Thermodynamic Analysis of Typical Alloy Oxidation and Carburization in High-Temperature CO2 Atmosphere
Abstract
:1. Introduction
2. Analytic Methods
3. Results and Discussion
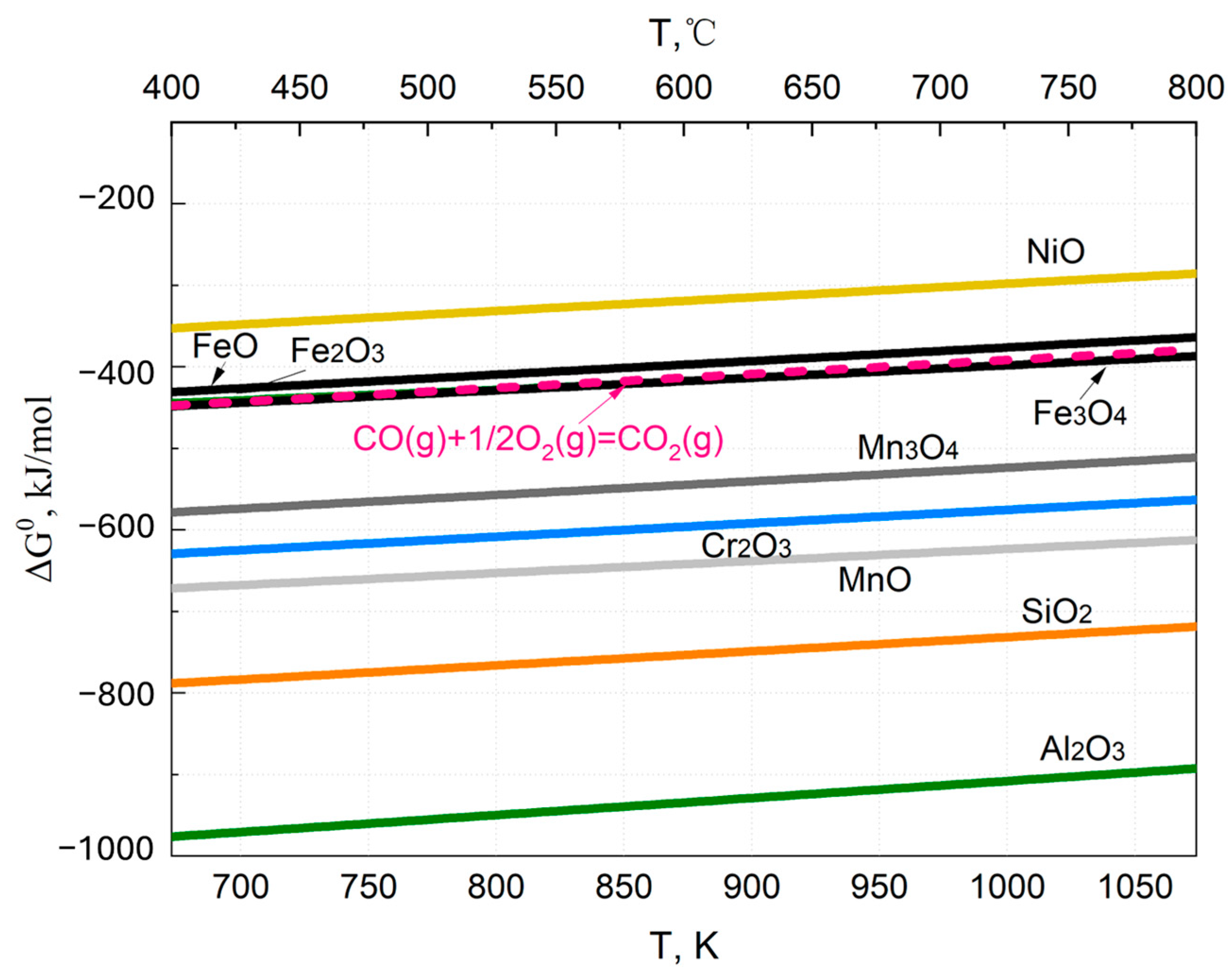
3.1. Oxidation Behavior of Alloys in a CO2 Environment
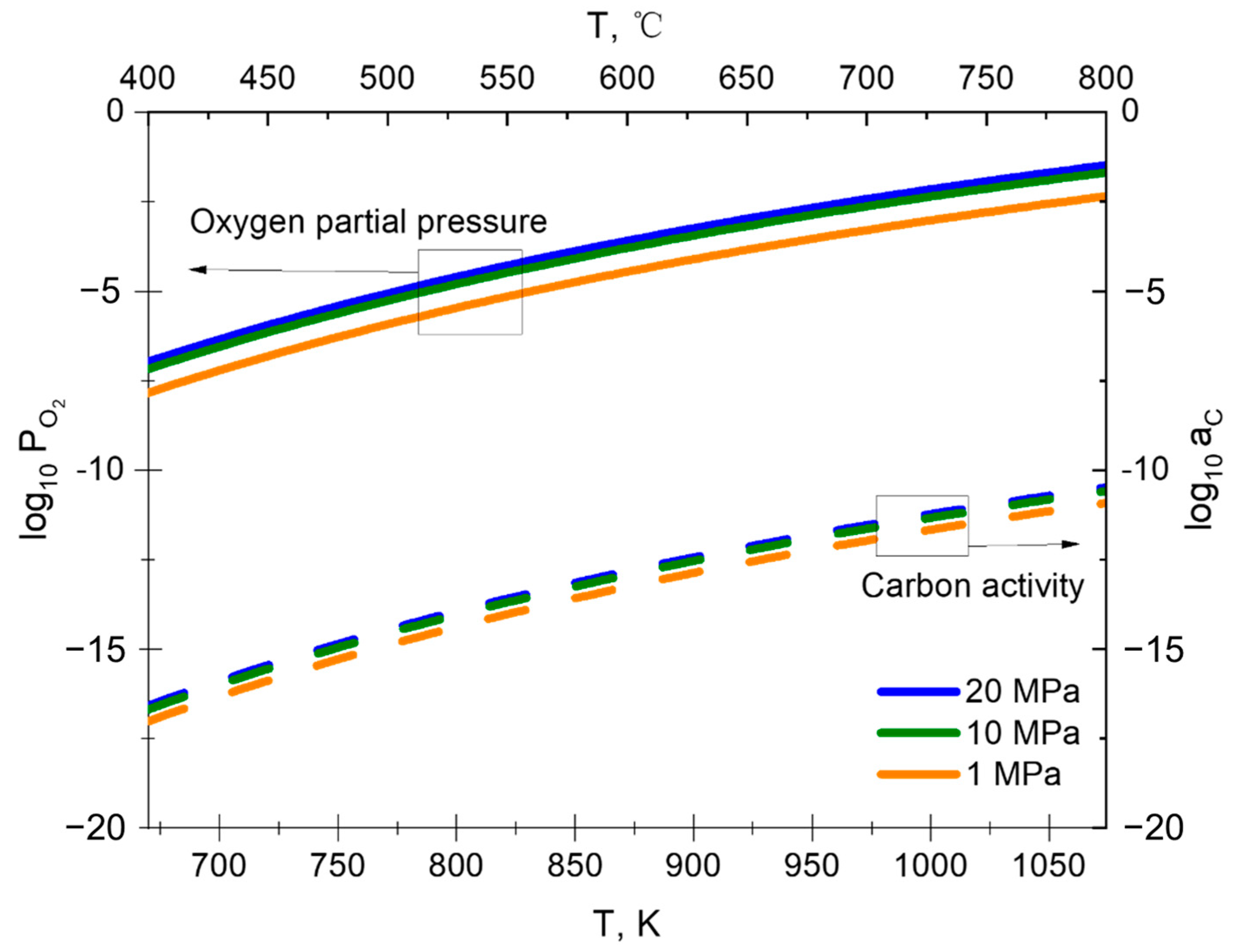
3.1.1. Equilibrium Decomposition of the CO2
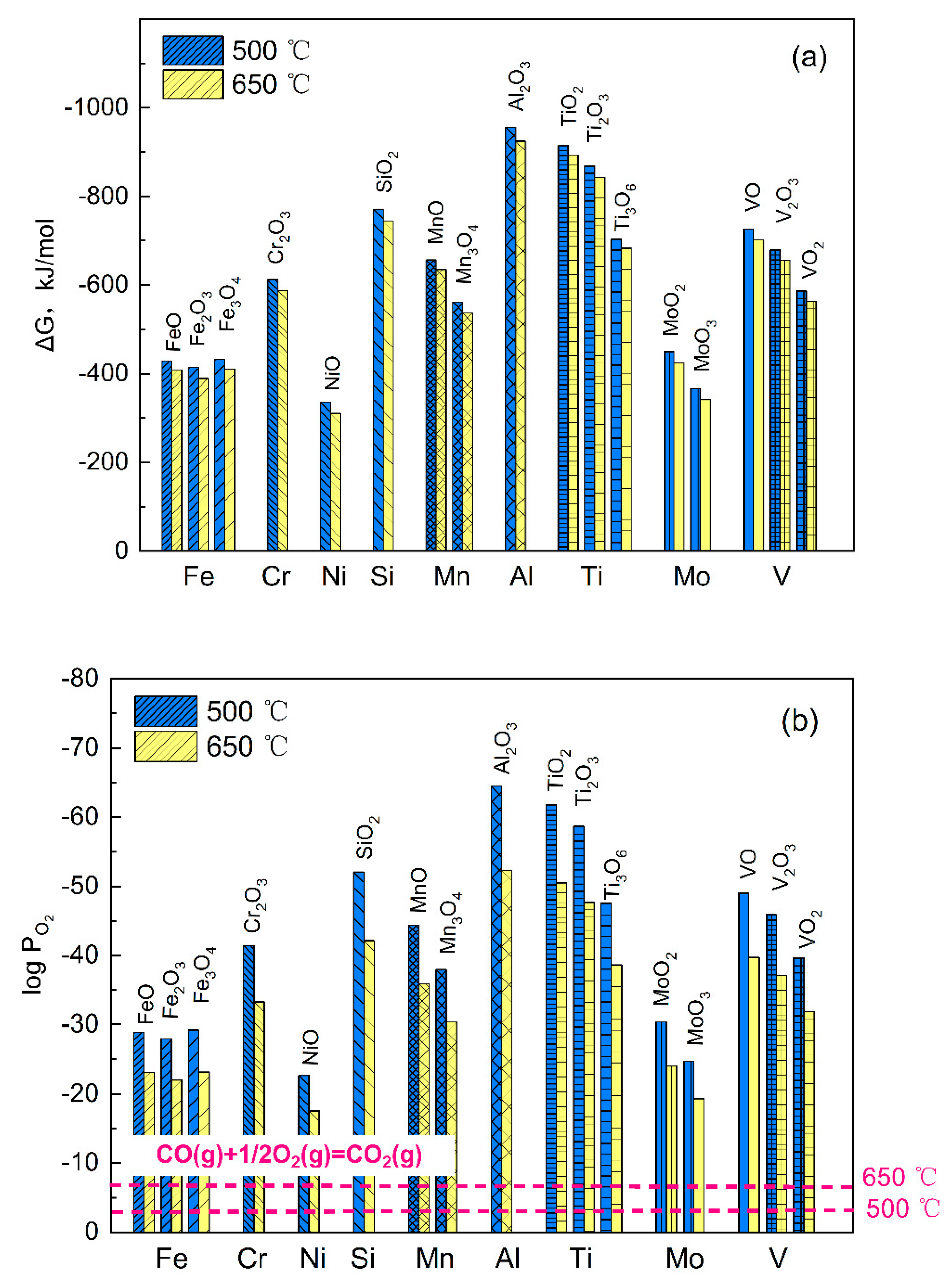
3.1.2. Oxidation of the Alloy
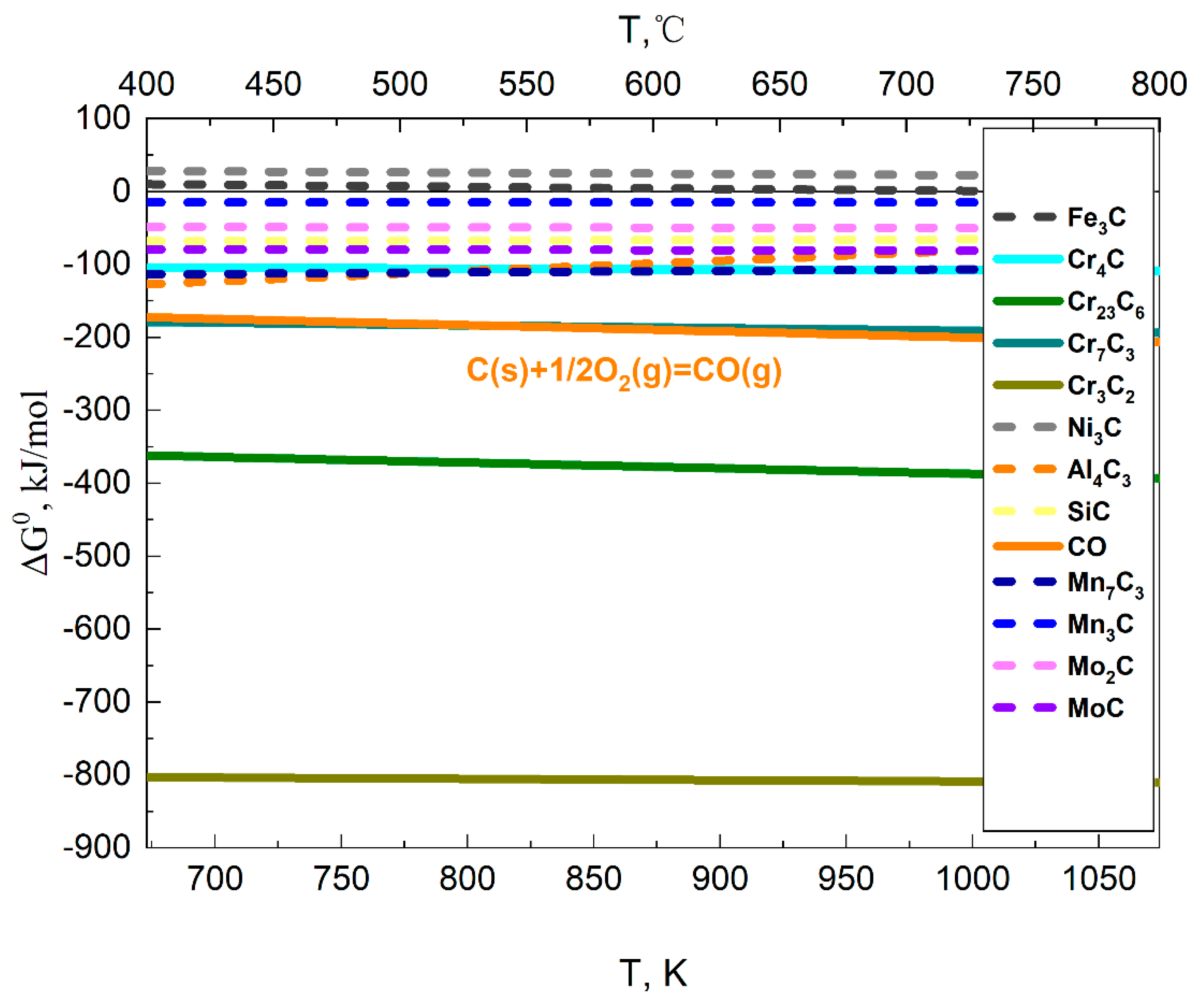
3.2. Thermodynamic Analysis of Alloys Carburization in CO2 Environment
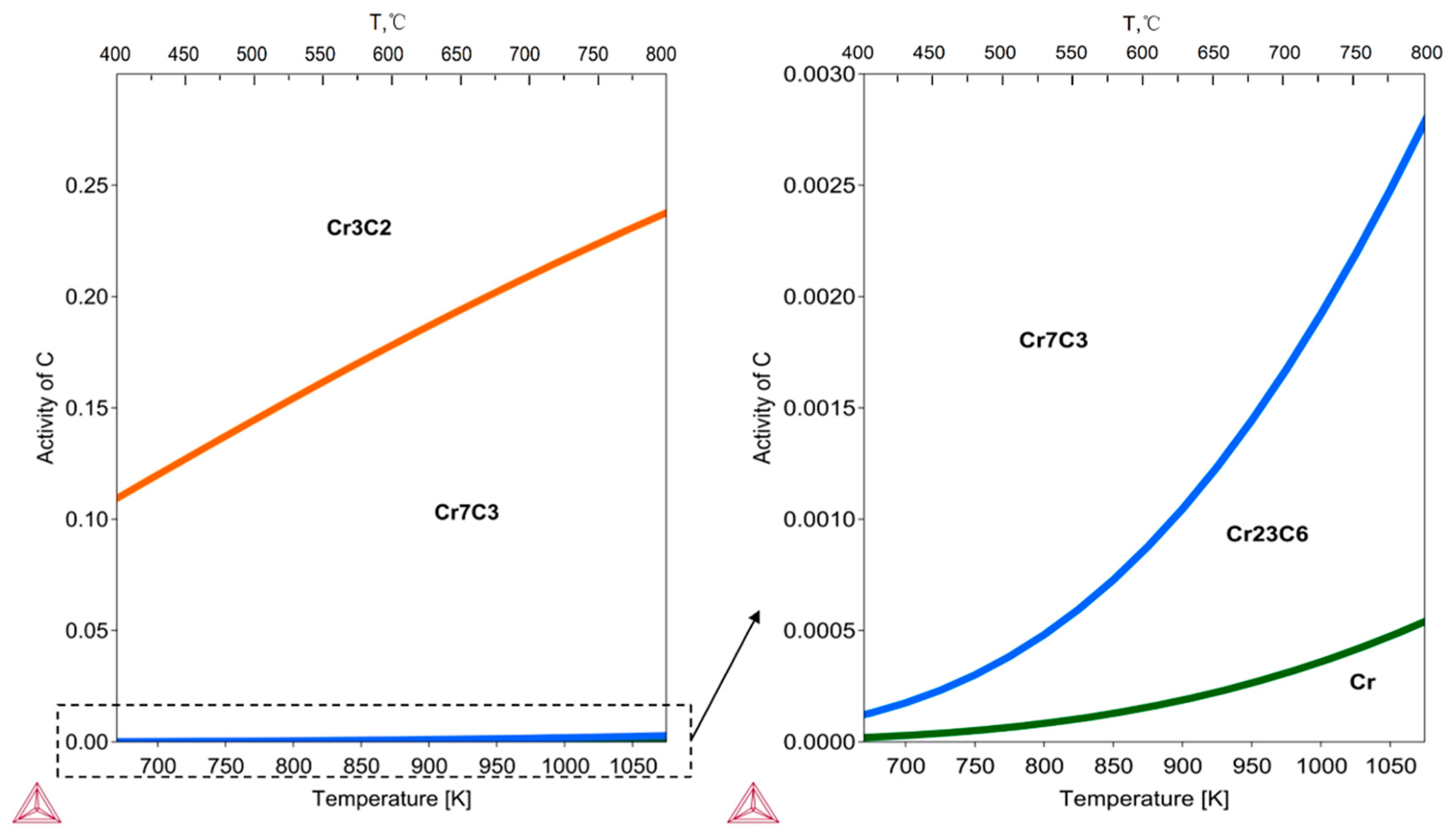
3.2.1. Carbon Activity in the Environment
3.2.2. Carburization of Alloy Materials
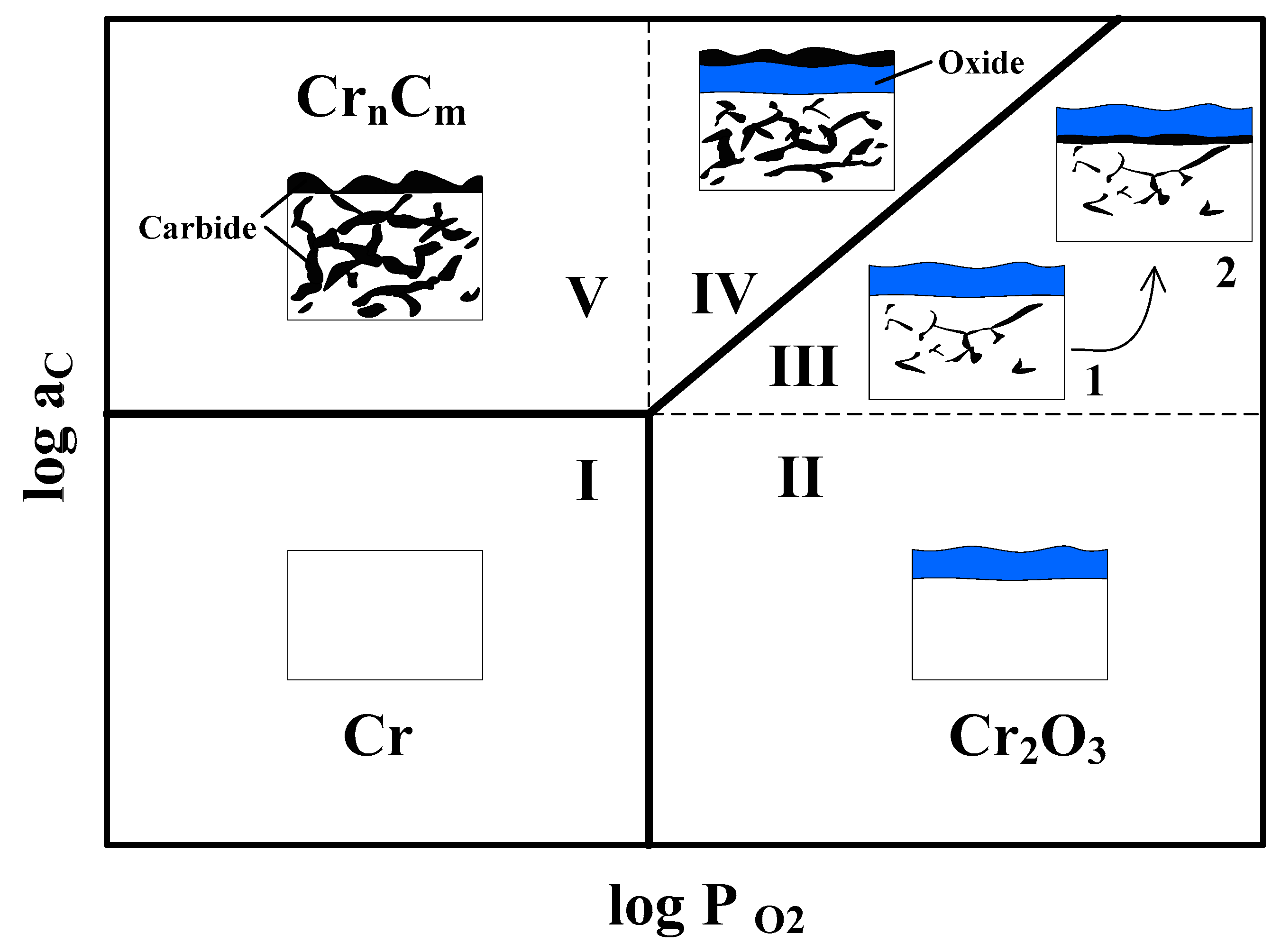
3.3. Decarburization and Carburization Behavior of Alloys
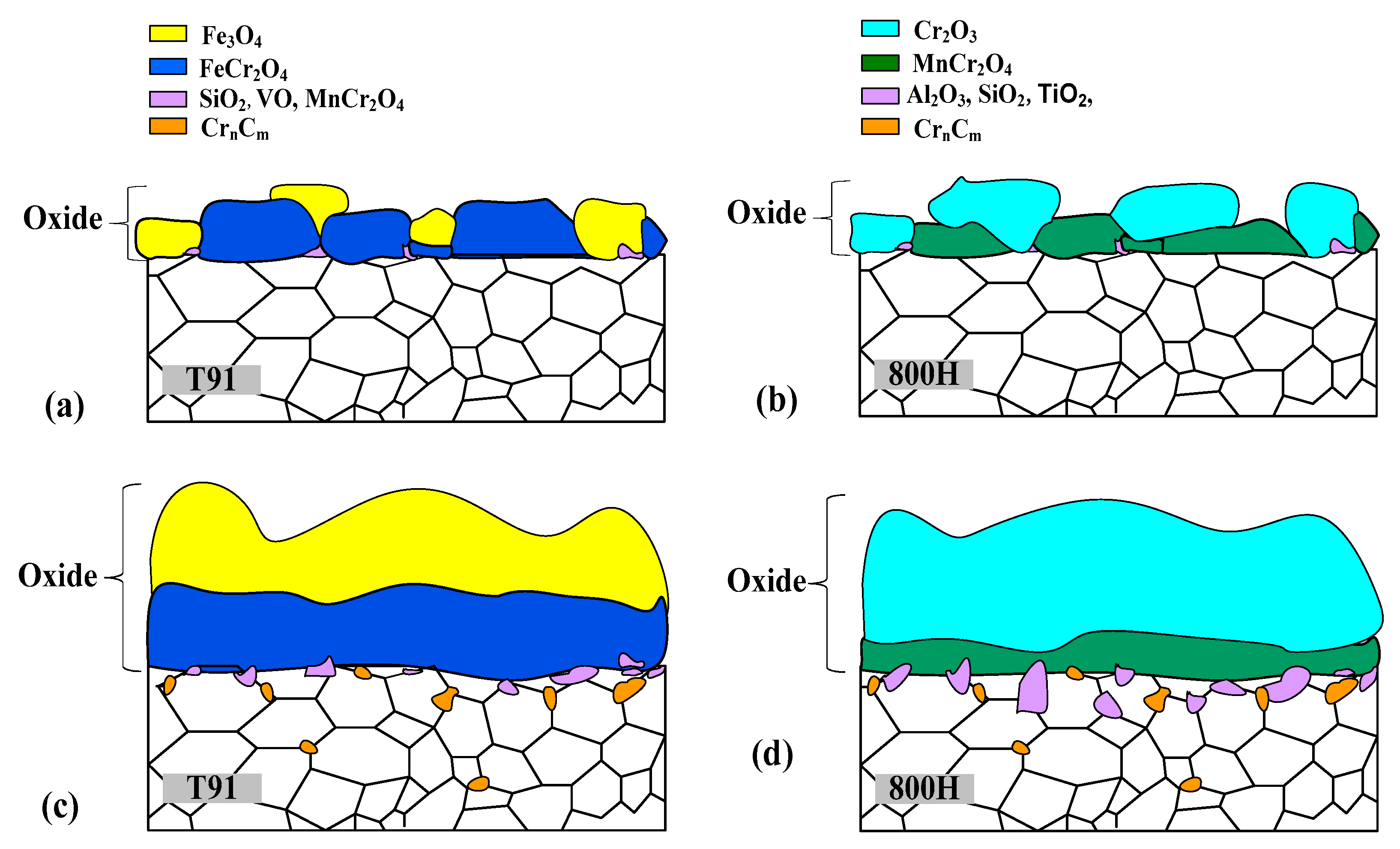
3.4. Oxidation and Carburization Analysis of T91 and 800H
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, S.; Zhang, D.; Li, X.; He, X.; Tian, W.; Qiu, S.; Su, G.H.; Zhao, Q. Superstructure design and optimization on closed Brayton cycle system of fluoride-salt-cooled high-temperature advanced reactor. Appl. Energy 2023, 347, 121404. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Z.; Xiao, B.; Luo, J.-L.; Zhang, N. State of the art overview material degradation in high-temperature supercritical CO2 environments. Prog. Mater. Sci. 2023, 136, 101107. [Google Scholar] [CrossRef]
- Liang, Z.; Gui, Y.; Wang, Y.; Zhao, Q. Corrosion performance of heat-resisting steels and alloys in supercritical carbon dioxide at 650 °C and 15 MPa. Energy 2019, 175, 345–352. [Google Scholar] [CrossRef]
- Teeter, L.; Adam, B.; Wood, T.; Tucker, J.D. Comparison of the corrosion of materials in supercritical carbon dioxide, air, and argon environments. Corros. Sci. 2021, 192, 109752. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.M.; Zheng, D.; Song, G.-L. The localized corrosion of mild steel in carbonated cement pore solution under supercritical carbon-dioxide in a simulated geothermal environment. Constr. Build. Mater. 2021, 274, 122035. [Google Scholar] [CrossRef]
- Gui, Y.; Zhao, Q.; Wang, S.; Liang, Z. Effect of shot-peening on corrosion behavior of austenitic steel in supercritical carbon dioxide at 700 °C. Corros. Sci. 2022, 199, 110180. [Google Scholar] [CrossRef]
- Tan, L.; Anderson, M.; Taylor, D.; Allen, T.R. Corrosion of austenitic and ferritic-martensitic steels exposed to supercritical carbon dioxide. Corros. Sci. 2011, 53, 3273–3280. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Jia, H.; Gao, R.; Ran, M.; Zheng, W.; Zhang, M.; Li, H.; Zhang, J.; Zeng, X.; et al. Understanding the excellent corrosion resistance of Fe-12Cr ODS alloys with and without Si in supercritical CO2 through advanced characterization. Corros. Sci. 2023, 210, 110827. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Gong, B.; Jiang, E.; Huang, Y.; Zhang, G.; Zhao, Y. Corrosion behavior of typical structural steels in 500 °C, 600 °C and high pressure supercritical carbon dioxide conditions. Corros. Sci. 2021, 192, 109801. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Xiao, B.; Khan, H.I.; Xu, H.; Zhang, N. Corrosion behavior of ferritic and ferritic-martensitic steels in supercritical carbon dioxide. Energy 2019, 175, 1075–1084. [Google Scholar] [CrossRef]
- Guo, T.; Xu, Y.; Liu, S.; Liang, Z.; Zhao, Q. Characteristics of the corrosion products on three scratched heat-resisting alloys in closed-loop supercritical and hightemperature CO2. Corros. Sci. 2022, 198, 110148. [Google Scholar] [CrossRef]
- Lee, H.; Subramanian, G.; Kim, S.; Jang, C. Effect of pressure on the corrosion and carburization behavior of chromia-forming heat-resistant alloys in hightemperature carbon dioxide environments. Corros. Sci. 2016, 111, 649–658. [Google Scholar] [CrossRef]
- Mahaffey, J.; Schroeder, A.; Adam, D.; Brittan, A.; Anderson, M.; Couet, A. Effects of CO and O2 Impurities on Supercritical CO2 Corrosion of Alloy 625. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 3703–3714. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.H.; Jang, C. Characterization of Alloy 600 joints exposed to a high-temperature supercritical-carbon dioxide environment. Mater. Charact. 2018, 138, 245–254. [Google Scholar] [CrossRef]
- Subramanian, G.; Lee, H.; Kim, S.; Jang, C. Corrosion and carburization behaviour of Ni-xCr binary alloys in a high-temperature supercritical-carbon dioxide environment. Oxid. Met. 2018, 89, 683–697. [Google Scholar] [CrossRef]
- Adam, B.; Teeter, L.; Mahaffey, J.; Anderson, M.; Árnadóttir, L.; Tucker, J. Effects of corrosion in supercritical CO2 on the microstructural evolution in 800H alloy. Oxid. Met. 2018, 90, 453–468. [Google Scholar] [CrossRef]
- Bidabadi, M.; Chandra-Ambhorn, S.; Rehman, A.; Zheng, Y.; Zhang, C.; Chen, H. Carbon depositions within the oxide scale and its effect on the oxidation behavior of low alloy steel in low (0.1 MPa), sub-(5 MPa) and supercritical (10 MPa) CO2 at 550 °C. Corros. Sci. 2020, 177, 108950. [Google Scholar] [CrossRef]
- Young, D.J. Corrosion by Carbon Dioxide. In High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 495–547. [Google Scholar]
- Turkdogan, E.T. Physical Chemistry of High Temperature Technology; Academic Press: New York, NY, USA, 1980; pp. 5–26. [Google Scholar]
- Rao, Y.K. Stoiochimetry and Thermodynamics of Metallurgical Processes; Cambridge University Press: Cambridge, CA, USA, 1985. [Google Scholar]
- Comrt, H.; Partt, J.N. The standard molar Gibbs free energy of formation of NiO from high-temperature emf measurements. J. Chem. Thermodyn. 1984, 16, 1145–1148. [Google Scholar] [CrossRef]
- Robie, E.A.; Hemingway, B.S. Low-temperature molar heat capacities and entropies of MnO2 (pyrolusite), Mn3O4 (hausmanite), and Mn2O3 (bixbyite). J. Chem. Thermodyn. 1985, 17, 165–181. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo–Calc databank system. CALPHAD Comput. Coupling Phase Diagr. Thermochem. 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Bidabadi, M.H.S.; Zhang, C.; Chen, H.; Yang, Z.-G. Temperature dependence of carbon deposits within oxide scale on CrMoV steel in atmospheric and supercritical CO2. Corros. Sci. 2022, 195, 109979. [Google Scholar] [CrossRef]
- Firouzdor, V.; Sridharan, K.; Cao, G.; Anderson, M.; Allen, T.R. Corrosion of a steel and nickel-based alloys in high temperature supercritical carbon dioxide environment. Corros. Sci. 2013, 69, 281–291. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Li, L.; Cheng, J.; Su, H.; Zhang, L. Revealing the long-term oxidation and carburization mechanism of 310S SS and Alloy 800H exposed to supercritical carbon dioxide. Mater. Charact. 2022, 183, 111603. [Google Scholar] [CrossRef]
- Olivares, R.I.; Young, D.J.; Marvig, P.; Stein, W. Alloys SS316 and Hastelloy-C276 in Supercritical CO2 at High Temperature. Oxid. Met. 2015, 84, 585–606. [Google Scholar] [CrossRef]
- Pint, B.A.; Su, Y.F.; Lance, M.J.; Pillai, R.; Keiser, J.R. Internal carburization and scale formation on austenitic steels in supercritical carbon dioxide. Mater. High. Temp. 2023, 40, 308–317. [Google Scholar] [CrossRef]
- Cao, G.; Firouzdor, V.; Sridharan, K.; Anderson, M.; Allen, T.R. Corrosion of austenitic alloys in high temperature supercritical carbon dioxide. Corros. Sci. 2012, 60, 246–255. [Google Scholar] [CrossRef]
- He, L.-F.; Roman, P.; Leng, B.; Sridharan, K.; Anderson, M.; Allen, T.R. Corrosion behavior of an alumina forming austenitic steel exposed to supercritical carbon dioxide. Corros. Sci. 2014, 82, 67–76. [Google Scholar] [CrossRef]
- Furukawa, T.; Inagaki, Y.; Aritomi, M. Compatibility of FBR structural materials with supercritical carbon dioxide. Prog. Nucl. Energy 2011, 53, 1050–1055. [Google Scholar] [CrossRef]
- Gui, Y.; Liang, Z.; Shao, H.; Zhao, Q. Corrosion behavior and lifetime prediction of VM12, Sanicro 25 and Inconel 617 in supercritical carbon dioxide at 600 °C. Corros. Sci. 2020, 175, 108870. [Google Scholar] [CrossRef]
- Gui, Y.; Liang, Z.; Wang, S.; Zhao, Q. Corrosion behavior of T91 tubing in high temperature supercritical carbon dioxide environment. Corros. Sci. 2023, 211, 110857. [Google Scholar] [CrossRef]
- Gheno, T.; Monceau, D.; Zhang, J.; Young, D.J. Carburisation of ferritic Fe–Cr alloys by low carbon activity gases. Corros. Sci. 2011, 53, 2767–2777. [Google Scholar] [CrossRef]
- Bowen, A.W.; Leak, G.M. Diffusion in Bcc Iron Base Alloys. Metall. Trans. 1970, 1, 2767–2773. [Google Scholar] [CrossRef]





| Alloy | Fe | Cr | Ni | C | Si | Mn | Al | Ti | Mo | Co | V | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T91 | 89.39 | 8.52 | 0.07 | 0.09 | 0.29 | 0.41 | <0.015 | - | 0.90 | - | 0.25 | 0.08 |
| 800H | 45.77 | 20.34 | 31.66 | 0.077 | 0.64 | 0.68 | 0.35 | 0.48 | - | - | - | - |
| T, °C | Oxygen Partial Pressure, Pa |
|---|---|
| 450 | 1.27 × 10−6 |
| 500 | 9.55 × 10−6 |
| 550 | 5.61 × 10−5 |
| 600 | 2.69 × 10−4 |
| 650 | 1.09 × 10−3 |
| 700 | 3.81 × 10−3 |
| 750 | 1.18 × 10−2 |
| 800 | 3.29 × 10−2 |
| Oxidation Reaction of Alloy Elements | Gibbs Free Energy of Reaction, J/mol | ||
|---|---|---|---|
| (14a) | [20] | ||
| (14b) | [20] | ||
| (14c) | [20] | ||
| (14d) | [19] | ||
| (14e) | [21] | ||
| (14f) | [20] | ||
| (14g) | [19] | ||
| (14h) | [22] | ||
| (14i) | [22] |
| Oxidation Reaction of Alloy Elements | Gibbs Free Energy of Reaction, J/mol | ||
|---|---|---|---|
| (23a) | [19] | ||
| (23b) | [19] | ||
| (23c) | [19] | ||
| (23d) | [19] | ||
| (23e) | [19] | ||
| (23f) | [19] | ||
| (23g) | [19] | ||
| (23h) | [20] | ||
| (23i) | [20] | ||
| (23j) | [20] | ||
| (23k) | [19] | ||
| (23l) | [19] |
| T, °C | Cr2O3 log10 PO2 | Cr2C3 log10 aC | Cr7C3 log10 aC | Cr23C6 log10 aC |
|---|---|---|---|---|
| 500 | −46.867 | −0.844 | −3.416 | −4.161 |
| 600 | −40.316 | −0.753 | −3.057 | −3.788 |
| 650 | −37.682 | −0.716 | −2.908 | −3.632 |
| Composite Oxide | Chemical Reaction | ΔG, J/mol [19] | 500 °C ΔG, kJ/mol | 650 °C ΔG, kJ/mol |
|---|---|---|---|---|
| FeCr2O4 | −260.588 | −249.699 | ||
| NiFe2O4 | −22.8142 | −23.3797 | ||
| NiCr2O4 | −47.1068 | −45.8468 | ||
| NiAl2O4 | −13.8812 | −15.7637 | ||
| NiSiO3 | −8.3884 | −7.0084 | ||
| MnCr2O4 | −1495500+321.07T * | −1247.31 * | −1199.15 * | |
| MnAl2O4 | −42.4571 | −41.3621 | ||
| MnSiO3 | −34.4837 | −30.7742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J. Thermodynamic Analysis of Typical Alloy Oxidation and Carburization in High-Temperature CO2 Atmosphere. Coatings 2024, 14, 869. https://doi.org/10.3390/coatings14070869
Xiong J. Thermodynamic Analysis of Typical Alloy Oxidation and Carburization in High-Temperature CO2 Atmosphere. Coatings. 2024; 14(7):869. https://doi.org/10.3390/coatings14070869
Chicago/Turabian StyleXiong, Jing. 2024. "Thermodynamic Analysis of Typical Alloy Oxidation and Carburization in High-Temperature CO2 Atmosphere" Coatings 14, no. 7: 869. https://doi.org/10.3390/coatings14070869






