Hierarchical Porous Activated Carbon Derived from Pleurotus Eryngii and the Influence of Pore Structural Parameters on Capacitance Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
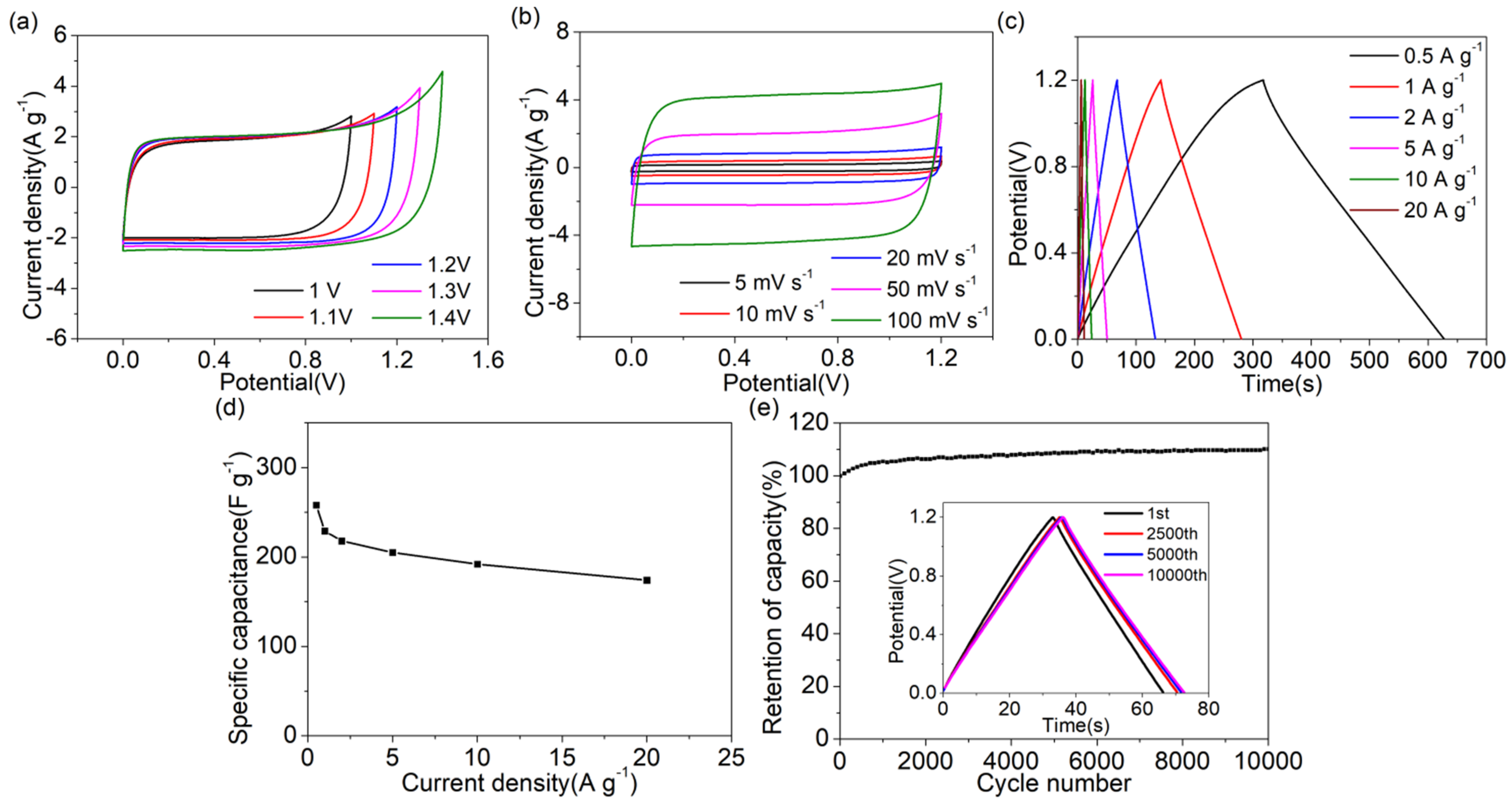
2.3. Electrochemical Testing
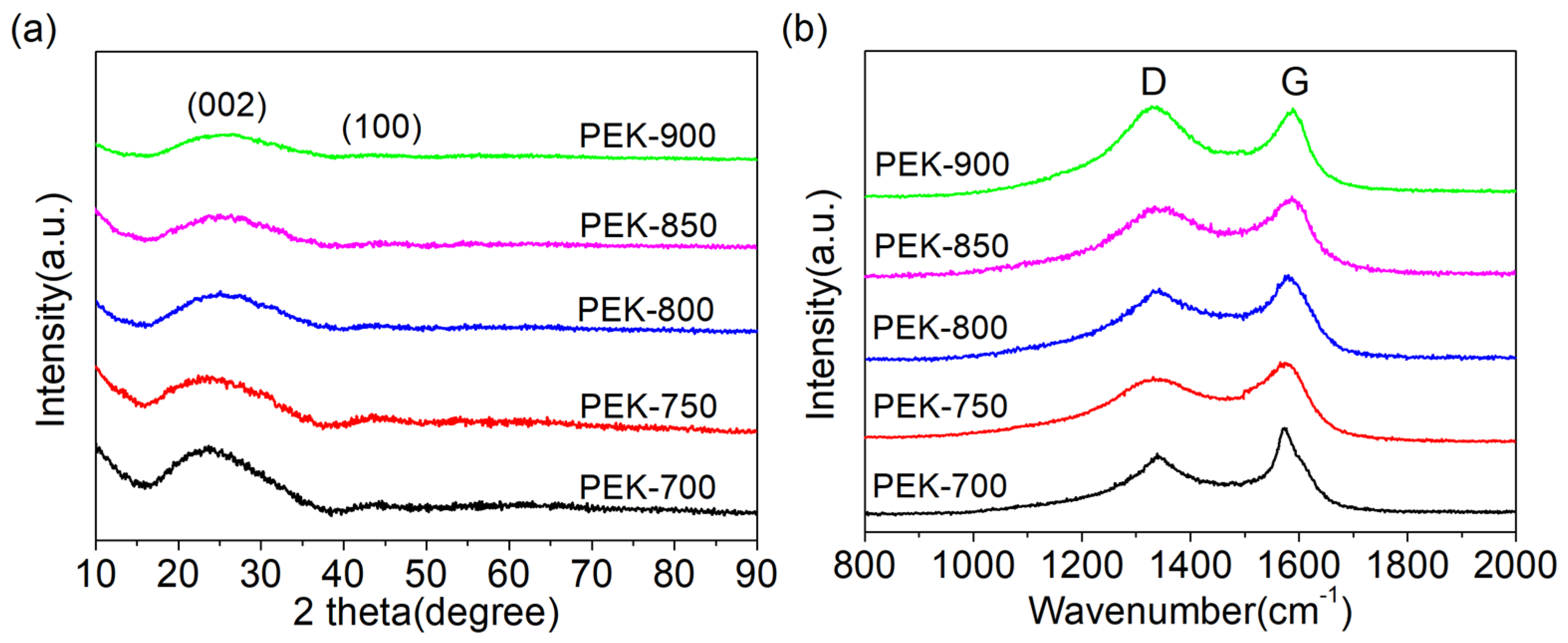
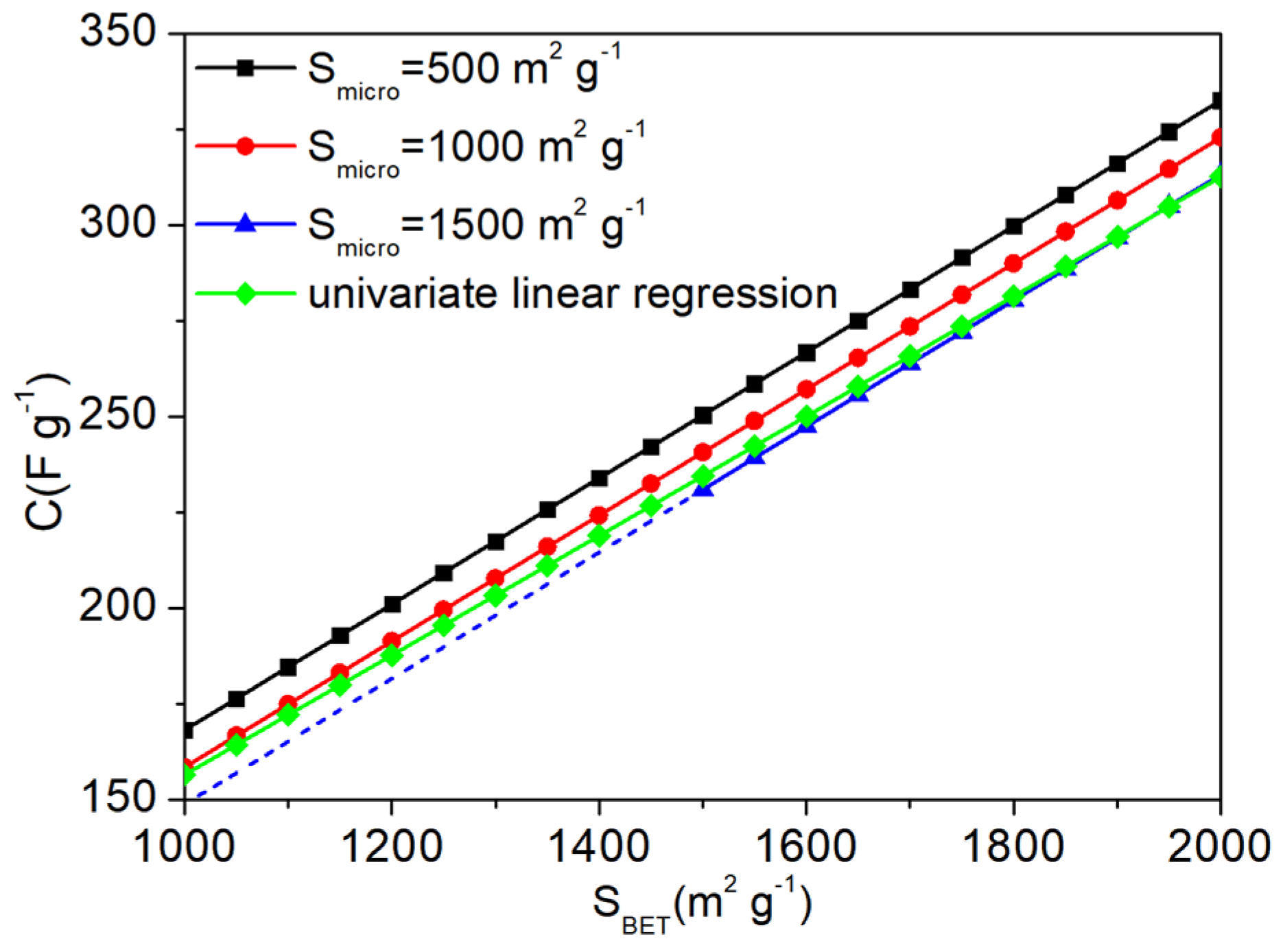
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ai, J.; Yang, S.; Sun, Y.; Liu, M.; Zhang, L.; Zhao, D.; Wang, J.; Yang, C.; Wang, X.; Cao, B. Corncob cellulose-derived hierarchical porous carbon for high performance supercapacitors. J. Power Sources 2021, 484, 229221. [Google Scholar] [CrossRef]
- Tai, C.-W.; Lu, Y.-T.; Yi, T.-Y.; Liu, Y.-C.; Chen, Y.-S.; Hu, C.-C. A Comprehensive Study on the Interactive Effects of Carbon Crystallinity and Electrochemical Activation for KOH-Modified Soft Carbons and Their High-Voltage Supercapacitor Application. J. Electrochem. Soc. 2023, 170, 040526. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Peng, J.; Jia, X.; Li, L.; Chuan, X. Porous Activity of Biomass-Activated Carbon Enhanced by Nitrogen-Dopant Towards High-Performance Lithium Ion Hybrid Battery-Supercapacitor. J. Electrochem. Soc. 2021, 168, 120537. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Li, N.; Hu, Y.; Zhao, C. Soybean root-derived N, O co-doped hierarchical porous carbon for supercapacitors. Appl. Surf. Sci. 2021, 555, 149726. [Google Scholar] [CrossRef]
- Liu, J.; Min, S.; Wang, F.; Zhang, Z. High-Performance Aqueous Supercapacitors Based on Biomass-Derived Multiheteroatom Self-Doped Porous Carbon Membranes. Energy Technol. 2020, 8, 2000391. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hosseini-Monfared, H.; Javanbakht, M.; Mahdi, F. Biomass-derived nanostructured carbon materials for high-performance supercapacitor electrodes. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Singh, A.; Ojha, A.K. Orange peel derived activated carbon for supercapacitor electrode material. J. Mater. Sci. Mater. Electron. 2023, 34, 1003. [Google Scholar] [CrossRef]
- Tian, X.; Ma, H.; Li, Z.; Yan, S.; Ma, L.; Yu, F.; Wang, G.; Guo, X.; Ma, Y.; Wong, C. Flute type micropores activated carbon from cotton stalk for high performance supercapacitors. J. Power Sources 2017, 359, 88–96. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, J.; Liang, Y.; Zheng, M.; Hu, H.; Dong, H.; Liu, Y.; Xiao, Y. The changing structure by component: Biomass-based porous carbon for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 585, 778–786. [Google Scholar] [CrossRef]
- Su, X.-L.; Cheng, M.-Y.; Fu, L.; Yang, J.-H.; Zheng, X.-C.; Guan, X.-X. Superior supercapacitive performance of hollow activated carbon nanomesh with hierarchical structure derived from poplar catkins. J. Power Sources 2017, 362, 27–38. [Google Scholar] [CrossRef]
- Shang, M.; Zhang, J.; Liu, X.; Liu, Y.; Guo, S.; Yu, S.; Filatov, S.; Yi, X. N, S self-doped hollow-sphere porous carbon derived from puffball spores for high performance supercapacitors. Appl. Surf. Sci. 2021, 542, 148697. [Google Scholar] [CrossRef]
- Cuong, D.V.; Wu, P.-C.; Liu, N.-L.; Hou, C.-H. Hierarchical porous carbon derived from activated biochar as an eco-friendly electrode for the electrosorption of inorganic ions. Sep. Purif. Technol. 2020, 242, 116813. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Abdolmaleki, H.; Doroudian, M.; Hosseini, S.H. Novel synthesis route for preparation of porous nitrogen-doped carbons from lignocellulosic wastes for high performance supercapacitors. J. Alloys Compd. 2020, 827, 154116. [Google Scholar] [CrossRef]
- Yao, L.; Yang, G.; Han, P.; Tang, Z.; Yang, J. Three-dimensional beehive-like hierarchical porous polyacrylonitrile-based carbons as a high performance supercapacitor electrodes. J. Power Sources 2016, 315, 209–217. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Chen, X.; Jiang, Z.; Wen, X.; Mijowska, E.; Tang, T. Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater. J. Mater. Chem. A 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Gong, J.; Michalkiewicz, B.; Chen, X.; Mijowska, E.; Liu, J.; Jiang, Z.; Wen, X.; Tang, T. Sustainable Conversion of Mixed Plastics into Porous Carbon Nanosheets with High Performances in Uptake of Carbon Dioxide and Storage of Hydrogen. ACS Sustain. Chem. Eng. 2014, 2, 2837–2844. [Google Scholar] [CrossRef]
- Liang, J.; Qu, T.; Kun, X.; Zhang, Y.; Chen, S.; Cao, Y.-C.; Xie, M.; Guo, X. Microwave assisted synthesis of camellia oleifera shell-derived porous carbon with rich oxygen functionalities and superior supercapacitor performance. Appl. Surf. Sci. 2018, 436, 934–940. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Y.; Yan, W.; Wu, X.; Wang, S.; Zhao, W. Two-step synthesis of B and N co-doped porous carbon composites by microwave-assisted hydrothermal and pyrolysis process for supercapacitor application. Electrochim. Acta 2020, 360, 137010. [Google Scholar] [CrossRef]
- Guo, M.; Guo, J.; Tong, F.; Jia, D.; Jia, W.; Wu, J.; Wang, L.; Sun, Z. Hierarchical porous carbon spheres constructed from coal as electrode materials for high performance supercapacitors. RSC Adv. 2017, 7, 45363–45368. [Google Scholar] [CrossRef]
- Song, Y.; Li, W.; Xu, Z.; Ma, C.; Liu, Y.; Xu, M.; Wu, X.; Liu, S. Hierarchical porous carbon spheres derived from larch sawdust via spray pyrolysis and soft-templating method for supercapacitors. SN Appl. Sci. 2018, 1, 122. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Liu, G.; Li, M. Tobacco-Stem-Derived Nitrogen-Enriched Hierarchical Porous Carbon for High-Energy Supercapacitor. ChemistrySelect 2021, 6, 532–537. [Google Scholar] [CrossRef]
- Hong, P.; Liu, X.; Zhang, X.; Peng, S.; Wang, Z.; Yang, Y.; Zhao, R.; Wang, Y. Hierarchically porous carbon derived from the activation of waste chestnut shells by potassium bicarbonate (KHCO3) for high-performance supercapacitor electrode. Int. J. Energy Res. 2019, 44, 988–999. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Feng, Z.; Li, X.; Yi, R.; Sun, W.; Zhao, C.; Yang, L. Nitrogen-Doped Hierarchical Porous Activated Carbon Derived from Paddy for High-Performance Supercapacitors. Materials 2021, 14, 318. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, F.; Long, G.; Zhang, T.; Leng, K.; Zhang, Y.; Huang, Y.; Ma, Y.; Zhang, M.; et al. Controlling the effective surface area and pore size distribution of sp2 carbon materials and their impact on the capacitance performance of these materials. J. Am. Chem. Soc. 2013, 135, 5921–5929. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Kong, L.-B.; Kang, L.; Yan, K.; Zhang, T.; Li, K.; Luo, Y.-C. Synthesis of a hierarchical nanoporous carbon material with controllable pore size and effective surface area for high-performance electrochemical capacitors. RSC Adv. 2017, 7, 14516–14527. [Google Scholar] [CrossRef]
- Niu, L.; Shen, C.; Yan, L.; Zhang, J.; Lin, Y.; Gong, Y.; Li, C.; Sun, C.Q.; Xu, S. Waste bones derived nitrogen-doped carbon with high micropore ratio towards supercapacitor applications. J. Colloid Interface Sci. 2019, 547, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, Y.; Liu, C.; Yang, L.; Zhao, C. Influence of KHCO3 Activation on Characteristics of Biomass-Derived Carbons for Supercapacitor. Coatings 2023, 13, 1236. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Zhou, J.; Wang, X.; Xu, J.; Tan, F. Nitrogen-doped lignin-derived electrode materials for supercapacitors were prepared using the domain-limited effect. Int. J. Biol. Macromol. 2024, 265 Pt 2, 130796. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Wang, S.; Xu, Y.; Zhang, L.; Wang, X.; Yu, H.; Ren, B. Starch-based carbon aerogels prepared by an innovative KOH activation method for supercapacitors. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128587. [Google Scholar] [CrossRef]
- Guo, C.; Tao, C.; Yu, F.; Zhao, Z.; Wang, Z.; Deng, N.; Huang, X. Ball-milled layer double hydroxide as persulfate activator for efficient degradation of organic: Alkaline sites-triggered non-radical mechanism. J. Hazard. Mater. 2024, 461, 132219. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Zhang, Q.; Zou, J.; Ai, J.; Qiao, D.; Zhong, W.; Liu, Y.; Jun, S.C.; Yamauchi, Y.; et al. Hierarchical Spatial Confinement Unlocking the Storage Limit of MoS2 for Flexible High-Energy Supercapacitors. ACS Nano 2024, 18, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; He, P.; Ding, S.; Zhang, G.; He, M.; Dong, F.; Wen, J.; Du, L.; Liu, M. Oxygen-doped activated carbons derived from three kinds of biomass: Preparation, characterization and performance as electrode materials for supercapacitors. RSC Adv. 2016, 6, 5949–5956. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Tao, S.; Zu, X.; Li, S.; Qiao, L. Nitrogen/oxygen co-doped carbon nanofoam derived from bamboo fungi for high-performance supercapacitors. J. Power Sources 2020, 479, 228835. [Google Scholar] [CrossRef]
- Sun, Z.; Masa, J.; Weide, P.; Fairclough, S.M.; Robertson, A.W.; Ebbinghaus, P.; Warner, J.H.; Tsang, S.C.E.; Muhler, M.; Schuhmann, W. High-quality functionalized few-layer graphene: Facile fabrication and doping with nitrogen as a metal-free catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 15444–15450. [Google Scholar] [CrossRef]
- Tao, H.; Yan, C.; Robertson, A.W.; Gao, Y.; Ding, J.; Zhang, Y.; Ma, T.; Sun, Z. N-Doping of graphene oxide at low temperature for the oxygen reduction reaction. Chem. Commun. 2017, 53, 873–876. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing Functionalized Graphene as Electrodes for High-Performance Supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Y.; Li, M.; Yang, R. High performance N-doped porous activated carbon based on chicken feather for supercapacitors and CO2 capture. RSC Adv. 2015, 5, 34803–34811. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. “Salt templating”: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Z.; Wang, G.; Chen, T. Biomass Porous Carbon Derived from Celery Leaves with High Capacitance for Supercapacitor. ChemistrySelect 2023, 8, e202204616. [Google Scholar] [CrossRef]

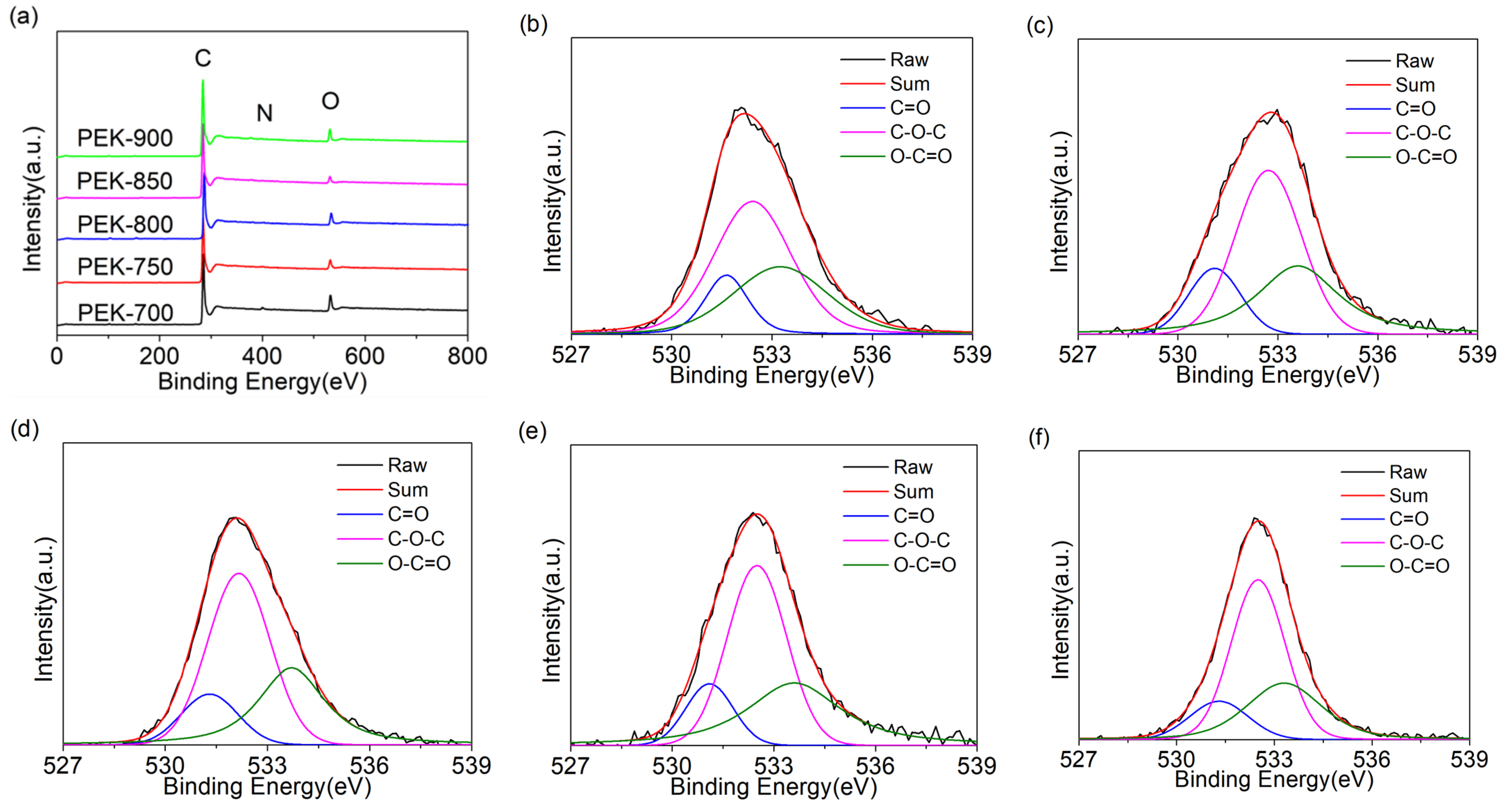



| Samples | C (Atom%) | O (Atom%) | N (Atom%) | C=O (%) | C-O-C (%) | O-C=O (%) |
|---|---|---|---|---|---|---|
| PEK-700 | 89.27 | 8.69 | 2.04 | 13.81 | 52.72 | 33.47 |
| PEK-750 | 92.89 | 5.71 | 1.4 | 15.74 | 49.83 | 34.43 |
| PEK-800 | 91.87 | 6.87 | 1.26 | 14.14 | 52.49 | 33.37 |
| PEK-850 | 95.13 | 3.94 | 0.93 | 13.52 | 49.28 | 37.20 |
| PEK-900 | 93.01 | 5.9 | 1.09 | 14.03 | 54.95 | 31.02 |
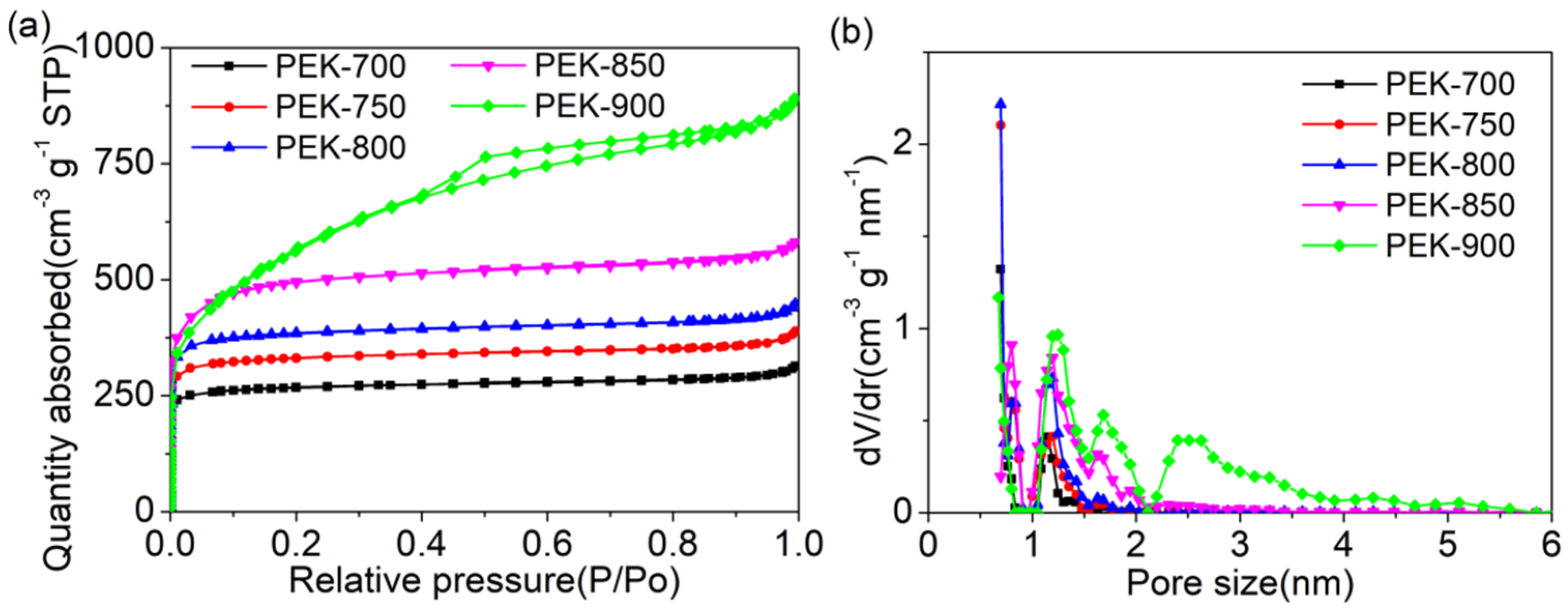
| Samples | SBET (m2 g−1) | Smicro (m2 g−1) | Vtotal (cm3 g−1) | Vmicro (cm3 g−1) |
|---|---|---|---|---|
| PEK-700 | 1049.1 | 1008.1 | 0.4853 | 0.4001 |
| PEK-750 | 1270.7 | 1229.0 | 0.6006 | 0.5024 |
| PEK-800 | 1502.4 | 1440.9 | 0.6920 | 0.5751 |
| PEK-850 | 1852.0 | 1759.5 | 0.8981 | 0.7426 |
| PEK-900 | 2002.2 | 1227.7 | 1.2963 | 0.6036 |
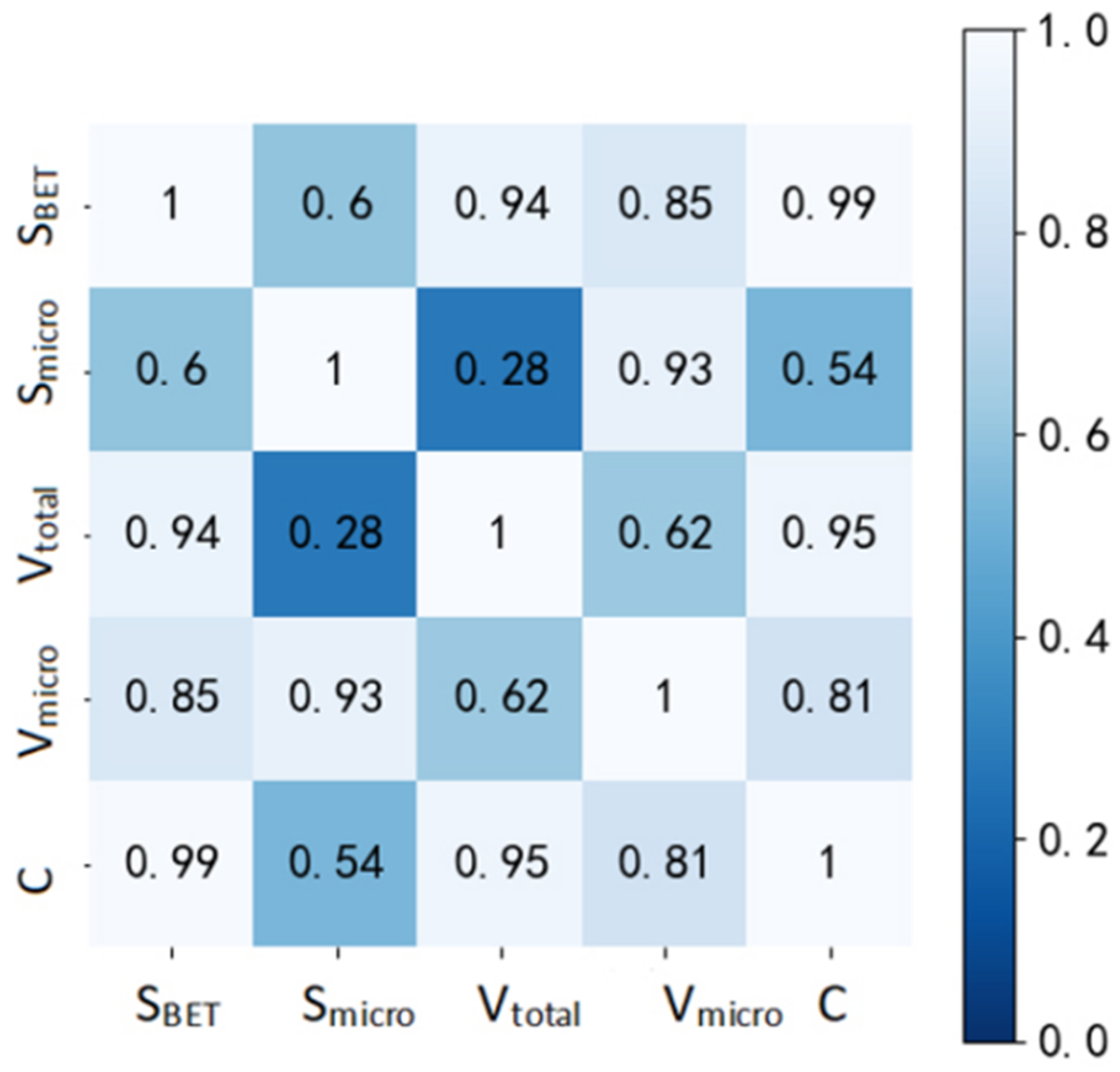
| SBET | Smicro | Vtotal | Vmicro | C | |
|---|---|---|---|---|---|
| SBET | 1.00 | 0.60 | 0.94 | 0.85 | 0.99 |
| Smicro | 0.60 | 1.00 | 0.28 | 0.93 | 0.54 |
| Vtotal | 0.94 | 0.28 | 1.00 | 0.62 | 0.95 |
| Vmicro | 0.85 | 0.93 | 0.62 | 1.00 | 0.81 |
| C | 0.99 | 0.54 | 0.95 | 0.81 | 1.00 |
| Parameters | SBET | Smicro | Vtotal | Vmicro |
|---|---|---|---|---|
| Coefficients | 0.164 | −0.019 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Sun, Y.; Liu, C.; Yang, L.; Zhao, C. Hierarchical Porous Activated Carbon Derived from Pleurotus Eryngii and the Influence of Pore Structural Parameters on Capacitance Performance. Coatings 2024, 14, 840. https://doi.org/10.3390/coatings14070840
Yuan Y, Sun Y, Liu C, Yang L, Zhao C. Hierarchical Porous Activated Carbon Derived from Pleurotus Eryngii and the Influence of Pore Structural Parameters on Capacitance Performance. Coatings. 2024; 14(7):840. https://doi.org/10.3390/coatings14070840
Chicago/Turabian StyleYuan, Yudan, Yi Sun, Chenguang Liu, Li Yang, and Cezhou Zhao. 2024. "Hierarchical Porous Activated Carbon Derived from Pleurotus Eryngii and the Influence of Pore Structural Parameters on Capacitance Performance" Coatings 14, no. 7: 840. https://doi.org/10.3390/coatings14070840





