Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Samples
2.2. In Situ Corrosion Morphology Investigation
2.3. Electrochemical Testing
3. Results
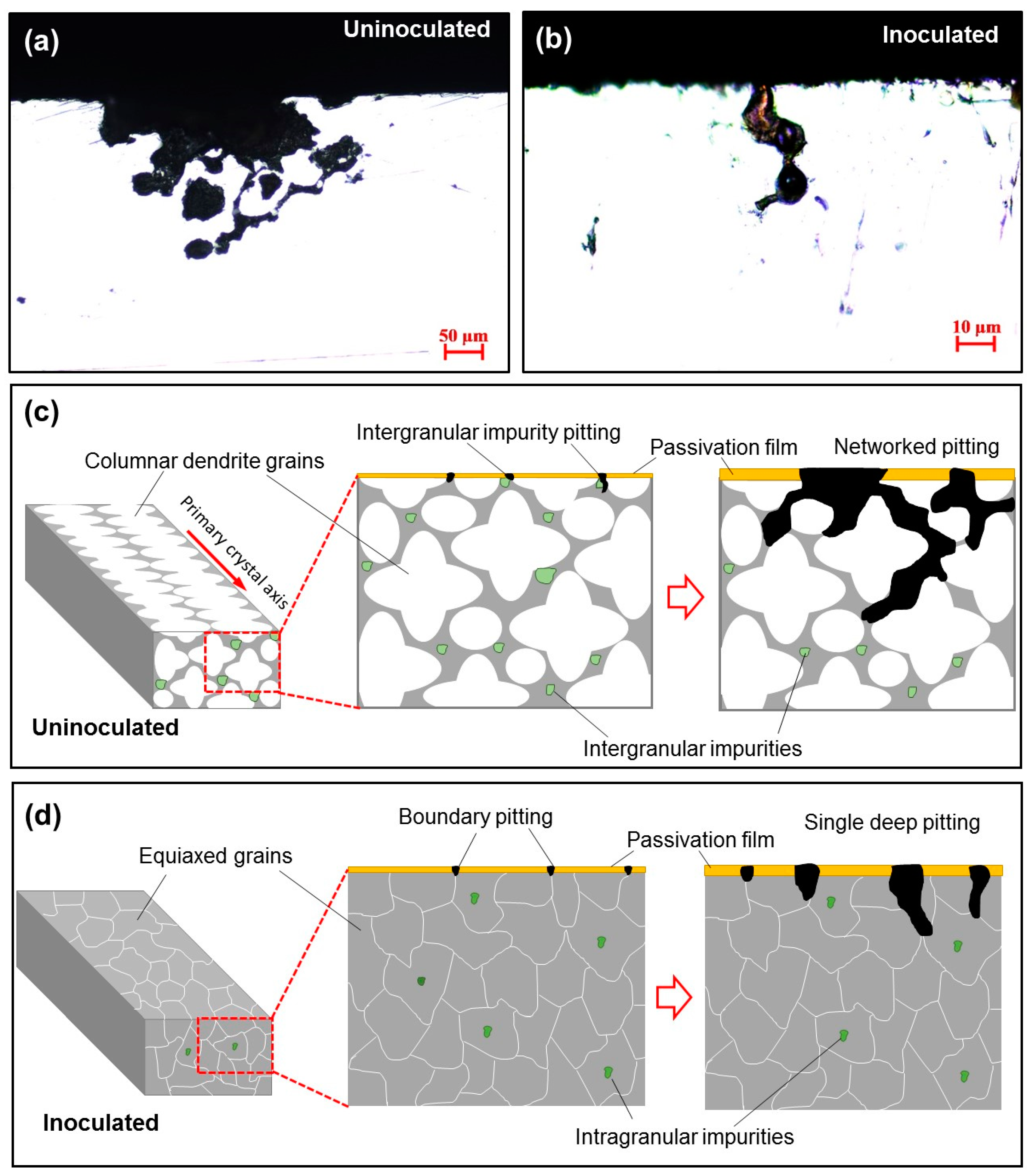
3.1. Microstructure of the As Cast GCr15 Steel
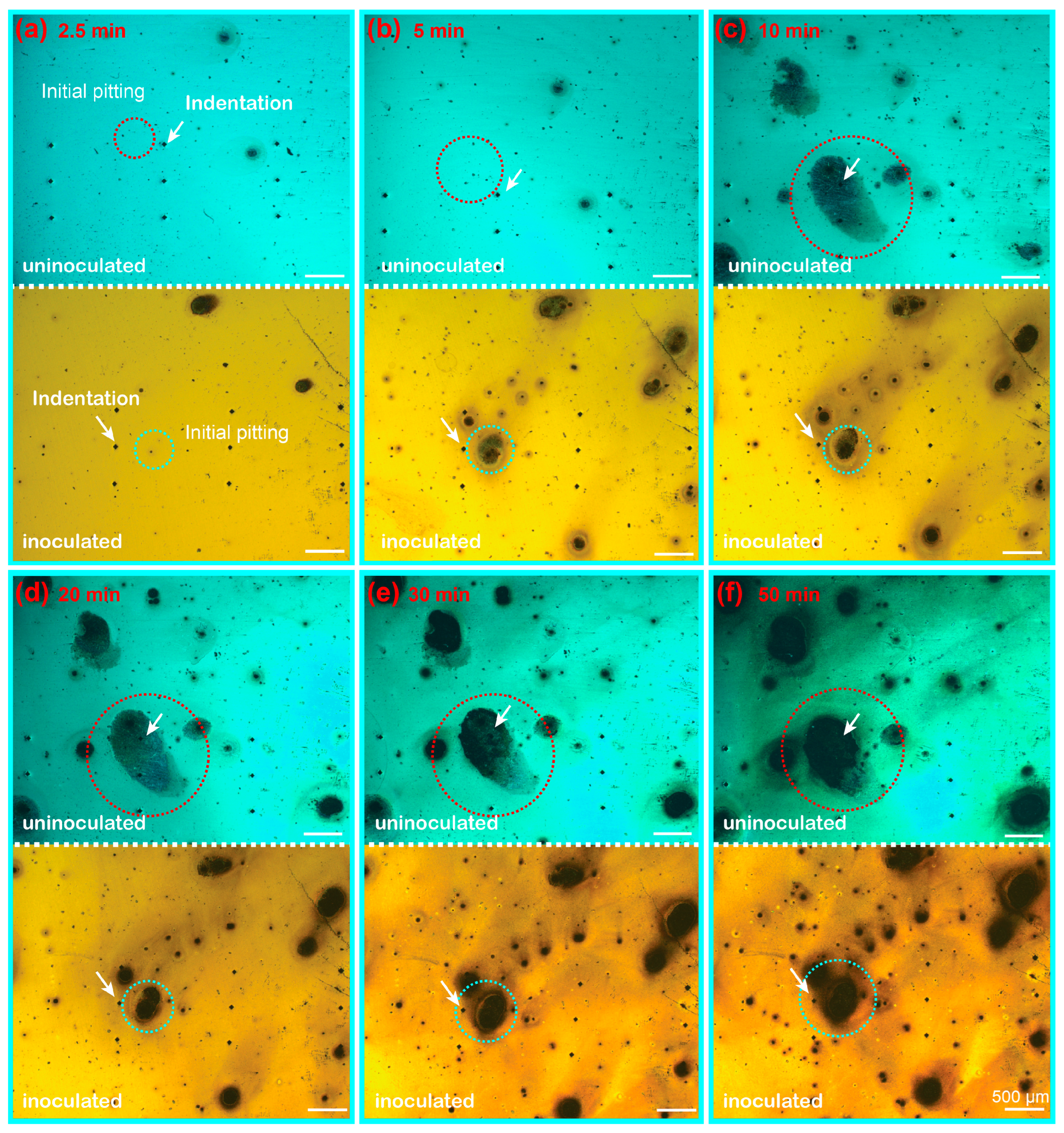
3.2. In Situ Corrosion Morphology
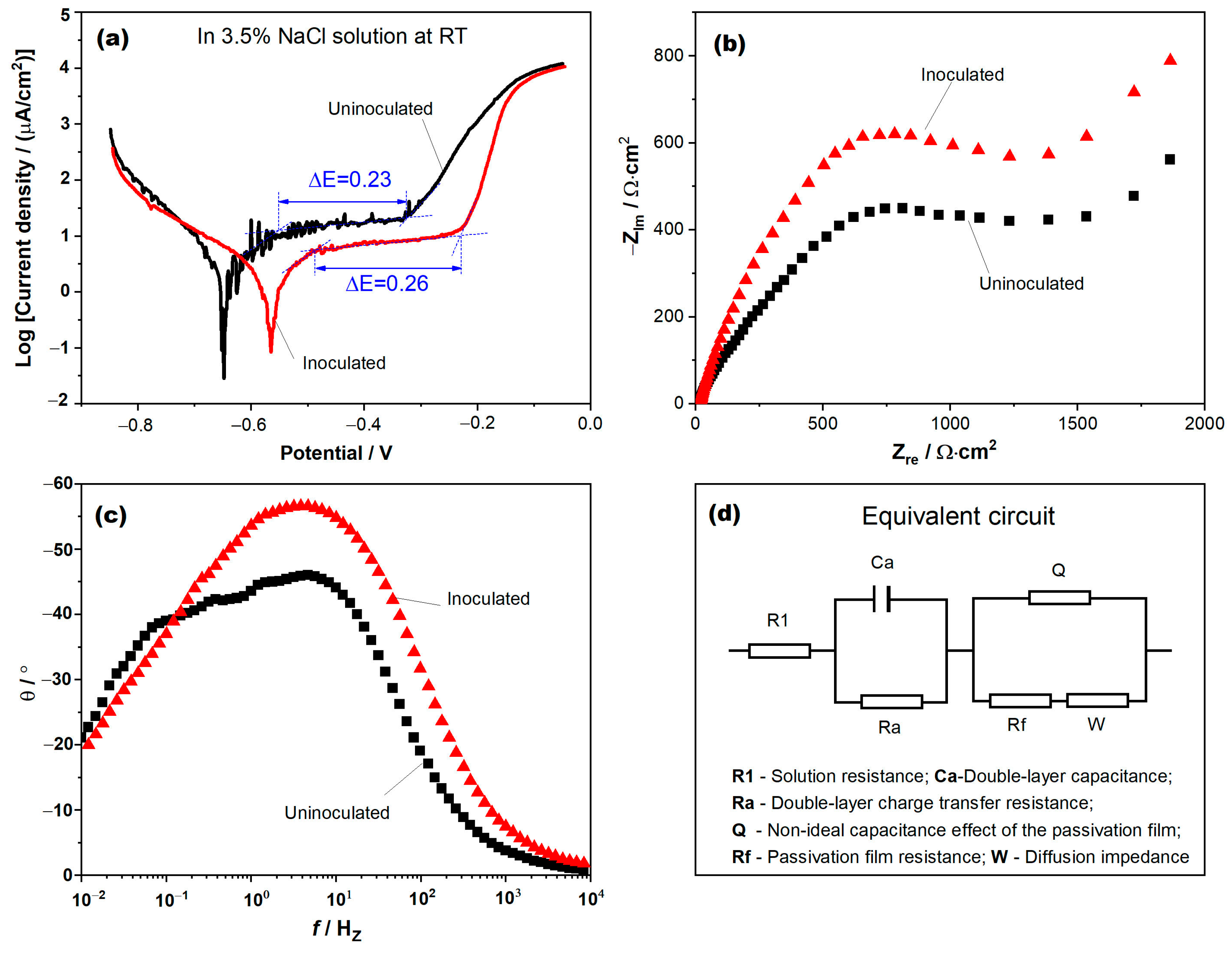
3.3. Electrochemical Analysis
4. Discussion
5. Conclusions
- (1)
- The NbC type inoculant can refine the as-cast structure of GCr15 steel from columnar grains to typical equiaxed grains, and effectively reduces the number of inclusions and casting pores on the grain boundary.
- (2)
- The in situ dynamic corrosion experiments show that the GCr15 steel after inoculation has more pitting sites but a lower tendency for pit growth, demonstrating that inoculation treatment can effectively reduce the pitting propagation tendency of cast steels.
- (3)
- By comparing to uninoculated samples, inoculated steel exhibits an increased corrosion potential, reduced passivation current density, expanded passivation zone width, and a larger impedance arc. It suggests that grain refinement enhances the stability of the passivation film, thereby increasing the corrosion resistance.
- (4)
- The cross-sectional morphology of corrosion pits in GCr15 steel samples before and after inoculation differs significantly. The columnar dendrites lead to networked pits, whereas the equiaxed grains tend to form isolated intergranular pits, suggesting that inoculation treatment can reduce the pitting trend of cast GCr15 steel. This study sheds lights on the development of corrosion-resistant high carbon cast steel and provides new insights into the design and manufacturing of long-life bearings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhadeshia, H. Steels for bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Zaretsky, E.V. Rolling bearing steels—A technical and historical perspective. Mater. Sci. Technol. 2012, 28, 58–69. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Xu, N.; Li, N.; Ding, Q.; Ding, N.; Hu, Z. Corrosion Behavior of High Strength Bearing Steel GCr15 in NaCl Solution. Corros. Prot. 2018, 39, 897–911. [Google Scholar] [CrossRef]
- Fu, J. Microstructure and corrosion behavior of hot-rolled GCr15 bearing steel. Appl. Phys. A Mater. Sci. Process. 2016, 122, 416. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H. Pitting Corrosion Behavior of GCr15 Bearing Steel in Hydrochloric Acid Solution. Corros. Prot. 2019, 40, 893–897. [Google Scholar] [CrossRef]
- Lu, H.; Su, H.; Mei, C.; Yang, Q.; Xu, Q.; Xiang, D.; Zhou, T. Effects of B, N, Cr and Mo ion implantation on the corrosion resistance of pure iron and its alloys (GCr15 and Cr4Mo4V). Vacuum 1989, 39, 187–189. [Google Scholar] [CrossRef]
- Xie, J.; Alpas, A.T.; Northwood, D.O. The role of heat treatment on the erosion–corrosion behavior of AISI 52100 steel. Mater. Sci. Eng. A 2005, 393, 42–50. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, C.; Niu, D.; Xu, J.; Li, M. Influence of alloyed chromium on the atmospheric corrosion resistance of weathering steels. Corros. Sci. 2013, 74, 424–429. [Google Scholar] [CrossRef]
- Zhao, Q.; Luo, H.; Pan, Z.; Wang, X.; Cheng, H. Study on mechanical properties of rare earth elements modified high carbon chromium bearing steel. Mater. Today. Commun. 2023, 34, 105329. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Li, B.; Chen, W.; Zhang, J.; Mao, J. Inclusions modification by rare earth in steel and the resulting properties: A review. J. Rare Earths 2024, 42, 431–445. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Pan, Z.; Wei, Y.; Cheng, H.; Ma, Y.; Luo, H.; Li, X. Effects of rare earth elements addition on mechanical properties and corrosion behavior of GCr15 bearing steel under different heat treatment conditions. Corros. Commun. 2023, 9, 65–76. [Google Scholar] [CrossRef]
- Lu, Y.C.; Ives, M.B. The improvement of the localized corrosion resistance of stainless steel by cerium. Corros. Sci. 1993, 34, 1773–1785. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Tang, M.; Zhang, X.; Wu, K. Role of rare earth elements on the improvement of corrosion resistance of micro-alloyed steels in 3.5 wt.% NaCl solution. J. Mater. Res. Technol. 2021, 11, 519–534. [Google Scholar] [CrossRef]
- Han, Y.; Hao, L.; Wang, J.; Ke, W. Effect of rare earth addition on corrosion sensitivity of GCr15 bearing steel in marine environment. Mater. Lett. 2023, 333, 133693. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Zhao, Y.; Zhang, Q.; Bai, L. Structure and properties of GCr15 modified by multiphase ceramic nanoparticles/Fe-C composite inoculants. Mat. Sci. Eng. A 2018, 738, 63–74. [Google Scholar] [CrossRef]

| Sample | Elements/wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Mn | Si | Al | Mg | Nb | S | P | C | |
| uninoculated | 97.8 | 1.54 | 0.252 | 0.200 | 0.096 | 0.0451 | — | 0.0219 | 0.0103 | — |
| inoculated | 97.5 | 1.64 | 0.267 | 0.195 | 0.139 | 0.0171 | 0.168 | 0.0225 | 0.0105 | — |
| Sample | OCP /V | Ecorr /V | Eb /V | icorr /Log(μA/cm2) | ip /Log(μA/cm2) |
|---|---|---|---|---|---|
| uninoculated | −0.45 | −0.65 | −0.33 | 0.63 | 1.10 |
| inoculated | −0.43 | −0.57 | −0.23 | 0.28 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, Q.; Zhao, L.; Yang, W.; Wang, X. Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study. Coatings 2024, 14, 836. https://doi.org/10.3390/coatings14070836
Liu J, Liu Q, Zhao L, Yang W, Wang X. Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study. Coatings. 2024; 14(7):836. https://doi.org/10.3390/coatings14070836
Chicago/Turabian StyleLiu, Jiacheng, Qingao Liu, Lichen Zhao, Wei Yang, and Xin Wang. 2024. "Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study" Coatings 14, no. 7: 836. https://doi.org/10.3390/coatings14070836
APA StyleLiu, J., Liu, Q., Zhao, L., Yang, W., & Wang, X. (2024). Reducing Pitting Corrosion Trend of Cast GCr15 Steel by Inoculation: An In Situ Corrosion Morphology Study. Coatings, 14(7), 836. https://doi.org/10.3390/coatings14070836







