Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
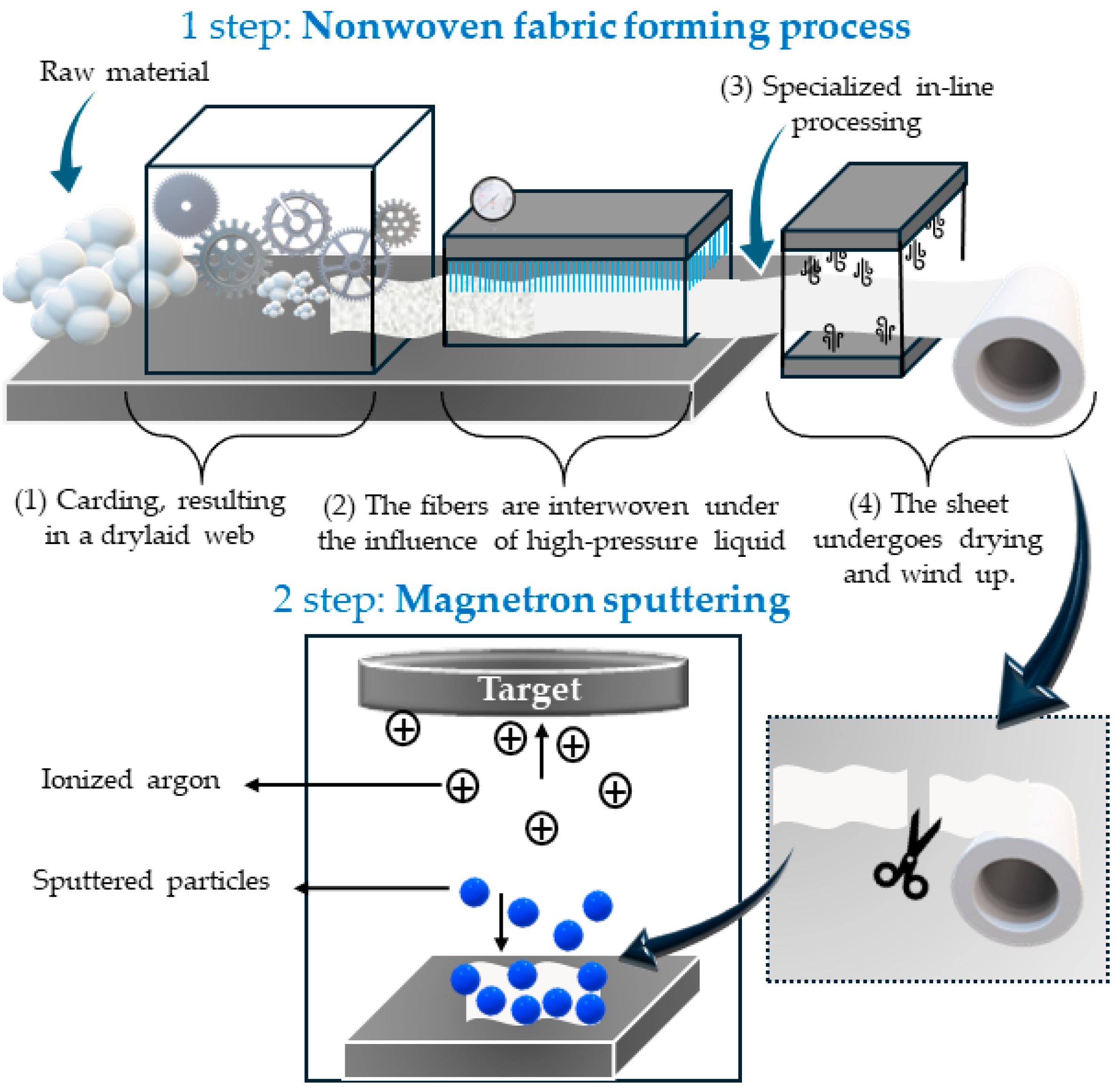
2.2.1. Aqua-Jet/Spunlace Technique
2.2.2. Magnetron Sputtering
2.2.3. Microscopy Analysis
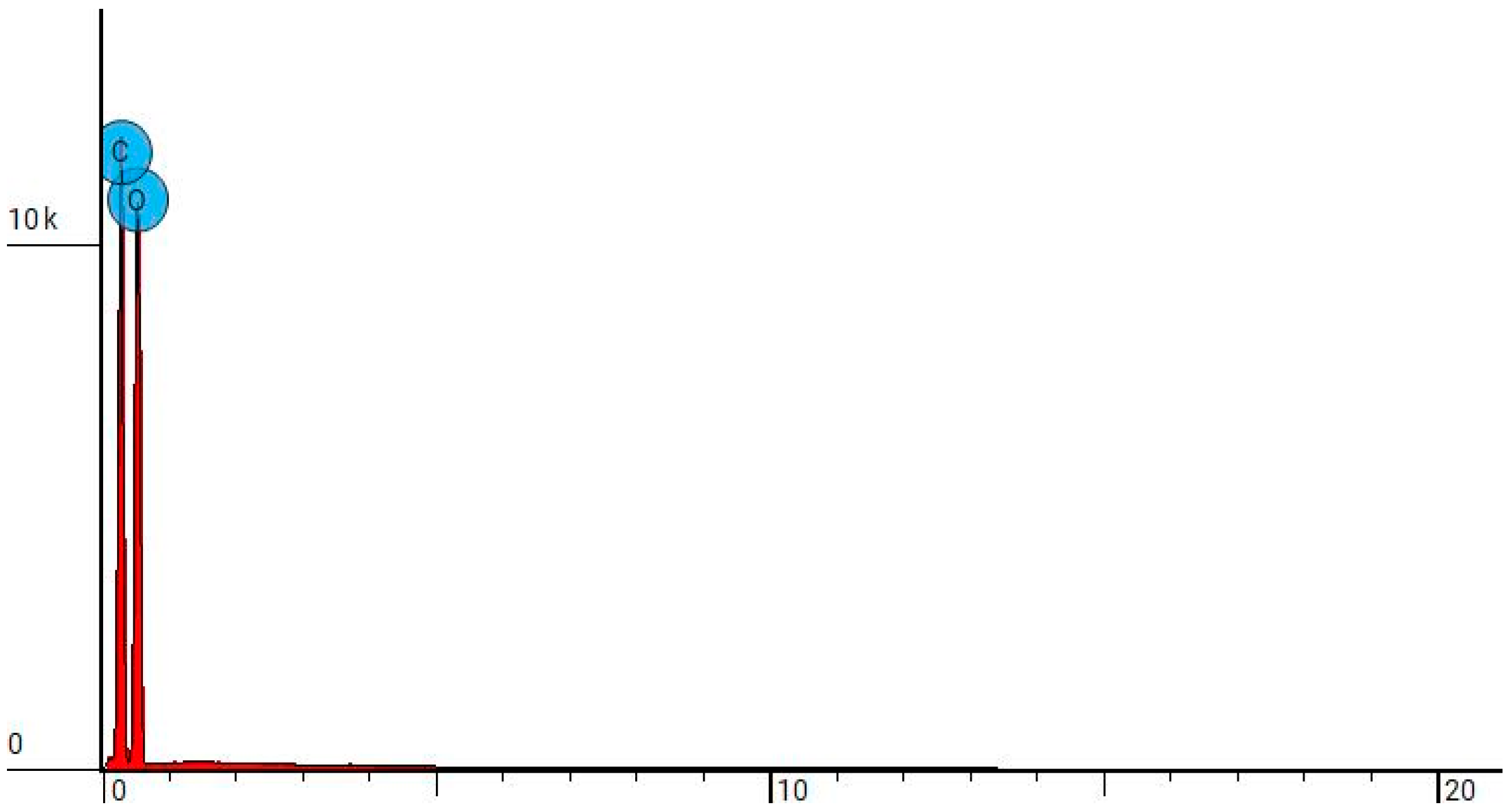
2.2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.2.5. UV-Vis Analysis
2.2.6. Flame Atomic Absorption Spectrometry (FAAS)-Copper Content Assessment
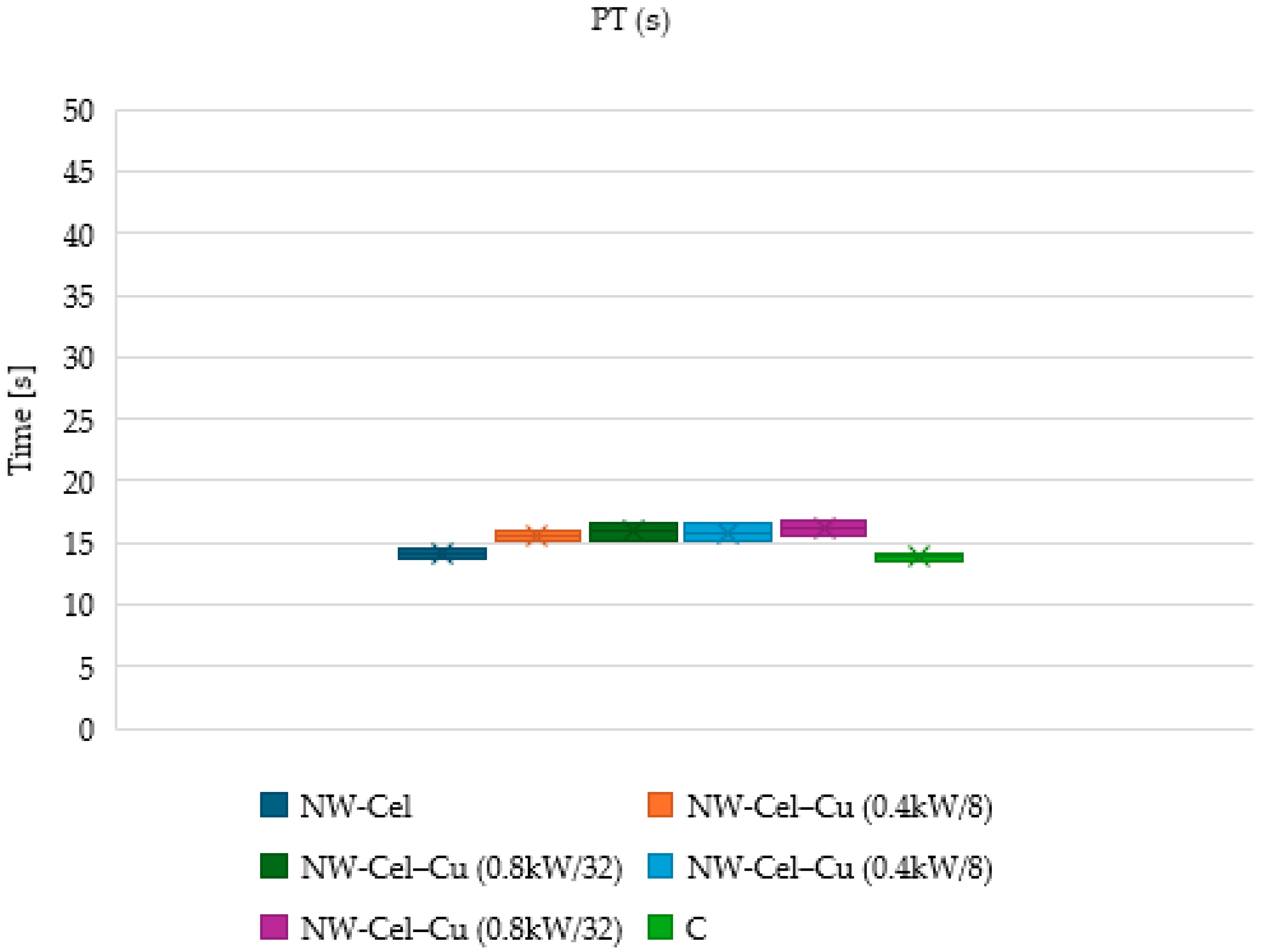
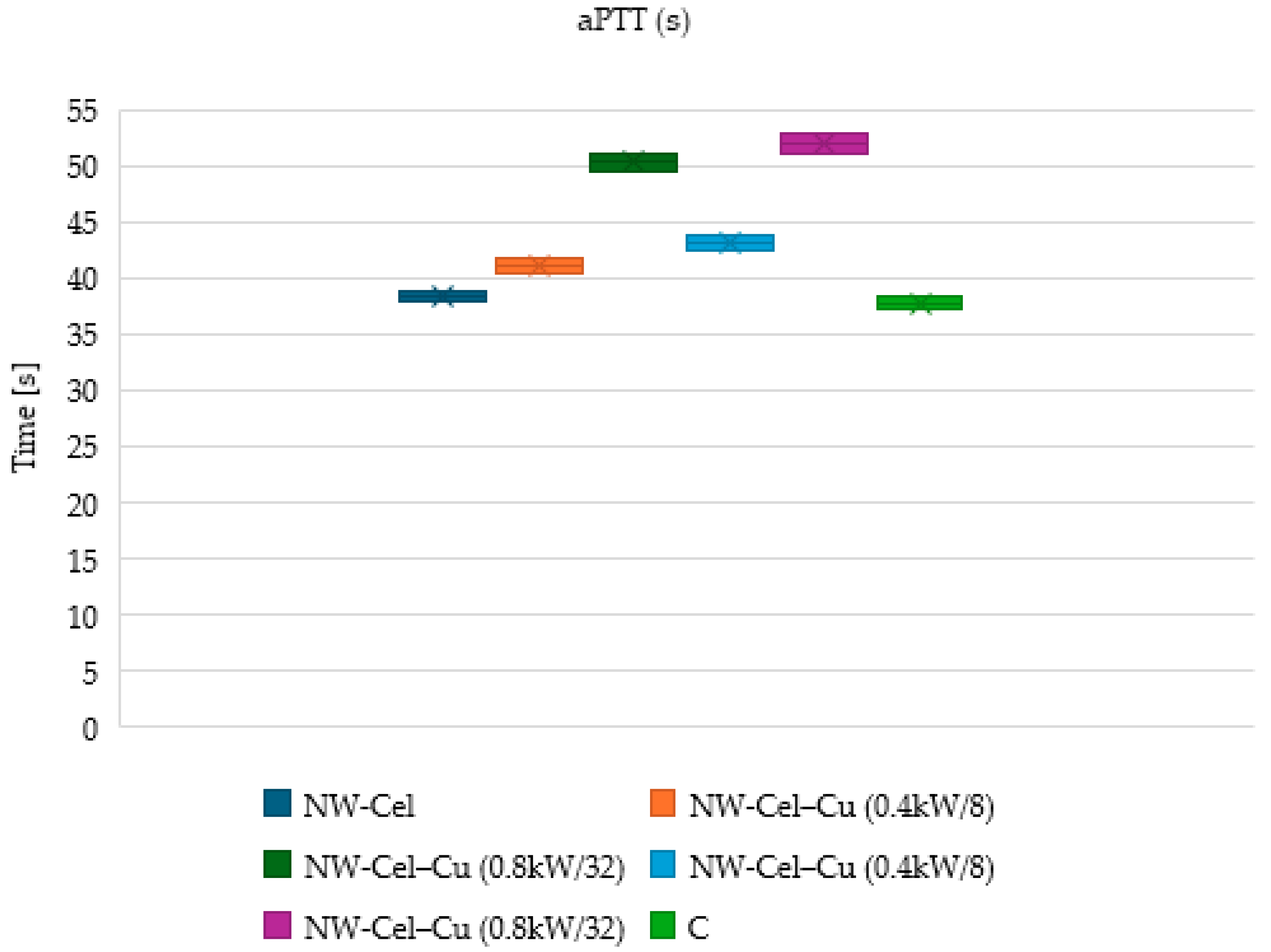
2.2.7. Measurement of Blood Clotting Factors, including aPTT and PT
2.2.8. Antibacterial and Antifungal Tests
3. Results
3.1. Preparation of Nonwoven Cellulose-Copper Composites
3.2. Determination of Copper Content
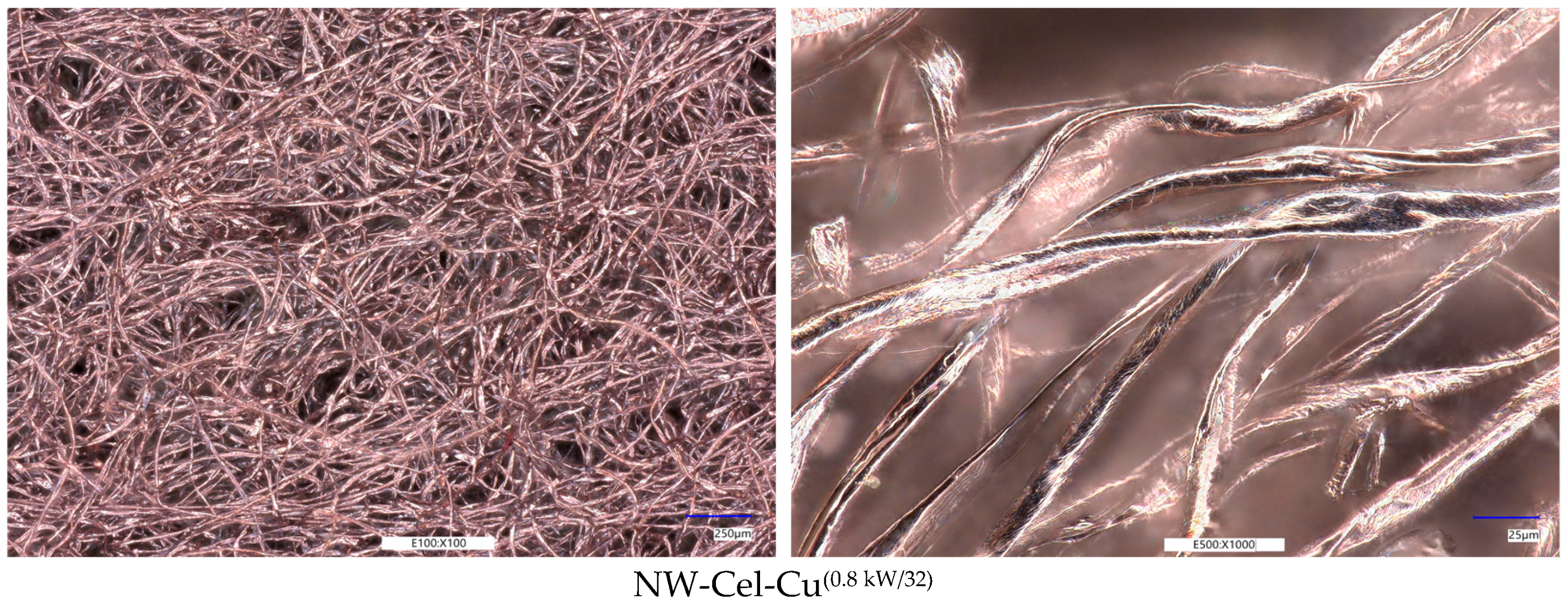
Optical Microscopy Analysis
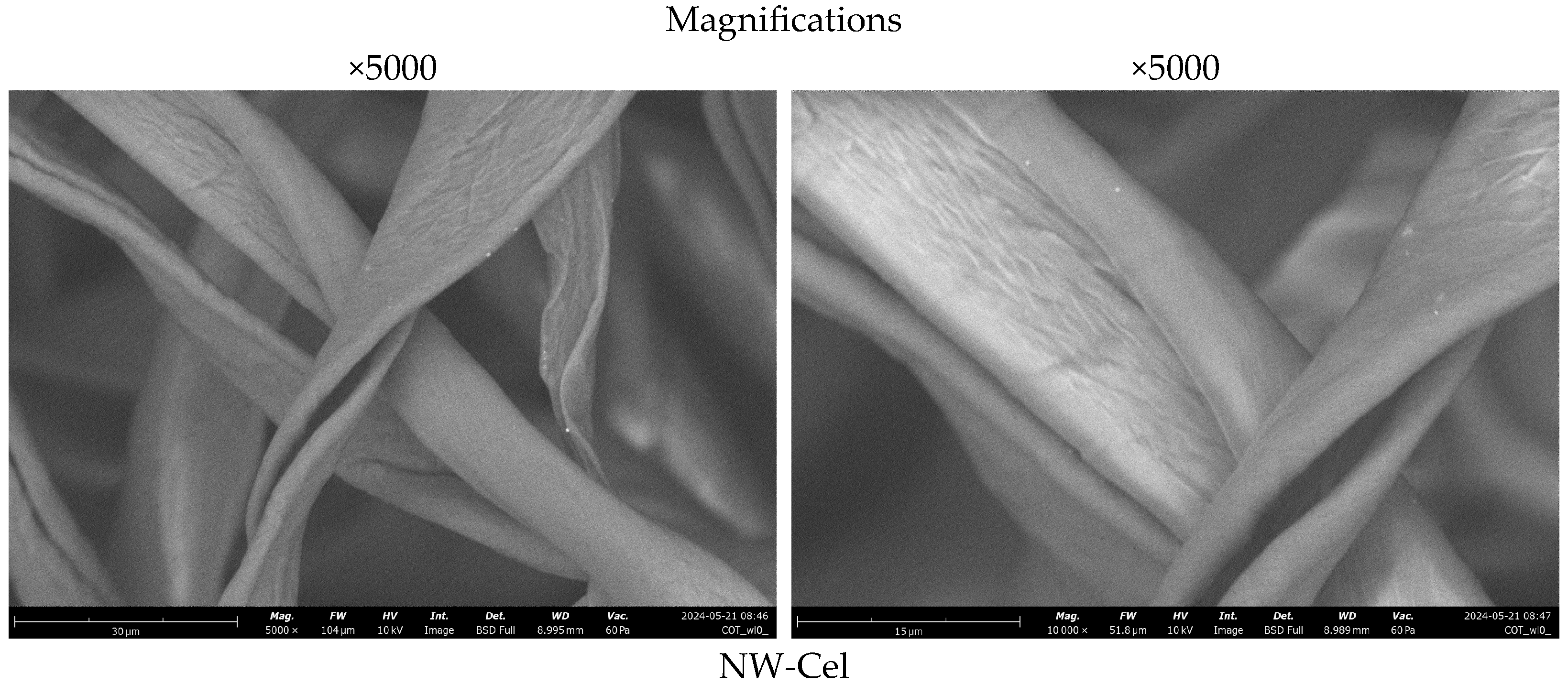
3.3. Scanning Electron Microscopy
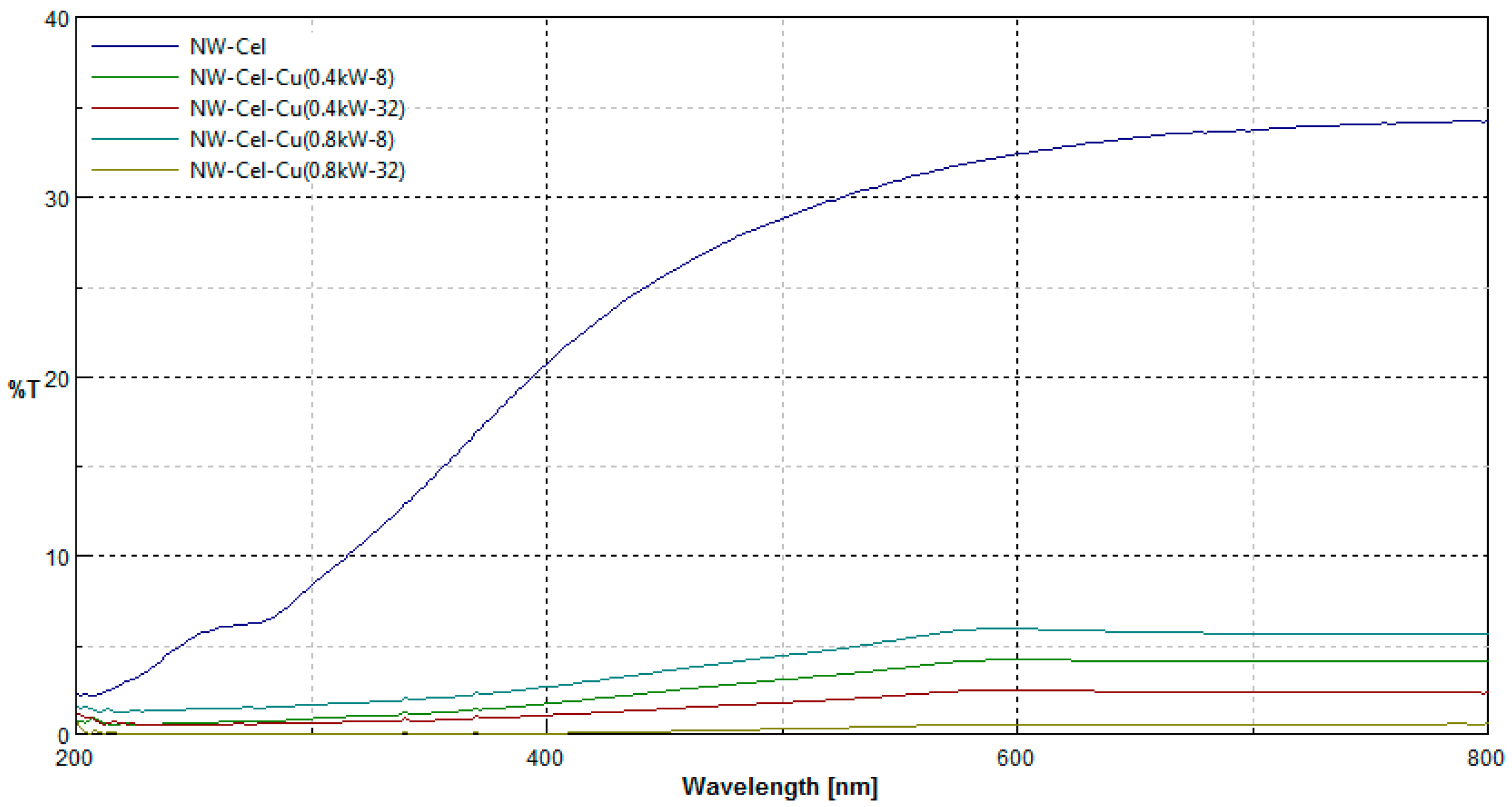
3.4. UV/Vis Transmittance
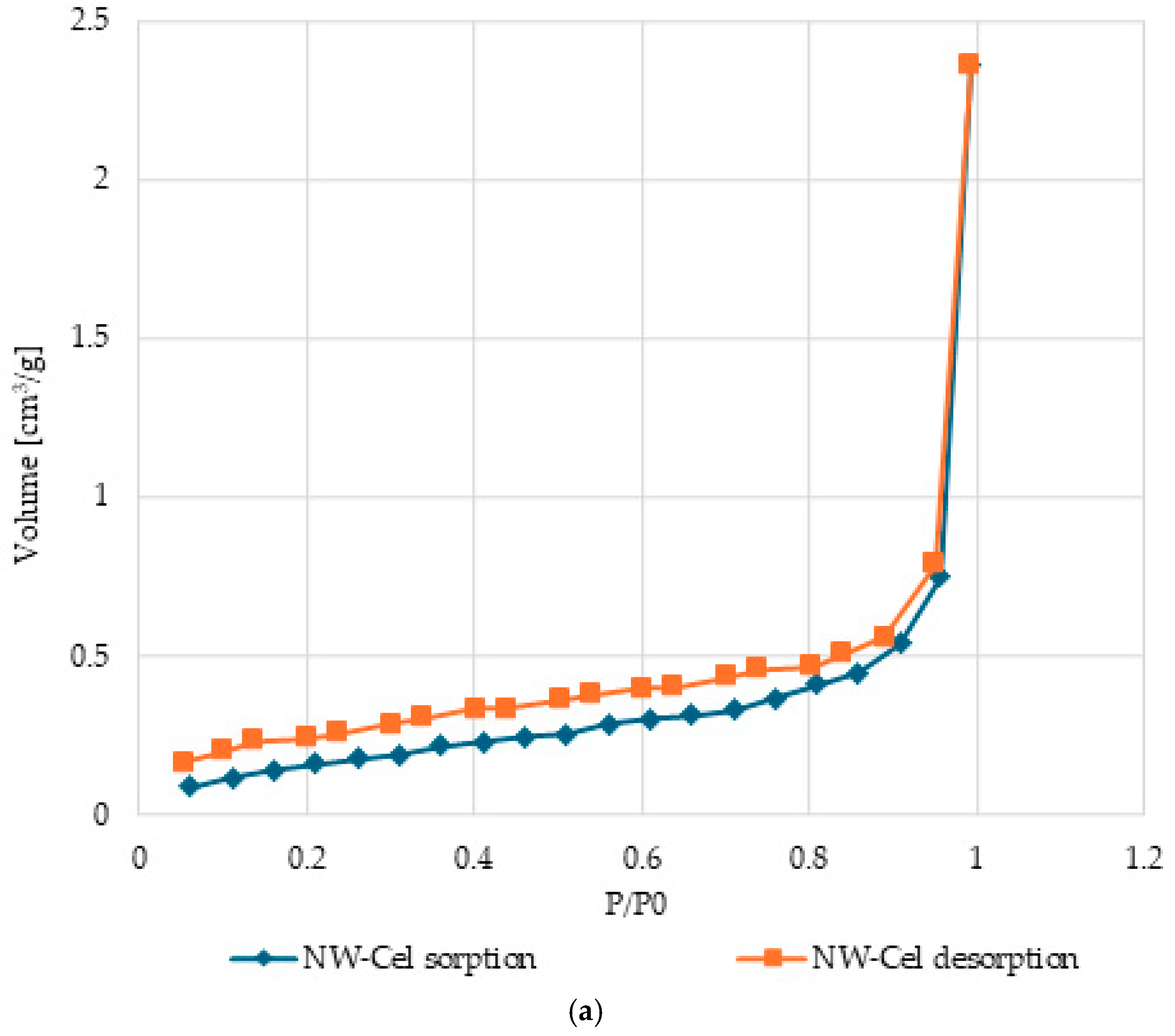
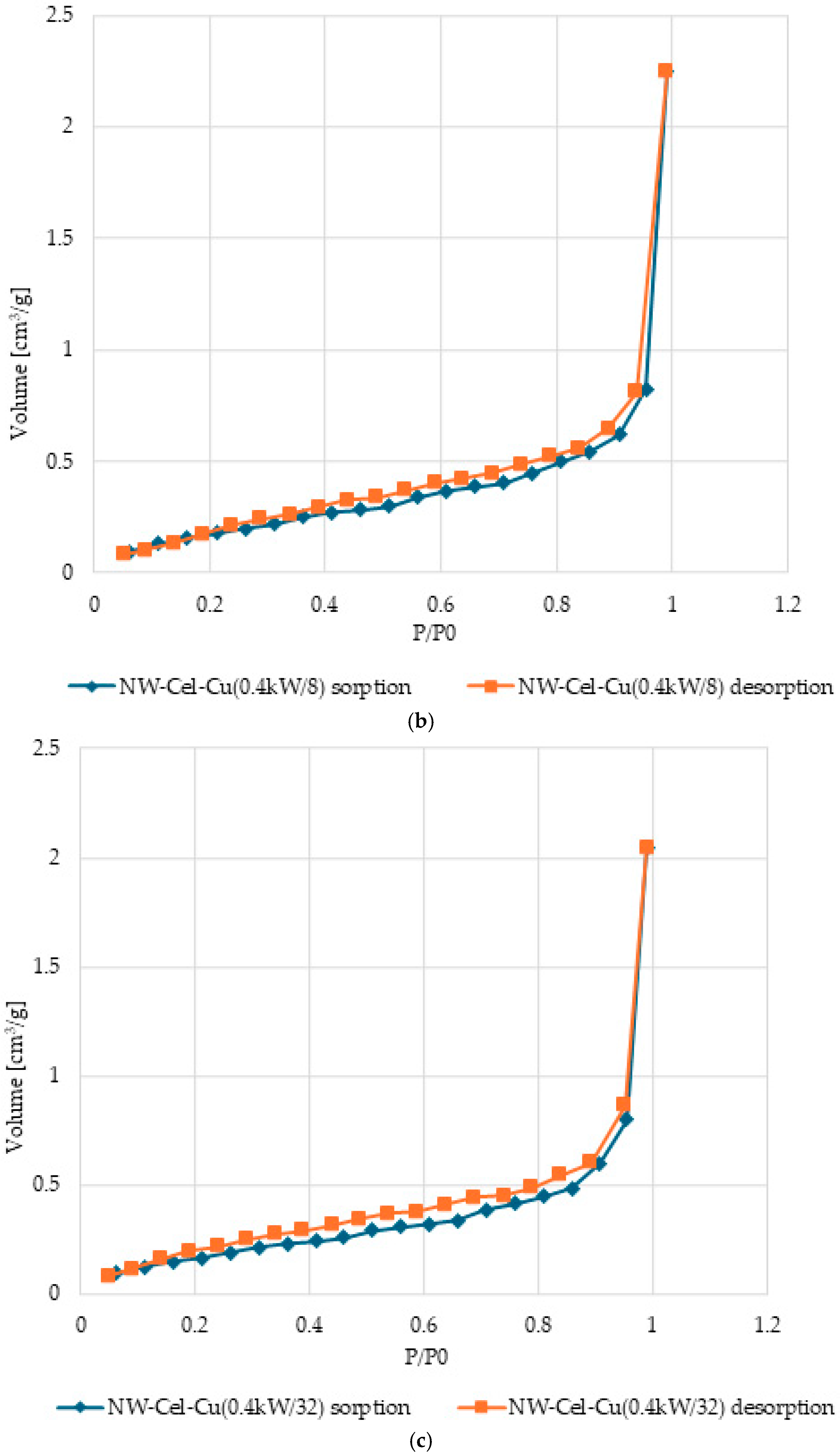
3.5. Surface Characteristics and Pore Volume in Cel-Cu Samples
3.6. Measurements of aPTT and PT Times
Antibacterial Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable Cellulose and Its Derivatives for Promising Biomedical Applications. Prog. Mater. Sci. 2023, 138, 101152. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K.; Gupta, P.K.; et al. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; IntechOpen: Houston, TX, USA, 2019; ISBN 978-1-83968-057-1. [Google Scholar]
- Carolin, C.F.; Kamalesh, T.; Kumar, P.S.; Hemavathy, R.V.; Rangasamy, G. A Critical Review on Sustainable Cellulose Materials and Its Multifaceted Applications. Ind. Crops Prod. 2023, 203, 117221. [Google Scholar] [CrossRef]
- Dufresne, A. Chapter 19—Cellulose-Based Composites and Nanocomposites. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 401–418. ISBN 978-0-08-045316-3. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Li, X.; Wan, C.; Tao, T.; Chai, H.; Huang, Q.; Chai, Y.; Wu, Y. An Overview of the Development Status and Applications of Cellulose–Based Functional Materials. Cellulose 2024, 31, 61–99. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, C.; Zhou, D.; Chen, S.; Zou, L.; Wang, H.; Wu, J. Hydrophobic, Breathable Cellulose Nonwoven Fabrics for Disposable Hygiene Applications. Carbohydr. Polym. 2022, 288, 119367. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Sánchez–López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Calabrese, C.; La Parola, V.; Testa, M.L.; Liotta, L.F. Antifouling and Antimicrobial Activity of Ag, Cu and Fe Nanoparticles Supported on Silica and Titania. Inorganica Chim. Acta 2022, 529, 120636. [Google Scholar] [CrossRef]
- Brandelli, A.; Ritter, A.C.; Veras, F.F. Antimicrobial Activities of Metal Nanoparticles. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 337–363. ISBN 978-3-319-63790-7. [Google Scholar]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial Metals and Alloys for Potential Biomedical Implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Hossein Mohammadi, A.; Sobhani-Nasab, A.; Nejati, M.; Hadi, S.; Behjati, M.; Mirzaii-Dizgah, I.; Moradi Hasan-Abad, A.; Karami, M. Preparation and Characterization of CuO, Ag2O and ZnO Nanoparticles and Investigation of Their Antibacterial and Anticancer Properties on HCT-116 and C26 Cells. Inorg. Chem. Commun. 2023, 149, 110404. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact Killing and Antimicrobial Properties of Copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef] [PubMed]
- Alselami, A.; Drummond, R.A. How Metals Fuel Fungal Virulence, yet Promote Anti-Fungal Immunity. Dis. Model. Mech. 2023, 16, dmm050393. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper as a Biocidal Tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New Insights on Antimicrobial Efficacy of Copper Surfaces in the Healthcare Environment: A Systematic Review. Clin. Microbiol. Infect. 2018, 24, 1130–1138. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Maliki, M.; Ifijen, I.H.; Ikhuoria, E.U.; Jonathan, E.M.; Onaiwu, G.E.; Archibong, U.D.; Ighodaro, A. Copper Nanoparticles and Their Oxides: Optical, Anticancer and Antibacterial Properties. Int. Nano Lett. 2022, 12, 379–398. [Google Scholar] [CrossRef]
- Li, X.; Cong, Y.; Ovais, M.; Cardoso, M.B.; Hameed, S.; Chen, R.; Chen, M.; Wang, L. Copper-Based Nanoparticles against Microbial Infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1888. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xu, H.; Liao, Z.; Liu, K.; Chaima, M.; Zhang, X.; Hu, Y.; Wei, Y.; Huang, D. Copper-Based Nanoparticles as Antibacterial Agents. Eur. J. Inorg. Chem. 2023, 26, e202200614. [Google Scholar] [CrossRef]
- Cortes, A.A.; Zuñiga, J.M. The Use of Copper to Help Prevent Transmission of SARS-Coronavirus and Influenza Viruses. A General Review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115176. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, L.V.; Kiseleva, I.V.; Polishchuk, E.V.; Broggini, M.; Ilyechova, E.Y. The Crossroads between Host Copper Metabolism and Influenza Infection. Int. J. Mol. Sci. 2021, 22, 5498. [Google Scholar] [CrossRef] [PubMed]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential Molecular Mechanisms of Zinc- and Copper-Mediated Antiviral Activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Pieretti, J.C.; Rubilar, O.; Fernández-Baldo, M.; Benavides-Mendoza, A.; Diez, M.C.; Seabra, A.B. Silver, Copper and Copper Oxide Nanoparticles in the Fight against Human Viruses: Progress and Perspectives. Crit. Rev. Biotechnol. 2022, 42, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, O.V.; Vasyukova, I.A.; Gusev, A.A. Metal-Based Nanoparticles for the Diagnostics, Therapy, and Prevention of Viral Infections. Nanotechnol. Russia 2023, 18, 165–188. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in Fungal Virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal-Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Carreira, T.S.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. Int. J. Mol. Sci. 2022, 23, 1165. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. A Comprehensive Review on Potential Applications of Metallic Nanoparticles as Antifungal Therapies to Combat Human Fungal Diseases. Saudi Pharm. J. 2023, 31, 101733. [Google Scholar] [CrossRef] [PubMed]
- Jelis, E.; Kristol, D.; Arora, R.R.; Spillert, C.R. The Effect of Copper Ion on Blood Coagulation. In Proceedings of the IEEE 30th Annual Northeast Bioengineering Conference, Springfield, MA, USA, 17–18 April 2004; p. 127. [Google Scholar]
- Muthulakshmi, L.; Varada Rajalu, A.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of Cellulose/Copper Nanoparticles Bionanocomposite Films Using a Bioflocculant Polymer as Reducing Agent for Antibacterial and Anticorrosion Applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar] [CrossRef]
- Royer, A.; Sharman, T. Copper Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Alkhanjaf, A.A.M.; Sharma, S.; Sharma, M.; Kumar, R.; Arora, N.K.; Kumar, B.; Umar, A.; Baskoutas, S.; Mukherjee, T.K. Microbial Strategies for Copper Pollution Remediation: Mechanistic Insights and Recent Advances. Environ. Pollut. 2024, 346, 123588. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Erişen, D.E.; Yang, K.; Zhang, B.; Guan, H.; Chen, S. Anticoagulation and Antibacterial Functional Coating on Vascular Implant Interventional Medical Catheter. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2868–2877. [Google Scholar] [CrossRef]
- EN ISO 20645:2006; Textile Fabrics. Determination of Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- EN 14119: 2005 Point 10.5 (B2); Testing of Textiles. Evaluation of the Action of Microfungi. Visual Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed If Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore Characterization of Different Types of Coal from Coal and Gas Outburst Disaster Sites Using Low Temperature Nitrogen Adsorption Approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kudzin, M.H.; Ponczek, M.B.; Kaczmarek, A.; Król, P.; Lisiak-Kucińska, A.; Żyłła, R.; Walawska, A. Biochemical Approach to Poly(Lactide)-Copper Composite—Impact on Blood Coagulation Processes. Materials 2024, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4020-2303-3. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279Ca. [Google Scholar] [CrossRef]
- Medrano, V.G.B.; Celis, V.N.; Giraldo, R.I. Systematic Analysis of the Nitrogen Adsorption-Desorption Isotherms Recorded for a Series of Microporous-Mesoporous Amorphous Aluminosilicates Using Classical Methods. J. Chem. Eng. Data 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- Mutch, N.J.; Waters, E.K.; Morrissey, J.H. Immobilized Transition Metal Ions Stimulate Contact Activation and Drive Factor XII-mediated Coagulation. J. Thromb. Haemost. 2012, 10, 2108–2115. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Ponczek, M.; Kaczmarek, A.; Boguń, M.; Sulak, E.; Kudzin, M.H. Blood Coagulation Activities of Cotton-Alginate-Copper Composites. Mar. Drugs 2023, 21, 625. [Google Scholar] [CrossRef]




| Parameter | Range |
|---|---|
| Gas Pressure | 2.3 × 10−3 mbar |
| Magnetron Power | 0.4 kW and 0.8 kW |
| Sample Name | Process Duration (Magnetron Power) |
| NW-Cel-Cu(0.4 kW/8) | 8 min (0.4 kW) |
| NW-Cel-Cu(0.4 kW/32) | 32 min (0.4 kW) |
| NW-Cel-Cu(0.8 kW/8) | 8 min (0.8 kW) |
| NW-Cel-Cu(0.8 kW/32) | 32 min (0.8 kW) |
| Sample Name | Cu Concentration [g/kg] |
|---|---|
| NW-Cel | 0 |
| NW-Cel-Cu(0.4 kW/8) | 9.59 |
| NW-Cel-Cu(0.4 kW/32) | 26.13 |
| NW-Cel-Cu(0.8 kW/8) | 14.35 |
| NW-Cel-Cu(0.8 kW/32) | 28.11 |
| The results have been measured in triplicate and are presented as a mean value with ± deviation equal to approximately 2%. | |
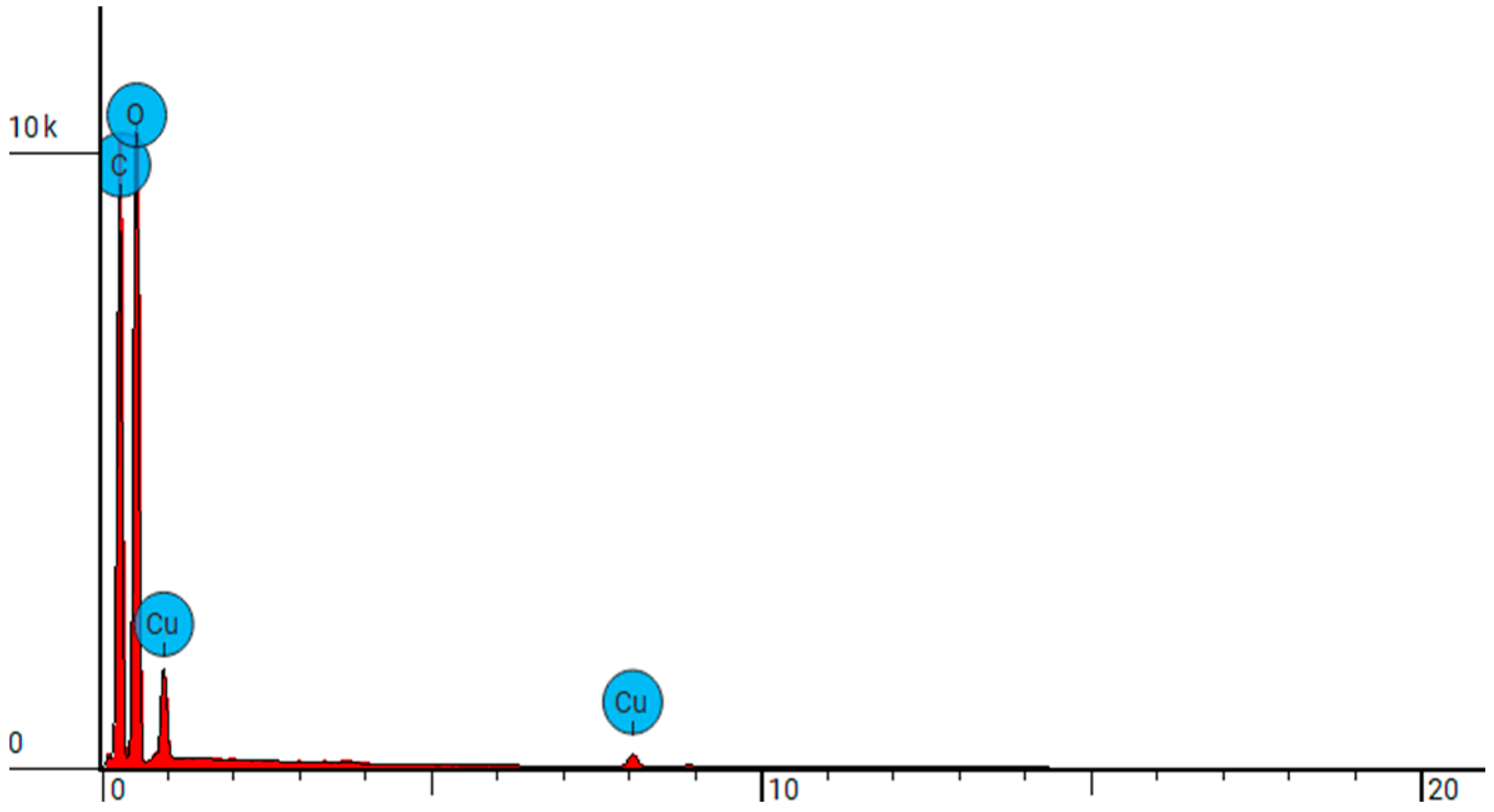
| Sample Name | Element Symbol | Element Name | Atomic Conc. | Weight Conc. |
|---|---|---|---|---|
| NW-Cel | C | Carbon | 45.265 | 38.300 |
| O | Oxygen | 54.735 | 61.700 | |
| NW-Cel-Cu(0.4 kW/8) | C | Carbon | 45.274 | 35.500 |
| O | Oxygen | 52.364 | 54.700 | |
| Cu | Copper | 2.362 | 9.800 | |
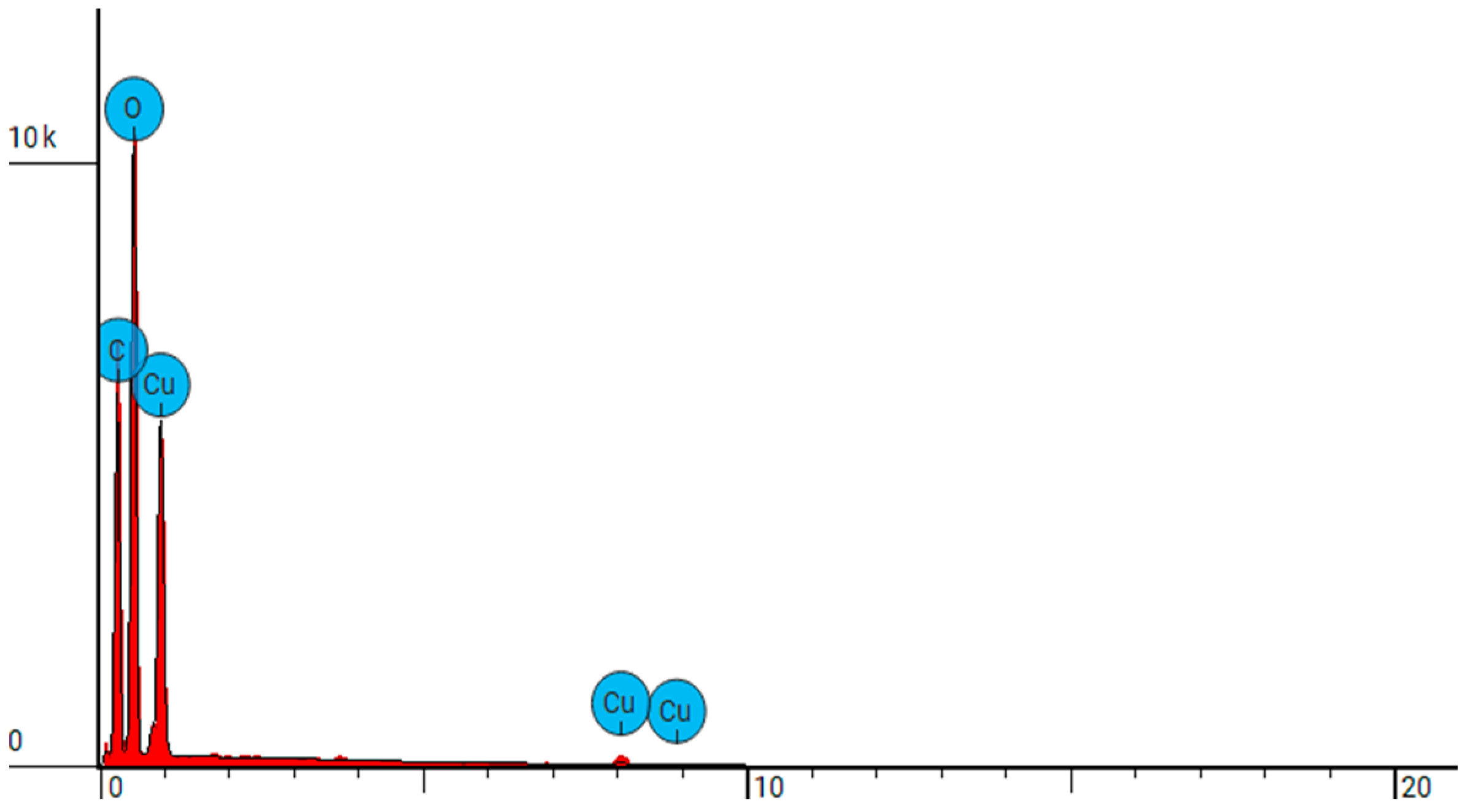
| NW-Cel-Cu(0.4 kW/32) | C | Carbon | 41.086 | 26.374 |
| O | Oxygen | 49.765 | 42.557 | |
| Cu | Copper | 9.148 | 31.069 | |
| NW-Cel-Cu(0.8 kW/8) | C | Carbon | 39.137 | 24.200 |
| O | Oxygen | 50.378 | 41.500 | |
| Cu | Copper | 10.485 | 34.300 | |
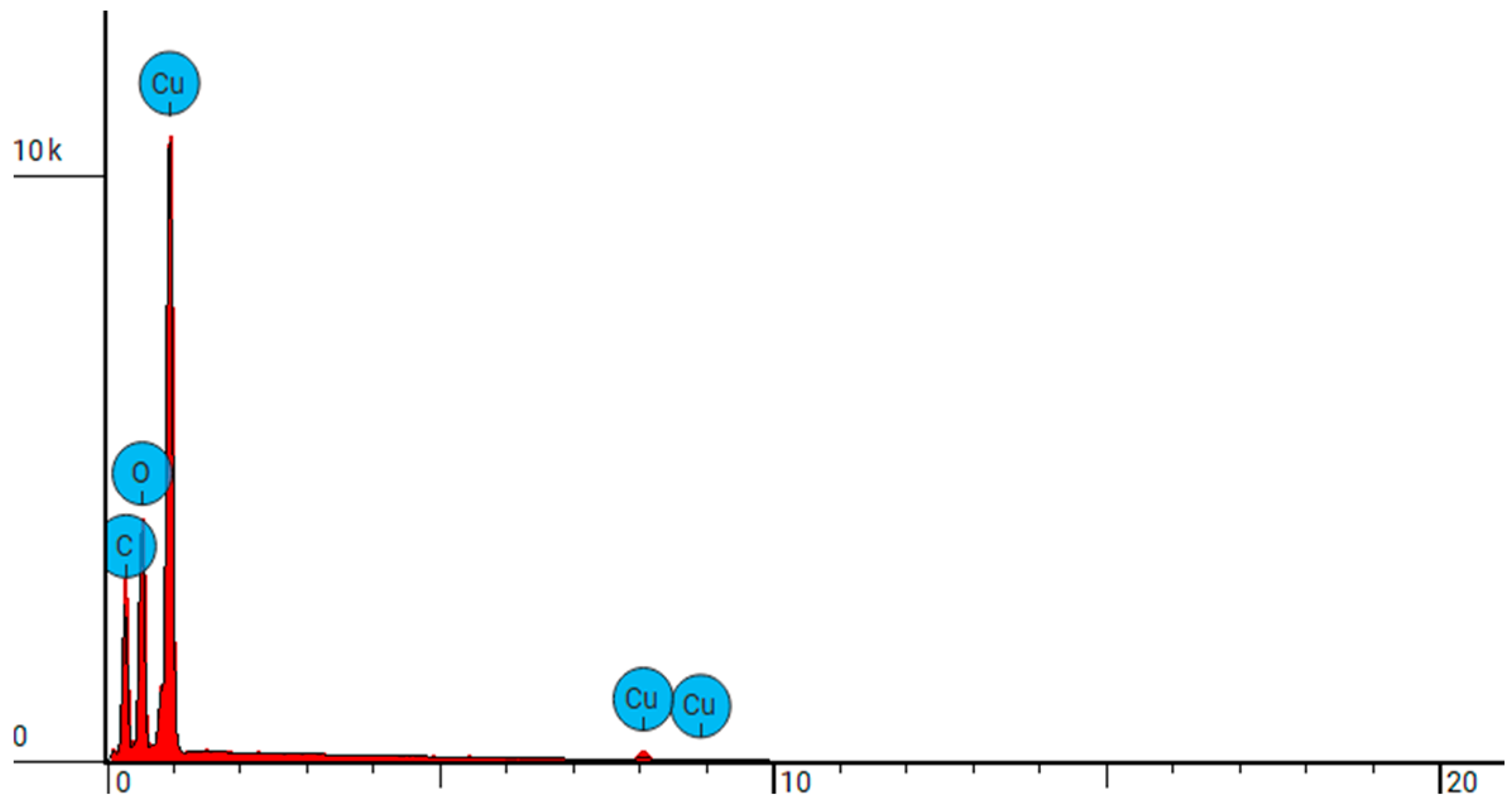
| NW-Cel-Cu(0.8 kW/32) | C | Carbon | 37.277 | 15.415 |
| O | Oxygen | 32.160 | 17.718 | |
| Cu | Copper | 30.563 | 66.867 |
| Sample Name | Total Pore Volume (TPV) | Specific Surface Area (SSA) |
|---|---|---|
| cm3/g | m2/g | |
| NW-Cel | 0.6021 | 3.660 × 10−3 |
| NW-Cel-Cu(0.4 kW/8) | 0.7985 | 3.483 × 10−3 |
| NW-Cel-Cu(0.4 kW/32) | 0.7256 | 3.172 × 10−3 |
| NW-Cel-Cu(0.8 kW/8) | 0.7717 | 3.508 × 10−3 |
| NW-Cel-Cu(0.8 kW/32) | 0.7425 | 3.243 × 10−3 |
| Sample Name | Average Inhibition Zone (mm) | |||
|---|---|---|---|---|
| E. Coli | S. aureus | A. Niger | C. Globosum | |
| NW-Cel | 0 | 0 | 0 | 0 |
| NW-Cel-Cu(0.4 kW/8) | 2 | 1 | 1 | 2 |
| NW-Cel-Cu(0.4 kW/32) | 3 | 2 | 3 | 2 |
| NW-Cel-Cu(0.8 kW/8) | 2 | 1 | 2 | 2 |
| NW-Cel-Cu(0.8 kW/32) | 3 | 2 | 3 | 2 |
| Concentration of inoculum [CFU/mL]: E. coli: = 1.5 × 108; S. aureus: = 1.3 × 108; A.Niger: 1.8 × 106; C. Globosum: 2.1 × 106. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczyńska, M.; Mrozińska, Z.; Lisiak-Kucińska, A.; Walawska, A.; Kudzin, M.H. Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology. Coatings 2024, 14, 843. https://doi.org/10.3390/coatings14070843
Świerczyńska M, Mrozińska Z, Lisiak-Kucińska A, Walawska A, Kudzin MH. Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology. Coatings. 2024; 14(7):843. https://doi.org/10.3390/coatings14070843
Chicago/Turabian StyleŚwierczyńska, Małgorzata, Zdzisława Mrozińska, Agnieszka Lisiak-Kucińska, Anetta Walawska, and Marcin H. Kudzin. 2024. "Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology" Coatings 14, no. 7: 843. https://doi.org/10.3390/coatings14070843
APA StyleŚwierczyńska, M., Mrozińska, Z., Lisiak-Kucińska, A., Walawska, A., & Kudzin, M. H. (2024). Biochemical Evaluation and Structural Characteristics of Copper Coating Cellulose Nonwovens Prepared by Magnetron Sputtering Technology. Coatings, 14(7), 843. https://doi.org/10.3390/coatings14070843


.jpg)



