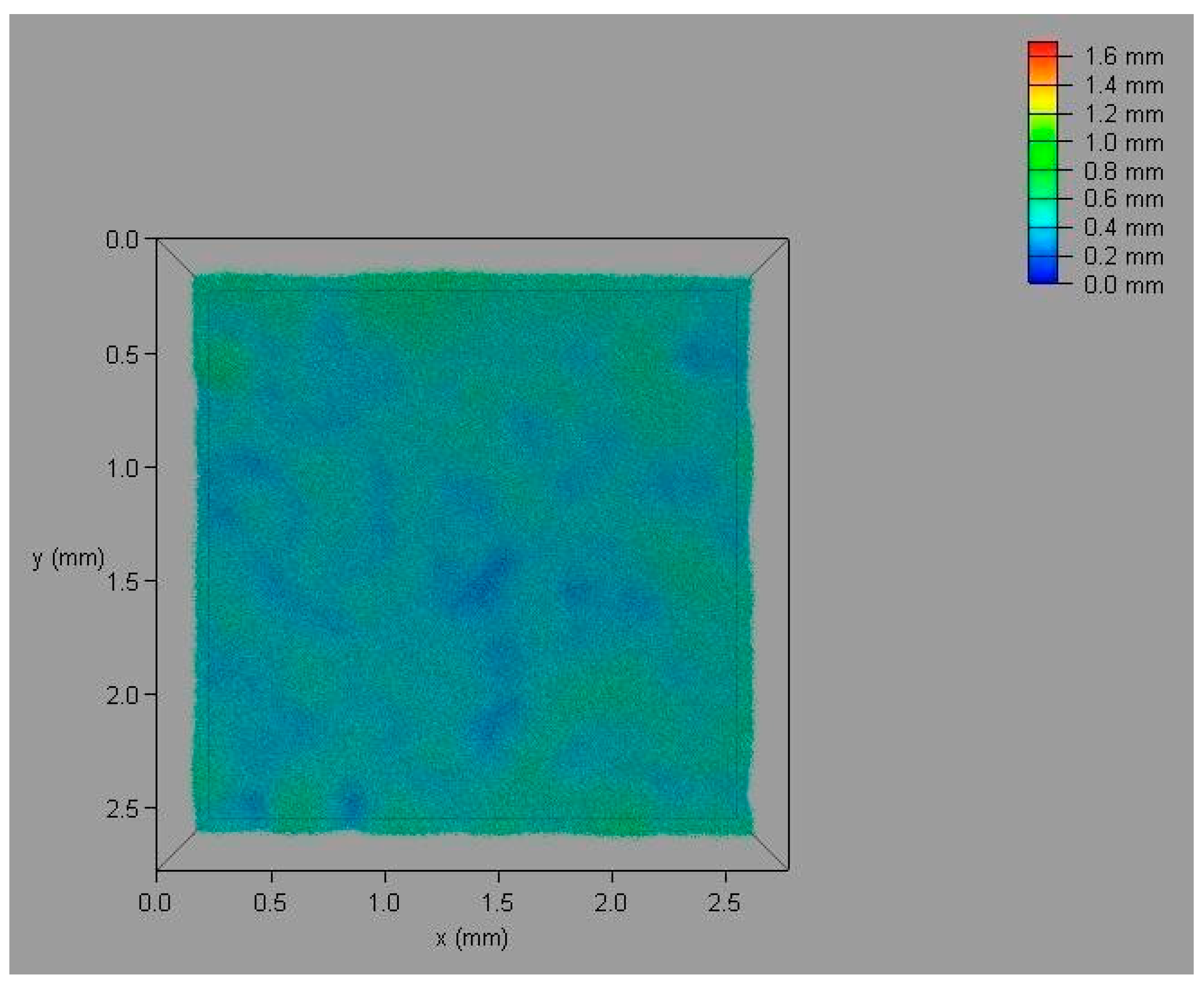
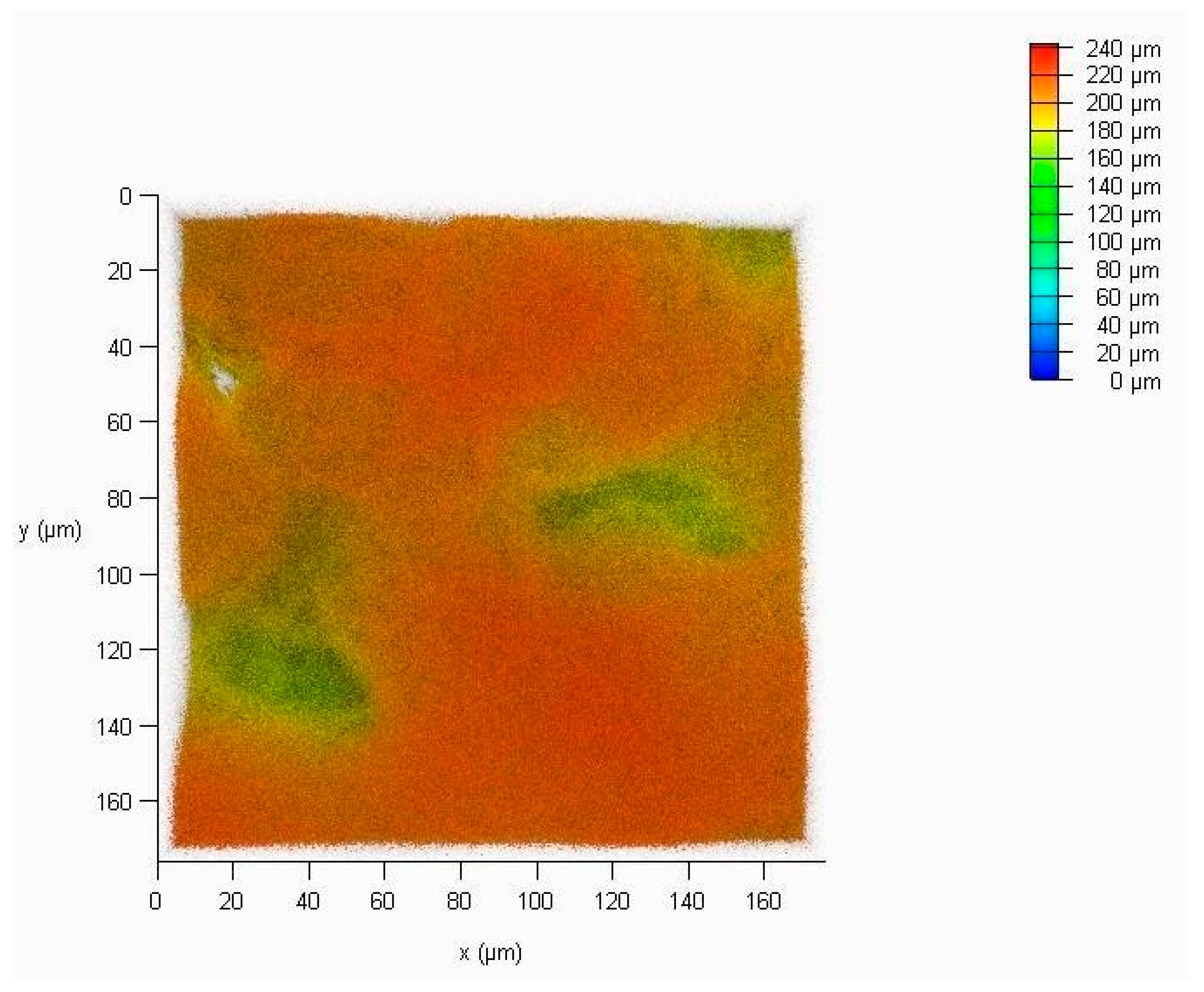
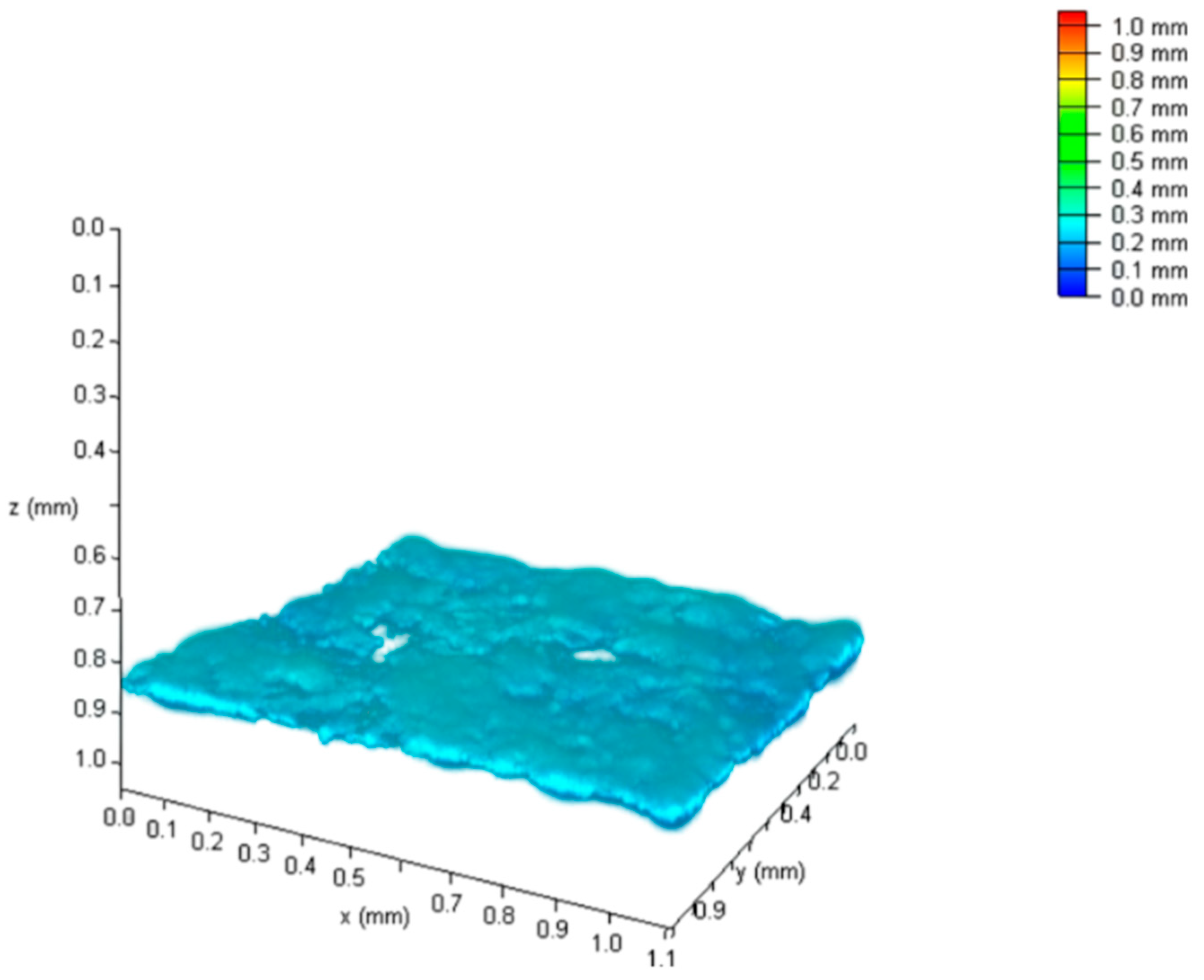
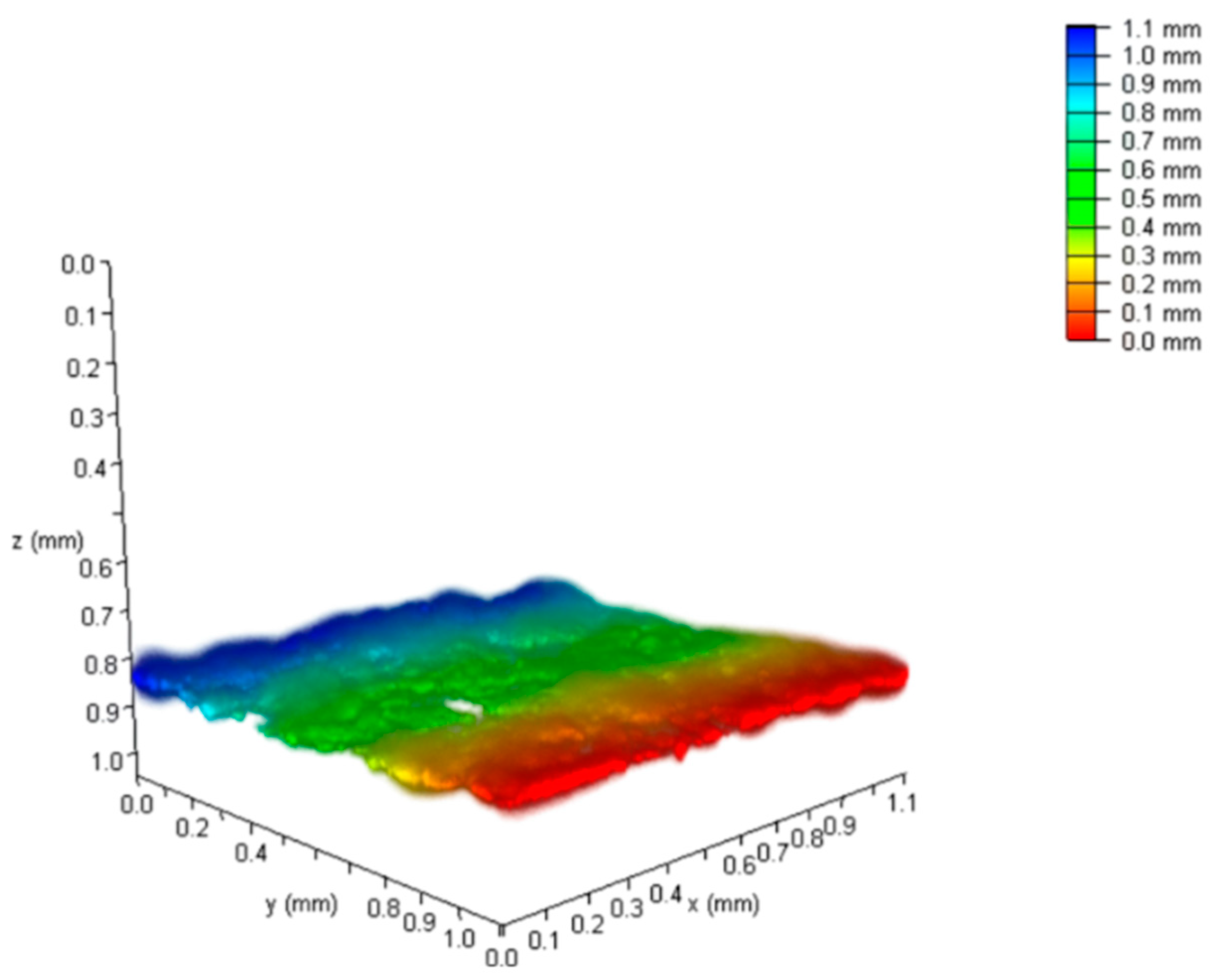
4.1.2. Laser Scanning Confocal Microscopy
4.1.3. Scanning Electron Microscopy
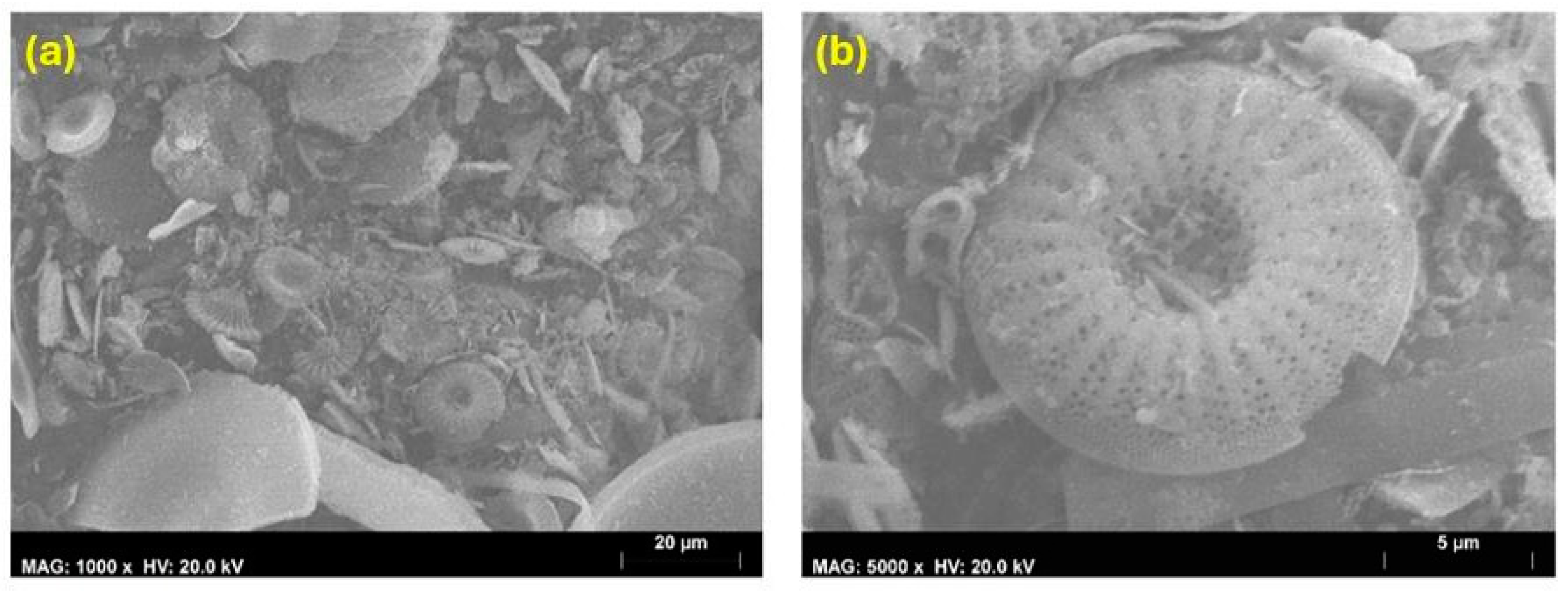
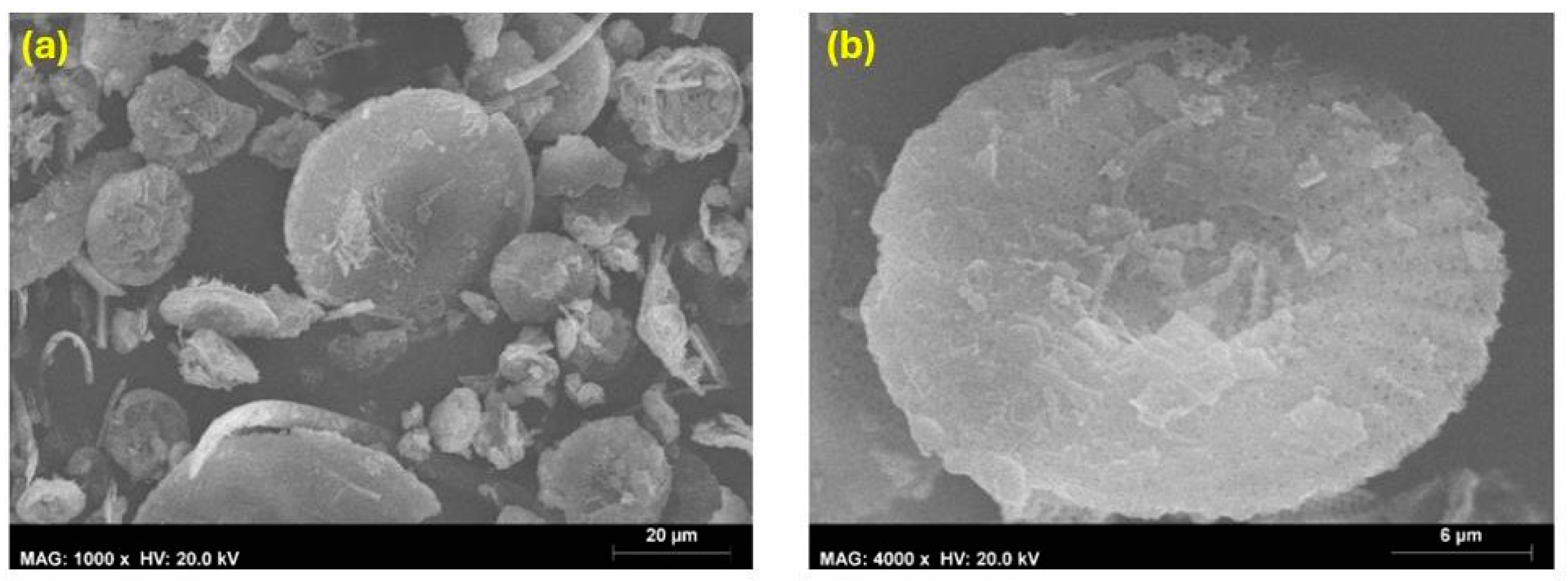
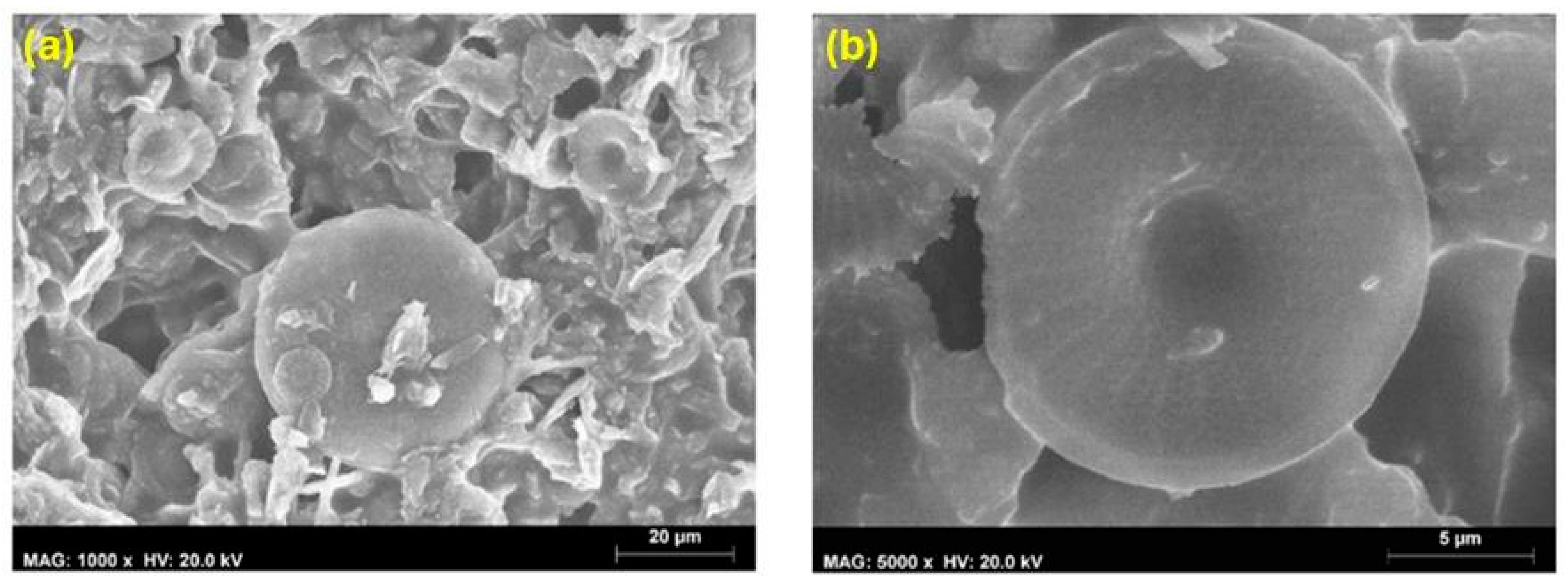
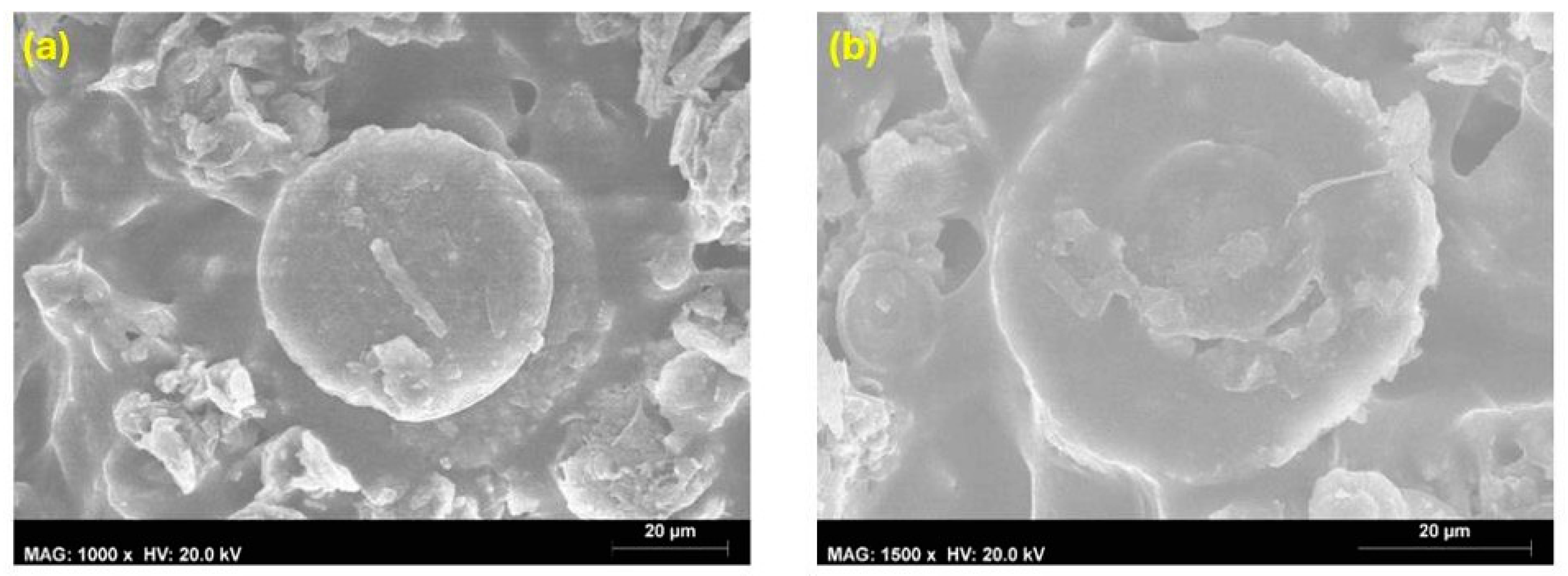


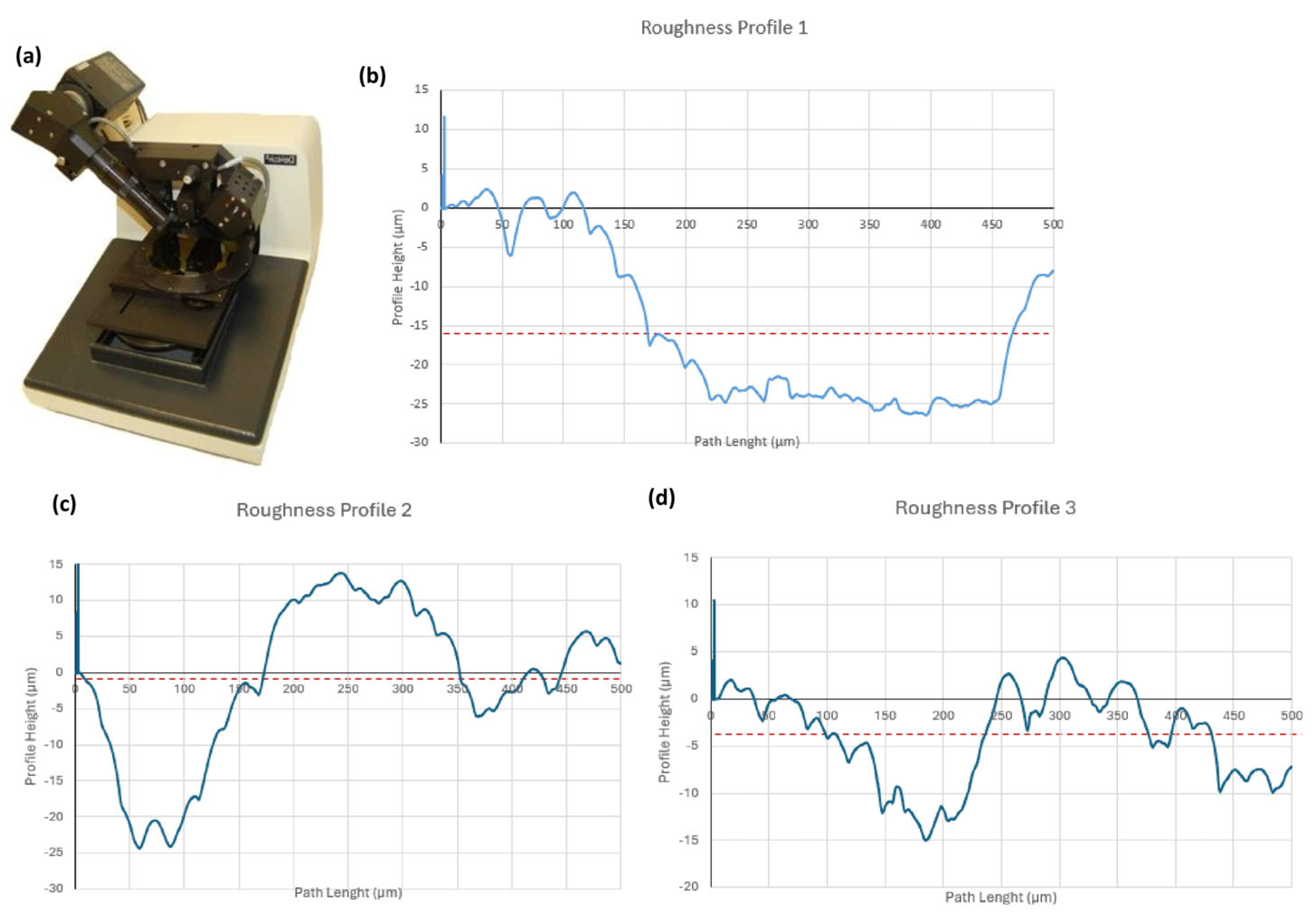
4.1.4. Roughness Measurement
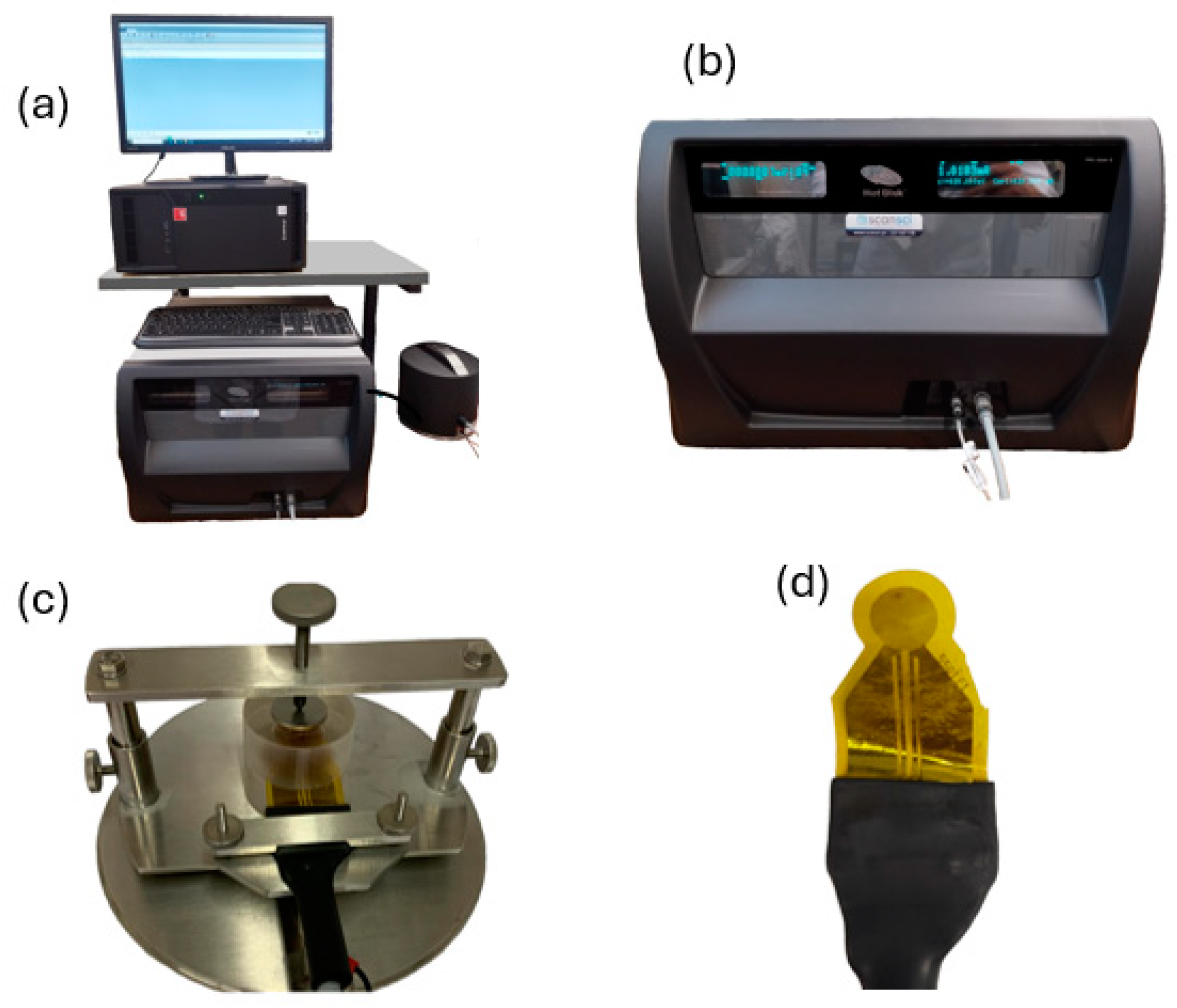
4.1.5. Contact Angle Measurement
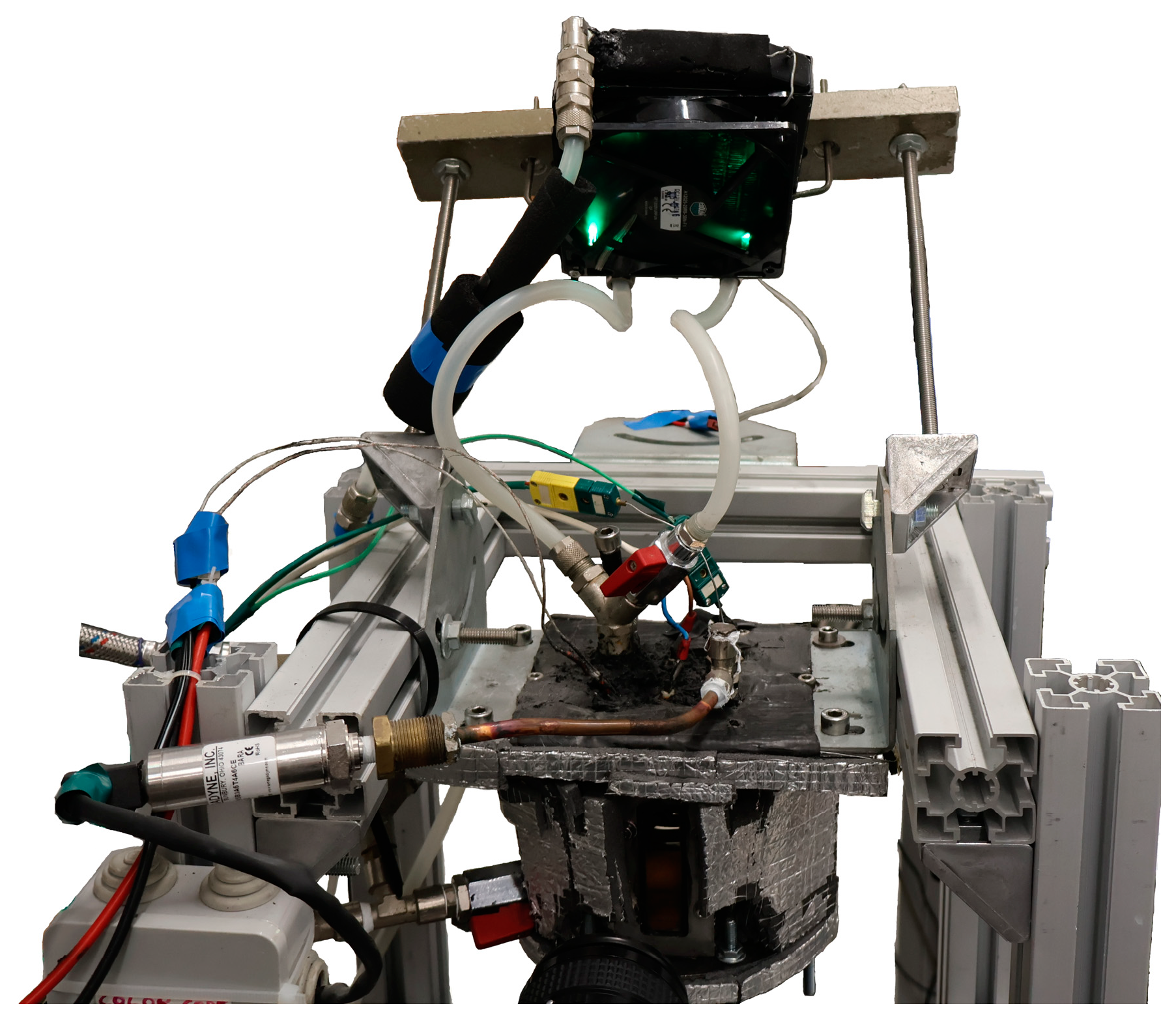
4.1.6. Thermal Conductivity Measurement
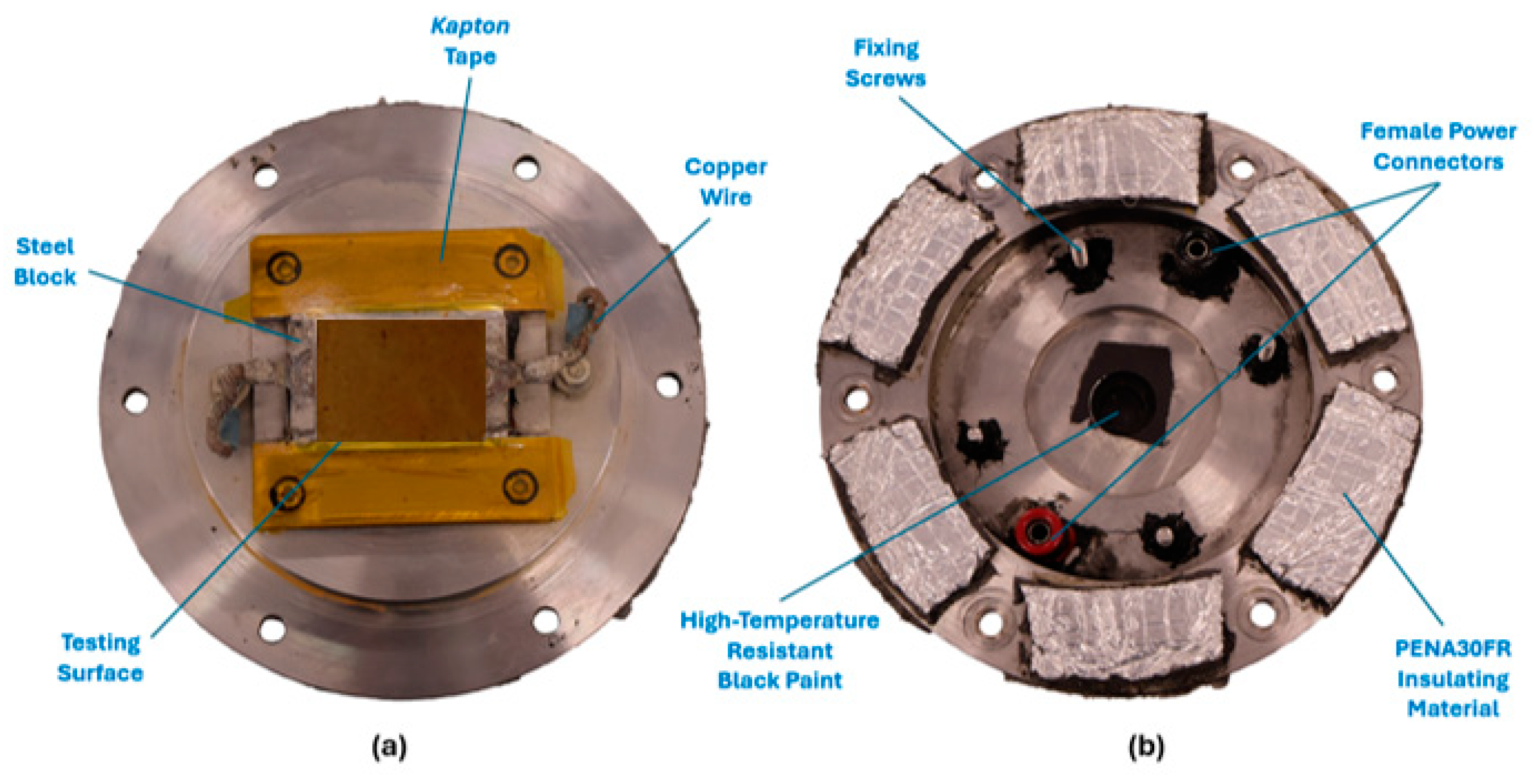
5. Durability Tests
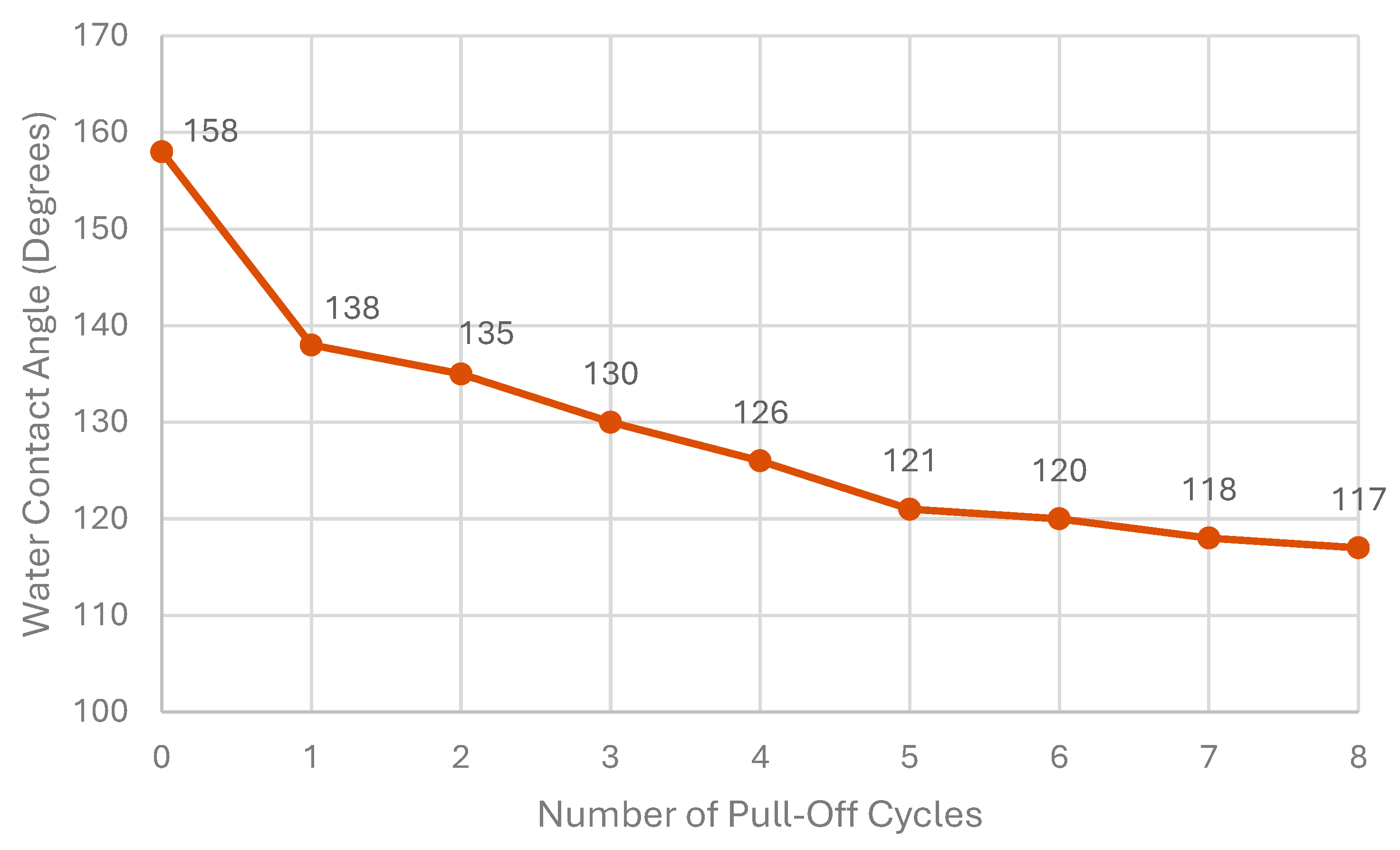
5.1. Adhesion Resistance Test (Peel-Off Test)
- A peeling tape is applied and carefully pressed down with a metal cylinder onto the surface to be tested to eliminate any air entrapment and to ensure uniform contact between the tape and testing coating;
- The tape is peeled off from the surface vertically from one end, the after-peeled condition of the coating is evaluated, and it is observed whether the coating was completely removed or, alternatively, only partially detached;
- The procedure of points one and two completes one peeling cycle, and after each cycle, the wettability of the coating should be characterized using a contact angle measurement.
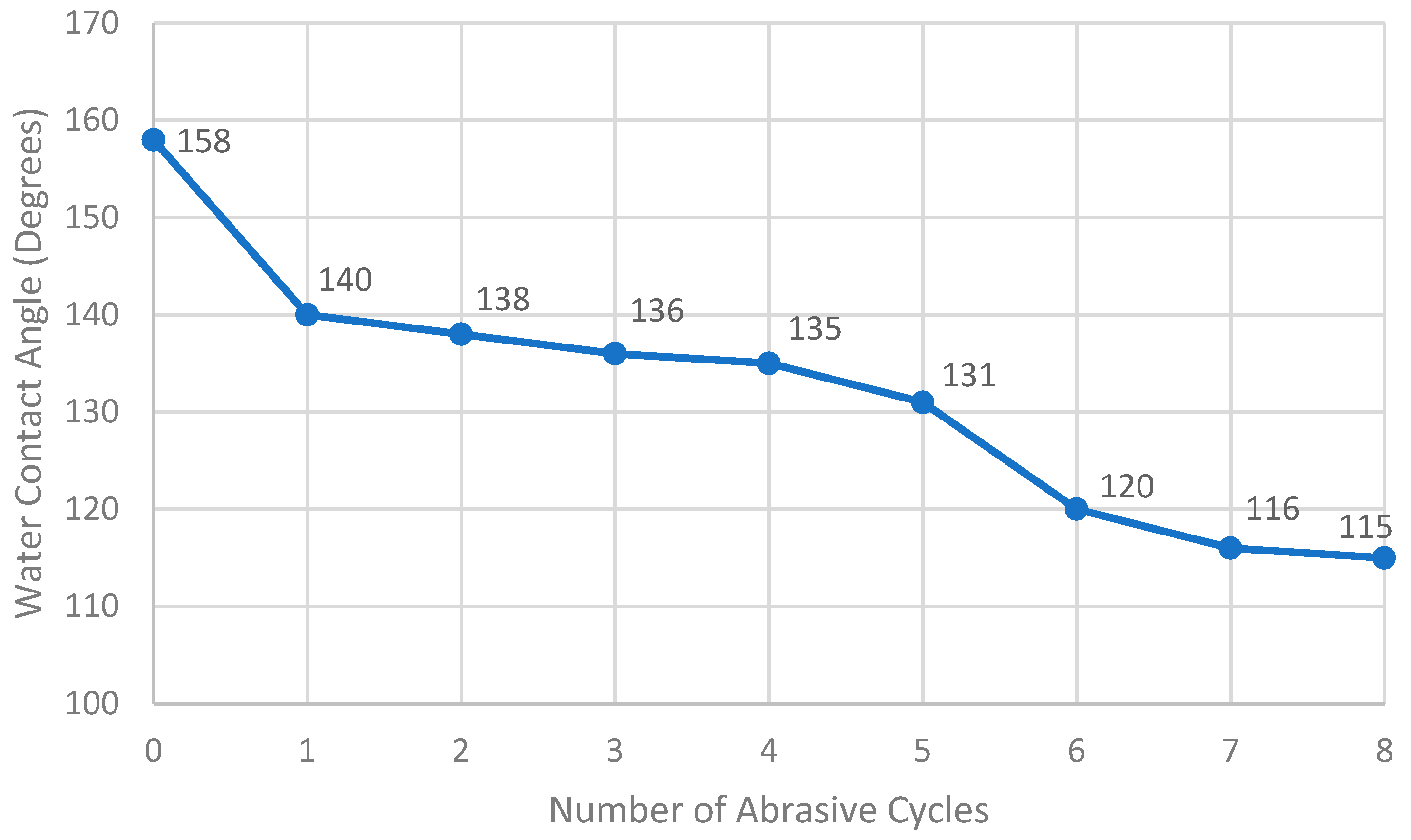
5.2. Abrasion Resistance Test
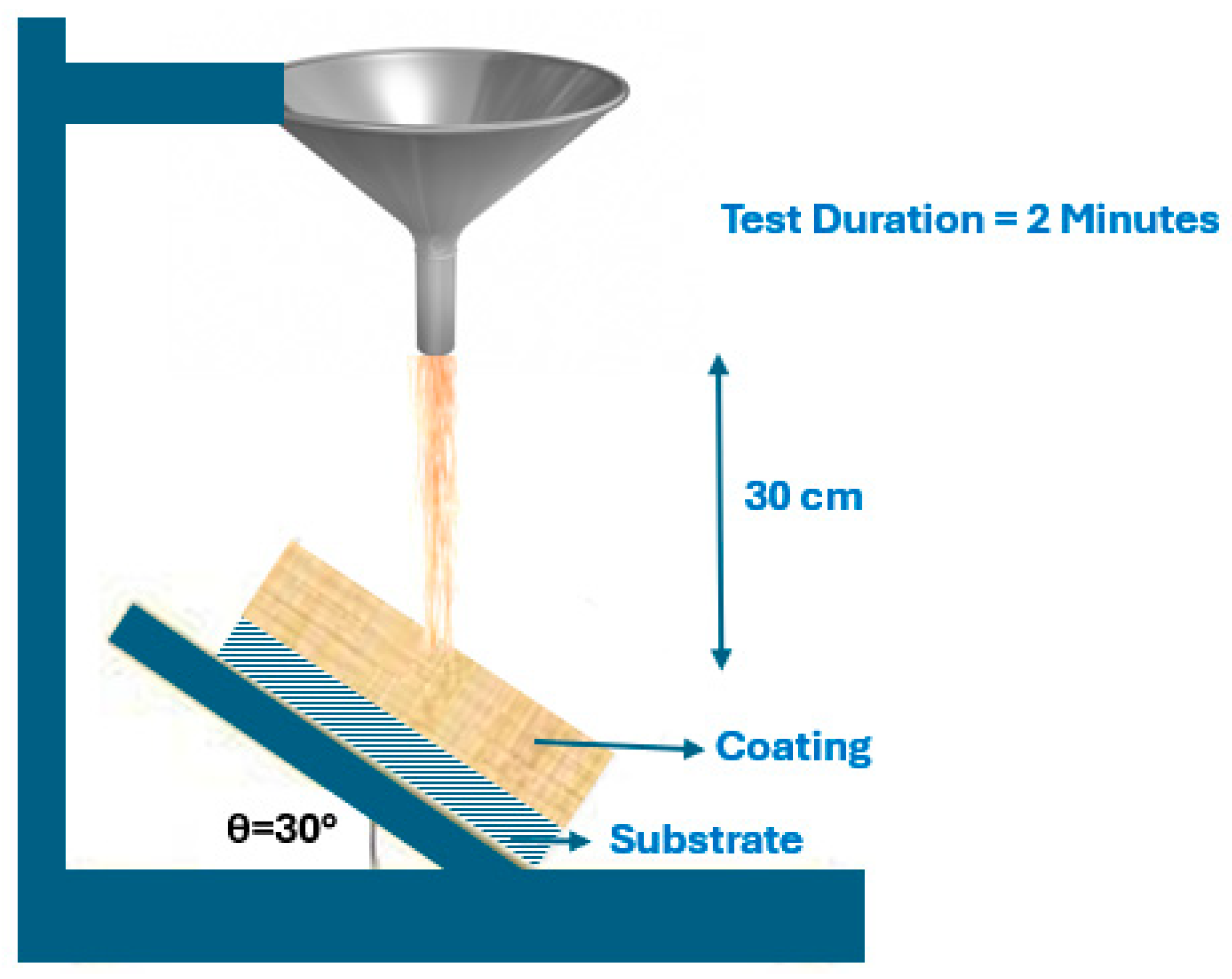
5.3. Solid Impact Resistance Test
5.4. Liquid Impact Resistance Test
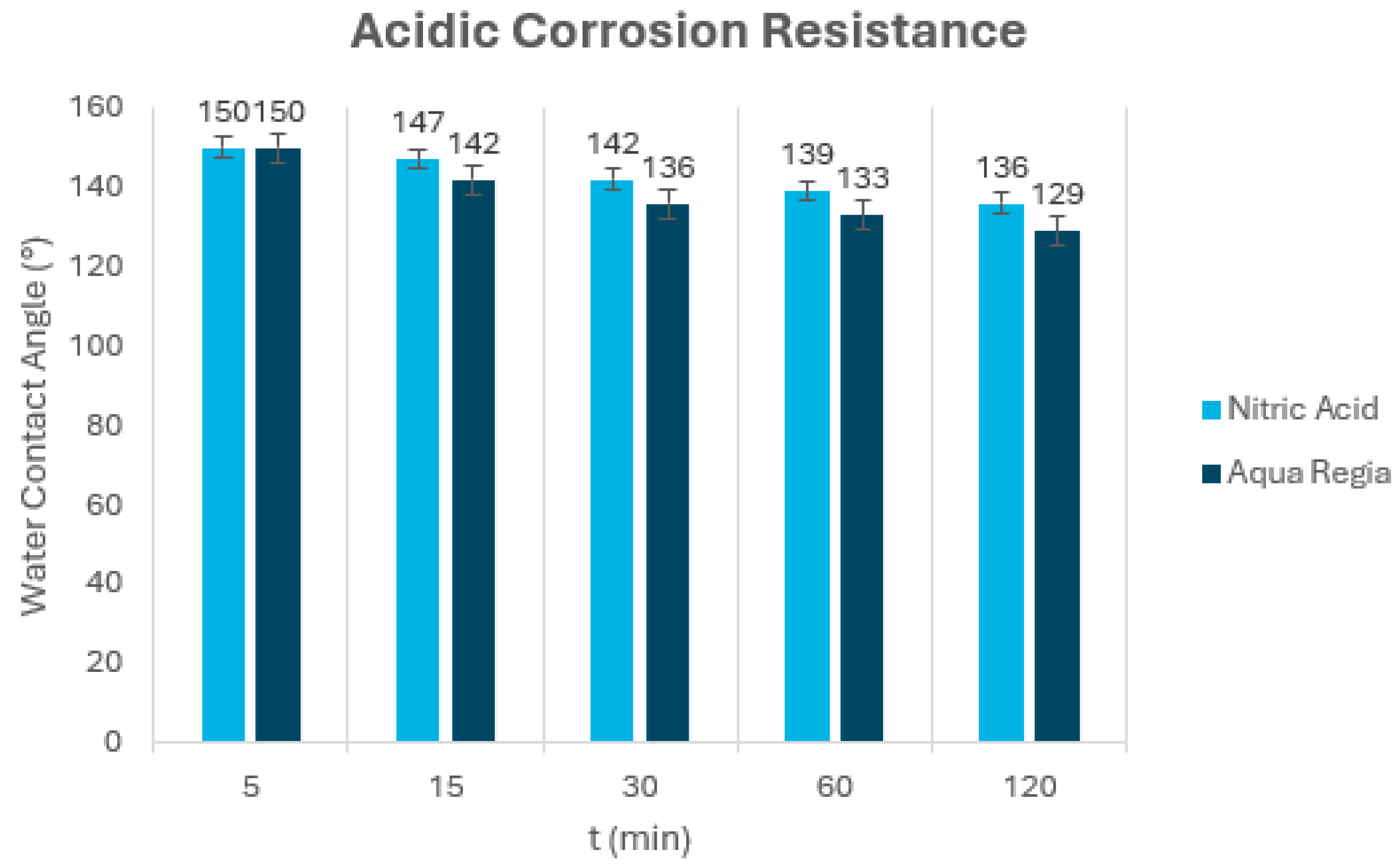
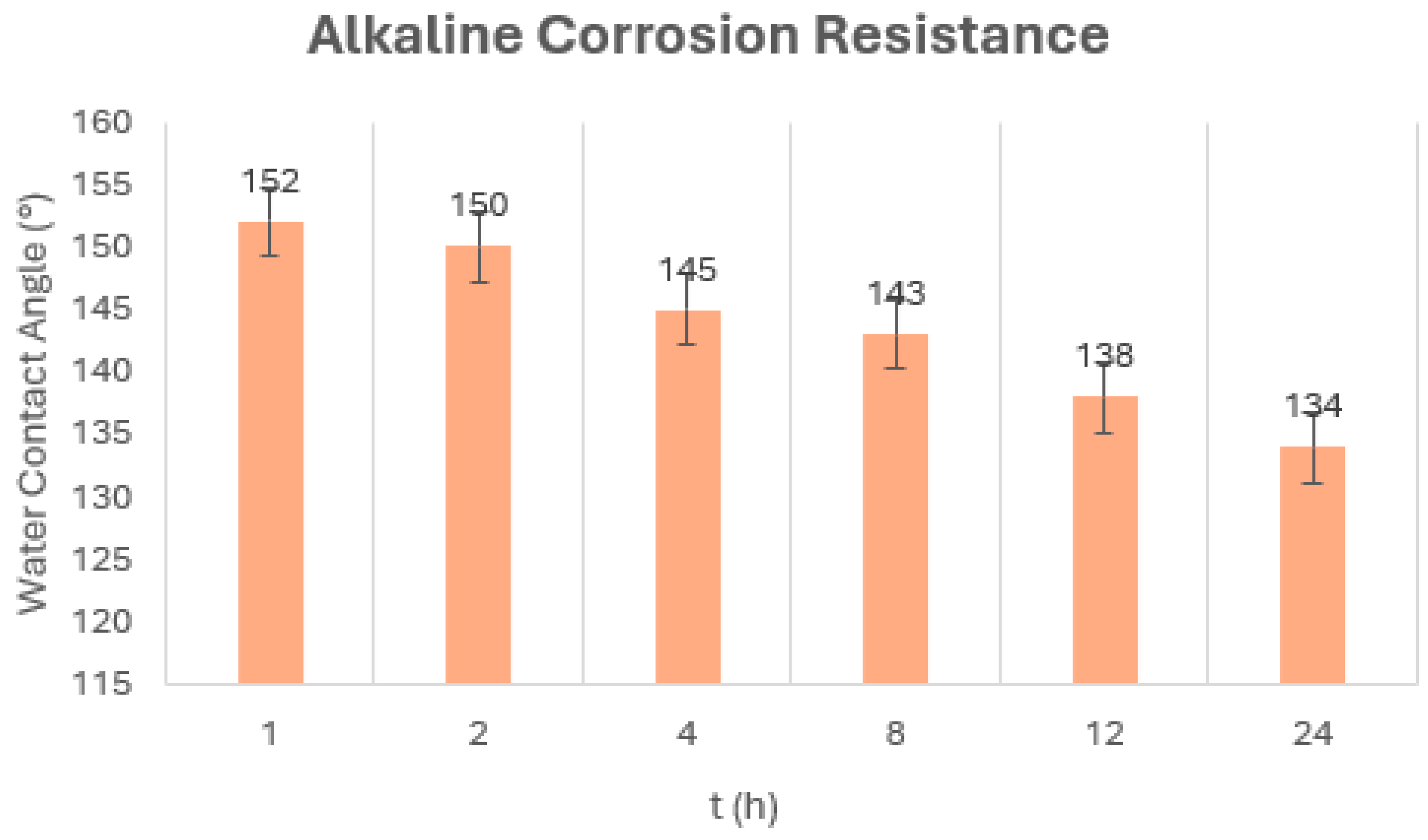
5.5. Corrosion Resistance Test
5.6. Air Durability Test
5.7. Water Durability Test
5.8. Aging Durability Test
6. Conclusions
- The water contact angle measurement gave relatively standard deviations from point to point. This was mainly due to the surface heterogeneities caused by the different surface roughness, thickness, porosity, and topographical features of the six measuring spots across the coatings;
- The dip-coating methodology has some limitations, such as the lack of total uniformity of the coatings if it is not subjected to a proper control. Hence, this method may impart non-uniform spreading of the coating particles, which may negatively influence the consistency of the experimental results;
- Since the nanopowders and nanomaterials commonly exhibit an agglomerating trend, special care should be given to the preparation of the coating solutions, always attempting to obtain the most uniform dispersion of the nanopowders in the solvents. Otherwise, the coating solution may result in the formation of local agglomerates and surface heterogeneities when applied to the substrate, whatever the coating method;
- However, in terms of scalability, all the referred issues are possible to mitigate, or even eliminate, in large-scale industrial production with ameliorated and rapid coating fabrication methods;
- The solutions with better wettability performance that obtained average contact angles superior to 135° (considered as quasi-superhydrophobic or even superhydrophobic by some authors) were DE–APTES–EPOXY, two-layer DE–PFOTS–EPOXY, and DE–PFOTS–APTES–EPOXY;
- It appears that the overlap of one or more coating layers did not entail any considerable wettability improvement. The superposition of coating layers even deteriorated the wettability measured for only one applied coating layer, as in the DE–APTES–EPOXY case;
- None of the coating alternatives presented superior robustness and durability characteristics in all the tested parameters according to the performed resistance assays. Nonetheless, the conducted qualitative analysis gave strong indications to conclude that the solutions with better wettability performance in the superhydrophobicity near range were the ones with better durability;
- Even considering the most performant coating formulations, these had only satisfactory performance in the robustness and durability tests, namely, in the more exigent ones like the abrasion resistance test;
- Despite the non-ideal results, the characterization and robustness test results of the coatings indicate at least satisfactory behavior under pool boiling conditions in the form of biphilic boiling surfaces;
- In further research, it is recommended to prepare biphilic boiling surfaces with different configurations of the developed coating of the superhydrophobic regions and test them under pool boiling conditions with the capture of high-speed images of the bubble dynamics and infrared thermographic images of the associated thermal phenomena.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cp | Specific heat capacity [J·Kg−1·K−1] |
| k | Thermal conductivity [Wm−1·K−1] |
| D | Thermal diffusivity [m2/s] |
| µ | Viscosity of suspensions [Pa·s] |
| Ra | Mean arithmetic rugosity [µm] |
| Rz | Mean peak-to-peak rugosity [µm] |
| Ts | Surface temperature (K) |
| Tsat | Saturation temperature of the liquid (K) |
Appendix A
| Coating | 1 Cycle | 2 Cycles | 3 Cycles | 4 Cycles | 5 Cycles |
|---|---|---|---|---|---|
| DE–APTES–EPOXY | √ | × | × | × | × |
| DE–APTES–EPOXY Two Layers | √ | √ | × | × | × |
| DE–APTES–EPOXY Three Layers | √ | √ | × | × | × |
| DE–PFOTS–EPOXY | √ | √ | × | × | × |
| DE–PFOTS–EPOXY Two Layers | √ | √ | √ | × | × |
| DE–PFOTS–APTES– EPOXY | √ | √ | × | × | × |
| DE–PFOTS–APTES– EPOXY Two Layers | √ | √ | √ | × | × |
| Coating | 10 kPa | 100 kPa |
|---|---|---|
| DE–APTES–EPOXY | √ | × |
| DE–APTES–EPOXY Two Layers | √ | √ |
| DE–APTES–EPOXY Three Layers | √ | √ |
| DE–PFOTS–EPOXY | √ | × |
| DE–PFOTS–EPOXY Two Layers | √ | × |
| DE–PFOTS–EPOXY Three Layers | √ | × |
| DE–PFOTS–APTES– EPOXY | √ | √ |
| DE–PFOTS–APTES– EPOXY Two Layers | √ | × |
| Coating | 24 h | 48 h | 96 h | 192 h | 384 h | 762 h |
|---|---|---|---|---|---|---|
| DE–APTES–EPOXY | √ | √ | √ | √ | × | × |
| DE–APTES–EPOXY Two Layers | √ | √ | √ | √ | √ | √ |
| DE–APTES–EPOXY Three Layers | √ | √ | √ | √ | √ | √ |
| DE–PFOTS–EPOXY | √ | √ | √ | √ | × | × |
| DE–PFOTS–EPOXY Two layers | √ | √ | √ | √ | √ | √ |
| DE–PFOTS–APTES– EPOXY | √ | √ | √ | √ | × | × |
| DE–PFOTS–APTES– EPOXY Two Layers | √ | √ | √ | √ | √ | √ |
| Coating | 24 h | 48 h | 96 h | 192 h | 384 h | 762 h |
|---|---|---|---|---|---|---|
| DE–APTES–EPOXY | √ | √ | √ | × | × | × |
| DE–APTES–EPOXY Two Layers | √ | √ | √ | √ | √ | × |
| DE–APTES–EPOXY Three Layers | √ | √ | √ | √ | √ | × |
| DE–PFOTS–EPOXY | √ | √ | √ | × | × | × |
| DE–PFOTS–EPOXY Two layers | √ | √ | √ | √ | √ | × |
| DE–PFOTS–APTES– EPOXY | √ | √ | √ | × | × | × |
| DE–PFOTS–APTES– EPOXY Two Layers | √ | √ | √ | √ | √ | × |
| Coating | Acetic Acid, pH = 3 | Sodium Hydroxide, pH = 13 |
|---|---|---|
| DE–APTES–EPOXY | × | × |
| DE–APTES–EPOXY Two Layers | √ | × |
| DE–APTES–EPOXY Three Layers | √ | √ |
| DE–PFOTS–EPOXY | √ | × |
| DE–PFOTS–EPOXY Two layers | √ | × |
| DE–PFOTS–APTES– EPOXY | √ | × |
| DE–PFOTS–APTES– EPOXY Two Layers | √ | × |
Appendix B

References
- Yuan, X.; Du, Y.; Fei, G.; Yang, R.; Wang, C.; Xu, Q.; Li, C. Experimental study on developing in-situ hierarchical micro/nanocrystals for improved capillary wicking. Microfluid. Nanofluid. 2023, 27, 31. [Google Scholar] [CrossRef]
- Vrieling, E.G.; Beelen, T.P.M.; van Santen, R.A.; Gieskes, W.W.C. Diatom silicon biomineralization as an inspirational source of new approaches to silica production. J. Biotechnol. 1999, 70, 39–51. [Google Scholar] [CrossRef]
- Gulturk, E.A.; Guden, M.; Tasdemirci, A. Calcined and natural frustules filled epoxy matrices: The effect of volume fraction on the tensile and compression behavior. Compos. B Eng. 2013, 44, 491–500. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Johnson, L.; Tran, D.N.H.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Tan, D.-Y.; Liu, K.-K.; Yu, H.-G.; Zhong, Y.-H.; Yuan, A.-H.; Yu, W.-B.; He, H.-P. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater. 2013, 170, 9–19. [Google Scholar] [CrossRef]
- Vasani, R.B.; Losic, D.; Cavallaro, A.; Voelcker, N.H. Fabrication of stimulus-responsive diatom biosilica microcapsules for antibiotic drug delivery. J. Mater. Chem. B 2015, 3, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Shibuichi, S.; Yamamoto, T.; Onda, T.; Tsujii, K. Super Water- and Oil-Repellent Surfaces Resulting from Fractal Structure. J. Colloid Interface Sci. 1998, 208, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Roethel, S.; Breedveld, V.; Hess, D.W. Creation of low hysteresis superhydrophobic paper by deposition of hydrophilic diamond-like carbon films. Cellulose 2013, 20, 3219–3226. [Google Scholar] [CrossRef]
- Nystrom, D.; Lindqvist, J.; Ostmark, E.; Hult, A.; Malmstrom, E. Superhydrophobic bio-fibre surfaces via tailored grafting architecture. Chem. Commun. 2006, 34, 3594–3596. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Oner, D.; Youngblood, J.; McCarthy, T. Ultrahydrophobic and Ultralyophobic Surfaces: Some Comments and Examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Callies, M.; Chen, Y.; Marty, F.; Pépin, A.; Quére, D. Microfabricated textured surfaces for super-hydrophobicity investigations. Microelectron. Eng. 2005, 78–79, 100–105. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Perry, C.C.; Roach, P. Porous materials show superhydrophobic to superhydrophilic switching. Chem. Commun. 2005, 25, 3135–3137. [Google Scholar] [CrossRef]
- Puretskiy, N.; Chanda, J.; Stoychev, G.; Synytska, A.; Ionov, L. Anti-Icing Superhydrophobic Surfaces Based on Core-Shell Fossil Particles. Adv. Mater. Interfaces 2015, 2, 1500124. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Reis, R.L.; Mano, J.F. Superhydrophobic Surfaces Engineered Using Diatomaceous Earth. ACS Appl. Mater. Interfaces 2013, 5, 10. [Google Scholar] [CrossRef]
- Polizos, G.; Winter, K.; Lance, M.J.; Meyer, H.M.; Armstrong, B.L.; Schaeffer, D.A.; Simpson, J.T.; Hunter, S.R.; Datskos, P.G. Scalable superhydrophobic coatings based on fluorinated diatomaceous earth: Abrasion resistance versus particle geometry. Appl. Surf. Sci. 2014, 292, 563–569. [Google Scholar] [CrossRef]
- Perera, H.J.; Khatiwada, B.K.; Paul, A.; Mortazavian, H.; Blum, F.D. Superhydrophobic surfaces with silane- treated diatomaceous earth/resin systems. J. Appl. Polym. Sci. 2016, 133, 44072. [Google Scholar] [CrossRef]
- Moita, A.; Moreira, A.; Pereira, J. Nanofluids for the Next Generation Thermal Management of Electronics: A Review. Symmetry 2021, 13, 1362. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Latthe, S.; Li, G.; An, S.; Yoon, S.; Liu, B.; Xing, R. Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, H.; Qing, Y.; Zhuo, Z.; Mo, Q. Formation of SiO2/polytetrafluoroethylene hybrid superhydrophobic coating. Appl. Surf. Sci. 2021, 258, 9859–9863. [Google Scholar] [CrossRef]
- Ipekci, H.; Harun, H.; Serdar, M.; Hancer, M. Superhydrophobic coatings with improved mechanical robustness based on polymer brushes. Surf. Coat. Technol. 2016, 299, 162–168. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Tang, A.-Q.; Huang, Q.-L.; Jia, H.; Mao, J.-H.; Ge, B.; Bai, H. Novel strategy in increasing stability and corrosion resistance for super-hydrophobic coating on aluminum alloy surfaces. Appl. Surf. Sci. 2011, 258, 580–585. [Google Scholar] [CrossRef]
- Kucuk, F.; Sismanoglu, S.; Kanbur, Y.; Tayfun, U. Effect of silane-modification of diatomite on its composites with thermoplastic polyurethane. Mater. Chem. Phys. 2020, 256, 123683. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Z.; Guo, Z.; Wang, Y.; Wang, J.; Liu, W.-B.; Derradji, M.; Qiu, J. Diatomite-filled epoxy resin composites: Curing behavior, dielectric, and thermal properties. Polym. Compos. 2022, 43, 422–429. [Google Scholar] [CrossRef]
- DIN 4768; Standard—Determination of Values of Surface Roughness Parameters Ra. Rz, Rmax Using Electrical Contact (Stylus) Instruments Concepts and Measuring Conditions. Deutsches Institut fur Normung: Berlin, Germany, 1990.
- ASTM D3359-02; Standard—Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM D4060-19; Standard—Standard Test Method for Abrasion Resistance of Organic Coatings by the Taber Abraser. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D968-22; Standard—Standard Test Methods for Abrasion Resistance of Organic Coatings by Falling Abrasive. ASTM International: West Conshohocken, PA, USA, 2022.












| Material-Function | Designation | Properties | Brand |
|---|---|---|---|
| Stainless Steel Foil—Substrate | AISI-304 Stainless Steel Foil | 20 µm-thick | Commercially available from several brands |
| Diatomite—Base Powder | Diatomaceous Earth, Kieselguhr | Sigma-Aldrich®, St. Louis, MO, USA | |
| PS | 192,000, granulated | Sigma-Aldrich® | |
| Epoxy Resin DGEBA—Binder | DGEBA: Bisphenol A diglycidyl ether C21H24O4 | Sigma-Aldrich® | |
| Epoxy Resin TEPA—Binder | TEPA: Tetraethylenepentamine C8H23N5 | Sigma-Aldrich® | |
| APTES—Silanization | 3-Aminopropyltriethoxy silane APTES, C9H23NO3Si, | ThermoScientific®, Waltham, MA, USA | |
| PFOTS—Silanization | 3-Aminopropyltriethoxy silane (PFOTS), C14H19F13O3Si | ThermoScientific® |
| 1 | 2 | 3 | 4 | 5 | Average | Standard Deviation | |
|---|---|---|---|---|---|---|---|
| Ra | 4.24 | 8.32 | 2.53 | 2.30 | 4.13 | 4.30 | 2.415 |
| Rz | 11.70 | 18.10 | 8.11 | 7.02 | 10.5 | 11.09 | 3.881 |
| Solution | Contact Angle Left (°) | Contact Angle Right (°) | Contact Angle (°) | Droplet Volume (µL) |
|---|---|---|---|---|
| DE–APTES–EPOXY | 135.7 ± 2.8 | 136.1 ± 3.4 | 135.9 ± 3.1 | 5.7 ± 0.2 |
| DE–APTES–EPOXY—two layers | 80.1 ± 4.8 | 80.1 ± 4.7 | 80.1 ± 4.7 | 4.9 ± 0.4 |
| DE–APTES–EPOXY—three layers | 62.3 ± 6.9 | 62.7 ± 6.7 | 62.5 ± 6.8 | 6.2 ± 0.8 |
| DE–PFOTS–EPOXY | 125.8 ± 7.2 | 126.0 ± 7.8 | 125.9 ± 7.4 | 4.7 ± 0.7 |
| DE–PFOTS–EPOXY—Two layers | 136.4 ± 4.2 | 135.6 ± 5.1 | 136.0 ± 4.5 | 5.3 ± 0.6 |
| DE–APTES–PFOTS–EPOXY | 138.8 ± 5.0 | 138.8 ± 7.1 | 138.8 ± 5.3 | 6.1 ± 0.4 |
| DE–APTES–PFOTS–EPOXY—Two Layers | 134.4 ± 6.1 | 134.8 ± 6.7 | 134.6 ± 6.4 | 5.1 ± 0.6 |
| Stainless Steel Substrate | 66.5 ± 3.0 | 67.5 ± 2.9 | 66.6 ± 3.4 | 4.6 ± 0.7 |
| Sample | Thermal Conductivity (W/m·K) | Thermal Diffusivity (mm2/s) | Specific Heat (MJ/m3·K) | Penetration Depth (mm) |
|---|---|---|---|---|
| PL = 25% | 0.12 ± 0.008 | 0.06 ± 0.007 | 2.11 ± 0.113 | 4.33 ± 0.270 |
| PL = 35% | 0.23 ± 0.004 | 0.16 ±0.006 | 1.49 ± 0.036 | 7.06 ± 0.140 |
| PL = 45% | 0.12 ± 0.004 | 0.13 ±0.008 | 0.90 ± 0.024 | 6.46 ± 0.190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Souza, R.; Moreira, A.; Moita, A. Durable and High-Temperature-Resistant Superhydrophobic Diatomite Coatings for Cooling Applications. Coatings 2024, 14, 805. https://doi.org/10.3390/coatings14070805
Pereira J, Souza R, Moreira A, Moita A. Durable and High-Temperature-Resistant Superhydrophobic Diatomite Coatings for Cooling Applications. Coatings. 2024; 14(7):805. https://doi.org/10.3390/coatings14070805
Chicago/Turabian StylePereira, José, Reinaldo Souza, António Moreira, and Ana Moita. 2024. "Durable and High-Temperature-Resistant Superhydrophobic Diatomite Coatings for Cooling Applications" Coatings 14, no. 7: 805. https://doi.org/10.3390/coatings14070805
APA StylePereira, J., Souza, R., Moreira, A., & Moita, A. (2024). Durable and High-Temperature-Resistant Superhydrophobic Diatomite Coatings for Cooling Applications. Coatings, 14(7), 805. https://doi.org/10.3390/coatings14070805










