Magnetron Sputtered Low-Platinum Loading Electrode as HER Catalyst for PEM Electrolysis
Abstract
1. Introduction
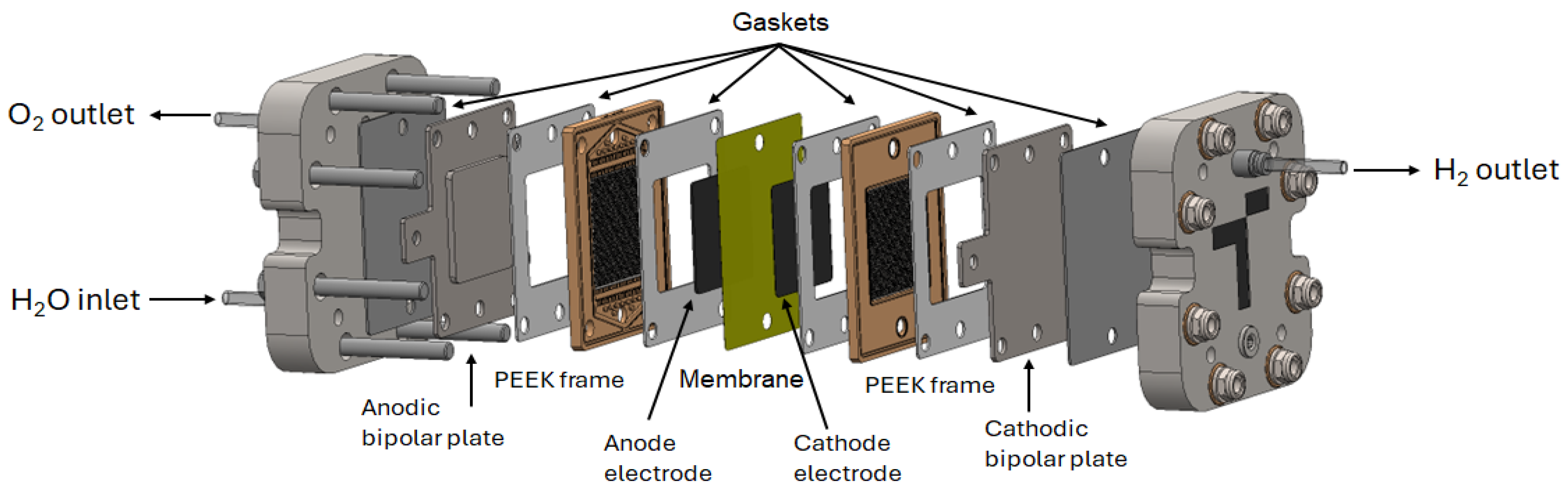
2. Materials and Methods
2.1. Platinum Nanoparticles Deposition
2.2. Characterization Methods
2.3. Electrochemical Characterization
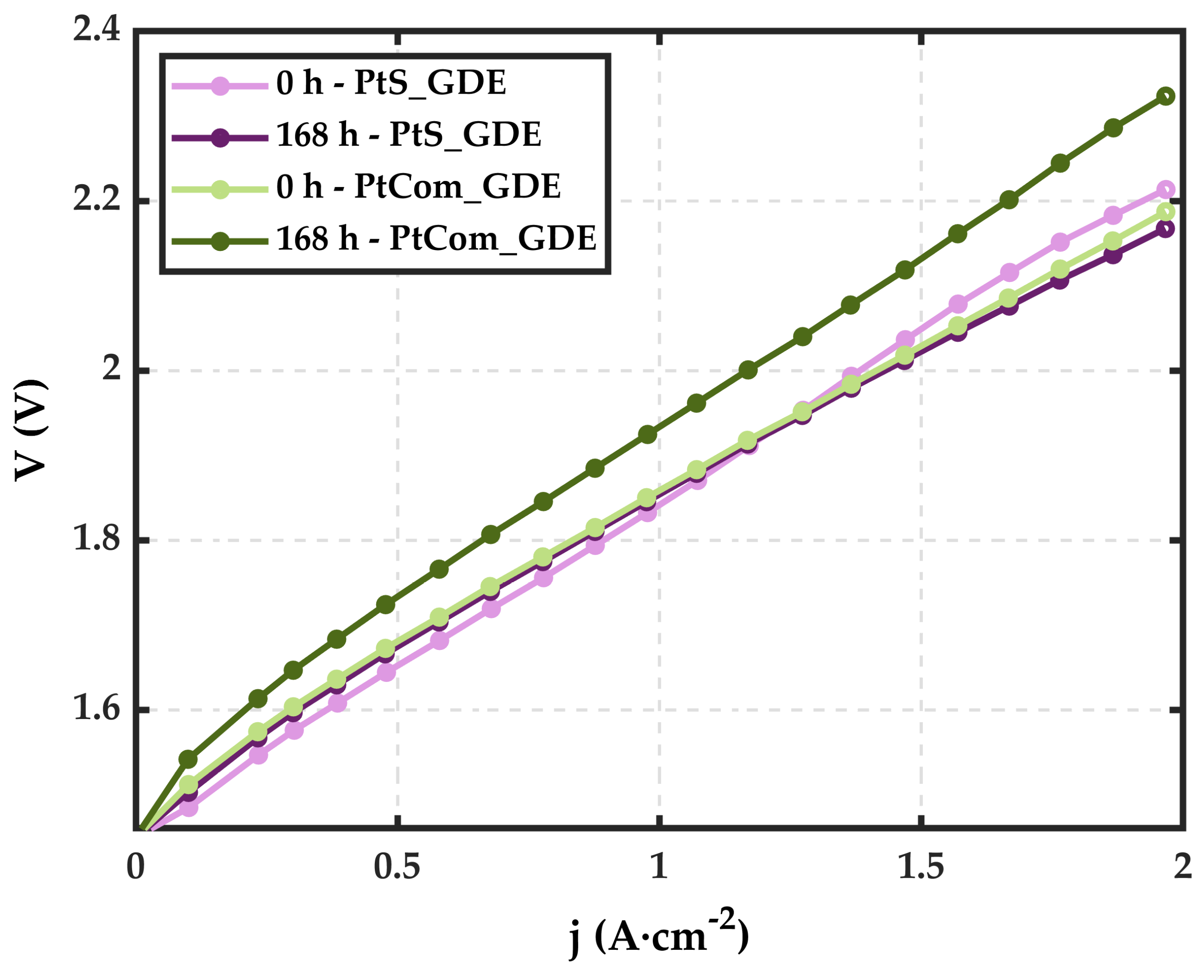
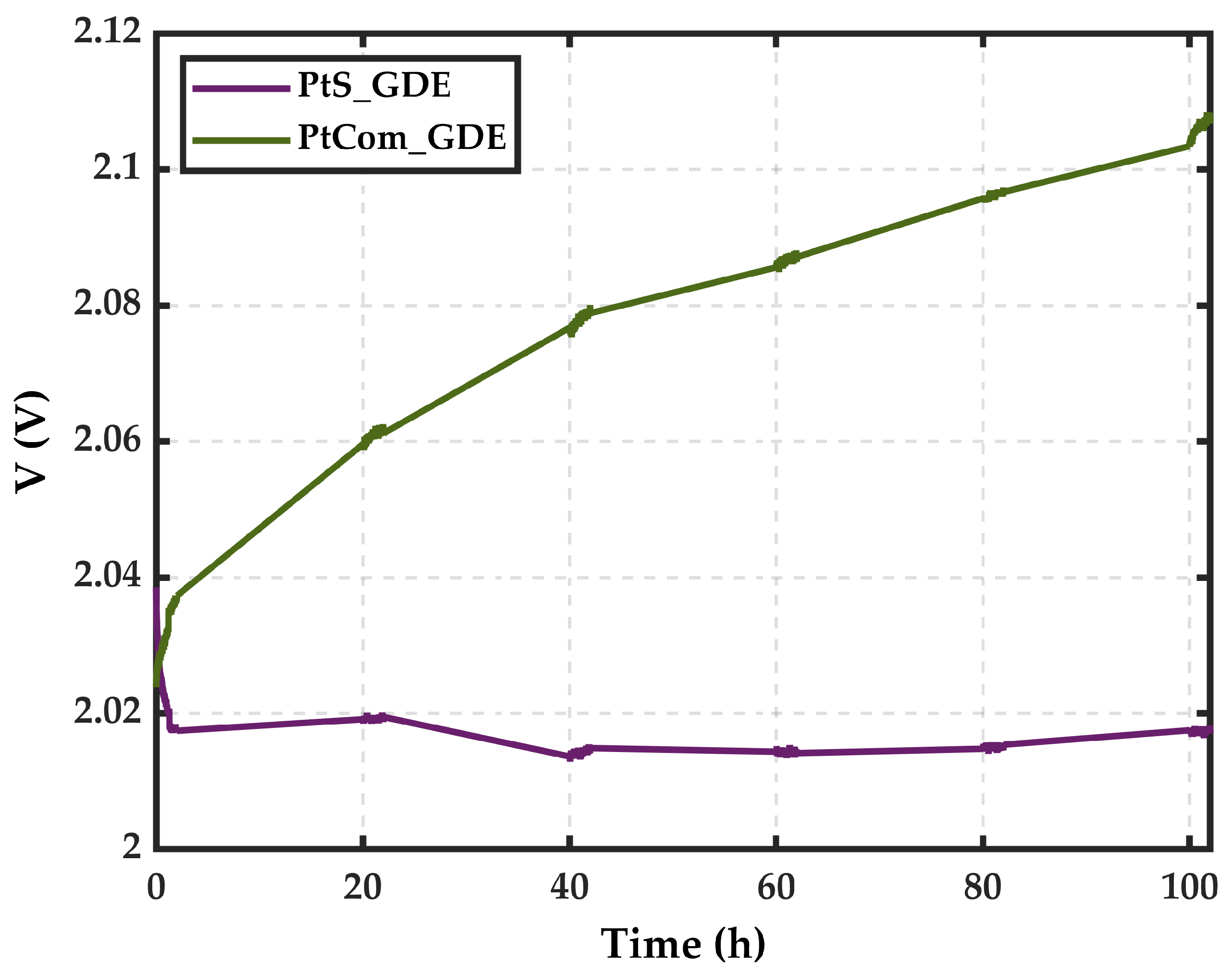
3. Results and Discussion
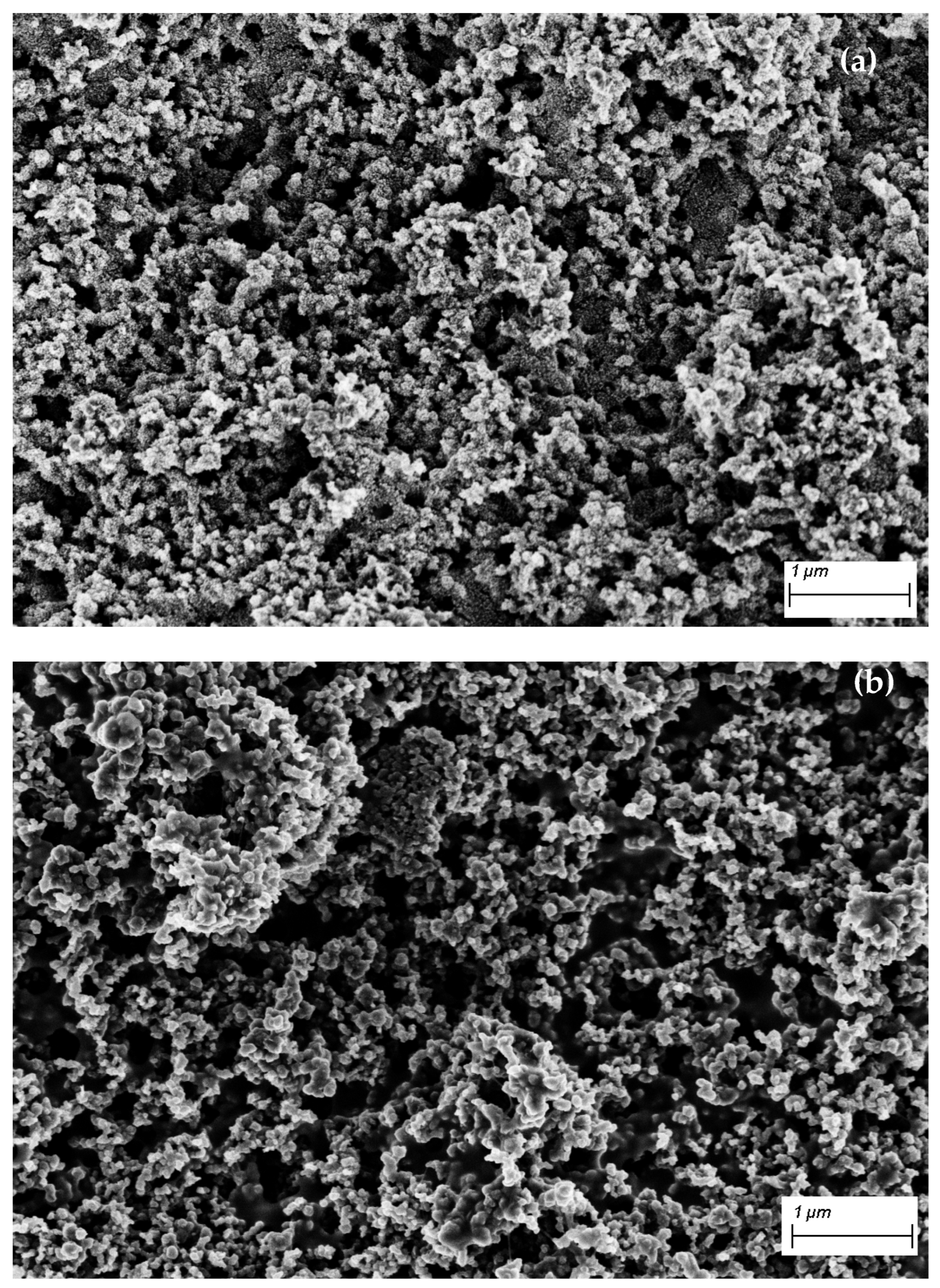
3.1. Characterization
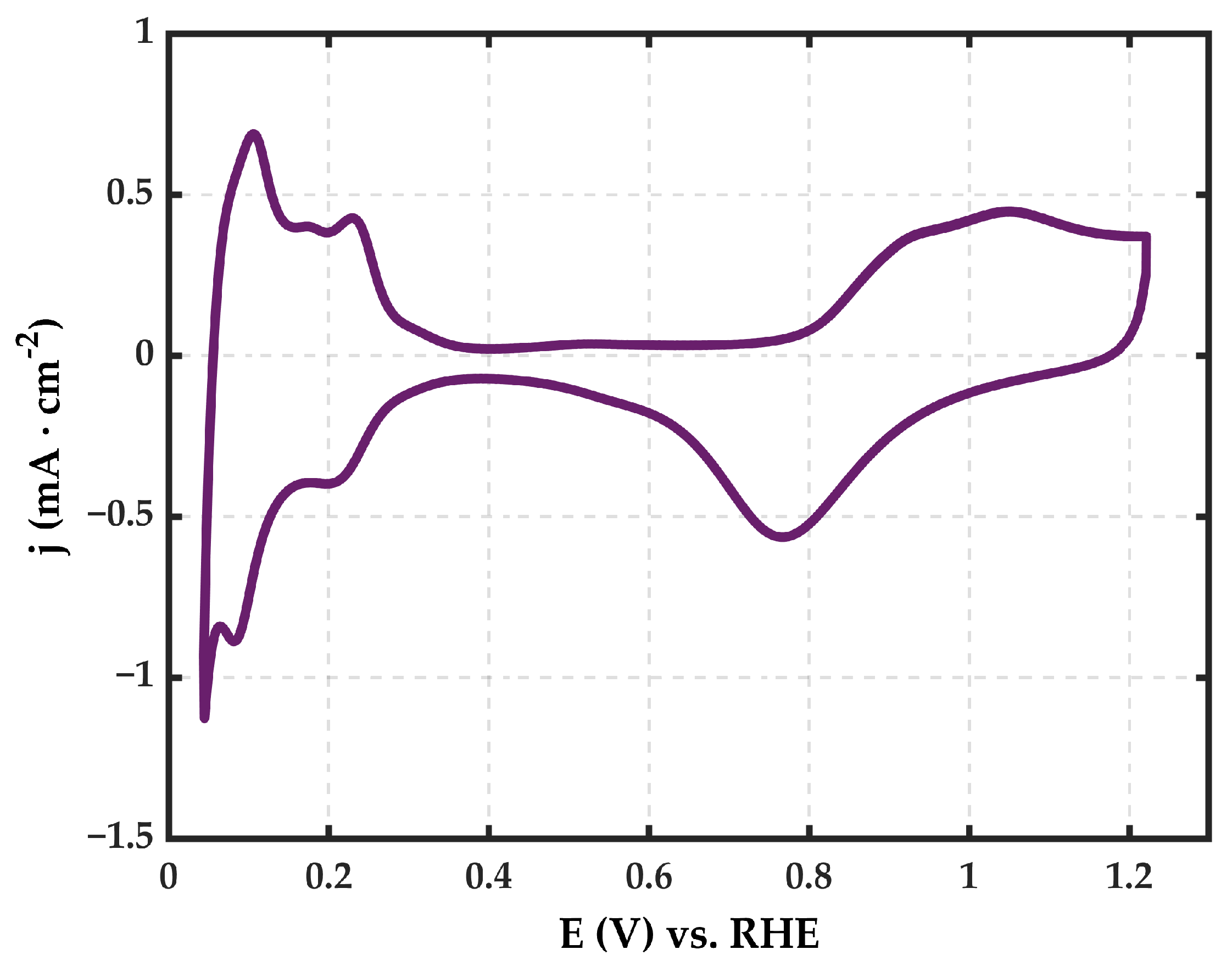
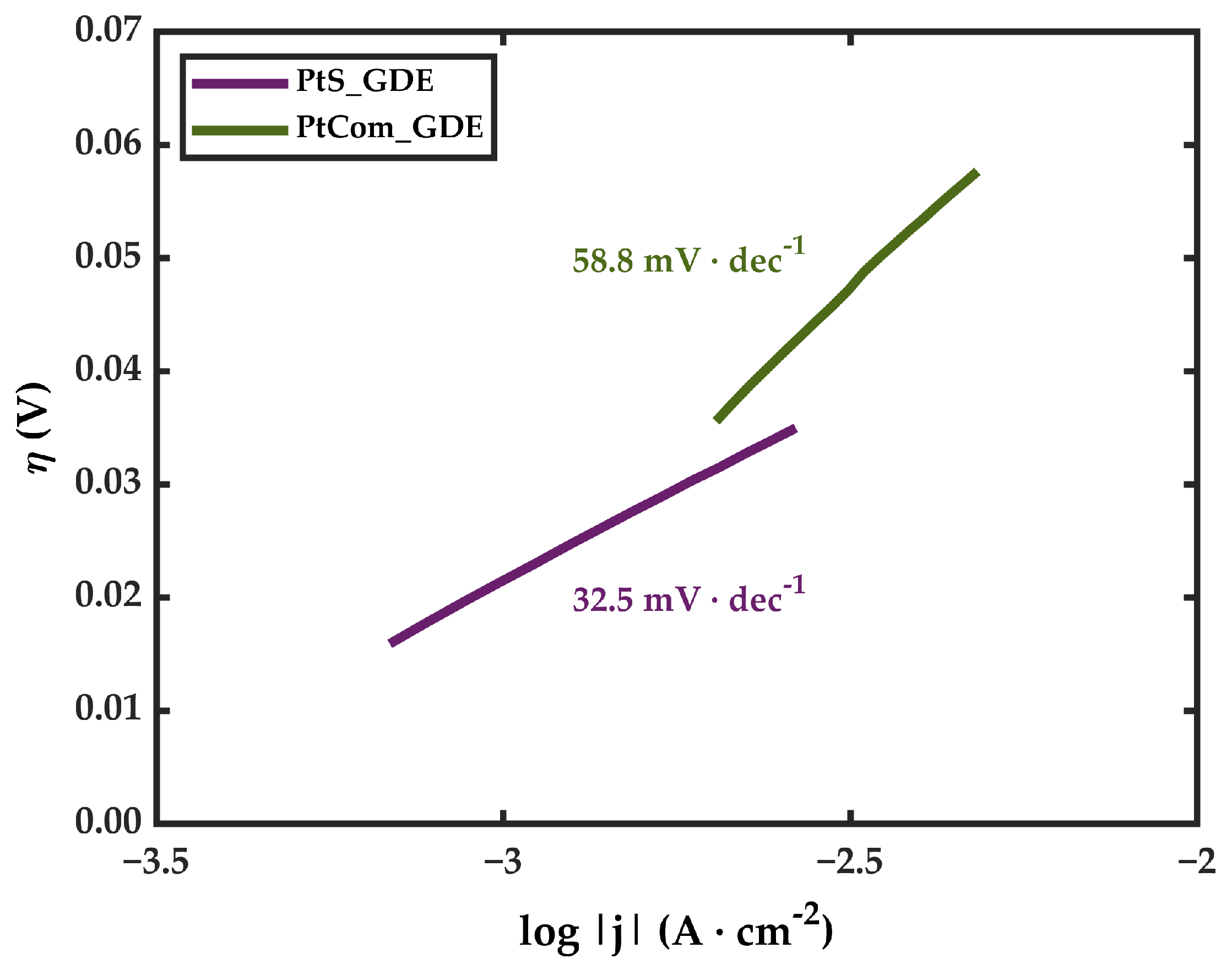
3.2. Electrochemical Characterization
4. Conclusions
- The amount of Pt is not directly related to an improvement in cell performance. The exposition of Pt active sites seems to be much more relevant than the Pt present in the catalytic coating.
- MSGA is confirmed as a practical and feasible manufacturing method for developing Pt HER electrodes. This technique successfully produces effective, stable, and effective electrodes in a step process, directly depositing pure Pt nanoparticles on the GDL.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baykara, S.Z. Hydrogen as fuel: A critical technology? Int. J. Hydrogen Energy 2005, 30, 545–553. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 1–19. [Google Scholar] [CrossRef]
- Ayers, K.E.; Renner, J.N.; Danilovic, N.; Wang, J.X.; Zhang, Y.; Maric, R.; Yu, H. Pathways to ultra-low platinum group metal catalyst loading in proton exchange membrane electrolyzers. Catal. Today 2016, 262, 121–132. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- Kamaroddin, M.F.A.; Sabli, N.; Abdullah, T.A.T.; Siajam, S.I.; Abdullah, L.C.; Jalil, A.A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef]
- Hancke, R.; Holm, T.; Ulleberg, Ø. The case for high-pressure PEM water electrolysis. Energy Convers. Manag. 2022, 261, 115642. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Rajendran, S.; Khusnun, N.F.; Bahari, M.B.; Johari, A.; Kamaruddin, M.J.; Ismail, M. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrogen Energy 2024, 52, 420–441. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Tian, R.; Ye, L.; Zhang, J.; Rao, M.; Li, G. Platinum-Group Metals: Demand, Supply, Applications and Their Recycling from Spent Automotive Catalysts. J. Environ. Chem. Eng. 2023, 11, 110237. [Google Scholar] [CrossRef]
- Villamayor, A.; Galyamin, D.; Barrio, L.V.; Berasategui, E.G.; Rojas, S. Highly active ultralow loading Pt electrodes for hydrogen evolution reaction developed by magnetron sputtering. Int. J. Hydrogen Energy 2024, 64, 50–57. [Google Scholar] [CrossRef]
- Durst, J.; Simon, C.; Hasché, F.; Gasteiger, H.A. Hydrogen Oxidation and Evolution Reaction Kinetics on Carbon Supported Pt, Ir, Rh, and Pd Electrocatalysts in Acidic Media. J. Electrochem. Soc. 2015, 162, F190–F203. [Google Scholar] [CrossRef]
- Bernt, M.; Siebel, A.; Gasteiger, H.A. Analysis of Voltage Losses in PEM Water Electrolyzers with Low Platinum Group Metal Loadings. J. Electrochem. Soc. 2018, 165, F305–F314. [Google Scholar] [CrossRef]
- Zhang, Z.; Baudy, A.; Testino, A.; Gubler, L. Cathode Catalyst Layer Design in PEM Water Electrolysis toward Reduced Pt Loading and Hydrogen Crossover. ACS Appl. Mater. Interfaces 2024, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, L.; Hamdy, M.S.; Zhang, K.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, 9120032. [Google Scholar] [CrossRef]
- Hrbek, T.; Kúš, P.; Kosto, Y.; Rodríguez, M.G.; Matolínová, I. Magnetron-sputtered thin-film catalyst with low-Ir-Ru content for water electrolysis: Long-term stability and degradation analysis. J. Power Sources 2023, 556, 232375. [Google Scholar] [CrossRef]
- Nefedkin, S.I.; Ryabukhin, A.V.; Eletskikh, V.E.; Boldin, R.G.; Mikhnevich, V.D.; Klimova, M.A. Magnetron Technology for Manufacturing of Electrodes for Electrolyzers with Proton-Exchange Membranes. Russ. J. Electrochem. 2024, 60, 200–210. [Google Scholar] [CrossRef]
- Jiménez, P.C.; Sievers, G.; Quade, A.; Brüser, V.; Pittkowski, R.K.; Arenz, M. Gas diffusion electrode activity measurements of iridium-based self-supported catalysts produced by alternated physical vapour deposition. J. Power Sources 2023, 569, 232990. [Google Scholar] [CrossRef]
- Bernsmeier, D.; Selve, S.; Nissen, J.; Maticiuc, N.; Ibaceta-Jaña, J.; Szyszka, B.; Muydinov, R. Gas Flow Sputtering of Pt/C Films and Their Performance in Electrocatalytic Hydrogen Evolution Reaction. ChemPhysChem 2023, 24, e202200650. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Kukueva, E.V.; Shapir, B.L.; Kudinova, E.S.; Akel’kina, S.V.; Alekseeva, O.K. Russian Text © The Author(s), 2020, published in Rossiiskie Nanotekhnologii. Nanotechnol. Russ. 2020, 15, 741–748. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Alekseeva, O.K.; Fateev, V.N.; Shapir, B.L.; Spasov, D.D.; Nikitin, S.M.; Presnyakov, M.Y.; Kolobylina, N.N.; Soloviev, M.A.; Mikhalev, A.I.; et al. Activity and durability of electrocatalytic layers with low platinum loading prepared by magnetron sputtering onto gas diffusion electrodes. Int. J. Hydrogen Energy 2019, 44, 29529–29536. [Google Scholar] [CrossRef]
- Alekseeva, O.K.; Mikhalev, A.I.; Lutikova, E.K.; Porembsky, V.I.; Presnyakov, M.Y.; Fateev, V.N.; Shapir, B.L.; Grigoriev, S.A. Structural and Electrocatalytic Properties of Platinum and Platinum-Carbon Layers Obtained by Magnetron-Ion Sputtering. Catalysts 2018, 8, 665. [Google Scholar] [CrossRef]
- Alekseeva, O.K.; Lutikova, E.K.; Markelov, V.V.; Porembsky, V.I.; Fateev, V.N. Stationary and Pulsed Magnetron Sputtering Technologies for Protective/Catalyst Layer Production for PEM Systems. Int. J. Electrochem. Sci. 2018, 13, 797–811. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, G.; Mo, J.; Li, Y.; Yu, S.; Cullen, D.A.; Retterer, S.T.; Toops, T.J.; Bender, G.; Pivovar, B.S.; et al. Novel thin/tunable gas diffusion electrodes with ultra-low catalyst loading for hydrogen evolution reactions in proton exchange membrane electrolyzer cells. Nano Energy 2018, 47, 434–441. [Google Scholar] [CrossRef]
- Fedotov, A.A.; Grigoriev, S.A.; Lyutikova, E.K.; Millet, P.; Fateev, V.N. Characterization of carbon-supported platinum nano-particles synthesized using magnetron sputtering for application in PEM electrochemical systems. Int. J. Hydrogen Energy 2013, 38, 426–430. [Google Scholar] [CrossRef]
- Huttel, Y. (Ed.) Gas-Phase Synthesis of Nanoparticles; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Martínez, L.; Díaz, M.; Román, E.; Ruano, M.; Llamosa P, D.; Huttel, Y. Generation of nanoparticles with adjustable size and controlled stoichiometry: Recent advances. Langmuir 2012, 28, 11241–11249. [Google Scholar] [CrossRef] [PubMed]
- Popok, V.N.; Kylián, O. Gas-Phase Synthesis of Functional Nanomaterials. Appl. Nano 2020, 1, 25–58. [Google Scholar] [CrossRef]
- Acsente, T.; Dobrea, M.C.; Satulu, V.; Bita, B.; Dinescu, G. Operation of a magnetron sputtering gas aggregation cluster source in a plasma jet regime for synthesis of core–shell nanoparticles. J. Phys. D Appl. Phys. 2020, 54, 02LT01. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kuzminova, A.; Kylián, O.; Biederman, H. Comparison of magnetron sputtering and gas aggregation nanoparticle source used for fabrication of silver nanoparticle films. Surf. Coatings Technol. 2015, 275, 296–302. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. iR drop correction in electrocatalysis: Everything one needs to know! J. Mater. Chem. A 2022, 10, 9348–9354. [Google Scholar] [CrossRef]
- Chen, D.; Tao, Q.; Liao, L.W.; Liu, S.X.; Chen, Y.X.; Ye, S. Determining the Active Surface Area for Various Platinum Electrodes. Electrocatalysis 2011, 2, 207–219. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31, e1806296. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-Based Catalysts with Platinum-like Activity and Higher Durability for the Hydrogen Evolution Reaction at All pH Values. Angew. Chem. Int. Ed. 2017, 56, 11559–11564. [Google Scholar] [CrossRef]
- Liu, Y.; Mustain, W.E. Evaluation of tungsten carbide as the electrocatalyst support for platinum hydrogen evolution/oxidation catalysts. Int. J. Hydrogen Energy 2012, 37, 8929–8938. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamayor, A.; Alba, A.; Barrio, L.V.; Rojas, S.; Gutierrez-Berasategui, E. Magnetron Sputtered Low-Platinum Loading Electrode as HER Catalyst for PEM Electrolysis. Coatings 2024, 14, 868. https://doi.org/10.3390/coatings14070868
Villamayor A, Alba A, Barrio LV, Rojas S, Gutierrez-Berasategui E. Magnetron Sputtered Low-Platinum Loading Electrode as HER Catalyst for PEM Electrolysis. Coatings. 2024; 14(7):868. https://doi.org/10.3390/coatings14070868
Chicago/Turabian StyleVillamayor, Antía, Alonso Alba, Laura V. Barrio, Sergio Rojas, and Eva Gutierrez-Berasategui. 2024. "Magnetron Sputtered Low-Platinum Loading Electrode as HER Catalyst for PEM Electrolysis" Coatings 14, no. 7: 868. https://doi.org/10.3390/coatings14070868
APA StyleVillamayor, A., Alba, A., Barrio, L. V., Rojas, S., & Gutierrez-Berasategui, E. (2024). Magnetron Sputtered Low-Platinum Loading Electrode as HER Catalyst for PEM Electrolysis. Coatings, 14(7), 868. https://doi.org/10.3390/coatings14070868









