Corrosion of Anodized Titanium Alloys
Abstract
:1. Introduction
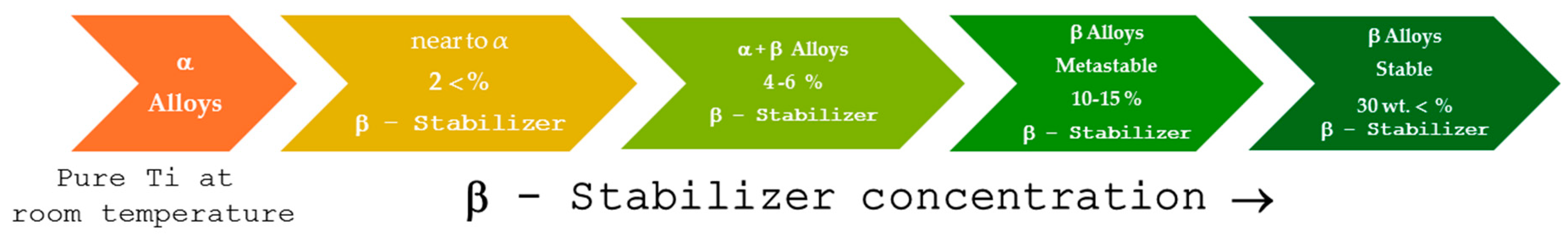
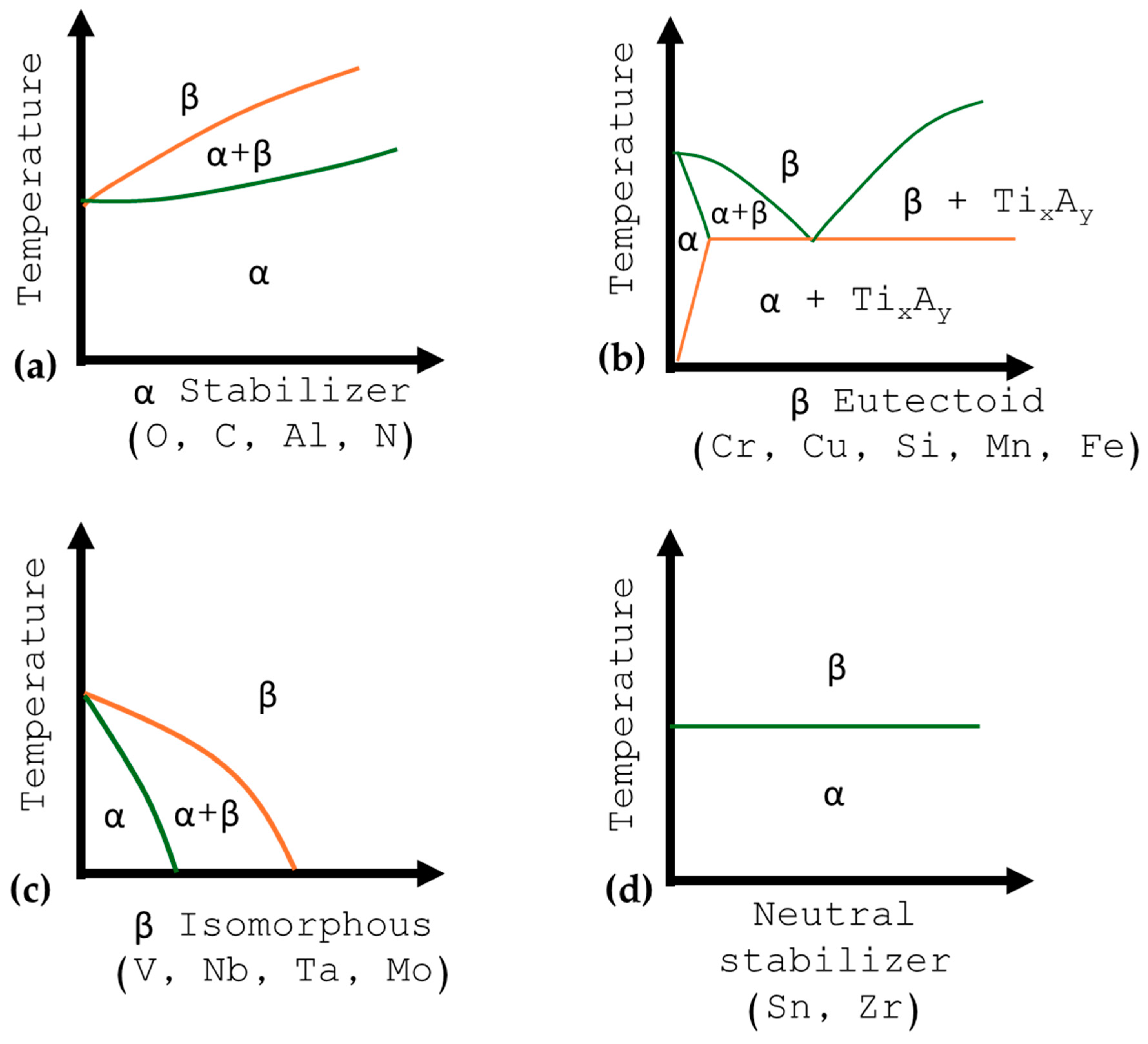
2. Titanium Classification
2.1. Titanium Alpha (α)
2.2. Titanium near to α
2.3. Titanium α + β
2.4. Titanium Beta (β)
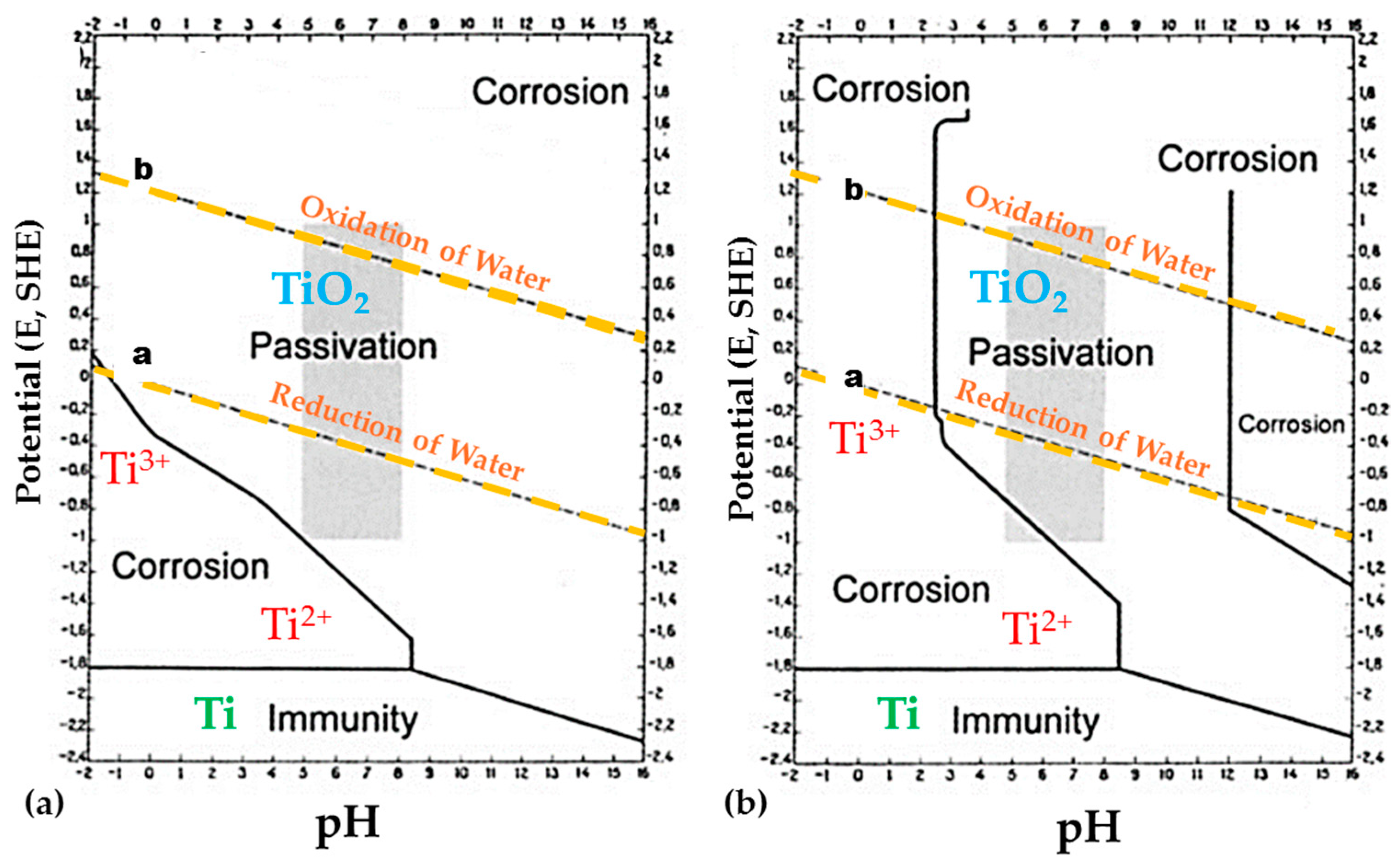
3. Corrosion in Titanium
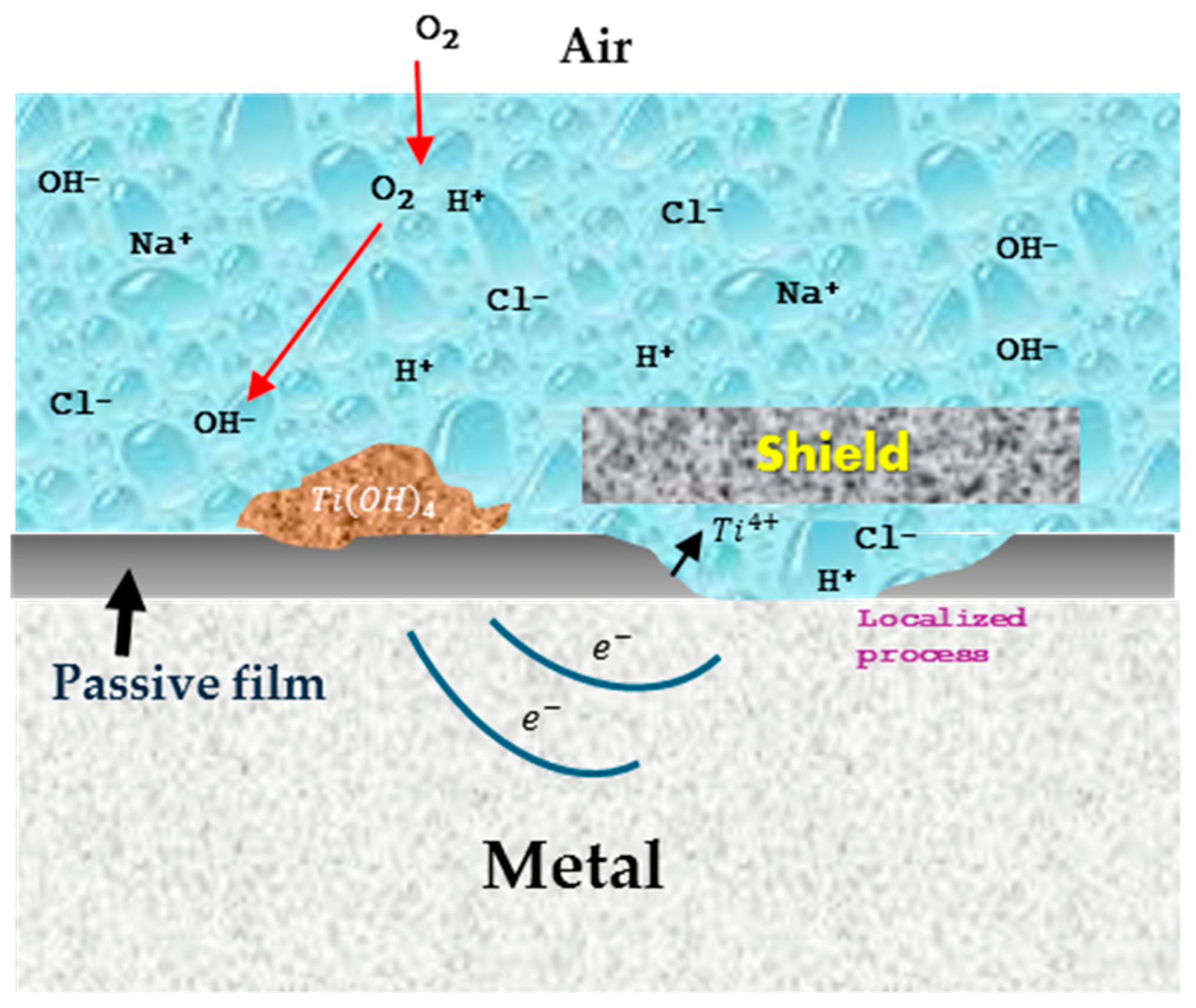
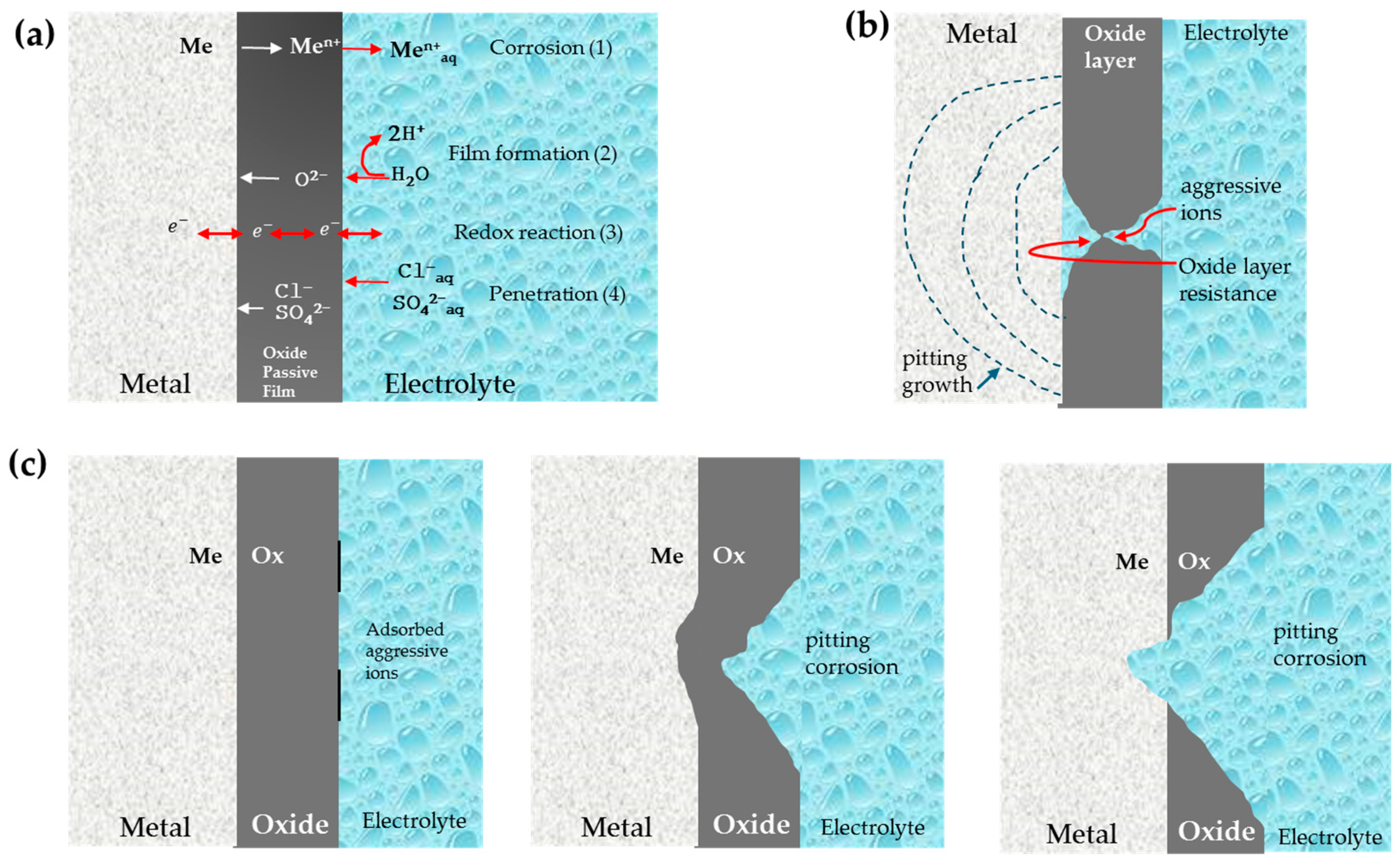
3.1. Corrosion Mechanisms
3.2. Crevice Corrosion
3.3. Pitting Corrosion
4. Coatings
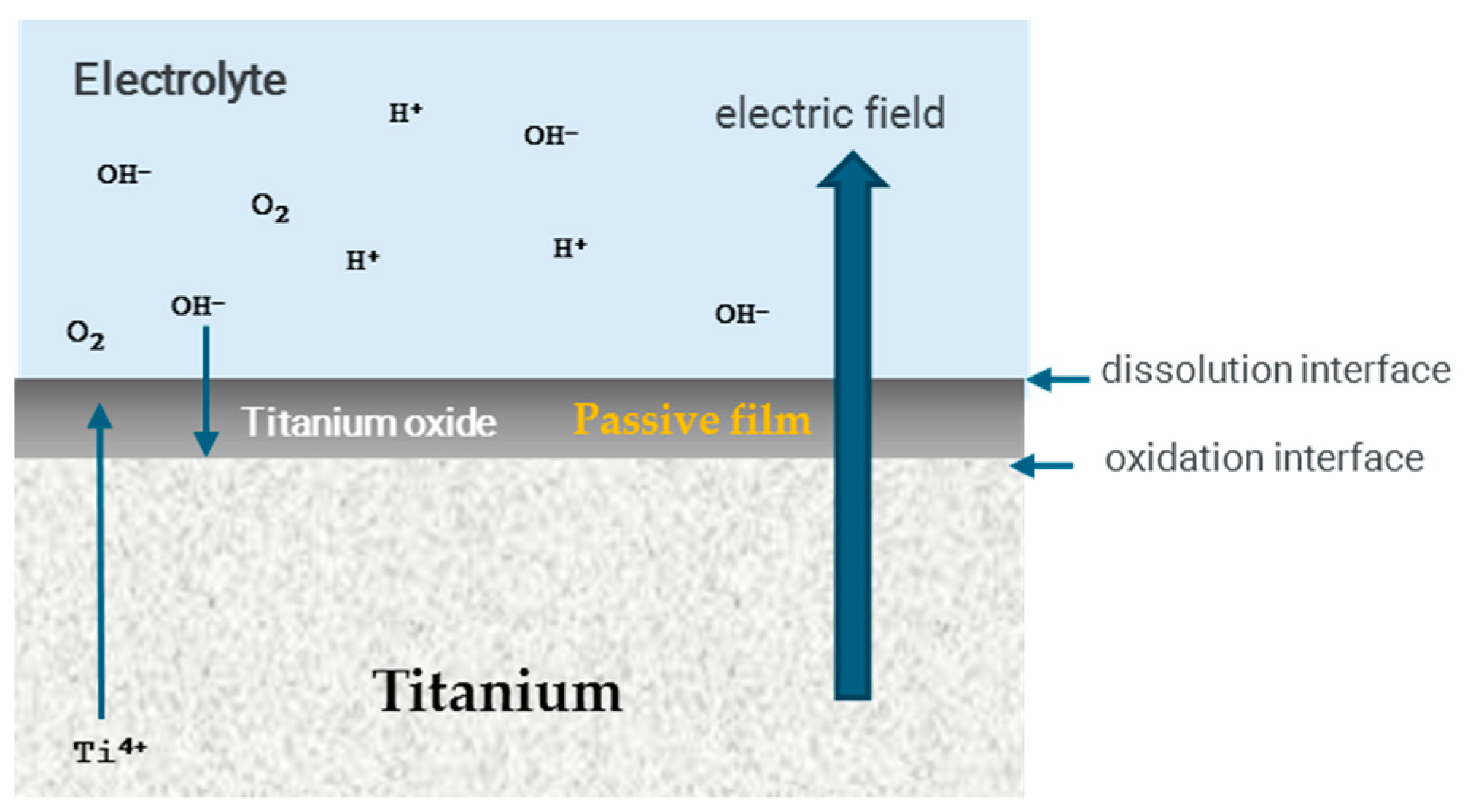
4.1. Anodizing
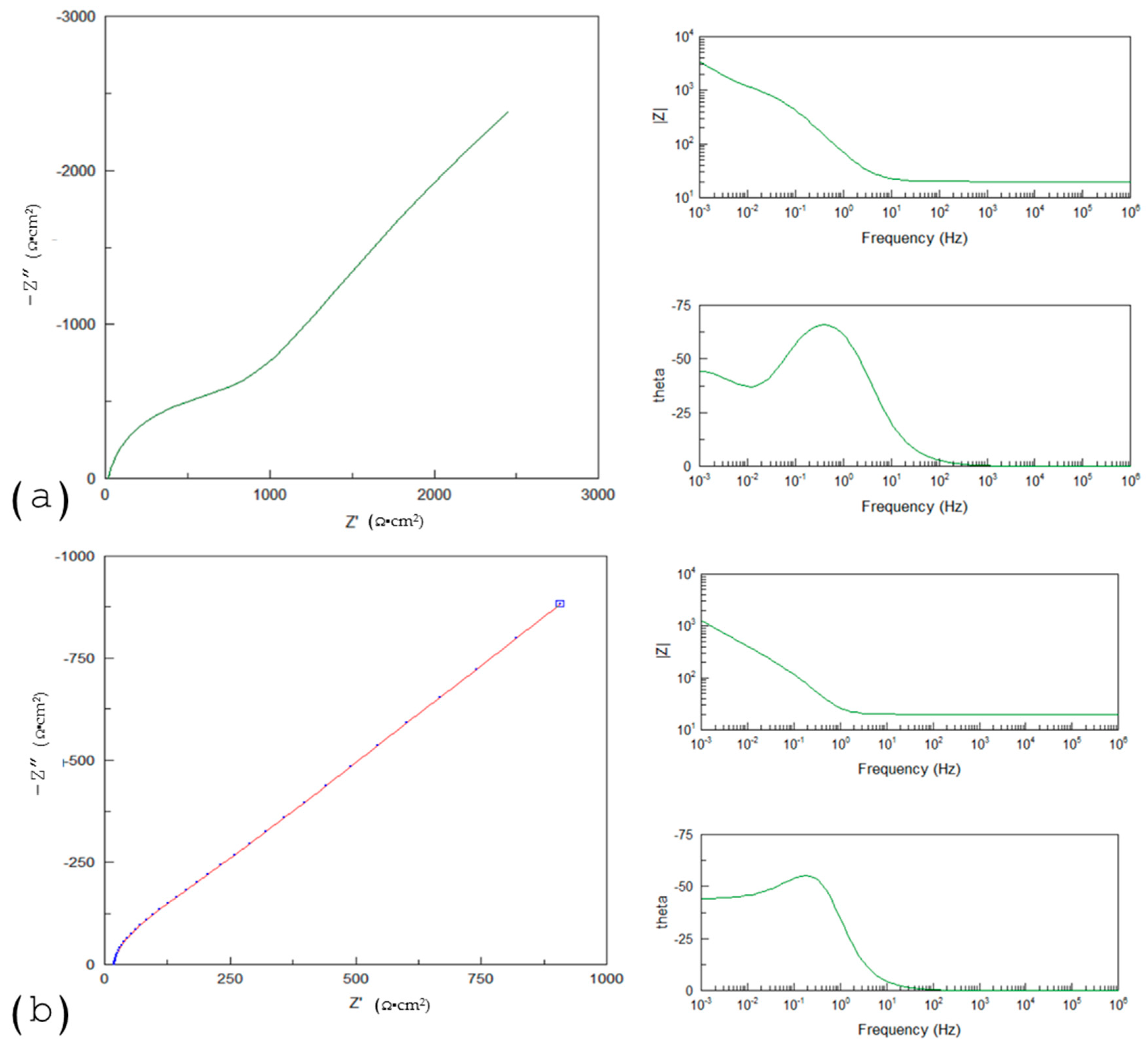
4.2. Corrosion in Anodizing Titanium
5. Future Directions for Anodized Titanium
6. Conclusions
- The research results indicate that in an anodizing process, the chemical composition of the alloy is one of the first variables to be considered. The chemical composition can determine the phases of the alloy and the type of coating that will form.
- ○
- Anodization shows better behavior against corrosion when it is carried out in acidic electrolytes. Alkaline media tend to generate a heterogeneous surface, while acidic media generate a uniform surface.
- ○
- The biomedical industry tends to develop anodization in neutral media because a compact layer with nanometric porosities is required. One of the most used electrolytes is ethylene glycol and some anodizing salts.
- ○
- Temperature influences anodization rates, porosity diameters, and anodization hardness. At high temperatures, it helps increase anodization rates; however, the anodization will present high porosity.
- Recent research showed that two-step anodization (TSA) performs better in developing a homogenous anodized layer than conventional anodizing. Also, the TSA presented high resistance to Cl− ion penetration.
- To generate a thin and compact oxide layer by anodization, it is necessary to apply a voltage between 10 and 50 V. Any voltage over that range will generate porosities on the anodized surface. This anodization voltage is used for biomedical Ti alloys.
- Titanium alloys and their anodized variants will present corrosion susceptibility when exposed to solution with halides. In the environment, Cl generates a corrosion process in Ti alloys and its anodized variants.
- Elements such as V generate an oxide layer susceptible to corrosion attacks; however, elements as Zr, Mo, Nb, and Cr help to generate a more stable oxide layer that avoids the corrosion process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sam Froes, F.H.; Qian, M.; Niinomi, M. An Introduction to Titanium in Consumer Applications. In Titanium for Consumer Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–12. [Google Scholar] [CrossRef]
- Sha, W.; Malinov, S. Titanium Alloys: Modelling of Microstructure, Properties and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; ISBN 9781845693756. [Google Scholar]
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys. In Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030244392. [Google Scholar]
- Mouritz, A.P. Introduction to Aerospace Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2012; ISBN 9781855739468. [Google Scholar]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Titanium and Titanium Alloys, 1st ed.; Leyens, C., Peters, M., Eds.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; De Los Rios, J.P.F.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Chien, C.S.; Hung, Y.C.; Hong, T.F.; Wu, C.C.; Kuo, T.Y.; Lee, T.M.; Liao, T.Y.; Lin, H.C.; Chuang, C.H. Preparation and Characterization of Porous Bioceramic Layers on Pure Titanium Surfaces Obtained by Micro-Arc Oxidation Process. Appl. Phys. A Mater. Sci. Process. 2017, 123, 204. [Google Scholar] [CrossRef]
- Yang, X.; Liu, C.R. Machining Titanium and Its Alloys. Mach. Sci. Technol. 1999, 3, 107–139. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Seetharaman, V.; Weiss, I. The Thermomechanical Processing of Alpha/Beta Titanium Alloys. Miner. Met. Mater. Soc. 1997, 49, 33–39. [Google Scholar] [CrossRef]
- Contu, F.; Elsener, B.; Böhni, H. A Study of the Potentials Achieved during Mechanical Abrasion and the Repassivation Rate of Titanium and Ti6Al4V in Inorganic Buffer Solutions and Bovine Serum. Electrochim. Acta 2004, 50, 33–41. [Google Scholar] [CrossRef]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface Damage of Dental Implant Systems and Ions Release after Exposure to Fluoride and Hydrogen Peroxide. J. Periodontal Res. 2019, 54, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Oh, M.C.; Kim, J.T.; Srivastava, A.K.; Ahn, B. Investigation of Electrochemical Corrosion Behavior of Additive Manufactured Ti–6Al–4V Alloy for Medical Implants in Different Electrolytes. J. Alloys Compd. 2020, 830, 154620. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is There Scientific Evidence Favoring the Substitution of Commercially Pure Titanium with Titanium Alloys for the Manufacture of Dental Implants? Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Mehkri, S.; Abishek, N.R.; Sumanth, K.S.; Rekha, N. Study of the Tribocorrosion Occurring at the Implant and Implant Alloy Interface: Dental Implant Materials. Mater. Today Proc. 2021, 44, 157–165. [Google Scholar] [CrossRef]
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic Interactions between Corrosion and Wear at Titanium-Based Dental Implant Connections: A Scoping Review. J. Periodontal Res. 2017, 52, 946–954. [Google Scholar] [CrossRef]
- Dini, C.; Costa, R.C.; Sukotjo, C.; Takoudis, C.G.; Mathew, M.T.; Barão, V.A.R. Progression of Bio-Tribocorrosion in Implant Dentistry. Front. Mech. Eng. 2020, 6, 497882. [Google Scholar] [CrossRef]
- Diercks, D.R.; Loomis, B.A. Alloying and Impurity Effects in Vanadium-Base Alloys. J. Nucl. Mater. 1986, 141–143, 1117–1124. [Google Scholar] [CrossRef]
- Meng, Y.; Cui, J.; Zhao, Z.; Zuo, Y. Effect of Vanadium on the Microstructures and Mechanical Properties of an Al–Mg–Si–Cu–Cr–Ti Alloy of 6XXX Series. J. Alloys Compd. 2013, 573, 102–111. [Google Scholar] [CrossRef]
- Shen, J.; Nagasaka, T.; Tokitani, M.; Muroga, T.; Kasada, R.; Sakurai, S. Effects of Titanium Concentration on Microstructure and Mechanical Properties of High-Purity Vanadium Alloys. Mater. Des. 2022, 224, 111390. [Google Scholar] [CrossRef]
- Najafi, H.; Rassizadehghani, J. Effects of Vanadium and Titanium on Mechanical Properties of Low Carbon as Cast Microalloyed Steels. Int. J. Cast Met. Res. 2006, 19, 323–329. [Google Scholar] [CrossRef]
- Vaughan, J.; Alfantazi, A. Corrosion of Titanium and Its Alloys in Sulfuric Acid in the Presence of Chlorides. J. Electrochem. Soc. 2006, 153, B6. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Calderon-Moreno, J.M.; Drob, P.; Vasilescu, E. Characterisation of Passive Film and Corrosion Behaviour of a New Ti-Ta-Zr Alloy in Artificial Oral Media: In Time Influence of pH and Fluoride Ion Content. Mater. Corros. 2015, 66, 971–981. [Google Scholar] [CrossRef]
- Pushp, P.; Dasharath, S.M.; Arati, C. Classification and Applications of Titanium and Its Alloys. Mater. Today Proc. 2022, 54, 537–542. [Google Scholar] [CrossRef]
- Yadav, P.; Saxena, K.K. Effect of Heat-Treatment on Microstructure and Mechanical Properties of Ti Alloys: An Overview. Mater. Today Proc. 2020, 26, 2546–2557. [Google Scholar] [CrossRef]
- Tendero Lozano, I. Desarrollo de Aleaciones de Titanio-Manganeso Pulvimetalúrgicas Para Aplicaciones Biomédicas; Universitat Politècnica de València: Valencia, Spain, 2018. [Google Scholar]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Loureiro Properties and Applications of Titanium Alloys: A Brief Review. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Lütjering, G.; Williams, J.C. Titanium; Engineering Materials, Processes; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-71397-5. [Google Scholar]
- Abe, J.O.; Popoola, A.P.I.; Popoola, O.M. Influence of Varied Process Parameters on the Microstructure, Densification and Microhardness of Spark Plasma Sintered Ti-6Al-4V/h-BN Binary Composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 689, 012005. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Salleh, K.; Sahari, M.; Ishak, M.; Khidhir, B.A. Titanium and Its Alloy. Int. J. Sci. Res. 2014, 3, 1351–1361. [Google Scholar]
- Donachie, M.J. Titanium—A Technical Guide; ASM International: Detroit, MI, USA, 2000; Volume 55, ISBN 978-0-87170-686-7. [Google Scholar]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical Corrosion of Titanium and Titanium-Based Alloys. J. Prosthet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef]
- Pathania, A.; Kumar, S.A.; Nagesha, B.K.; Barad, S.; Suresh, T.N. Reclamation of Titanium Alloy Based Aerospace Parts Using Laser Based Metal Deposition Methodology. Mater. Today Proc. 2021, 45, 4886–4892. [Google Scholar] [CrossRef]
- Peel, C.J.; Gregson, P.J. Design Requirements for Aerospace Structural Materials. In High Performance Materials in Aerospace; Springer: Dordrecht, The Netherlands, 1995; pp. 1–48. [Google Scholar] [CrossRef]
- Barrington, N.; Black, M. Aerospace Materials and Manufacturing Processes at the Millennium. In Aerospace Materials; CRC Press: Boca Raton, FL, USA, 2020; Volume 1, pp. 15–26. [Google Scholar] [CrossRef]
- Dutta Majumdar, J.; Manna, I. Laser Surface Engineering of Titanium and Its Alloys for Improved Wear, Corrosion and High-Temperature Oxidation Resistance. In Laser Surface Engineering: Processes and Applications; Woodhead Publishing: Sawston, UK, 2015; pp. 483–521. ISBN 9781782420798. [Google Scholar]
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Sankaran, K.K.; Mishra, R.S. Metallurgy and Design of Alloys with Hierarchical Microstructures; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128120255. [Google Scholar]
- Bermingham, M.J.; StJohn, D.H.; Krynen, J.; Tedman-Jones, S.; Dargusch, M.S. Promoting the Columnar to Equiaxed Transition and Grain Refinement of Titanium Alloys during Additive Manufacturing. Acta Mater. 2019, 168, 261–274. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Bambach, M.D.; Seifert, D.; Sizova, I. Intensive Forming of Grade 5 Titanium Bars with Increased Performance for Aerospace Applications. Procedia Manuf. 2020, 47, 288–294. [Google Scholar] [CrossRef]
- Bermingham, M.J.; Kent, D.; Pace, B.; Cairney, J.M.; Dargusch, M.S. High Strength Heat-Treatable β-Titanium Alloy for Additive Manufacturing. Mater. Sci. Eng. A 2020, 791, 139646. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; Van Der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion Behavior of Titanium Alloys in Acidic and Saline Media: Role of Alloy Design, Passivation Integrity, and Electrolyte Modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Neacsu, E.I.; Mirza Rosca, J.C. Surface Analysis and Corrosion Resistance of a New Titanium Base Alloy in Simulated Body Fluids. Corros. Sci. 2012, 65, 431–440. [Google Scholar] [CrossRef]
- Castany, P.; Gordin, D.M.; Drob, S.I.; Vasilescu, C.; Mitran, V.; Cimpean, A.; Gloriant, T. Deformation Mechanisms and Biocompatibility of the Superelastic Ti–23Nb–0.7Ta–2Zr–0.5N Alloy. Shape Mem. Superelast. 2016, 2, 18–28. [Google Scholar] [CrossRef]
- Vasilescu, C.; Drob, S.I.; Osiceanu, P.; Moreno, J.M.C.; Prodana, M.; Ionita, D.; Demetrescu, I.; Marcu, M.; Popovici, I.A.; Vasilescu, E. Microstructure, Surface Characterization, and Electrochemical Behavior of New Ti-Zr-Ta-Ag Alloy in Simulated Human Electrolyte. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 513–523. [Google Scholar] [CrossRef]
- Coakley, J.; Vorontsov, V.A.; Littrell, K.C.; Heenan, R.K.; Ohnuma, M.; Jones, N.G.; Dye, D. Nanoprecipitation in a Beta-Titanium Alloy. J. Alloys Compd. 2015, 623, 146–156. [Google Scholar] [CrossRef]
- Wandelt, K. Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Brandoli, B.; de Geus, A.R.; Souza, J.R.; Spadon, G.; Soares, A.; Rodrigues, J.F.; Komorowski, J.; Matwin, S. Aircraft Fuselage Corrosion Detection Using Artificial Intelligence. Sensors 2021, 21, 4026. [Google Scholar] [CrossRef]
- Coakley, J.; Isheim, D.; Radecka, A.; Dye, D.; Stone, H.J.; Seidman, D.N. Microstructural Evolution in a Superelastic Metastable Beta-Ti Alloy. Scr. Mater. 2017, 128, 87–90. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.C.; Dos Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6Al-4V and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Schweitzer, P.E. Paint and Coatings; CRC Press: Boca Raton, FL, USA, 2005; Volume 35. [Google Scholar]
- Schweitzer, P.E.P.A. Corrosion Engineering Handbook—3 Volume Set; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9780429188084. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2008; ISBN 9780471732792. [Google Scholar]
- Hebert, K.; Alkire, R. Dissolved Metal Species Mechanism for Initiation of Crevice Corrosion of Aluminum: II. Math. Model. J. Electrochem. Soc. 1983, 130, 1007–1014. [Google Scholar] [CrossRef]
- Rashidi, N.; Alavi-Soltani, S.R.; Asmatulu, R. Crevice Corrosion Theory, Mechanisms and Prevention Methods; Wichita State University, Graduate School: Wichita, KS, USA, 2007. [Google Scholar]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion Mechanisms in Titanium Oxide-Based Films Produced by Anodic Treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef]
- Zieliński, A.; Sobieszczyk, S. Corrosion of Titanium Biomaterials, Mechanisms, Effects and Modelisation. Corros. Rev. 2008, 26, 1–22. [Google Scholar] [CrossRef]
- Pourbaix, M.; Burbank, J. Atlas D-Equilibres Electrochimiques. J. Electrochem. Soc. 1964, 111, 14C. [Google Scholar] [CrossRef]
- Demo, J.; Friedersdorf, F. Aircraft Corrosion Monitoring and Data Visualization Techniques for Condition Based Maintenance. In Proceedings of the 2015 IEEE Aerospace Conference, Big Sky, MT, USA, 8 June 2015. [Google Scholar] [CrossRef]
- Schenk, R. The Corrosion Properties of Titanium and Titanium Alloys; Springer: Berlin/Heidelberg, Germany, 2001; pp. 145–170. [Google Scholar]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In Vivo Severe Corrosion and Hydrogen Embrittlement of Retrieved Modular Body Titanium Alloy Hip-Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef]
- Laurindo, C.A.H.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on Anodized Titanium Surface: Effect of High Current Density. Mater. Sci. Eng. C 2014, 37, 223–231. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An Overview of the Corrosion Aspect of Dental Implants (Titanium and Its Alloys). Indian J. Dent. Res. 2009, 20, 91–98. [Google Scholar] [CrossRef]
- Betts, A.J.; Boulton, L.H. Crevice Corrosion: Review of Mechanisms, Modelling, and Mitigation. Br. Corros. J. 1993, 28, 279–295. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H.; Botello, M.A. Failure of the Metallic Structures Due to Microbiologically Induced Corrosion and the Techniques for Protection. In Handbook of Materials Failure Analysis; Butterworth-Heinemann: Oxford, UK, 2018; pp. 1–18. [Google Scholar] [CrossRef]
- Strehblow, H.H.; Marcus, P. Mechanisms of Pitting Corrosion. In Corrosion Mechanisms in Theory and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 349–394. ISBN 9781420094633. [Google Scholar]
- Sato, N.; Kudo, K.; Noda, T. The Anodic Oxide Film on Iron in Neutral Solution. Electrochim. Acta 1971, 16, 1909–1921. [Google Scholar] [CrossRef]
- Sato, N. A Theory for Breakdown of Anodic Oxide Films on Metals. Electrochim. Acta 1971, 16, 1683–1692. [Google Scholar] [CrossRef]
- Galvele, J.R. 1-Pitting Corrosion. Treatise Mater. Sci. Technol. 1983, 23, 1–57. [Google Scholar]
- Soltis, J. Passivity Breakdown, Pit Initiation and Propagation of Pits in Metallic Materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals: A Review of the Critical Factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Isaacs, H.S. The Localized Breakdown and Repair of Passive Surfaces during Pitting. Corros. Sci. 1989, 29, 313–323. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A Point Defect Model for Anodic Passive Films: III. Impedance Response. J. Electrochem. Soc. 1982, 129, 1874–1879. [Google Scholar] [CrossRef]
- Macdonald, D.D. The Point Defect Model for the Passive State. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Macdonald, D.D. Review of Mechanistic Analysis by Electrochemical Impedance Spectroscopy. Electrochim. Acta 1990, 35, 1509–1525. [Google Scholar] [CrossRef]
- Engelhardt, G.R.; Macdonald, D.D. Monte-Carlo Simulation of Pitting Corrosion with a Deterministic Model for Repassivation. J. Electrochem. Soc. 2020, 167, 013540. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and Characterization of TiO2 Thin Films by Sol-Gel Method. J. Sol.-Gel. Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Codeluppi, S.; Cordioli, A.; Pedeferri, M.P. Effect of Thermal Oxidation on Titanium Oxides’ Characteristics. J. Exp. Nanosci. 2009, 4, 365–372. [Google Scholar] [CrossRef]
- Löbl, P.; Huppertz, M.; Mergel, D. Nucleation and Growth in TiO2 Films Prepared by Sputtering and Evaporation. Thin Solid Filmds 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Dziewoński, P.M.; Grzeszczuk, M. Deposition of Thin TiO2 Layers on Platinum by Means of Cyclic Voltammetry of Selected Complex Ti(IV) Media Leading to Anatase. Electrochim. Acta 2009, 54, 4045–4055. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment. Int. J. Corros. 2020, 2020, 1678615. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Reactivity of Porous Titanium Oxide Film and Chitosan Layer Electrochemically Formed on Ti-6Al-4V Alloy in Biological Solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Cheng, Y.; Zuo, X.; Wang, Y.; Yuan, X.; Huang, H. Effect of Temperature on the Corrosion Behavior and Corrosion Resistance of Copper–Aluminum Laminated Composite Plate. Materials 2022, 15, 1621. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Igual-Muñoz, A.; Antón, J.G.; García-García, D.M. Galvanic Corrosion of Titanium Coupled to Welded Titanium in LiBr Solutions at Different Temperatures. Corros. Sci. 2009, 51, 1095–1102. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Corrosion Behavior and Surface Characterization of Titanium in Solution Containing Fluoride and Albumin. Biomaterials 2005, 26, 829–837. [Google Scholar] [CrossRef]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Nieves-Mendoza, D.; Lopez-Leon, L.D.; Jaquez-Muñoz, J.M.; Estupiñán-López, F.; et al. Use of Electrochemical Noise for the Study of Corrosion by Passivated CUSTOM 450 and AM 350 Stainless Steels. Metals 2024, 14, 341. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Łazińska, M.; Łukasiewicz, J.; Mol, J.M.C.; Durejko, T. Self-Organized Anodic Oxides on Titanium Alloys Prepared from Glycol- and Glycerol-Based Electrolytes. Materials 2020, 13, 4743. [Google Scholar] [CrossRef]
- Guo, T.; Ivanovski, S.; Gulati, K. Fresh or Aged: Short Time Anodization of Titanium to Understand the Influence of Electrolyte Aging on Titania Nanopores. J. Mater. Sci. Technol. 2022, 119, 245–256. [Google Scholar] [CrossRef]
- Gulati, K.; Aw, M.S.; Findlay, D.; Losic, D. Local Drug Delivery to the Bone by Drug-Releasing Implants: Perspectives of Nano-Engineered Titania Nanotube Arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef]
- Luz, A.R.; Santos, L.S.; Lepienski, C.M.; Kuroda, P.B.; Kuromoto, N.K. Characterization of the Morphology, Structure and Wettability of Phase Dependent Lamellar and Nanotube Oxides on Anodized Ti-10Nb Alloy. Appl. Surf. Sci. 2018, 448, 30–40. [Google Scholar] [CrossRef]
- Dikici, T.; Erol, M.; Toparli, M.; Celik, E. Characterization and Photocatalytic Properties of Nanoporous Titanium Dioxide Layer Fabricated on Pure Titanium Substrates by the Anodic Oxidation Process. Ceram. Int. 2014, 40, 1587–1591. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; Garza-Montes-de-Oca, N.; Gaona-Tiburcio, C.; Almeraya-Calderón, F. Corrosion mechanism of advanced high strength dual-phase steels by electrochemical noise analysis in chloride solutions. Mater. Today Commun. 2023, 35. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous Anodic Alumina: Fabrication, Characterization and Applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Xu, Y.; Thompson, G.E.; Wood, G.C.; Bethune, B. Anion Incorporation and Migration during Barrier Film Formation on Aluminium. Corros. Sci. 1987, 27, 83–102. [Google Scholar] [CrossRef]
- Saenz de Miera, M.; Curioni, M.; Skeldon, P.; Thompson, G.E. The Behaviour of Second Phase Particles during Anodizing of Aluminium Alloys. Corros. Sci. 2010, 52, 2489–2497. [Google Scholar] [CrossRef]
- Saenz de Miera, M.; Curioni, M.; Skeldon, P.; Thompson, G.E. Modelling the Anodizing Behaviour of Aluminium Alloys in Sulphuric Acid through Alloy Analogues. Corros. Sci. 2008, 50, 3410–3415. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Mazzarolo, A.; Curioni, M.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Anodic Growth of Titanium Oxide: Electrochemical Behaviour and Morphological Evolution. Electrochim. Acta 2012, 75, 288–295. [Google Scholar] [CrossRef]
- Torrescano-Alvarez, J.M.; Curioni, M.; Skeldon, P. Effects of Oxygen Evolution on the Voltage and Film Morphology during Galvanostatic Anodizing of AA 2024-T3 Aluminium Alloy in Sulphuric Acid at −2 and 24 °C. Electrochim. Acta 2018, 275, 172–181. [Google Scholar] [CrossRef]
- Ono, S.; Ichinose, H.; Kawaguchi, T.; Masuko, N. The Observation of Anodic Oxide Films on Aluminum by High Resolution Electron Microscopy. Corros. Sci. 1990, 31, 249–254. [Google Scholar] [CrossRef]
- Palibroda, E.; Marginean, P. Considerations on the Adsorbed Water Concentration of Sulfuric Porous Aluminium Oxide. Thin Solid Films 1994, 240, 73–75. [Google Scholar] [CrossRef]
- Voon, C.H.; Derman, M.N.; Hashim, U.; Ahmad, K.R.; Foo, K.L. Effect of Temperature of Oxalic Acid on the Fabrication of Porous Anodic Alumina from Al-Mn Alloys. J. Nanomater. 2013, 2013, 167047. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhou, X.; Lim, C.V.S.; Boyer, R.R.; Williams, J.C.; Wu, X. A Strong and Ductile Ti-3Al-8V-6Cr-4Mo-4Zr (Beta-C) Alloy Achieved by Introducing Trace Carbon Addition and Cold Work. Scr. Mater. 2020, 178, 124–128. [Google Scholar] [CrossRef]
- Taveira, L.V.; Macák, J.M.; Tsuchiya, H.; Dick, L.F.P.; Schmuki, P. Initiation and Growth of Self-Organized TiO2 Nanotubes Anodically Formed in NH4F(NH4)2SO4 Electrolytes. J. Electrochem. Soc. 2005, 152, B405. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Taveira, L.; Balaur, E.; Ghicov, A.; Sirotna, K.; Schmuki, P. Self-Organized TiO2 Nanotubes Prepared in Ammonium Fluoride Containing Acetic Acid Electrolytes. Electrochem. Commun. 2005, 7, 576–580. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Kurokawa, T.; Miyabe, S.; Fujimoto, S. Fast Current-Controlled Polarization for the Analysis of Rapid Cathodic Process on Anodized Metal. J. Electrochem. Soc. 2019, 166, C3443–C3447. [Google Scholar] [CrossRef]
- Guerra Neto, C.L.B.; Da Silva, M.A.M.; Alves, C. In Vitro Study of Cell Behaviour on Plasma Surface Modified Titanium. Surf. Eng. 2009, 25, 146–150. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 2: Effects of Surface Treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef]
- Salman, S.A.; Okido, M. Anodization of Magnesium (Mg) Alloys to Improve Corrosion Resistance. Corros. Prev. Magnes. Alloys 2013, 197–231. [Google Scholar] [CrossRef]
- David, T.M.; Priya, D.R.; Wilson, P.; Sagayaraj, P.; Mathews, T. A Critical Review on the Variations in Anodization Parameters toward Microstructural Formation of TiO2 Nanotubes. Electrochem. Sci. Adv. 2022, 2, e202100083. [Google Scholar] [CrossRef]
- Raj, V.; Rajaram, M.P.; Balasubramanian, G.; Vincent, S.; Kanagaraj, D. Pulse Anodizing—An Overview. Trans. IMF 2017, 81, 114–121. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of Titanium Alloys for Orthopedic Applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Aerts, T.; Jorcin, J.B.; De Graeve, I.; Terryn, H. Comparison between the Influence of Applied Electrode and Electrolyte Temperatures on Porous Anodizing of Aluminium. Electrochim. Acta 2010, 55, 3957–3965. [Google Scholar] [CrossRef]
- Kumar, A. Anodization of Titanium Alloy (Grade 5) to Obtain Nanoporous Surface Using Sulfuric Acid Electrolyte. IETE J. Res. 2020, 68, 3855–3861. [Google Scholar] [CrossRef]
- Olmo Martinez, R.D.; Munirathinam, B.; Michalska-Domańska, M. Biomedical Application of Anodic Nanomaterials. Synth. Bionano. Mater. Biomed. Appl. 2023, 395–441. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lockman, Z.; Hazan, R.; Tasbihi, M.; Tong, L.K.; Mohamed, A.R. Influence of Electrolyte pH on TiO2 Nanotube Formation by Ti Anodization. J. Alloys Compd. 2009, 485, 478–483. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T.J. Anodization: A Promising Nano-Modification Technique of Titanium Implants for Orthopedic Applications. J. Nanosci. Nanotechnol. 2006, 6, 2682–2692. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell Response of Anodized Nanotubes on Titanium and Titanium Alloys. J. Biomed. Mater. Res. Part A 2013, 101A, 2726–2739. [Google Scholar] [CrossRef]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, J.; Gaona-Tiburcio, C.; Silva-Vidaurri, L.; Cabral-Miramontes, J.; Bastidas, J.M.; Chacón-Nava, J.G.; Bastidas, D.M. Efecto del tratamiento de sellado en el comportamiento frente a corrosión de la aleación anodizada de aluminio-litio AA2099. Rev. Met. 2020, 56, e180. [Google Scholar] [CrossRef]
- De Graeve, I.; Terryn, H.; Thompson, G.E. AC-Anodising of Aluminium: Contribution to Electrical and Efficiency Study. Electrochim. Acta 2006, 52, 1127–1134. [Google Scholar] [CrossRef]
- Chung, C.K.; Liao, M.W.; Chang, H.C.; Lee, C.T. Effects of Temperature and Voltage Mode on Nanoporous Anodic Aluminum Oxide Films by One-Step Anodization. Thin Solid Films 2011, 520, 1554–1558. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Effect of Cathode Material on the Morphology and Photoelectrochemical Properties of Vertically Oriented TiO2 Nanotube Arrays. Sol. Energy Mater. Sol. Cells 2008, 92, 1468–1475. [Google Scholar] [CrossRef]
- Indira, K.; Ningshen, S.; Mudali, U.K.; Rajendran, N. Effect of Anodization Parameters on the Structural Morphology of Titanium in Fluoride Containing Electrolytes. Mater. Charact. 2012, 71, 58–65. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Macak, J.M.; Ghicov, A.; Räder, A.S.; Taveira, L.; Schmuki, P. Characterization of Electronic Properties of TiO2 Nanotube Films. Corros. Sci. 2007, 49, 203–210. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Gaber, A. Parametric Study on the Anodization of Pure Aluminum Thin Film Used in Fabricating Nano-Pores Template. Int. J. Electrochem. Sci. 2013, 8, 9741–9751. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth Anodic TiO2 Nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Anodic Formation of Ordered TiO2 Nanotube Arrays: Effects of Electrolyte Temperature and Anodization Potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Lin, J. Self-Assembled TiO2 Nanotube Arrays with U-Shaped Profile by Controlling Anodization Temperature. J. Nanomater. 2010, 2010, 753253. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of Electrolyte Temperature and Anodization Time on Formation of TiO2 Nanotubes for Biomedical Applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Yun, K.C.; Chen, Y.C.; Kuo, M.Y.; Wang, H.W.; Lu, Y.F.; Chung, J.C.; Liu, Y.C.; Zeng, Y.Z. Synthesis and Characterization of Highly Ordered TiO2 Nanotube Arrays for Hydrogen Generation via Water Splitting. Mater. Chem. Phys. 2011, 129, 35–39. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Han, J.; Ji, L.; Wang, J.; Chen, J.; Wang, Y. Controllable Preparation, Growth Mechanism and the Properties Research of TiO2 Nanotube Arrays. Appl. Surf. Sci. 2014, 297, 103–108. [Google Scholar] [CrossRef]
- Joo, S.; Muto, I.; Hara, N. In Situ Ellipsometric Analysis of Growth Processes of Anodic TiO2 Nanotube Films. J. Electrochem. Soc. 2008, 155, C154. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R.; Krzywicka, M. Porous Titanium Materials and Applications. In Titanium for Consumer Applications: Real-World Use of Titanium; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–75. ISBN 9780128158203. [Google Scholar]
- Eah, K.H.W.; Thampuran, R.; Teoh, S.H. The Influence of Pore Morphology on Corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Dabrowski, B.; Kaminski, J.; Swieszkowski, W.; Kurzydlowski, K.J. Porous Titanium Scaffolds for Biomedical Applications: Corrosion Resistance and Structure Investigation. Mater. Sci. Forum 2011, 674, 41–46. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In Vitro Studying Corrosion Behavior of Porous Titanium Coating in Dynamic Electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef]
- Ye, D.; Wang, W.; Xu, Z.; Yin, C.; Zhou, H.; Li, Y. Prediction of Thermal Barrier Coatings Microstructural Features Based on Support Vector Machine Optimized by Cuckoo Search Algorithm. Coatings 2020, 10, 704. [Google Scholar] [CrossRef]
- Karballaeezadeh, N.; Danial, M.S.; Moazemi, D.; Band, S.S.; Mosavi, A.; Reuter, U. Smart Structural Health Monitoring of Flexible Pavements Using Machine Learning Methods. Coatings 2020, 10, 1100. [Google Scholar] [CrossRef]
- Singh, A.V.; Jahnke, T.; Wang, S.; Xiao, Y.; Alapan, Y.; Kharratian, S.; Onbasli, M.C.; Kozielski, K.; David, H.; Richter, G.; et al. Anisotropic Gold Nanostructures: Optimization via in Silico Modeling for Hyperthermia. ACS Appl. Nano Mater. 2018, 1, 6205–6216. [Google Scholar] [CrossRef]
- Thampi, V.V.A.; Ramanathan, S. Corrosion Behavior of Anodized Ti-Ta Binary Surface Alloys in Various Physiological Fluids for Implant Applications. Corros. Sci. 2023, 219, 111233. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Bastidas, D.M.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderón, F.M.; Gaona-Tiburcio, C. Corrosion Behavior of Zn-TiO2 and Zn-ZnO Electrodeposited Coatings in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Eavers, J.A.; Durr, C.L.; Thompson, N.G. Unique Interpretations of Potentiodynamic Polarization Technique. In Proceedings of the NACE—International Corrosion Conference Series, San Diego, CA, USA, 22–27 March 1998. [Google Scholar]
- Cabral Miramontes, J.A.; Barceinas Sánchez, J.D.O.; Almeraya Calderón, F.; Martínez Villafañe, A.; Chacón Nava, J.G. Effect of Boron Additions on Sintering and Densification of a Ferritic Stainless Steel. J. Mater. Eng. Perform. 2010, 19, 880–884. [Google Scholar] [CrossRef]
- Ramgopal, T.; Schmutz, P.; Frankel, G.S. Electrochemical Behavior of Thin Film Analogs of Mg(Zn, Cu, Al)2. J. Electrochem. Soc. 2001, 148, B348. [Google Scholar] [CrossRef]
- Montoya-Rangel, M.; de Oca, N.G.M.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical Noise Measurements of Advanced High-Strength Steels in Different Solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Ispas, A.; Bund, A.; Vrublevsky, I. Investigations on Current Transients in Porous Alumina Films during Re-Anodizing Using the Electrochemical Quartz Crystal Microbalance. J. Solid State Electrochem. 2010, 14, 2121–2128. [Google Scholar] [CrossRef]
- Huang, Y.S.; Shih, T.S.; Chou, J.H. Electrochemical Behavior of Anodized AA7075-T73 Alloys as Affected by the Matrix Structure. Appl. Surf. Sci. 2013, 283, 249–257. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Gurgul, M.; Dziurka, M.; Nowak, M.; Gilek, D.; Sulka, G.D. Influence of Anodizing Conditions on Generation of Internal Cracks in Anodic Porous Tin Oxide Films Grown in NaOH Electrolyte. Appl. Surf. Sci. 2018, 439, 672–680. [Google Scholar] [CrossRef]
- Gawalt, E.S.; Brault-Rios, K.; Dixon, M.S.; Tang, D.C.; Schwartz, J. Enhanced Bonding of Organometallics to Titanium via a Titanium(III) Phosphate Interface. Langmuir 2001, 17, 6743–6745. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization Parameters Influencing the Morphology and Electrical Properties of TiO2 Nanotubes for Living Cell Interfacing and Investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- El-Taib Heakal, F.; Mogoda, A.S.; Mazhar, A.A.; El-Basiouny, M.S. Kinetic Studies on the Dissolution of the Anodic Oxide Film on Titanium in Phosphoric Acid Solutions. Corros. Sci. 1987, 27, 453–462. [Google Scholar] [CrossRef]
- Parse, H.; Patil, I.M.; Swami, A.S.; Kakade, B.A. TiO2-Decorated Titanium Carbide MXene Co-Doped with Nitrogen and Sulfur for Oxygen Electroreduction. ACS Appl. Nano Mater. 2021, 4, 1094–1103. [Google Scholar] [CrossRef]
- Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. [Google Scholar] [CrossRef]
- Legat, A.; Doleček, V. Corrosion Monitoring System Based on Measurement and Analysis of Electrochemical Noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Xia, D.H.; Qin, Z.; Song, S.; Macdonald, D.; Luo, J.L. Combating Marine Corrosion on Engineered Oxide Surface by Repelling, Blocking and Capturing Cl−: A Mini Review. Corros. Commun. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Martínez-Villafañe, A.; Almeraya-Calderón, M.F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1997, 7, 108–113. [Google Scholar] [CrossRef]
- Pan, L.; Ding, W.; Ma, W.; Hu, J.; Pang, X.; Wang, F.; Tao, J. Galvanic Corrosion Protection and Durability of Polyaniline-Reinforced Epoxy Adhesive for Bond-Riveted Joints in AA5083/Cf/Epoxy Laminates. Mater. Des. 2018, 160, 1106–1116. [Google Scholar] [CrossRef]
- Sadek Mogoda, A.; Zohdy, K.M. Electrochemical Behavior of Titanium in NaF Solutions and Characterization of Oxide Film Formed on Its Surface. Int. J. Electrochem. Sci. 2020, 15, 8070–8085. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Zheng, H.; Latham, K.; Wlodarski, W.; Kalantar-Zadeh, K. Anodization of Ti Thin Film Deposited on ITO. Langmuir 2009, 25, 509–514. [Google Scholar] [CrossRef]
- Contreras, A.; Salazar, M.; Carmona, A.; Galván-Martínez, R. Electrochemical Noise for Detection of Stress Corrosion Cracking of Low Carbon Steel Exposed to Synthetic Soil Solution. Mater. Res. 2017, 20, 1201–1210. [Google Scholar] [CrossRef]
- Galvan-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion Study of the X52 Steel Immersed in Seawater with a Corrosion Inhibitor Using a Rotating Cylinder Electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Galván-Martínez, R.; Cabrera-de la Cruz, D.; Contreras, A.; Orozco-Cruz, R. A Novel Experimental Arrangement for Corrosion Study of X60 Pipeline Steel Weldments at Turbulent Flow Conditions. Corros. Eng. Sci. Technol. 2016, 51, 400–407. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.M.; Delgado, A.D.; Flores-De Los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure Commercial Titanium Color Anodizing and Corrosion Resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Application-Wise Nanostructuring of Anodic Films on Titanium: A Review. J. Exp. Nanosci. 2015, 10, 1285–1308. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Bolzoni, F.; Ormellese, M.; Pérez-Rosales, E.A.; Pedeferri, M.P. Characterisation of Titanium Oxide Films by Potentiodynamic Polarisation and Electrochemical Impedance Spectroscopy. Corros. Eng. Sci. Technol. 2010, 45, 428–434. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, M.K.; Jung, G.C.; Vang, M.S.; Park, Y.J. The Effects of Spark Anodizing Treatment of Pure Titanium Metals and Titanium Alloys on Corrosion Characteristics. Surf. Coat. Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Corrosion Characteristics of Anodized Ti-(10-40wt%)Hf Alloys for Metallic Biomaterials Use. J. Mater. Sci. Mater. Med. 2011, 22, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Prando, D.; Brenna, A.; Bolzoni, F.M.; Diamanti, M.V.; Pedeferri, M.; Ormellese, M. Electrochemical Anodizing Treatment to Enhance Localized Corrosion Resistance of Pure Titanium. J. Appl. Biomater. Funct. Mater. 2017, 15, e19–e24. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Pedeferri, M.P.; Ormellese, M. Pitting Corrosion on Anodized Titanium: Effect of Halides. Mater. Corros. 2018, 69, 1441–1446. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G.; Ke, W.; Qiao, Y.X. Comparison of the Corrosion Behavior of Pure Titanium and Its Alloys in Fluoride-Containing Sulfuric Acid. Corros. Sci. 2016, 103, 50–65. [Google Scholar] [CrossRef]
- Scully, J.C. The Electrochemical Parameters of Stress-Corrosion Cracking. Corros. Sci. 1968, 8, 513–523, IN13–IN18. [Google Scholar] [CrossRef]
- Casillas, N.; Charlebois, S.; Smyrl, W.H.; White, H.S. Pitting Corrosion of Titanium. J. Electrochem. Soc. 1994, 141, 636–642. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of Fluoride Concentration and pH on Corrosion Behavior of Titanium for Dental Use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Ittah, R.; Amsellem, E.; Itzhak, D. Pitting Corrosion Evaluation of Titanium in NH4Br Solutions by Electrochemical Methods. Int. J. Electrochem. Sci. 2014, 9, 633–643. [Google Scholar] [CrossRef]
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- Park, M.; Heo, A.; Shim, E.; Yoon, J.; Kim, H.; Joo, H. Effect of Length of Anodized TiO2 Tubes on Photoreactivity: Photocurrent, Cr(VI) Reduction and H2 Evolution. J. Power Sources 2010, 195, 5144–5149. [Google Scholar] [CrossRef]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark Anodization of Titanium–Zirconium Alloy: Surface Characterization and Bioactivity Assessment. J. Mater. Sci. Mater. Med. 2015, 26, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, J.; Yang, C.; Zhang, X.; Liu, R. Effect of Zr Addition on the Microstructure and Tribological Property of the Anodization of Ti-6Al-4V Alloy. Surf. Coat. Technol. 2018, 356, 38–48. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical Behavior of Ti–Mo Alloys Applied as Biomaterial. Corros. Sci. 2008, 50, 938–945. [Google Scholar] [CrossRef]
- Nguyen, P.M.H.; Won, D.H.; Kim, B.S.; Jang, Y.S.; Nguyen, T.D.T.; Lee, M.H.; Bae, T.S. The Effect of Two-Step Surface Modification for Ti-Ta-Mo-Zr Alloys on Bone Regeneration: An Evaluation Using Calvarial Defect on Rat Model. Appl. Surf. Sci. 2018, 442, 630–639. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, B.; Yuan, D.; Guo, Z. Construction of Porous Micro/Nano Structures on the Surface of Ti–Mo–Zr Alloys by Anodic Oxidation for Biomedical Application. J. Mater. Res. Technol. 2024, 30, 2986–2998. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olgui-Coca, J.; Lopez-Leon, L.D.; Estupiñán-López, F.; Lira-Martínez, A.; Gaona Tiburcio, C. Corrosion Resistance of Titanium Alloys Anodized in Alkaline Solutions. Metals 2023, 13, 1510. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Mukherjee, N.; Grimes, C.A. Fabrication of Tapered, Conical-Shaped Titania Nanotubes. J. Mater. Res. 2003, 18, 2588–2593. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Luo, J.-L.; Huet, F. Review—Electrochemical Noise Applied in Corrosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Casanova, L.; La Padula, M.; Pedeferri, M.P.; Diamanti, M.V.; Ormellese, M. An Insight into the Evolution of Corrosion Resistant Coatings on Titanium during Bipolar Plasma Electrolytic Oxidation in Sulfuric Acid. Electrochim. Acta 2021, 379, 138190. [Google Scholar] [CrossRef]
- Fekry, A.M. The Influence of Chloride and Sulphate Ions on the Corrosion Behavior of Ti and Ti-6Al-4V Alloy in Oxalic Acid. Electrochim. Acta 2009, 54, 3480–3489. [Google Scholar] [CrossRef]
- Puga, M.L.; Venturini, J.; ten Caten, C.S.; Bergmann, C.P. Influencing Parameters in the Electrochemical Anodization of TiO2 Nanotubes: Systematic Review and Meta-Analysis. Ceram. Int. 2022, 48, 19513–19526. [Google Scholar] [CrossRef]
- Degirmenci, K.; Saridag, S. Influence of Anodized Titanium Abutment Backgrounds on the Color Parameters of Different Zirconia Materials. Am. J. Dent. 2021, 34, 39–43. [Google Scholar]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E, M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- Hernández-López, J.M.; Conde, A.; de Damborenea, J.; Arenas, M.A. Correlation of the Nanostructure of the Anodic Layers Fabricated on Ti13Nb13Zr with the Electrochemical Impedance Response. Corros. Sci. 2015, 94, 61–69. [Google Scholar] [CrossRef]
- Shukla, A.K.; Balasubramaniam, R. Effect of Surface Treatment on Electrochemical Behavior of CP Ti, Ti–6Al–4V and Ti–13Nb–13Zr Alloys in Simulated Human Body Fluid. Corros. Sci. 2006, 48, 1696–1720. [Google Scholar] [CrossRef]
- Choi, Y.; Jeong, C. Influence of Electrolyte on the Shape and Characteristics of TiO2 during Anodic Oxidation of Titanium. Corros. Sci. Technol. 2023, 22, 193–200. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Marinko, Ž.; Ponikvar-Svet, M.; Tavčar, G.; Kovač, J.; Čeh, M. Influence of Anodization-Electrolyte Aging on the Photocatalytic Activity of TiO2 Nanotube Arrays. J. Phys. Chem. C 2020, 124, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, N.; Komiya, S.; Kodama, K. Effect of Electrolytes on Anodic Oxidation of Titanium for Fabricating Titanium Dioxide Photocatalyst. Thin Solid Films 2013, 534, 70–75. [Google Scholar] [CrossRef]
- Choi, Y.; Jeong, C. Investigating the Influence of Pore-Widening Time Control in Electrochemical Anodization for the Formation of Hybrid Titanium Nanostructures with Enhanced Superhydrophobicity for Improved Corrosion Resistance Efficiency. Electrochim. Acta 2024, 492, 144380. [Google Scholar] [CrossRef]
- Bouchama, L.; Bouznit, Y.; Boukmouche, N.; Irki, S. Two-Step vs. Single-Step Electrochemical Anodizing Process Regarding Anti-Corrosion Properties of Titanium. Anal. Bioanal. Electrochem. 2023, 15, 264–279. [Google Scholar] [CrossRef]
- Winiarski, J.; Niciejewska, A.; Górnik, M.; Jakubowski, J.; Tylus, W.; Szczygieł, B. Titanium Anodizing in a Choline Dihydrogencitrate Salt–Oxalic Acid Deep Eutectic Solvent: A Step towards Green Chemistry in Surface Finishing of Titanium and Its Alloys. RSC Adv. 2021, 11, 21104–21115. [Google Scholar] [CrossRef]
- Lazarouk, S.K.; Sasinovich, D.A.; Kupreeva, O.V.; Orehovskaia, T.I.; Rochdi, N.; D’Avitaya, F.A.; Borisenko, V.E. Effect of the Electrolyte Temperature on the Formation and Structure of Porous Anodic Titania Film. Thin Solid Films 2012, 526, 41–46. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral Microbiome and Peri-Implant Diseases: Where Are We Now? Ther. Clin. Risk Manag. 2017, 13, 1529. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, N.; Lai, W.H.; Liu, Y.; Chou, S.L.; Liu, H.K.; Dou, S.X.; Wang, Y.X. Anode Optimization Strategies for Aqueous Zinc-Ion Batteries. Chem. Sci. 2022, 13, 14246–14263. [Google Scholar] [CrossRef]



| Media | Reaction |
|---|---|
| Acid (oxygen reduction) | |
| Neutral/alkaline (oxygen reduction) | |
| Chloride reduction (acid) | |
| Hypochlorite reduction (near to neutral) | |
| Hypochlorite reduction (alkaline) | |
| Sulfur reduction | |
| Thiosulfate reduction | |
| Hydrogen evolution (discharge) | |
| Hydrogen evolution (neutral/alkaline) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Mendez-Ramirez, C.T.; Carrera-Ramirez, M.G.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Lara-Banda, M.; Estupiñan-Lopez, F.; Nieves-Mendoza, D.; Almeraya-Calderon, F. Corrosion of Anodized Titanium Alloys. Coatings 2024, 14, 809. https://doi.org/10.3390/coatings14070809
Jáquez-Muñoz JM, Gaona-Tiburcio C, Mendez-Ramirez CT, Carrera-Ramirez MG, Baltazar-Zamora MA, Santiago-Hurtado G, Lara-Banda M, Estupiñan-Lopez F, Nieves-Mendoza D, Almeraya-Calderon F. Corrosion of Anodized Titanium Alloys. Coatings. 2024; 14(7):809. https://doi.org/10.3390/coatings14070809
Chicago/Turabian StyleJáquez-Muñoz, Jesús Manuel, Citlalli Gaona-Tiburcio, Ce Tochtli Mendez-Ramirez, Martha Guadalupe Carrera-Ramirez, Miguel Angel Baltazar-Zamora, Griselda Santiago-Hurtado, Maria Lara-Banda, Francisco Estupiñan-Lopez, Demetrio Nieves-Mendoza, and Facundo Almeraya-Calderon. 2024. "Corrosion of Anodized Titanium Alloys" Coatings 14, no. 7: 809. https://doi.org/10.3390/coatings14070809






