Revealing the Mechanical Impact of Biomimetic Nanostructures on Bacterial Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bionanostructure Preparation Process
2.3. Surface Characterization
2.4. Cu2+ Release Test (ICP)
2.5. In Vitro Antibacterial Test
2.6. Finite Element Model (FEM)
2.7. In Vitro Cytocompatibility Assay
2.8. Statistical Analysis
3. Results
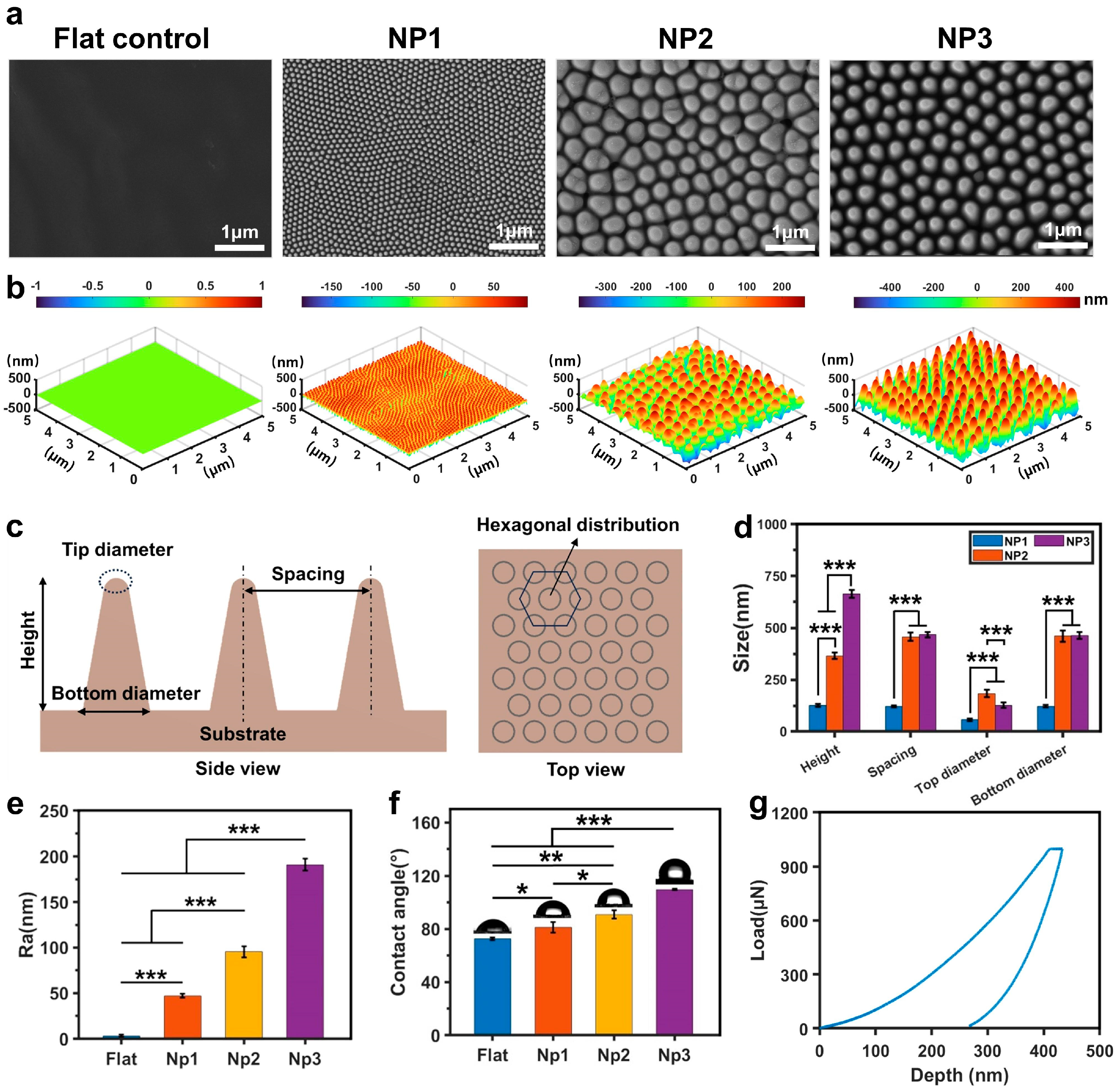
3.1. Preparation and Characterization of Biomimetic Cicada-Wing Nanostructures on Polymer Surface
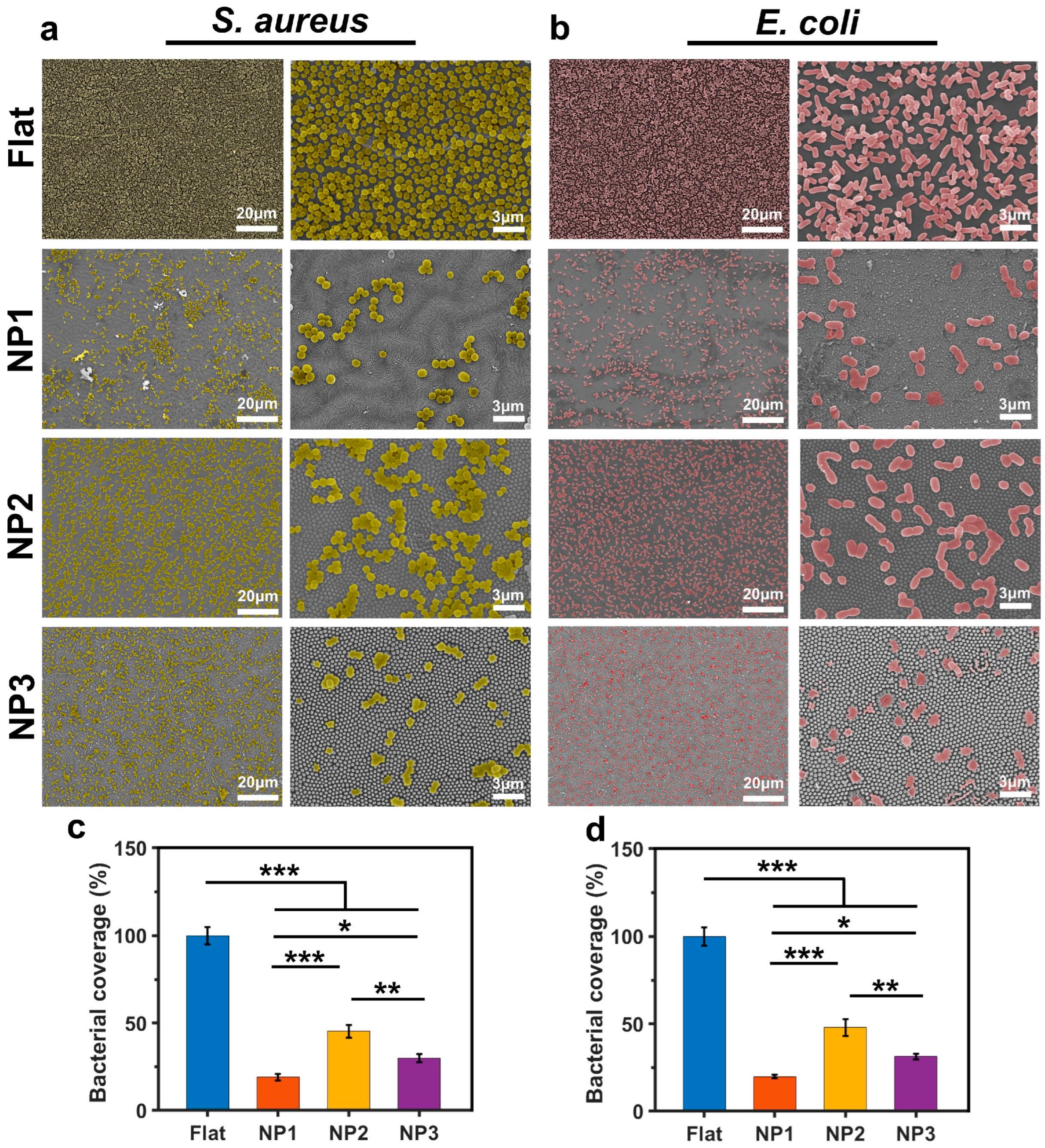
3.2. Bacteriostatic Properties of Different Structured Surfaces
3.3. Simulation of Bacteria-Surface Mechanical Interaction Process
3.4. In Vitro Cytocompatibility Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Asri, L.A.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.; Busscher, H.J. A shape-adaptive, antibacterial-coating of immobilized quaternary-ammonium compounds tethered on hyperbranched polyurea and its mechanism of action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.-M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S. Mediating bone regeneration by means of drug eluting implants: From passive to smart strategies. Mater. Sci. Eng. C 2017, 71, 1241–1252. [Google Scholar] [CrossRef]
- Liao, H.; Zhao, Q.; Cui, P.; Chen, Z.; Yu, Z.; Geisen, S.; Friman, V.-P.; Zhou, S. Efficient reduction of antibiotic residues and associated resistance genes in tylosin antibiotic fermentation waste using hyperthermophilic composting. Environ. Int. 2019, 133, 105203. [Google Scholar] [CrossRef]
- Gallardo-Godoy, A.; Muldoon, C.; Becker, B.; Elliott, A.G.; Lash, L.H.; Huang, J.X.; Butler, M.S.; Pelingon, R.; Kavanagh, A.M.; Ramu, S. Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B. J. Med. Chem. 2016, 59, 1068–1077. [Google Scholar] [CrossRef]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489. [Google Scholar] [CrossRef]
- Nouri-Goushki, M.; Mirzaali, M.; Angeloni, L.; Fan, D.; Minneboo, M.; Ghatkesar, M.; Staufer, U.; Fratila-Apachitei, L.; Zadpoor, A. 3D printing of large areas of highly ordered submicron patterns for modulating cell behavior. ACS Appl. Mater. Interfaces 2019, 12, 200–208. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef]
- Hazell, G.; Fisher, L.E.; Murray, W.A.; Nobbs, A.H.; Su, B. Bioinspired bactericidal surfaces with polymer nanocone arrays. J. Colloid Interface Sci. 2018, 528, 389–399. [Google Scholar] [CrossRef]
- Sjöström, T.; Nobbs, A.H.; Su, B. Bactericidal nanospike surfaces via thermal oxidation of Ti alloy substrates. Mater. Lett. 2016, 167, 22–26. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, T.; Li, X.; Song, K.; Ge, D. Nanopillared polycarbonate surfaces having variable feature parameters as bactericidal coatings. ACS Appl. Nano Mater. 2020, 3, 4599–4609. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Werner, M.; Baulin, V.A.; Truong, V.K.; Kobaisi, M.A.; Nguyen, S.H.; Balcytis, A.; Juodkazis, S.; Wang, J.Y.; Mainwaring, D.E. Subtle variations in surface properties of black silicon surfaces influence the degree of bactericidal efficiency. Nano-Micro Lett. 2018, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Werner, M.; Baulin, V.A.; Xu, X.; Vrancken, N.; Rubanov, S.; Hanssen, E.; Wandiyanto, J.; Truong, V.K. The multi-faceted mechano-bactericidal mechanism of nanostructured surfaces. Proc. Natl. Acad. Sci. USA 2020, 117, 12598–12605. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Ishak, M.I.; Eales, M.; Gholinia, A.; Kulkarni, S.; Keller, T.F.; May, P.W.; Nobbs, A.H.; Su, B. Resolving physical interactions between bacteria and nanotopographies with focused ion beam scanning electron microscopy. iScience 2021, 24, 102818. [Google Scholar] [CrossRef]
- Ye, J.; Li, B.; Zheng, Y.; Wu, S.; Chen, D.; Han, Y. Eco-friendly bacteria-killing by nanorods through mechano-puncture with top selectivity. Bioact. Mater. 2022, 15, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Lin, N.; Bryche, J.-F.; McKay, G.; Canva, M.; Charette, P.G.; Nguyen, D.; Moraes, C.; Tufenkji, N. Hydrophilic mechano-bactericidal nanopillars require external forces to rapidly kill bacteria. Nano Lett. 2020, 20, 5720–5727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xu, Z.; Wei, Z.; Sun, Y.; Zhou, Q. Nature-inspired mechano-bactericidal nanostructured surfaces with photothermally enhanced antibacterial performances. Prog. Org. Coat. 2023, 182, 107599. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Wang, S.; Dou, H.; Fan, Y.; Tian, L.; Zhao, J.; Ren, L. Bio-inspired self-adaptive nanocomposite array: From non-antibiotic antibacterial actions to cell proliferation. ACS Nano 2022, 16, 16549–16562. [Google Scholar] [CrossRef]
- Alameda, M.T.; Osorio, M.R.; Pedraz, P.; Rodríguez, I. Mechano-Dynamic Analysis of the Bactericidal Activity of Bioinspired Moth-Eye Nanopatterned Surfaces. Adv. Mater. Interfaces 2022, 9, 2200608. [Google Scholar] [CrossRef]
- Oehrlein, G.S.; Phaneuf, R.J.; Graves, D.B. Plasma-polymer interactions: A review of progress in understanding polymer resist mask durability during plasma etching for nanoscale fabrication. J. Vac. Sci. Technol. B 2011, 29, 010801. [Google Scholar] [CrossRef]
- Bierbaum, S.; Mulansky, S.; Bognár, E.; Kientzl, I.; Nagy, P.; Vrana, N.E.; Weszl, M.; Boschke, E.; Scharnweber, D.; Wolf-Brandstetter, C. Osteogenic nanostructured titanium surfaces with antibacterial properties under conditions that mimic the dynamic situation in the oral cavity. Biomater. Sci. 2018, 6, 1390–1402. [Google Scholar] [CrossRef]
- Truong, V.K.; Pham, V.T.; Medvedev, A.; Lapovok, R.; Estrin, Y.; Lowe, T.C.; Baulin, V.; Boshkovikj, V.; Fluke, C.J.; Crawford, R.J. Self-organised nanoarchitecture of titanium surfaces influences the attachment of Staphylococcus aureus and Pseudomonas aeruginosa bacteria. Appl. Microbiol. Biotechnol. 2015, 99, 6831–6840. [Google Scholar] [CrossRef]
- Stolzoff, M.; Burns, J.E.; Aslani, A.; Tobin, E.J.; Nguyen, C.; De La Torre, N.; Golshan, N.H.; Ziemer, K.S.; Webster, T.J. Decreased bacterial growth on titanium nanoscale topographies created by ion beam assisted evaporation. Int. J. Nanomed. 2017, 12, 1161–1169. [Google Scholar] [CrossRef][Green Version]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef]
- Linklater, D.P.; De Volder, M.; Baulin, V.A.; Werner, M.; Jessl, S.; Golozar, M.; Maggini, L.; Rubanov, S.; Hanssen, E.; Juodkazis, S. High aspect ratio nanostructures kill bacteria via storage and release of mechanical energy. Acs Nano 2018, 12, 6657–6667. [Google Scholar] [CrossRef] [PubMed]
- Zahir, T.; Pesek, J.; Franke, S.; Van Pee, J.; Rathore, A.; Smeets, B.; Ramon, H.; Xu, X.; Fauvart, M.; Michiels, J. Model-driven controlled alteration of Nanopillar Cap Architecture reveals its effects on bactericidal activity. Microorganisms 2020, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Gambacorta, F.; Divan, R.; Aranson, I.S.; Sokolov, A.; Noirot, P.; Laible, P.D. Tuning antimicrobial properties of biomimetic nanopatterned surfaces. Nanoscale 2018, 10, 6639–6650. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Niu, S.; Yankova, M.; Mecklenburg, M.; King, S.M.; Ravichandran, J.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Setlow, P. Analysis of killing of growing cells and dormant and germinated spores of Bacillus species by black silicon nanopillars. Sci. Rep. 2017, 7, 17768. [Google Scholar] [CrossRef] [PubMed]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Modaresifar, K.; Kunkels, L.B.; Ganjian, M.; Tümer, N.; Hagen, C.W.; Otten, L.G.; Hagedoorn, P.-L.; Angeloni, L.; Ghatkesar, M.K.; Fratila-Apachitei, L.E. Deciphering the roles of interspace and controlled disorder in the bactericidal properties of nanopatterns against Staphylococcus aureus. Nanomaterials 2020, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Mainwaring, D.E.; Nguyen, S.H.; Webb, H.; Jakubov, T.; Tobin, M.; Lamb, R.N.; Wu, A.H.-F.; Marchant, R.; Crawford, R.J.; Ivanova, E.P. The nature of inherent bactericidal activity: Insights from the nanotopology of three species of dragonfly. Nanoscale 2016, 8, 6527–6534. [Google Scholar] [CrossRef]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada wing surface topography: An investigation into the bactericidal properties of nanostructural features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef]
- Ivanova, E.; Crawford, R. Antibacterial Surfaces; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 052419. [Google Scholar] [CrossRef]
- Xue, F.; Liu, J.; Guo, L.; Zhang, L.; Li, Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015, 385, 1–7. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; Crawford, R.J. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Lazzini, G.; Lutey, A.; Romoli, L.; Fuso, F. Molecular dynamics model for the antibactericity of textured surfaces. Colloids Surf. B Biointerfaces 2021, 199, 111504. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, T.; Li, X.; Zhao, L.; Wu, Q.; Wang, X.; Song, K.; Ge, D. Validation of the mechano-bactericidal mechanism of nanostructured surfaces with finite element simulation. Colloids Surf. B Biointerfaces 2021, 206, 111929. [Google Scholar] [CrossRef]
- Velic, A.; Hasan, J.; Li, Z.; Yarlagadda, P.K. Mechanics of bacterial interaction and death on nanopatterned surfaces. Biophys. J. 2021, 120, 217–231. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Song, L.; Chen, Y.; Tian, L.; Zhao, J.; Ren, L. Biocompatible mechano-bactericidal nanopatterned surfaces with salt-responsive bacterial release. Acta Biomater. 2022, 141, 198–208. [Google Scholar] [CrossRef]
- Touhami, A.; Jericho, M.H.; Beveridge, T.J. Atomic force microscopy of cell growth and division in Staphylococcus aureus. J. Bacteriol. 2004, 186, 3286–3295. [Google Scholar] [CrossRef]
- Harris, L.G.; Foster, S.; Richards, R.G. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cell Mater. 2002, 4, 100–120. [Google Scholar] [CrossRef]
- Maleki, E.; Mirzaali, M.J.; Guagliano, M.; Bagherifard, S. Analyzing the mechano-bactericidal effect of nano-patterned surfaces on different bacteria species. Surf. Coat. Technol. 2021, 408, 126782. [Google Scholar] [CrossRef]
- Tuson, H.H.; Auer, G.K.; Renner, L.D.; Hasebe, M.; Tropini, C.; Salick, M.; Crone, W.C.; Gopinathan, A.; Huang, K.C.; Weibel, D.B. Measuring the stiffness of bacterial cells from growth rates in hydrogels of tunable elasticity. Mol. Microbiol. 2012, 84, 874–891. [Google Scholar] [CrossRef]
- Yao, X.; Jericho, M.; Pink, D.; Beveridge, T. Thickness and elasticity of gram-negative murein sacculi measured by atomic force microscopy. J. Bacteriol. 1999, 181, 6865–6875. [Google Scholar] [CrossRef]
- Amir, A.; Babaeipour, F.; McIntosh, D.B.; Nelson, D.R.; Jun, S. Bending forces plastically deform growing bacterial cell walls. Proc. Natl. Acad. Sci. USA 2014, 111, 5778–5783. [Google Scholar] [CrossRef]
- Bavi, N.; Nakayama, Y.; Bavi, O.; Cox, C.D.; Qin, Q.-H.; Martinac, B. Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc. Natl. Acad. Sci. USA 2014, 111, 13864–13869. [Google Scholar] [CrossRef]
- Fowler, L.; Engqvist, H.; Öhman-Mägi, C. Effect of copper ion concentration on bacteria and cells. Materials 2019, 12, 3798. [Google Scholar] [CrossRef]
- Yang, K.; Wang, L.; Zou, X.; Wang, H.; Liang, C.; Zhang, D.; Wang, L.-N. Modeling bacterial adhesion on the nanopatterned surface by varying contact area. J. Mater. Sci. Technol. 2024, 196, 137–147. [Google Scholar] [CrossRef]
- Valiei, A.; Lin, N.; McKay, G.; Nguyen, D.; Moraes, C.; Hill, R.J.; Tufenkji, N. Surface wettability is a key feature in the mechano-bactericidal activity of nanopillars. ACS Appl. Mater. Interfaces 2022, 14, 27564–27574. [Google Scholar] [CrossRef]
- Thwaites, J.; Surana, U.; Jones, A. Mechanical properties of Bacillus subtilis cell walls: Effects of ions and lysozyme. J. Bacteriol. 1991, 173, 204–210. [Google Scholar] [CrossRef]
- Stocks, S.; Thomas, C. Viability, strength, and fragmentation of Saccharopolyspora erythraea in submerged fermentation. Biotechnol. Bioeng. 2001, 75, 702–709. [Google Scholar] [CrossRef]
- Stocks, S.M.; Thomas, C.R. Strength of mid-logarithmic and stationary phase Saccharopolyspora erythraea hyphae during a batch fermentation in defined nitrate-limited medium. Biotechnol. Bioeng. 2001, 73, 370–378. [Google Scholar] [CrossRef]
- Thwaites, J.; Surana, U. Mechanical properties of Bacillus subtilis cell walls: Effects of removing residual culture medium. J. Bacteriol. 1991, 173, 197–203. [Google Scholar] [CrossRef][Green Version]
- Zeng, J.; Wang, Y.; Sun, Z.; Chang, H.; Cao, M.; Zhao, J.; Lin, K.; Xie, Y. A novel biocompatible PDA/IR820/DAP coating for antibiotic/photodynamic/photothermal triple therapy to inhibit and eliminate Staphylococcus aureus biofilm. Chem. Eng. J. 2020, 394, 125017. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef]
- Higgins, S.G.; Becce, M.; Belessiotis-Richards, A.; Seong, H.; Sero, J.E.; Stevens, M.M. High-aspect-ratio nanostructured surfaces as biological metamaterials. Adv. Mater. 2020, 32, 1903862. [Google Scholar] [CrossRef]
- Tay, A. The benefits of going small: Nanostructures for mammalian cell transfection. ACS Nano 2020, 14, 7714–7721. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cui, Q.; Wu, Q.; Li, X.; Song, K.; Ge, D.; Guan, S. Mechanism study of bacteria killed on nanostructures. J. Phys. Chem. B 2019, 123, 8686–8696. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Phillips, K.S.; Gu, H.; Kazemzadeh-Narbat, M.; Ren, D. How microbes read the map: Effects of implant topography on bacterial adhesion and biofilm formation. Biomaterials 2021, 268, 120595. [Google Scholar] [CrossRef]
- Elbourne, A.; Crawford, R.J.; Ivanova, E.P. Nano-structured antimicrobial surfaces: From nature to synthetic analogues. J. Colloid Interface Sci. 2017, 508, 603–616. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zou, X.; Wang, D.; Li, M.; Zhao, B.; Xia, Y.; Wang, H.; Liang, C. Revealing the Mechanical Impact of Biomimetic Nanostructures on Bacterial Behavior. Coatings 2024, 14, 860. https://doi.org/10.3390/coatings14070860
Wu X, Zou X, Wang D, Li M, Zhao B, Xia Y, Wang H, Liang C. Revealing the Mechanical Impact of Biomimetic Nanostructures on Bacterial Behavior. Coatings. 2024; 14(7):860. https://doi.org/10.3390/coatings14070860
Chicago/Turabian StyleWu, Xin, Xianrui Zou, Donghui Wang, Mingjun Li, Bo Zhao, Yi Xia, Hongshui Wang, and Chunyong Liang. 2024. "Revealing the Mechanical Impact of Biomimetic Nanostructures on Bacterial Behavior" Coatings 14, no. 7: 860. https://doi.org/10.3390/coatings14070860
APA StyleWu, X., Zou, X., Wang, D., Li, M., Zhao, B., Xia, Y., Wang, H., & Liang, C. (2024). Revealing the Mechanical Impact of Biomimetic Nanostructures on Bacterial Behavior. Coatings, 14(7), 860. https://doi.org/10.3390/coatings14070860





